Abstract
Some animals have evolved chemical weapons to deter predators. Bombardier beetles (Coleoptera: Carabidae: Brachininae: Brachinini) can eject toxic chemicals at temperatures of 100 °C from the tips of their abdomens, ‘bombing’ the attackers. Although some bombardier beetles can reportedly deter predators, few studies have tested whether bombing is essential for successful defence. Praying mantises (Mantodea) are ambush predators that attack various arthropods. However, it is unclear whether bombardier beetles deter mantises. To test the defensive function of bombing against praying mantises, I observed three mantis species, Tenodera sinensis, Tenodera angustipennis, and Hierodula patellifera (Mantidae), attacking the bombardier beetle Pheropsophus jessoensis (Carabidae: Brachininae: Brachinini) under laboratory conditions. All mantises easily caught the beetles using their raptorial forelegs, but released them immediately after being bombed. All of the counterattacked mantises were observed to groom the body parts sprayed with hot chemicals after releasing the beetles. When treated P. jessoensis that were unable to eject hot chemicals were provided, all mantises successfully caught and devoured the treated beetles. Therefore, bombing is essential for the successful defence of P. jessoensis against praying mantises. Consequently, P. jessoensis can always deter mantises.
Keywords: Bombardier beetles, Brachinini, Carabidae, Chemical defences, Mantodea, Predator, Prey
Introduction
Prey animals escape from predators in various ways (Edmunds, 1974; Sugiura, 2020a). Prey must evade predators at a stage in the predator behavioural sequence ‘encounter’, ‘detection’, ‘identification’, ‘approach’, ‘subjugation’, and ‘consumption’ (Endler, 1991). Many studies have shown that prey can evade predators before subjugation (Edmunds, 1974; Ruxton, Sherratt & Speed, 2004), and recent studies have indicated that prey can escape predators after subjugation (Umbers & Mappes, 2015; Sugiura & Sato, 2018; Sugiura, 2020a, 2020b).
Many animal species have evolved chemical weapons to defend themselves against predators (Eisner, 1970; Eisner, Eisner & Siegler, 2005). For example, some insects produce or sequester toxic chemicals that prevent predators from swallowing them (Eisner, 1970; Nishida, 2002; Eisner, Eisner & Siegler, 2005). In many chemically defended prey, contact with a predator triggers the ejection of defensive chemicals (Eisner, 2003; Jones & Bulbert, 2020). Because toxic chemicals can damage the predator digestive systems (Whitman & Vincent, 2008; Perelman & Chikatunov, 2010; Sugiura & Sato, 2018), many predators reject the chemically defended prey before swallowing them (Dean, 1980a; Taniguchi et al., 2005; Whitman & Vincent, 2008; Matsubara & Sugiura, 2017; Sugiura, 2018). However, predators with toxin tolerance can eat the chemically defended prey (Dean, 1980a; Fink & Brower, 1981; Sugiura & Sato, 2018). Therefore, the effectiveness of chemical defences depends on the predator species and individuals (Sugiura & Sato, 2018).
Adults of the beetle tribe Brachinini Bonelli (Coleoptera: Carabidae) can eject toxic chemicals at temperatures of approximately 100 °C (i.e., bombing) from the tips of their abdomens in response to a predator attack (Aneshansley et al., 1969; Dean, 1979; Eisner, 2003; Eisner, Eisner & Siegler, 2005; Arndt et al., 2015). Beetles of the subfamily Brachininae Bonelli, which comprises the tribes Brachinini (645 species in 8 genera) and Crepidogastrini Basilewsky (123 species in 6 genera), are called ‘bombardier beetles’ (Arndt, Beutel & Will, 2016; Anichtchenko et al., 2021). Bombardier beetles of the tribe Brachinini store hydroquinone and hydrogen peroxide separately in two reservoirs in the abdomen (Eisner, 2003). When the aqueous solutions of hydroquinones and hydrogen peroxide reach the reaction chamber from each reservoir, enzymes (catalysts) facilitate oxidation of the hydroquinones and decomposition of the hydrogen peroxide (Eisner, 2003). An explosive reaction ejects the reactants and boiling water. Some bombardier beetles can aim the hot chemicals in virtually any direction (Eisner & Aneshansley, 1999). Although many studies have investigated whether bombardier beetles can defend against predators (Eisner, 1958, 2003; Eisner & Meinwald, 1966; Eisner & Dean, 1976; Dean, 1980a; Conner & Eisner, 1983; Nowicki & Eisner, 1983; Eisner, Eisner & Aneshansley, 2005; Eisner et al., 2006; Sugiura & Sato, 2018; Sugiura, 2018; Kojima & Yamamoto, 2020), only a few studies have demonstrated that bombing is essential for the successful defence of bombardier beetles against predators (Sugiura & Sato, 2018). To test the effectiveness of bombing, it is necessary to use control beetles that can eject hot chemicals and treated beetles that cannot (Sugiura & Sato, 2018). Clarifying the importance of bombing would contribute to understanding the evolution of chemical defence mechanisms in bombardier beetles.
Bombardier beetles can successfully defend themselves against insectivorous animals such as toads, birds, and arthropods (Eisner, 1958, 2003; Eisner & Meinwald, 1966; Eisner & Dean, 1976; Dean, 1980a; Conner & Eisner, 1983; Eisner et al., 2006; Sugiura & Sato, 2018; Sugiura, 2018; Kojima & Yamamoto, 2020). Individuals of some vertebrate species are able to consume bombardier beetles (Dean, 1980a; Sugiura, 2018; Sugiura & Sato, 2018; Kojima & Yamamoto, 2020), whereas several invertebrate species such as spiders always reject them (Eisner & Dean, 1976; Eisner et al., 2006). Therefore, bombardier beetles may deter invertebrate predators more effectively than vertebrate predators. Testing this hypothesis would allow us to identify the types of predators that impose selective pressure on the evolution of anti-predator defences in bombardier beetles.
