Abstract
An asymmetric synthesis of C14-desmethylene corialactone D is described on the basis of strategic application of a metallacycle-mediated annulative cross-coupling reaction, a Still [2,3]-Wittig rearrangement, and Morken’s hydroxyl-directed diboration reaction. While representing a convenient approach to access novel compositions of matter inspired by the sesquiterpenoid natural product class (including classic natural product synthesis targets including the picrotaxanes and dendrobine), these studies have led to the discovery of natural product-inspired agents that inhibit nerve growth factor (NGF)-mediated neurite outgrowth in PC-12 cells.
Graphical Abstract
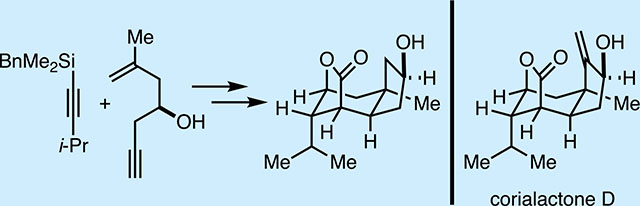
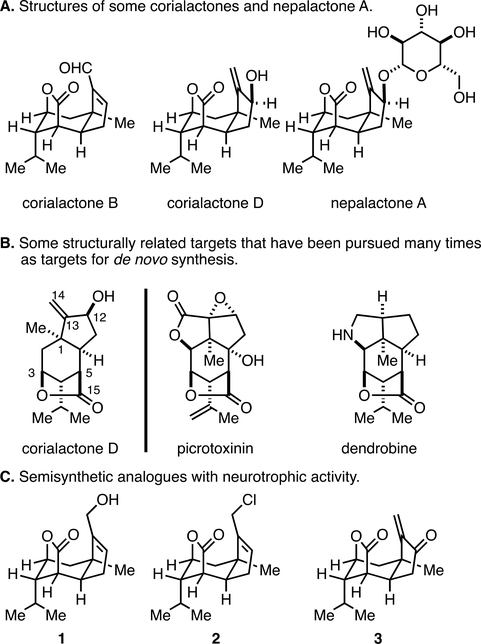
Small molecule modulators of nerve growth factor (NGF) are of great current interest in biology and medicine.1 While neurotrophic agents that promote regeneration and growth of neurons in the presence of NGF are thought to be of value in chronic neurodegenerative disease (conditions marked by axon degeneration and neuronal atrophy) and in acute spinal cord injury,1a,b,2 inhibitors of NGF are currently thought to be of potential value as nonopiate analgesic agents.1c,d As such, rare natural products shown to possess NGF-modulating properties have been the subject of much investigation in laboratories engaged in natural product synthesis efforts, including our own.3 Recently, neurotrophic sesquiterpenoid natural products represented by the corialactones (Figure 1A) have become of interest to our laboratory because of their structural similarity to classic targets in natural product synthesis (Figure 1B),4 and an interest in exploring the value of recently developed synthetic methods in target-oriented synthesis and the biological properties associated with compositions of matter in this class. Corialactones have been found in terrestrial Himalayan shrubs (Coriaria nepalensis) that have been used as a Chinese herbal medicine for the treatment of numbness, toothache, traumatic injury, and acute conjunctivitis, among other ailments.5 Notably, two sesquiterpenes identified from this terrestrial source have been claimed to be clinically useful for the treatment of schizophrenia.5,6 Despite the structural similarity of corialactone sesquiterpene natural products to the potent GABA antagonist picrotoxinin (described as one of the most toxic agents of plant origin)7 and the modestly analgesic alkaloid dendrobine (Figure 1B),7,8 sesquiterpenes from the roots of Coriaria nepalensis appear to have a unique and nontoxic pharmacological profile in vitro at concentrations up to 10 μM [PC-12 (neuro), HCT-8 (colon), HepG2 (liver), BGC-823 (gastric), A549 (lung epithelia), and SKOV3 (ovarian) - MTT assays].5a Piquing our interest in corialactones as synthetic targets, glycosides of sesquiterpenes in this class, as well as semisynthetic variants (1–3; Figure 1A), have been reported as neurotrophic agents, despite a variable nature of C14 (i.e., active agents are reported that contain an allylic alcohol, allylic halide, or an electrophilic enone in this region of the natural product structure).5b Due to this apparent tolerability of disparate functionality at this location of the tricyclic corialactone nucleus, we opted to pursue the construction of novel compositions of matter that simply lack the C14 carbon altogether. These efforts have led to establishing an asymmetric synthesis of C14-desmethylene corialactone D and the discovery of several new natural product-inspired compositions of matter that have distinct and opposite activity to that reported for semisynthetic agents 1–3; they significantly inhibit NGF-promoted nerve outgrowth in PC-12 cells at concentrations as low as 3 μM. The synthetic chemistry that fueled these advances was based on strategic application of an alkoxide-directed metallacycle-mediated annulative cross-coupling reaction,9 a Still [2,3]-Wittig reaction,10 and Morken’s hydroxyl-directed diboration reaction.11
Figure 1.