The bombardier beetle Pheropsophus jessoensis Morawitz (Brachininae: Brachinini) is common in farmland, grassland, and forest edges in East Asia, including Japan (Habu & Sadanaga, 1965; Ueno, Kurosawa & Sato, 1985; Yahiro et al., 1992; Ishitani & Yano, 1994; Fujisawa, Lee & Ishii, 2012; Ohwaki, Kaneko & Ikeda, 2015), South Korea (Jung et al., 2012), and China (Li et al., 2012). Like other bombardier species, P. jessoensis discharges quinones (1,4-benzoquinone and 2-methyl-1,4-benzoquinone) at a temperature of approximately 100 °C when stimulated (Video S1; Kanehisa & Murase, 1977; Kanehisa, 1996). Studies have tested how P. jessoensis can defend against toads (Sugiura & Sato, 2018), frogs (Sugiura, 2018), and birds (Kojima & Yamamoto, 2020). Adult P. jessoensis were easily swallowed by the toads Bufo japonicus Temminck & Schlegel and Bufo torrenticola Matsui (Sugiura & Sato, 2018). However, the swallowed P. jessoensis ejected chemicals inside the toad bodies causing 34.8% of the B. japonicus and 57.1% of the B. torrenticola to vomit 12–94 and 15–107 min after being swallowed, respectively (Sugiura & Sato, 2018). Sugiura & Sato (2018) used treated P. jessoensis that could not eject hot chemicals to show that bombing is essential for the successful escape of P. jessoensis from toads. Adult P. jessoensis were also rejected by the pond frog Pelophylax nigromaculatus (Hallowell) (Anura: Ranidae) (Sugiura, 2018) and quail Coturnix japonica Temminck & Schlegel (Galliformes: Phasianidae) (Kojima & Yamamoto, 2020). However, most of the frogs and quails rejected P. jessoensis adults before being bombed, suggesting that bombing is not essential for the successful defence of P. jessoensis against attacks by frogs and quails (Sugiura, 2018; Kojima & Yamamoto, 2020). Therefore, adult P. jessoensis can effectively defend themselves against vertebrate predators. However, the effectiveness of P. jessoensis defences against invertebrate predators remains unexplored.
In this study, I investigated the defence of P. jessoensis against praying mantises (Insecta: Mantodea) under laboratory conditions. Praying mantises are sit-and-wait (ambush) predators that attack various arthropods (Reitze & Nentwig, 1991) and small vertebrates (Nyffeler, Maxwell & Remsen, 2017; Valdez, 2020). Mantises recognise prey by movement and catch them using their raptorial forelegs (Rilling, Mittelstaedt & Roeder, 1959; Corrette, 1990). Mantises have powerful mouthparts and can devour tough prey (Reitze & Nentwig, 1991). Although praying mantises have been used to investigate the effectiveness of anti-predator defences in many insect species (Berenbaum & Miliczky, 1984; Reitze & Nentwig, 1991; Honma, Oku & Nishida, 2006; Whitman & Vincent, 2008; Rafter, Agrawal & Preisser, 2013; Mebs, Yotsu-Yamashita & Arakawa, 2016; Mebs et al., 2017; Rafter et al., 2017a, 2017b; Mebs, Wunder & Toennes, 2019; Prudic et al., 2019), only one study has used the mantis as a model predator of bombardier beetles. Eisner (1958) provided an adult female mantis [Hierodula patellifera (Audinet-Serville) (Mantidae)] with three adult bombardier beetles (Brachinus tenuicollis LeConte) under laboratory conditions; two of the three beetles successfully defended themselves against the mantis, while the mantis ate the third. Because the sample size was very small, the defence effectiveness of bombardier beetles against mantises remains unclear. To test whether bombardier beetles can effectively defend themselves against praying mantises, I quantified the defensive behaviour of the bombardier beetle P. jessoensis against three mantis species: Tenodera sinensis Saussure, Tenodera angustipennis Saussure, and H. patellifera (all Mantidae). I tested whether bombing is essential for the successful defence of P. jessoensis against a mantis attack experimentally.
Materials and Methods
Study organisms
I collected 60 adult P. jessoensis from grasslands and forest edges in the Kinki region (Hyogo and Shiga Prefectures) of Japan, in May 2018, May–September 2019, and July–September 2020 (cf. Sugiura & Sato, 2018; Sugiura, 2018). The beetles were kept individually in plastic cases (diameter 85 mm; height 25 mm) with wet tissue paper under laboratory conditions (25 ± 1 °C; Sugiura & Sato, 2018; Sugiura, 2018). Dead Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) larvae were provided as food (Sugiura & Sato, 2018; Sugiura, 2018). Before the experiments, I weighed the beetles to the closest 0.1 mg using an electronic balance (PA64JP; Ohaus, Tokyo, Japan) and measured the body length to the closest 0.01 mm using slide callipers. Beetles were not used repeatedly in different feeding experiments. I conducted the following experiments 57.1 ± 5.0 (range: 5–162) days after collecting the beetles.
I also collected 60 adult mantises (Tenodera sinensis, 10 males, 10 females; Tenodera angustipennis, 7 males, 13 females; Hierodula patellifera, 20 females) from grasslands and forest edges in the Kinki region (Hyogo, Osaka, Japan and Shiga Prefectures) in October 2018, August–October 2019, and September–November 2020 (Sugiura et al., 2019; Sakagami, Funamoto & Sugiura, 2021). In Japan, adult T. sinensis and T. angustipennis are common on grasses and herbs at grasslands and forest edges (Watanabe, Miyamoto & Yano, 2013; Sakagami, Funamoto & Sugiura, 2021) where the bombardier beetle P. jessoensis is also found. Although H. patellifera adults are also found at forest edges where P. jessoensis is abundant, this mantis species is arboreal (Watanabe & Yano, 2009; Sakagami, Funamoto & Sugiura, 2021). Therefore, the bombardier beetle species P. jessoensis, which walks on the ground below grasses and herbs, potentially encounters T. sinensis and T. angustipennis adults, but not H. patellifera adults under field conditions.
Mantises were kept individually in plastic cases (diameter 100 mm; height 100 mm) with wet tissue paper under laboratory conditions (25 ± 1 °C). Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae) and wild-caught insects (e.g., grasshoppers) were provided as food. The mantises were starved for 24 h before the feeding experiments to standardise their hunger level (cf. Sugiura, 2018). I weighed them to the closest 0.1 mg using an electronic balance (PA64JP; Ohaus, Tokyo, Japan) and measured the body length to the closest 0.01 mm using slide callipers. As with the bombardier beetles, individual mantises were not used repeatedly. I conducted the following experiments 11.3 ± 1.5 (range 1–45) days after I collected the mantises.
Experiments
To test the effectiveness of the anti-predator defences of P. jessoensis against praying mantises, I conducted the behavioural experiments under laboratory conditions (25 ± 1 °C).
First, I placed an adult mantis on a plastic net in a transparent plastic case (length × width × height, 120 × 85 × 130 mm), so that the mantis hung its head down below its legs (Fig. 1). Then, I placed a live P. jessoensis (‘control’ beetle) on the bottom of the case. Mantises that did not respond to the beetle were not used for the experiments. When a mantis displayed attacking behaviour (i.e., shooting out its forelegs to capture the prey), I recorded the behaviour on video using a digital camera (iPhone XS; Apple Inc., Cupertino, CA, USA) at 240 frames per second. If the mantis rejected the beetle after attacking it, I observed whether the mantis reattacked the same beetle within 1 min. Rejected beetles were also checked for injuries. If a mantis started to eat the beetle, I recorded the feeding time. I also weighed any uneaten beetle parts and calculated the percentage of the beetle eaten. In total, 30 control beetles and 30 mantises (10 T. sinensis, 10 T. angustipennis, and 10 H. patellifera) were used in the experiments.
Figure 1.
Experimental arena. Each mantis hung from a plastic net in a transparent plastic case (length × width × height, 120 × 85 × 130 mm) with its head below its legs. Photo credit: Shinji Sugiura.
To test whether the bombing response of P. jessoensis plays an essential role in deterring a mantis, I provided the mantises with treated P. jessoensis that were unable to eject hot chemicals. Following the method of Sugiura & Sato (2018), I repeatedly stimulated an adult P. jessoensis with forceps; the simulated attacks forced them to exhaust their chemicals (i.e., ‘treated’ beetles). Then, I observed whether an adult mantis successfully attacked the treated beetle in a transparent plastic case (length × width × height, 120 × 85 × 130 mm) using the same procedure as for the control beetles. In total, 30 treated beetles and 30 mantises (10 T. sinensis, 10 T. angustipennis, and 10 H. patellifera) were used in the experiments.
All experiments were performed in accordance with Kobe University Animal Experimentation Regulations (Kobe University Animal Care and Use Committee, No. 30–01).
Data analysis
I used Fisher’s exact test to compare reattack rates between mantis males and females and successful escape rates between control and treated P. jessoensis from each mantis species and all mantis species combined. I used Student’s t-test to compare the body sizes of P. jessoensis and mantises between the control and treatment experiments. All analyses were conducted using R ver. 3.5.2 (R Core Team, 2018).
Results
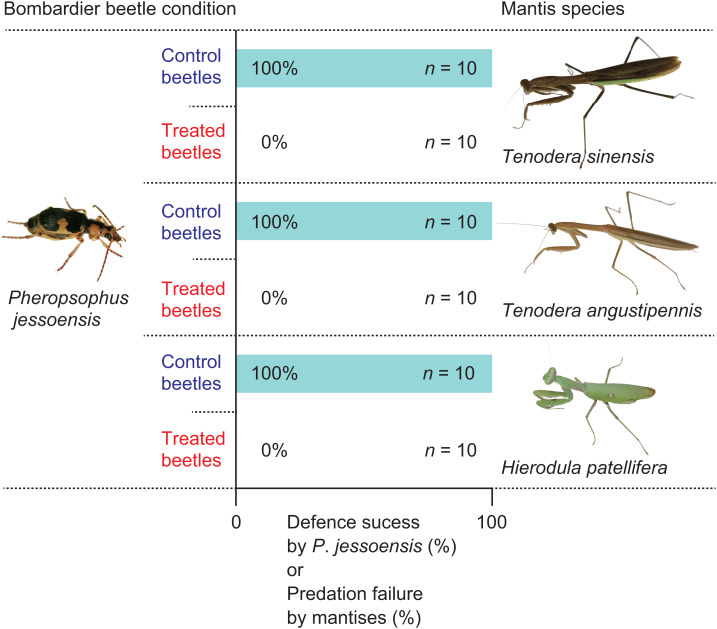
All mantises used their raptorial forelegs to capture P. jessoensis. However, all of the control beetles ejected hot chemicals immediately after being captured and the mantises released the beetles immediately after being bombed (n = 30; Figs. 2 and 3; Video S2). The chemicals ejected by P. jessoensis were sprayed on the head, forelegs, and/or thorax of each mantis. In T. sinensis, 60% of females (n = 5) and 20% of males (n = 5) reattacked P. jessoensis within 1 min after releasing them. In T. angustipennis, 33.3% females (n = 6) and 0% of males (n = 4) reattacked P. jessoensis. In H. patellifera, 20% of females (n = 10) reattacked P. jessoensis. Mantis females reattacked P. jessoensis more frequently than mantis males; however, these differences were not significant (Fisher’s exact test; T. sinensis, P = 0.5238; T. angustipennis, P = 0.4667; T. sinensis plus T. angustipennis, P = 0.1571; all species combined, P = 0.3742). All reattacking mantises rejected P. jessoensis again after being bombed. No mantis successfully preyed on control beetles (Fig. 2). After releasing the beetles, all of the mantises were observed to groom the body parts sprayed with hot chemicals. No released P. jessoensis was injured; all were active (n = 30).