Introduction to the corialactones and structurally related natural products
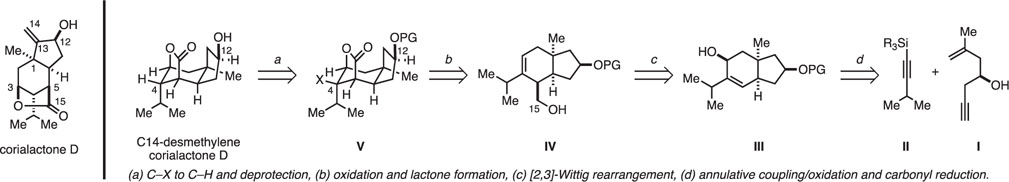
It was reasoned that C14-desmethylene corialactone (Figure 2) could be prepared from an intermediate of general structure V through sequential deprotection of the C12 alcohol and reductive cleavage of the C-X bond at C4. It was then thought that V could be accessed by way of intermediate IV through oxidation of C15 and lactonization. Finally, IV was reasoned to be accessible by Still [2,3]-Wittig rearrangement of the secondary allylic alcohol III, a structure that was reasoned to be derived from a sequence of stereoselective metallacycle-mediated annulative cross-coupling of a suitably silylated alkyne II and the chiral enyne I, a substrate ultimately available through simple functionalization of (R)-(−)-epichlorohydrin.
Figure 2.
Retrosynthetic strategy for C14-desmethylene corialactone D.
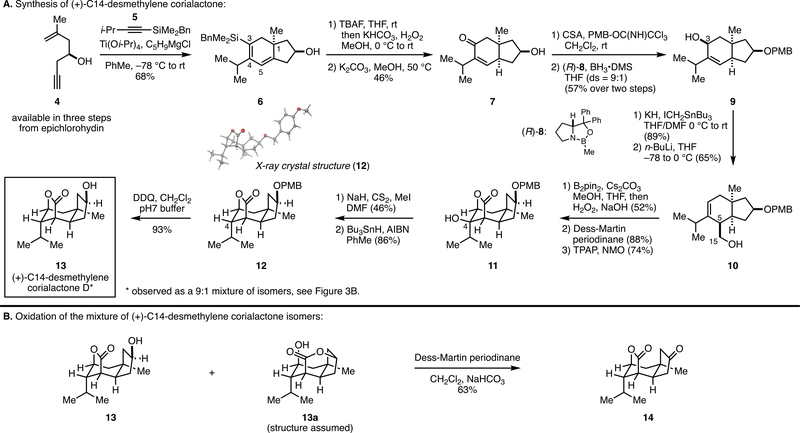
As illustrated in Figure 3A, titanium alkoxide-mediated and hydroxyl-directed coupling of enyne 4 with the silylated alkyne 5 delivered hydrindane 6 as a single regio- and stereoisomer in 68% yield (i.e., BnMe2Si— located at C3, i-Pr at C4, and establishment of the C1 quaternary center).12 Next oxidation of the C—Si bond at C3 and base-mediated equilibration delivered the cis-fused biyclic ketone 7 in a modest 46% yield over the two-step sequence. Protection of the secondary alcohol as its corresponding p-methoxybenzyl (PMB) ether and diastereoselective CBS13 reduction of the C3 ketone then delivered the stereodefined secondary allylic alcohol 9 in 57% yield over two steps (ds = 9:1). Still [2,3]-Wittig rearrangement then proceeded smoothly to deliver the stereodefined homoallylic alcohol 10 as a single stereoisomer.
Figure 3.
Asymmetric synthesis of (+)-C14-desmethylene corialactone D.
A variety of strategies were considered for conversion of 10 to the tricyclic lactone skeleton common to the corialactones, including oxidation to the β,γ-unsaturated carboxylic acid and 5-endo halonium ion-induced lactonization.14 Ultimately, it was found that Morken’s hydroxyl-directed diboration was particularly well suited to convert 10 to an intermediate of value for this synthesis campaign. As depicted, treatment of 10 with bis(pinacolato)diboron and cesium carbonate followed by oxidation with basic hydrogen peroxide delivered the formal product of directed dihydroxylation in 52% yield. Selective oxidation of the primary alcohol at C15 was then accomplished by treatment with Dess—Martin periodinane (88%), and subsequent oxidation of the intermediate lactol with TPAP and NMO delivered the lactone intermediate in 74% yield. Next deoxygenation at C4 of 11 was achieved through standard Barton—McCombie conditions15 to deliver the protected corialactone intermediate 12, the structure of which was confirmed through X-ray crystallography. Finally, deprotection of the PMB ether with DDQ delivered C14-desmethylene corialactone D (13), surprisingly as a 9:1 mixture of isomers. While not reported in the isolation and characterization of corialactone D, we note that compound 13 was formed as a mixture of lactone isomers. Notably, oxidation of this mixture with Dess—Martin periodinane delivered a single tricyclic ketone 14 (Figure 3B).
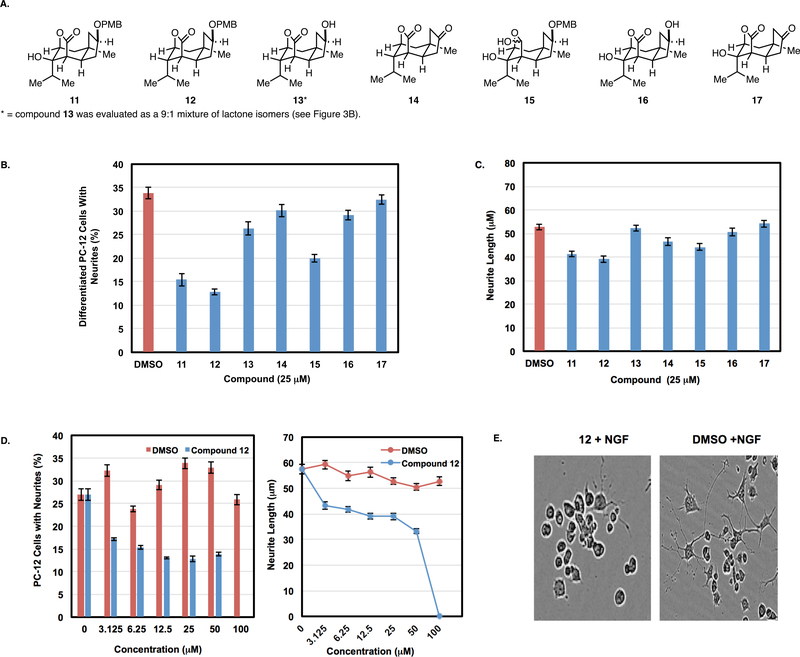
Next, a collection of synthetic agents bearing the central tricyclic lactone resident in the corialactones (Figure 4A) was assembled and advanced for evaluation in vitro. Specifically, an established neuronal cell model16 was employed to investigate the impact that these novel natural product-inspired agents have on nerve growth factor (NGF)-induced neurite outgrowth in PC-12 cells.16 Based on both differentiation (Figure 4B) and neurite length (Figure 4C), compounds 11–17 did not demonstrate NGF-induced neurotrophic properties compared to the vehicle control17 (i.e., these synthetic agents did not enhance NGF-promoted neurite outgrowth). Interestingly, and distinct from reports describing the biological activity of sesquiterpenoids related to the corialactones, all p-methoxybenzyl (PMB)-functionalized compounds 11, 12, and 15 had a significant inhibitory effect on NGF-induced neurite outgrowth. While exposure to 25 μM concentrations of these agents resulted in a significant decrease in number of differentiated cells (i.e., up to a 63% reduction; Figure 4B), by 72 h the small subpopulation of cells that could be observed as having some neurite outgrowth were markedly shorter than vehicle control (decrease in neurite length by 21.8%, 26.2%, and 16.3%; Figure 4C). A second treatment of compounds administered at 72 h further inhibited NGF-induced neurite outgrowth, and at 96 h from initial dosing a complete retraction of neurites was observed in cells treated with compounds 12 and 15 (Supplementary Figure 1A); importantly, none of the tricyclic lactones tested (11–17) had a cytotoxic effect on PC-12 cells at 25 μM after 96 h of treatment (Supplementary Figure 1B).
Figure 4.