Figure 2.
Defensive success of the bombardier beetle Pheropsophus jessoensis and praying mantis predation failure. Control and treated beetles were P. jessoensis adults that were able and unable to eject defensive chemicals, respectively. Photo credit: Shinji Sugiura.
Figure 3.
Temporal sequence of the mantis Hierodula patellifera attacking a control adult Pheropsophus jessoensis. (A) 0, (B) 50, (C) 75, (D) 125, (E) 250, (F) 325, and (G) 375 ms. (H) Close-up view (F), with the arrow indicating bombing (i.e., the ejected chemicals aimed at the mantis) from the tip of abdomen of the adult P. jessoensis. The mantis caught the beetle with its raptorial forelegs, but released it immediately after being bombed (see Video S2). Photo credit: Shinji Sugiura.
When treated P. jessoensis that were unable to eject hot chemicals were provided, all the mantises successfully caught the treated beetles using their raptorial forelegs (Video S3). All of the mantises devoured the treated beetles (n = 30; Figs. 2 and 4; Video S3). The mantises consumed 90.5% of the body (mainly the thorax and abdomen) of treated P. jessoensis, while parts of the elytra, legs, and antennae were not eaten (Table 1; Fig. 4). The mean ± standard error feeding time was 52.3 ± 6.6 min (Table 1).
Figure 4.
The mantis Tenodera angustipennis feeding on a treated adult Pheropsophus jessoensis. (A) Mantis feeding on the beetle body. (B) Leftover antennae, legs, elytra, and hindwings of the beetle. The treated beetle was unable to eject hot chemicals because they had been exhausted by repeated stimulation before the experiment. Photo credit: Shinji Sugiura.
Table 1. Consumption of treated Pheropsophus jessoensis by praying mantises.
| Mantis species | Leftover (mg) | Consumption rate (%) | Feeding time (min) |
|---|---|---|---|
| Tenodera sinensis | 25.1 ± 4.8 | 89.0 ± 2.0 | 56. 0 ± 14.7 |
| Tenodera angustipennis | 20.2 ± 3.8 | 90.2 ± 2.2 | 50.3 ± 8.1 |
| Hierodula patellifera | 18.1 ± 4.7 | 92.3 ± 2.0 | 50.6 ± 11.7 |
| All species combined | 21.2 ± 2.6 | 90.5 ± 1.2 | 52.3 ± 6.6 |
Note:
Values are the mean ± SE.
The rates of successful escape from mantises significantly differed between the control and treated P. jessoensis (Fisher’s exact test; T. sinensis, P < 0.0001; T. angustipennis, P < 0.0001; H. patellifera, P < 0.0001; all species combined, P < 0.0001). The mean body sizes (lengths and weights) of mantises that attacked control and treated beetles did not differ significantly (Table 2). The mean body sizes (lengths and weights) of control and treated beetles did not differ significantly (Table 2). Therefore, the bombing responses of adult P. jessoensis deterred mantises of all species, sexes, and sizes.
Table 2. Sizes of the bombardier beetle, Pheropsophus jessoensis, and praying mantises.
| Mantis species | Body size | Treatment | Statistical comparison | ||
|---|---|---|---|---|---|
| Control beetles | Treated beetles | t value | P value | ||
| Tenodera sinensis | Mantis body length (mm) | 80.2 ± 1.8 | 82.1 ± 1.7 | −0.75 | 0.46 |
| Mantis body weight (mg) | 2234.2 ± 637.3 | 2273.9 ± 475.7 | −0.05 | 0.96 | |
| Beetle body length (mm) | 17.9 ± 0.3 | 18.2 ± 0.4 | −0.58 | 0.57 | |
| Beetle body weight (mg) | 252.1 ± 17.6 | 229.6 ± 13.9 | 1.01 | 0.33 | |
| Tenodera angustipennis | Mantis body length (mm) | 76.3 ± 2.2 | 79.1 ± 1.7 | −0.98 | 0.34 |
| Mantis body weight (mg) | 1622.4 ± 333.2 | 1829.3 ± 243.2 | −0.50 | 0.62 | |
| Beetle body length (mm) | 17.1 ± 0.5 | 17.9 ± 0.5 | −1.19 | 0.25 | |
| Beetle body weight (mg) | 216.0 ± 19.5 | 222.6 ± 21.7 | −0.23 | 0.82 | |
| Hierodula patellifera | Mantis body length (mm) | 58.3 ± 0.6 | 56.2 ± 0.9 | 1.83 | 0.09 |
| Mantis body weight (mg) | 1736.4 ± 120.0 | 1850.0 ± 108.6 | −0.70 | 0.49 | |
| Beetle body length (mm) | 17.6 ± 0.5 | 17.7 ± 0.5 | −0.07 | 0.94 | |
| Beetle body weight (mg) | 247.7 ± 23.1 | 225.9 ± 20.0 | 0.71 | 0.49 | |
| All species combined | Mantis body length (mm) | 71.6 ± 2.0 | 72.4 ± 2.3 | −0.27 | 0.79 |
| Mantis body weight (mg) | 1864.3 ± 239.6 | 1984.4 ± 179.4 | −0.40 | 0.69 | |
| Beetle body length (mm) | 17.5 ± 0.3 | 17.9 ± 0.3 | −1.08 | 0.28 | |
| Beetle body weight (mg) | 238.6 ± 11.6 | 226.0 ± 10.5 | 0.80 | 0.43 | |
Note:
Values are the mean ± SE.