Evaluation of synthetic corialactones for NGF-modulating properties: (A) panel of natural product inspired tricyclic lactones prepared by the synthetic route presented in Figure 3. (B, C) Compounds 11–17 inhibit NGF-induced neurotrophic properties. Number of differentiated PC-12 cells (B) and neurite length in PC-12 cells (C) treated with 25 μM of compounds 11–17 or vehicle control DMSO for 72 h in the presence of 50 ng/mL of NGF. (D) NGF-induced neurite outgrowth is inhibited in a dose-dependent manner by 12: PC-12 cells were treated with 12 or DMSO at various concentrations in the presence of 50 ng/mL of NGF. The number of differentiated cells and neurite lengths were measured after 72 h. (E) NGF-induced neurite outgrowth is inhibited by 12: Phase-contrast images of PC-12 cells treated with 3.125 μM of 12 and DMSO in the presence of 50 ng/mL of NGF.
Further analyses of 12 revealed dose-dependent inhibition of NGF-induced neurite outgrowth. As illustrated in Figures 4D,E, concentrations as low as 3.1 μM of 12 result in a substantial decrease in the number of NGF-induced differentiated cells and length of neurites observed. Importantly, these actions are not due to a cytotoxic effect, as toxicity was not observed even at concentrations up to 100 μM (Supplementary Figure 2).
Overall, we report the development of an asymmetric synthesis of C14-desmethylene corialactone that proceeds by way of strategic application of an alkoxide-directed metallacycle-mediated annulative cross-coupling reaction, a Still [2,3]-Wittig rearrangement process, and strategic application of Morken’s hydroxyl-directed diboration reaction. The tricyclic picrotoxane skeleton of the corialactones was forged in 13 steps from the chiral enyne 4, and the synthetic route was employed to access a collection of tricyclic natural product-inspired agents for evaluation as potential modulators of nerve growth factor. While none of the unique compositions of matter prepared and evaluated here were found to have neurotrophic properties, our studies have led to the discovery that the p-methoxybenzyl-substituted species 11, 12, and 15 have activity as inhibitors of NGF-mediated neurite outgrowth in PC-12 cells—a biological profile that is opposite to what has been reported for some corialactone natural products and related semisynthetic agents. Due to the current pharmaceutical interest in developing NGF-inhibitors as nonopioid analgesics and the reality that current clinical agents are biologics (monoclonal antibodies), this discovery of a unique class of natural product-inspired inhibitors of NGF could be of interest for further clinical evaluation.18 Certainly, there remains great uncertainty associated with the mechanism of action of corialactone congeners previously reported as neurotrophic agents, as well as the manner in which the structurally unique tricyclic lactone 12 discovered in this program inhibits NGF-mediated neurite outgrowth in PC-12 cells. We look forward to future advances and discoveries that grow from the foundation of science described here.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from Dartmouth College, Mr. Don Bell, and the National Institutes of Health (GM080266). We also acknowledge the Irradiation, Preclinical Imaging and Microscopy Shared Resource (IPIMSR) in the Norris Cotton Cancer Center at Dartmouth College with NCI Cancer Center Support Grant 5P30 CA023108-37.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b00921.
Procedures and spectroscopic data (PDF)
Cell line data (PDF)
Accession Codes
CCDC 1869261 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UIC; fax: +44 1223 336033.
REFERENCES
- (1).(a) Koliatsos VE; Price DL; Clatterbuck RE; Markowska AL; Olton DS; Wilcox BJ Neurotrophic Strategies for Treating Alzheimer’s Disease: Lessons from Basic Neurobiology and Animal Models. Ann. N. Y. Acad. Sci. 1993, 695, 292–299. [DOI] [PubMed] [Google Scholar]; (b) Akagi M; Matsui N; Akae H; Hirashima N; Fukuishi N; Fukuyama Y; Akagi R Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer’s disease. J. Pharmacol. Sci. 2015, 127, 155–163. [DOI] [PubMed] [Google Scholar]; (c) Chang DS; Hsu E; Hottinger DG; Cohen SP Anti-nerve growth factor in pain management: current evidence. J. Pain Res. 2016, 9, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Seidel MF; Wise BL; Lane NE Nerve growth factor: an update on the science and therapy. Osteoarthr. Cartilage 2013, 21, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Price RD; Milne SA; Sharkey J; Matsuoka N Advances in small molecules promoting neurotrophic function. Pharmacol. Ther. 2007, 115, 292–306. [DOI] [PubMed] [Google Scholar]; (b) Dakas P-Y; Parga JA; Höing S.j; Schöler HR; Sterneckert J; Kumar K; Waldmann H. Discovery of Neuritogenic Compound Classes Inspired by Natural Products. Angew. Chem., Int. Ed. 2013, 52, 9576–9581. [DOI] [PubMed] [Google Scholar]; (c) Burch P; Binaghi M; Scherer M; Wentzel C; Bossert D; Eberhardt L; Neuburger M; Scheiffele P; Gademann K Total Synthesis of Gelsemiol. Chem. - Eur. J. 2013, 19, 2589–2591. [DOI] [PubMed] [Google Scholar]
- (3).Cheng X; Micalizio GC Synthesis of Neurotrophic Seco-prezizaane Sesquiterpenes (1R,10S)-2-Oxo-3,3-dehydroneomajucin, (2S)-Hydroxy-3,4,-dehydroneomajucin, and (−)-Jiadifenin. J. Am. Chem. Soc. 2016, 138, 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Yamada K; Suzuki M; Hayakawa Y; Aoki K; Nakamura H; Nagase H; Hirata Y Total synthesis of (±)-dendrobine. J. Am. Chem. Soc. 1972, 94, 8278–8280. [DOI] [PubMed] [Google Scholar]; (b) Inubushi Y; Kikuchi T; Ibuka T; Tanaka K; Saji I; Tokane K Total synthesis of the alkaloid (±)-dendrobine. J. Chem. Soc., Chem. Commun. 1972, 1252–1253. [Google Scholar]; (c) Inubushi Y; Kikuchi T; Ibuka T; Tanaka K; Saji I; Tokane K Total Synthesis of the Alkaloid (±)-Dendrobine. Chem. Pharm. Bull. 1974, 22, 349–367. [Google Scholar]; (d) Kende AS; Bentley TJ; Mader RA; Ridge D Simple total synthesis of (+)-dendrobine. J. Am. Chem. Soc. 1974, 96, 4332–4334. [DOI] [PubMed] [Google Scholar]; (e) Roush WR Total Synthesis of (±)-Dendrobine. J. Am. Chem. Soc. 1978, 100, 3599–3601. [Google Scholar]; (f) Corey EJ; Pearce HL Total Synthesis of Picrotoxinin. J. Am. Chem. Soc. 1979, 101, 5841–5843. [Google Scholar]; (g) Roush WR Total Synthesis of (±)-Dendrobine. J. Am. Chem. Soc. 1980, 102, 1390–1404. [Google Scholar]; (h) Corey EJ; Pearce HL Total Synthesis of Picrotin. Tetrahedron