Disussion
Some praying mantises can prey on well-defended insects, although the predation success rate varies among prey insect species (Reitze & Nentwig, 1991). The effectiveness of chemical defences by bombardier beetles against mantises remains unclear (Eisner, 1958). In this study, I tested the effectiveness of the defences of the bombardier beetle, P. jessoensis, against three mantis species under laboratory conditions (Fig. 2). My experiments demonstrated that bombing was essential for successful defence by P. jessoensis against mantises, which were always deterred (Fig. 2). To my knowledge, this is the first study to document a perfect defence against praying mantises by insects smaller than mantises.
Dean (1980b) experimentally investigated the relative importance of the toxic chemicals and heat produced by bombing for the successful defence of bombardier beetles against toads. Although the combination of chemicals and heat played an important role in deterring toads, the chemicals served as the primary defence and bombing as a secondary defence (Dean, 1980b). Toxic chemicals or other material on the body of P. jessoensis functioned as a primary deterrent against frogs (Sugiura, 2018) and birds (Kojima & Yamamoto, 2020), suggesting that bombing is not essential for the successful defence of P. jessoensis against frogs and birds. However, all praying mantises consumed the treated P. jessoensis (Fig. 2), suggesting that chemicals on the body of P. jessoensis could not deter mantises. Studies have indicated that chemically defended arthropods could not effectively deter mantises (Reitze & Nentwig, 1991). For example, mantises such as T. sinensis could consume toxic caterpillars after removing (‘gutting’) the midgut containing toxic plant material (Rafter, Agrawal & Preisser, 2013; Mebs et al., 2017; Mebs, Wunder & Toennes, 2019). Several mantis species could also tolerate noxious chemicals such as tetrodotoxin, cardenolides, and quinine used as anti-predator defences by toxic arthropods (Mebs, Yotsu-Yamashita & Arakawa, 2016; Mebs et al., 2017; Rafter et al., 2017a, 2017b; Mebs, Wunder & Toennes, 2019). Therefore, bombing plays an essential role in defending against mantis predation, although additional experiments are needed to test the importance of heat in the successful defence of P. jessoensis against mantises.
Some predators avoid attacking bombardier beetles after experiencing the toxic chemicals (Dean, 1980a; Kojima & Yamamoto, 2020). Dean (1980a) found that many American toads, Anaxyrus americanus (Holbrook) (Anura: Bufonidae), did not reattack bombardier beetles (Brachinus spp.) for at least 30 min after rejecting them. Kojima & Yamamoto (2020) observed that some quail exposed to live P. jessoensis avoided them for up to 5 weeks. In this study, 26.7% of mantises reattacked P. jessoensis within 1 min after being bombed; P. jessoensis mantises were reattacked more frequently by females than by males, although these differences were not significant. Hungrier mantises (starved >24 h) may be more likely to reattack P. jessoensis after being bombed. However, P. jessoensis should be capable of easy escape from mantises before they reattack under field conditions, because P. jessoensis can rapidly leave the site after release.
Chemically defended prey produce toxic chemicals that force predators to spit them out (Taniguchi et al., 2005; Whitman & Vincent, 2008; Matsubara & Sugiura, 2017). However, the first predator attack potentially damages the defended prey. Therefore, the chemically defended prey may have evolved tolerance for predator biting and other attacks (Sugiura & Sato, 2018; Sugiura, 2020a). In this study, none of the P. jessoensis released by mantises were injured, suggesting that P. jessoensis has a body tough enough to survive an attack by the raptorial forelegs of mantises.
Conclusions
While the hot chemicals ejected by bombardier beetles deter some vertebrate species, these species do not always reject the bombardier beetles; some individuals are able to consume the beetles (Dean, 1980a; Sugiura, 2018; Sugiura & Sato, 2018; Kojima & Yamamoto, 2020). Eisner & Dean (1976) reported that all individuals of an orb-weaving spider species Trichonephila clavipes (Linnaeus) rejected bombardier beetles (Brachinus spp.). Eisner et al. (2006) showed that the bombardier beetle Pheropsophus aequinoctialis (Linnaeus) always deterred the wolf spider Schizocosa ceratiola (Gertsch & Wallace) (Araneae: Lycosidae). In this study, P. jessoensis bombing always deterred praying mantises. Therefore, hot chemicals discharged by bombardier beetles may deter arthropod predators more effectively than vertebrate predators.
Supplemental Information
The beetle ejected hot chemicals when stimulated with forceps. This is the video in Sugiura (2018). Video credit: Shinji Sugiura.
The mantis caught the beetle using its raptorial forelegs but released it immediately after being bombed. Video credit: Shinji Sugiura.
The mantis caught this beetle using its raptorial forelegs and ate it. The treated beetle was unable to eject hot chemicals because they had been exhausted by repeated stimulation before the experiment. Video credit: Shinji Sugiura.
Acknowledgments
I am grateful to K. Sakagami, M. Tsujii, S. Matsubara, N. Shimada, M. Harada, K. Okai, D. Funamoto, H. Yamashita, and M. Nagao for helping to collect and maintain the study animals. I also thank the anonymous reviewers for their helpful advice on this manuscript.
Funding Statement
This study was supported by a Grant-in-Aid for Scientific Research (JSPS KAKENHI Grant number 19K06073). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Shinji Sugiura conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
My study was not conducted in any national parks or protected areas. Study insects were not protected species; no specific permissions are required to collect non-protected insects in non-protected areas in Japan.
Data Availability
The following information was supplied regarding data availability:
The raw data are available at figshare: Sugiura, Shinji (2021): Data from: Beetle bombing always deters praying mantises. figshare. Dataset. DOI 10.6084/m9.figshare.14443748.v1.