Lett. 1980, 21, 1823–1824. [Google Scholar]; (i) Tanaka K; Uchiyama F; Sakamoto K; Inubushi Y Stereocontrolled Total Synthesis of (±)-Coriamyrtin. J. Am. Chem. Soc. 1982, 104, 4965–4967. [Google Scholar]; (j) Tanaka K; Uchiyama F; Sakamoto K; Inubushi Y Synthetic Studies on a Picrotoxane Sesquiterpene, Coriamyrtin. III. Completion of the Stereocontrolled Total Synthesis of (±)-Coriamyrtin. Chem. Pharm. Bull. 1983, 31, 1972–1979. [Google Scholar]; (k) Niwa H; Wakamatsu K; Hida T; Niiyama K; Kigoshi H; Yamada M; Nagase H; Suzuki M; Yamada K Stereocontrolled Total Synthesis of (−)-Picrotoxinin and (+)-Coriamyrtin via a Common Isotwistane Intermediate. J. Am. Chem. Soc. 1984, 106, 4547–4552. [Google Scholar]; (l) Connolly PJ; Heathcock CH An Approach to the Total Synthesis of Dendrobine. J. Org. Chem. 1985, 50, 4135–4144. [Google Scholar]; (m) Wakamatsu K; Kigoshi H; Niiyama K; Niwa H; Yamada K Stereocontrolled Total Syntheses of (+)-Tutin and (+)-Asteromurin A, Toxic Picrotoxane Sesquiterpenes. Tetrahedron 1986, 42, 5551–5558. [Google Scholar]; (n) Miyashita M; Suzuki T; Yoshikoshi A Stereoselective Total Synthesis of (−)-Picrotoxinin and (−)-Picrotin. J. Am. Chem. Soc. 1989, 111, 3728–3734. [Google Scholar]; (o) Martin SF; Li W Application of Intramolecular Diels-Alder Reactions to Alkaloid Synthesis. A Formal Total Synthesis of (±)-Dendrobine. J. Org. Chem. 1991, 56, 642–650. [Google Scholar]; (p) Lee CH; Westling M; Livinghouse T; Williams AC Acylnitrilium Ion Initiated Heteroannulations in Alkaloid Synthesis. An Efficient, Stereocontrolled, Total Synthesis of the Orchidaceae Alkaloid (±)-Denrobine. J. Am. Chem. Soc. 1992, 114, 4089–4095. [Google Scholar]; (q) Mori M; Uesaka N; Shibasaki M Novel Synthesis of Nitrogen Heterocycles Using Zirconium-Promoted Reductive Coupling. Formal Total Synthesis of Dendrobine. J. Org. Chem. 1992, 57, 3519–3521. [Google Scholar]; (r) Mori M; Saitoh F; Uesaka N; Shibasaki M Formal Total Synthesis of (−)-Dendrobine Using Zirconium Promoted Cyclization. Determination of the Absolute Configuration of the Intermediary Tricyclic Ketone. Chem. Lett. 1993, 22, 213–216. [Google Scholar]; (s) Uesaka N; Saitoh F; Mori M; Shibasaki M; Okamura K; Date T Formal Total Synthesis of (−)-Dendrobine Using Zirconium-Promoted Reductive Cyclization. J. Org. Chem. 1994, 59, 5633–5642. [Google Scholar]; (t) Sha C-K; Chiu R-T; Yang C-F; Yao N-T; Tseng W-H; Liao F-L; Wang S-L Total Synthesis of (−)-Dendrobine via α-Carbonyl Radical Cyclization. J. Am. Chem. Soc. 1997, 119, 4130–4135. [Google Scholar]; (u) Trost BM; Krische MJ Palladium-Catalyzed Enyne Cycloisomerization Reaction in an Asymmetric Approach to the Picrotoxane Sesquiterpenes. 