References
- Aneshansley et al. (1969).Aneshansley DT, Eisner T, Widom JM, Widom B. Biochemistry at 100°C: explosive secretory discharge of bombardier beetles (Brachinus) Science. 1969;165(3888):61–63. doi: 10.1126/science.165.3888.61. [DOI] [PubMed] [Google Scholar]
- Anichtchenko et al. (2021).Anichtchenko A, Choi JB, Facchini S, Marrero J, Panin R, Potanin D, Roguet D, Solodovnikov I, Will KW. Carabidae of the World. 2021. https://carabidae.org/taxa/licinini-bonelli. [17 April 2021]. https://carabidae.org/taxa/licinini-bonelli
- Arndt et al. (2015).Arndt EM, Moore W, Lee WK, Ortiz C. Mechanistic origins of bombardier beetle (Brachinini) explosion-induced defensive spray pulsation. Science. 2015;348(6234):563–567. doi: 10.1126/science.1261166. [DOI] [PubMed] [Google Scholar]
- Arndt, Beutel & Will (2016).Arndt EM, Beutel RG, Will K. Carabidae Latreille. In: Beutel RG, Leschen RAB, editors. Coleoptera, Beetles. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim) Second Edition. Vol. 1. Berlin/Boston: Walter de Gruyter GmbH; 2016. pp. 162–190. [Google Scholar]
- Berenbaum & Miliczky (1984).Berenbaum MR, Miliczky E. Mantids and milkweed bugs: efficacy of aposematic coloration against invertebrate predators. American Midland Naturalist. 1984;111(1):64–68. [Google Scholar]
- Conner & Eisner (1983).Conner J, Eisner T. Capture of bombardier beetles by ant lion larvae. Psyche. 1983;90(1–2):175–178. doi: 10.1155/1983/25010. [DOI] [Google Scholar]
- Corrette (1990).Corrette BJ. Prey capture in the praying mantis Tenodera aridifolia sinensis: coordination of the capture sequence and strike movements. The Journal of Experimental Biology. 1990;148(1):147–180. doi: 10.1242/jeb.148.1.147. [DOI] [PubMed] [Google Scholar]
- Dean (1979).Dean J. Defensive reaction time of bombardier beetles: an investigation of the speed of a chemical defense. Journal of Chemical Ecology. 1979;5(5):691–701. doi: 10.1007/BF00986554. [DOI] [Google Scholar]
- Dean (1980a).Dean J. Encounters between bombardier beetles and two species of toads (Bufo americanus, B. marinus): speed of prey-capture does not determine success. Journal of Comparative Physiology. 1980a;133(1):41–50. doi: 10.1007/BF00660180. [DOI] [Google Scholar]
- Dean (1980b).Dean J. Effects of thermal and chemical components of bombardier beetle chemical defense: glossopharyngeal response in two species of toads (Bufo americanus, B. marinus) Journal of Comparative Physiology. 1980b;133(1):51–59. doi: 10.1007/BF00660181. [DOI] [Google Scholar]
- Edmunds (1974).Edmunds M. Defense in animals. Harlow: Longman; 1974. [Google Scholar]
- Eisner (1958).Eisner T. The protective role of the spray mechanism of the bombardier beetle, Brachynus ballistarius Lec. Journal of Insect Physiology. 1958;2(3):215–220. [Google Scholar]
- Eisner (1970).Eisner T. Chemical defense against predation in arthropods. In: Sondheimer E, Simeone JB, editors. Chemical Ecology. New York: Academic Press; 1970. pp. 157–217. [Google Scholar]
- Eisner (2003).Eisner T. For love of insects. Cambridge: The Belknap Press of the Harvard University Press; 2003. [Google Scholar]
- Eisner & Aneshansley (1999).Eisner T, Aneshansley DJ. Spray aiming in the bombardier beetle: photographic evidence. Proceeding of the National Academy of Sciences USA. 1999;96(17):9705–9709. doi: 10.1073/pnas.96.17.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner & Dean (1976).Eisner T, Dean J. Ploy and counterploy in predator-prey interactions: orb-weaving spiders versus bombardier beetles. Proceeding of the National Academy of Sciences USA. 1976;73(4):1365–1367. doi: 10.1073/pnas.73.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner & Meinwald (1966).Eisner T, Meinwald J. Defensive secretions of arthropods. Science. 1966;153(3742):1341–1350. doi: 10.1126/science.153.3742.1341. [DOI] [PubMed] [Google Scholar]
- Eisner, Eisner & Aneshansley (2005).Eisner T, Eisner M, Aneshansley DJ. Pre-ingestive treatment of bombardier beetles by jays: food preparation by anting and sand-wiping. Chemoecology. 2005;15(4):227–233. doi: 10.1007/s00049-005-0316-6. [DOI] [Google Scholar]
- Eisner, Eisner & Siegler (2005).Eisner T, Eisner M, Siegler M. Secret weapons: defenses of insects, spiders, scorpions, and other many-legged creatures. Cambridge: The Belknap Press of the Harvard University Press; 2005. [Google Scholar]
- Eisner et al. (2006).Eisner T, Aneshansley DJ, del Campo ML, Eisner M, Frank JH, Deyrup M. Effect of bombardier beetle spray on a wolf spider: repellency and leg autotomy. Chemoecology. 2006;16(4):185–189. doi: 10.1007/s00049-006-0346-8. [DOI] [Google Scholar]
- Endler (1991).Endler JA. Interactions between predators and prey. In: Krebs JR, Davies NB, editors. Behavioural ecology: an evolutionary approach. London, Paris, Berlin, Vienna: Blackwell; 1991. pp. 169–196. [Google Scholar]
- Fink & Brower (1981).Fink LS, Brower LP. Birds can overcome the cardenolide defence of monarch butterflies in Mexico. Nature. 1981;291(5810):67–70. doi: 10.1038/291067a0. [DOI] [Google Scholar]
- Fujisawa, Lee & Ishii (2012).Fujisawa T, Lee CM, Ishii M. Species diversity of ground beetle assemblages in the distinctive landscapes of the Yodo River flowing through northern Osaka Prefecture, central Japan. Japanese Journal of Environmental Entomology and Zoology. 2012;23(2):89–100. doi: 10.11257/jjeez.23.89. [DOI] [Google Scholar]
- Habu & Sadanaga (1965).Habu A, Sadanaga K. Illustrations for identification of larvae of the Carabidae found in cultivated fields and paddy-fields (III) Bulletin of the National Institute of Agricultural Sciences, Series C: Plant Pathology and Entomology. 1965;19:81–216. [in Japanese] [Google Scholar]
- Honma, Oku & Nishida (2006).Honma A, Oku S, Nishida T. Adaptive significance of death feigning posture as a specialized inducible defence against gape-limited predators. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1594):1631–1636. doi: 10.1098/rspb.2006.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani & Yano (1994).Ishitani M, Yano K. Species composition and seasonal activities of ground beetles (Coleoptera) in a fig orchard. Japanese Journal of Entomology. 1994;62(1):201–210. [Google Scholar]
- Jones & Bulbert (2020).Jones BR, Bulbert MW. Directed chemical spray of the peppermint stick insect (Megacrania batesii) is induced when predation risk is at its highest. Journal of Ethology. 2020;38(1):51–59. doi: 10.1007/s10164-019-00619-0. [DOI] [Google Scholar]
- Jung et al. (2012).Jung JK, Kim ST, Lee SY, Park CK, Lee EH, Lee JH. Ground beetle (Coleoptera: Carabidae) assemblage in the urban landscape. Korea Journal of Ecology and Field Biology. 2012;35(2):79–89. doi: 10.5141/JEFB.2012.012. [DOI] [Google Scholar]
- Kanehisa (1996).Kanehisa K. Secretion of defensive substance by Carabidae and Brachinidae. Bulletin of the Research Institute for Bioresources, Okayama University. 1996;4:9–23. [in Japanese with English summary] [Google Scholar]
- Kanehisa & Murase (1977).Kanehisa K, Murase M. Comparative study of the pygidial defensive systems of carabid beetles. Applied Entomology and Zoology. 1977;12(3):225–235. doi: 10.1303/aez.12.225. [DOI] [Google Scholar]
- Kojima & Yamamoto (2020).Kojima W, Yamamoto R. Defense of bombardier beetles against avian predators. The Science of Nature. 2020;107(5):36. doi: 10.1007/s00114-020-01692-z. [DOI] [PubMed] [Google Scholar]
- Li et al. (2012).Li X, Yin H, Li K, Gao X. Population genetic structure and historical demography of the ground beetle Pheropsophus jessoensis from the Tsinling-Dabashan Mountains, central China based on mitochondrial DNA analysis. Zoological Science. 2012;29(4):238–246. doi: 10.2108/zsj.29.238. [DOI] [PubMed] [Google Scholar]
- Matsubara & Sugiura (2017).Matsubara S, Sugiura S. Chemical defence of turnip sawfly larvae against Japanese tree frogs. Journal of Asia-Pacific Entomology. 2017;20(1):225–227. doi: 10.1016/j.aspen.2017.01.001. [DOI] [Google Scholar]
- Mebs, Yotsu-Yamashita & Arakawa (2016).Mebs D, Yotsu-Yamashita M, Arakawa O. The praying mantis (Mantodea) as predator of the poisonous red-spotted newt Notophthalmus viridescens (Amphibia: Urodela: Salamandridae) Chemoecology. 2016;26(3):121–126. doi: 10.1007/s00049-016-0211-3. [DOI] [Google Scholar]
- Mebs et al. (2017).Mebs D, Wunder C, Pogoda W, Toennes SW. Feeding on toxic prey: the praying mantis (Mantodea) as predator of poisonous butterfly and moth (Lepidoptera) caterpillars. Toxicon. 2017;131:16–19. doi: 10.1016/j.toxicon.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Mebs, Wunder & Toennes (2019).Mebs D, Wunder C, Toennes SW. Coping with noxious effects of quinine by praying mantids (Mantodea) and spiders (Araneae) Toxicon. 2019;162:57–60. doi: 10.1016/j.toxicon.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Nishida (2002).Nishida R. Sequestration of defensive substances from plants by Lepidoptera. Annual Review of Entomology. 2002;47(1):57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- Nyffeler, Maxwell & Remsen (2017).Nyffeler A, Maxwell MR, Remsen JVJ. Bird predation by praying mantises: a global perspective. The Wilson Journal of Ornithology. 2017;129(2):331–344. doi: 10.1676/16-100.1. [DOI] [Google Scholar]
- Nowicki & Eisner (1983).Nowicki S, Eisner T. Predatory capture of bombardier beetles by a tabanid fly larvae. Psyche. 1983;90(1–2):119–122. doi: 10.1155/1983/54806. [DOI] [Google Scholar]
- Ohwaki, Kaneko & Ikeda (2015).Ohwaki A, Kaneko Y, Ikeda H. Seasonal variability in the response of ground beetles (Coleoptera: Carabidae) to a forest edge in a heterogeneous agricultural landscape in Japan. European Journal of Entomology. 2015;112(1):135–144. doi: 10.14411/eje.2015.022. [DOI] [Google Scholar]
- Perelman & Chikatunov (2010).Perelman B, Chikatunov V. Intoxication of young crocodiles in captivity due to the ingestion of darkling beetles Blaps nitens laportei Ardoin (Coleoptera: Tenebrionidae) Israel Journal of Veterinary Medicine. 2010;65:100–102. [Google Scholar]
- Prudic et al. (2019).Prudic KL, Timmermann BN, Papaj DR, Ritland DB, Oliver JC. Mimicry in viceroy butterflies is dependent on abundance of the model queen butterfly. Communications Biology. 2019;2(1):68. doi: 10.1038/s42003-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafter, Agrawal & Preisser (2013).Rafter JL, Agrawal AA, Preisser EL. Chinese mantids gut toxic monarch caterpillars: avoidance of prey defence? Ecological Entomology. 2013;38(1):76–82. doi: 10.1111/j.1365-2311.2012.01408.x. [DOI] [Google Scholar]