2. Second-Generation Total Syntheses of Coranin, Picrotoxinin, Picrotin, and Methyl Picrotoxate. J. Am. Chem. Soc. 1999, 121, 6131–6142. [Google Scholar]; (v) Trost BM; Haffner CD; Jebaratnam DJ; Krische MJ; Thomas AP The Palladium-Catalyzed Enyne Cycloisomerization Reaction in a General Approach to the Asymmetric Syntheses of the Picrotoxane Sesquiterpenes. Part I. First-Generation Total Synthesis of Corianin and Formal Syntheses of Picrotoxinin and Picrotin. J. Am. Chem. Soc. 1999, 121, 6183–6192. [Google Scholar]; (w) Kreis LM; Carreira EM Total Synthesis of (−)-Dendrobine. Angew. Chem., Int. Ed. 2012, 51, 3436–3439. [DOI] [PubMed] [Google Scholar]; (x) Guo L; Frey W; Plietker B Catalytic Enantioselective Total Synthesis of the Picrotoxane Alkaloids (−)-Dendrobine, (−)-Mubironine B, and (−)-Dendroxine. Org. Lett. 2018, 20, 4328–4331. [DOI] [PubMed] [Google Scholar]
- (5).(a) Zhao F; Liu Y-B; Ma S-G; Qu J; Yu S-S; Fang Z-F; Li L; Si Y-K; Zhang J-J New sesquiterpenes from the roots of Coriaria nepalensis. Tetrahedron 2012, 68, 6204–6210. [Google Scholar]; (b) Wang Y-Y; Tian J-M; Zhang C-C; Luo B; Gao J-M Picrotoxane Sesquiterpene Glycosides and a Coumarin Derivative from Coriaria nepalensis and Their Neurotrophic Activity. Molecules 2016, 21, 1344. Notably, the enone-containing semisynthetic variant reported in ref 5b was described as being toxic to PC-12 cells at 20 μM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Okuda T; Yoshida T; Chen X-M; Xie J-X; Fukushima M Corianin from Coriaria japonica A. GRAY, and Sesquiterpene Lactones from Loranthus parasiticus MERR. Used for Treatment of Schizophrenia. Chem. Pharm. Bull. 1987, 35, 182–187. [DOI] [PubMed] [Google Scholar]
- (7).For a review of picrotoxinin and related substances, see: Porter LE Picrotoxinin and Related Substances. Chem. Rev. 1967, 67, 441–464. [DOI] [PubMed] [Google Scholar]
- (8).Chen KK; Chen AL The Pharmacological Action of Denrobine. The Alkaloid of Chin-Shih-Hu. J. Pharmacol. Exp. Ther. 1935, 55, 319–325. [Google Scholar]
- (9).Greszler SN; Reichard HA; Micalizio GC Asymmetric Synthesis of Dihydroindanes by Convergent Alkoxide-Directed Metallacycle-Mediated Bond Formation. J. Am. Chem. Soc. 2012, 134, 2766–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Still WC; Mitra A A Highly Stereoselective Synthesis of Z-Trisubstituted Olefins via [2,3]-Sigmatropic Rearrangement. Preference for a Pseudoaxially Substituted Transition State. J. Am. Chem. Soc. 1978, 100, 1927–1928. [Google Scholar]
- (11).Blaisdell TP; Caya TC; Zhang L; Sanz-Marco A; Morken JP Hydroxyl-Directed Stereoselective Diboration of Alkenes. J. Am. Chem. Soc. 2014, 136, 9264–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Micalizio GC; Mizoguchi H The Development of Alkoxide-Directed Metallacycle-Mediated Annulative Cross-Coupling Chemistry. Isr. J. Chem. 2017, 57, 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Corey EJ; Bakshi RK; Shibata S Highly Enantioselective Borane Reduction of Ketones Catalyzed by Chiral Oxazaborolidines. Mechanism and Syntehtic Implications. J. Am. Chem. Soc. 1987, 109, 5551–5553. [Google Scholar]; (b) Corey EJ; Helal CJ Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem., Int. Ed. 1998, 37, 1986–2012. [DOI] [PubMed] [Google Scholar]
- (14).For an example of 5-endo halo-lactonization, see:; (a) Schultz A; Kirincich S Asymmetric Total Synthesis of the Caribbean Fruit Fly Pheromone (+)-Epianastrephin. J. Org. Chem. 1996, 61, 5626–5630. For reported challenges associated with the control of regioselection in hydroboration reactions of homoallylic alcohols bearing a similarly substituted trisubstituted alkene, see: [Google Scholar]; (b) Paquette LA; Peng X; Yang J; Kang H-J The Carbohydrate—Sesquiterpene Interface. Directed Synthetic Routes to Both (+)- and (—)-Fomannosin from D-Glucose. J. Org. Chem. 2008, 73, 4548–4558. [DOI] [PubMed] [Google Scholar]; (c) Funk RL; Bolton GL; Daggett JU; Hansen MM; Horcher LHM Intramolecular Cycloaddition Reactions of Exocyclic Nitrones. Tetrahedron 1985, 41, 3479–3495. [Google Scholar]
- (15).(a) Barton DHR; McCombie SW A New Method for the Deoxygenation of Secondary Alcohols. J. Chem. Soc., Perkin Trans 11975, 1574–1585. For a procedure that is catalytic in BusSnH, see: [Google Scholar]; (b) Lopez RM; Hays DS; Fu GC Bu3SnH-Catalyzed Barton—McCombie Deoxygenation of Alcohols. J. Am. Chem. Soc. 1997, 119, 6949–6950. [Google Scholar]
- (16).Harrill JA; Mundy WR Quantitative Assessment of Neurite Outgrowth in PC12 Cells. Methods Mol. Biol. 2011, 758, 331–348. [DOI] [PubMed] [Google Scholar]
- (17).For the control, dimethyl sulfoxide (DMSO) was used at final volumes that were identical to those used to attain all concentrations tested.
- (18).For examples of structurally unrelated small molecules and natural products that have been shown to inhibit NGF, see:; (a) Owolabi JB; Rizkalla G; Tehim A; Ross GM; Riopelle RJ; Kamboj R; Ossipov M; Bian D; Wegert S; Porreca F; Lee DKH Characterization of Antiallodynic Actions of ALE-0540, a Novel Nerve Growth Factor Receptor Antagonist, in the Rat. J. Pharmacol. Exp. Ther. 1999, 289, 1271–1276. [PubMed] [Google Scholar]; (b) Koizumi S; Contreras ML; Matsuda Y; Hama T; Lazarovici P; Guroff G K-252a: A Specific Inhibitor of the Action of Nerve Growth Facton on PC 12 Cells. J. Neurosci. 1988, 8, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Miller RE; Malfait A-M; Block JA Current Status of Nerve Growth Factor Antibodies for the Treatment of Osteoarthritis Pain. Clin. Exp. Rheumatol. 2017, 35 (Suppl 107), 85–87. [PMC free article] [PubMed] [Google Scholar]; (d) Liu L; Shi X-W; Zong S-C; Tang J-J; Gao J-M Scabronine M, a novel inhibitor of NGF-induced neurite outgrowth from PC12 cells from the fungus Sarcodon scabrosus. Bioorg. Med. Chem. Lett. 2012, 22, 2401–2406. [DOI] [PubMed] [Google Scholar]; (e) Kennedy AE; Laamanen CA; Ross MS; Vohra R; Boreham DR; Scott JA; Ross GM Nerve Growth Factor Inhibitor with Novel-Binding Domain Demonstrates Nanomolar Efficacy in Both Cell-Based and Cell-Free Assay Systems. Pharmacol. Res. Perspect. 2017, 5, No. e00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.