- Rafter et al. (2017a).Rafter JL, Gonda-King L, Niesen D, Seeram NP, Rigsby CM, Preisser EL. Impact of consuming ‘toxic’ monarch caterpillars on adult Chinese mantis mass gain and fecundity. Insects. 2017a;8(1):23. doi: 10.3390/insects8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafter et al. (2017b).Rafter JL, Vendettuoli JF, Gonda-King L, Niesen D, Seeram NP, Rigsby CM, Preisser EL. Pretty picky for a generalist: impacts of toxicity and nutritional quality on mantid prey processing. Environmental Entomology. 2017b;46(3):626–632. doi: 10.1093/ee/nvx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018).R Core Team . R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2018. [Google Scholar]
- Reitze & Nentwig (1991).Reitze M, Nentwig W. Comparative investigations into the feeding ecology of six Mantodea species. Oecologia. 1991;86(4):568–574. doi: 10.1007/BF00318324. [DOI] [PubMed] [Google Scholar]
- Rilling, Mittelstaedt & Roeder (1959).Rilling S, Mittelstaedt H, Roeder KD. Prey recognition in the praying mantis. Behaviour. 1959;14(1–4):164–184. doi: 10.1163/156853959X00063. [DOI] [Google Scholar]
- Ruxton, Sherratt & Speed (2004).Ruxton GD, Sherratt TN, Speed MP. Avoiding attack: the evolutionary ecology of crypsis, aposematism, and mimicry. Oxford: Oxford University Press; 2004. [Google Scholar]
- Sakagami, Funamoto & Sugiura (2021).Sakagami K, Funamoto D, Sugiura S. Nocturnal ambush predators and their potential impact on flower-visiting moths. Ecology. 2021 doi: 10.1002/ecy.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura (2018).Sugiura S. Anti-predator defences of a bombardier beetle: is bombing essential for successful escape from frogs? PeerJ. 2018;6:e5942. doi: 10.7717/peerj.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura (2020a).Sugiura S. Predators as drivers of insect defenses. Entomological Science. 2020a;23(3):316–337. doi: 10.1111/ens.12423. [DOI] [Google Scholar]
- Sugiura (2020b).Sugiura S. Active escape of prey from predator vent via the digestive tract. Current Biology. 2020b;30(15):R867–R868. doi: 10.1016/j.cub.2020.06.026. [DOI] [PubMed] [Google Scholar]
- Sugiura & Sato (2018).Sugiura S, Sato T. Successful escape of bombardier beetles from predator digestive systems. Biology Letters. 2018;14(2):20170647. doi: 10.1098/rsbl.2017.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura et al. (2019).Sugiura S, Sakagami K, Harada M, Shimada N. Can praying mantises escape from spider webs? Ecology. 2019;100(11):e02799. doi: 10.1002/ecy.2799. [DOI] [PubMed] [Google Scholar]
- Taniguchi et al. (2005).Taniguchi K, Maruyama M, Ichikawa T, Ito F. A case of Batesian mimicry between a myrmecophilous staphylinid beetle, Pella comes, and its host ant, Lasius (Dendrolasius) spathepus: an experiment using the Japanese treefrog, Hyla japonica as a real predator. Insectes Sociaux. 2005;52:320–322. doi: 10.1007/s00040-005-0813-1. [DOI] [Google Scholar]
- Ueno, Kurosawa & Sato (1985).Ueno S, Kurosawa Y, Sato M. The Coleoptera of Japan in color. II. Osaka: Hoikusha; 1985. [in Japanese] [Google Scholar]
- Umbers & Mappes (2015).Umbers KDL, Mappes J. Postattack deimatic display in the mountain katydid, Acripeza reticulata. Animal Behaviour. 2015;100:68–73. doi: 10.1016/j.anbehav.2014.11.009. [DOI] [Google Scholar]
- Valdez (2020).Valdez JW. Arthropods as vertebrate predators: a review of global patterns. Global Ecology and Biogeography. 2020;29(10):1691–1703. doi: 10.1111/geb.13157. [DOI] [Google Scholar]
- Watanabe & Yano (2009).Watanabe H, Yano E. Behavioral response of mantid Hierodula patellifera to wind as an antipredator strategy. Annals of the Entomological Society of America. 2009;102(3):517–522. doi: 10.1603/008.102.0323. [DOI] [Google Scholar]
- Watanabe, Miyamoto & Yano (2013).Watanabe H, Miyamoto M, Yano E. Stage-specific site selection of the praying mantid Tenodera aridifolia. Annals of the Entomological Society of America. 2013;106(4):447–453. doi: 10.1603/AN12145. [DOI] [Google Scholar]
- Whitman & Vincent (2008).Whitman DW, Vincent S. Large size as an antipredator defense in an insect. Journal of Orthoptera Research. 2008;17(2):353–371. doi: 10.1665/1082-6467-17.2.353. [DOI] [Google Scholar]
- Yahiro et al. (1992).Yahiro K, Fujimoto T, Tokuda M, Yano K. Species composition and seasonal abundance of ground beetles (Coleoptera) in paddy fields. Japanese Journal of Entomology. 1992;60(4):805–813. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The beetle ejected hot chemicals when stimulated with forceps. This is the video in Sugiura (2018). Video credit: Shinji Sugiura.
The mantis caught the beetle using its raptorial forelegs but released it immediately after being bombed. Video credit: Shinji Sugiura.
The mantis caught this beetle using its raptorial forelegs and ate it. The treated beetle was unable to eject hot chemicals because they had been exhausted by repeated stimulation before the experiment. Video credit: Shinji Sugiura.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available at figshare: Sugiura, Shinji (2021): Data from: Beetle bombing always deters praying mantises. figshare. Dataset. DOI 10.6084/m9.figshare.14443748.v1.