Abstract
Endonuclease III from Escherichia coli is the prototype of a ubiquitous DNA repair enzyme essential for the removal of oxidized pyrimidine base damage. The yeast genome project has revealed the presence of two genes in Saccharomyces cerevisiae, NTG1 and NTG2, encoding proteins with similarity to endonuclease III. Both contain the highly conserved helix-hairpin-helix motif, whereas only one (Ntg2) harbors the characteristic iron-sulfur cluster of the endonuclease III family. We have characterized these gene functions by mutant and enzyme analysis as well as by gene expression and intracellular localization studies. Targeted gene disruption of NTG1 and NTG2 produced mutants with greatly increased spontaneous and hydrogen peroxide-induced mutation frequency relative to the wild type, and the mutation response was further increased in the double mutant. Both enzymes were found to remove thymine glycol and 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine (faPy) residues from DNA with high efficiency. However, on UV-irradiated DNA, saturating concentrations of Ntg2 removed only half of the cytosine photoproducts released by Ntg1. Conversely, 5-hydroxycytosine was removed efficiently only by Ntg2. The enzymes appear to have different reaction modes, as judged from much higher affinity of Ntg2 for damaged DNA and more efficient borhydride trapping of Ntg1 to abasic sites in DNA despite limited DNA binding. Northern blot and promoter fusion analysis showed that NTG1 is inducible by cell exposure to DNA-damaging agents, whereas NTG2 is constitutively expressed. Ntg2 appears to be a nuclear enzyme, whereas Ntg1 was sorted both to the nucleus and to the mitochondria. We conclude that functions of both NTG1 and NTG2 are important for removal of oxidative DNA damage in yeast.
DNA of all aerobic organisms suffers damage from reactive oxygen species (ROS), and oxidative DNA damage plays important roles in mutagenesis, carcinogenesis, and aging (1). However, the biological effects are prevented by DNA repair, and the base excision repair (BER) pathway appears to be the most important mechanism for removal of oxidative DNA damage (37). The first step in BER is the recognition and removal of the altered base by a DNA glycosylase activity, leaving an abasic site (AP site) in the DNA. The AP site is incised either by an AP-lyase activity associated with the DNA glycosylase itself or by an independent AP-endonuclease activity. The repair is completed by the sequential action of phosphodiesterase(s), DNA polymerase, and DNA ligase. Other accessory protein factors such as XRCC1 (11, 27) are also involved and possibly important for the coordination of the different steps in the BER pathway.
Several DNA glycosylases removing oxidized base residues have been identified. These can be classified into two functional subgroups, as exemplified by Escherichia coli Fpg (formamidopyrimidine DNA glycosylase) for the repair of oxidized purines and E. coli Nth (endonuclease III), for the repair of oxidized pyrimidines (13, 26). Fpg catalyzes the excision of 7,8-dihydro-8-oxoguanine (8-oxoG) and imidazole-ring fragmented formamidopyrimidine (faPy) residues from double-stranded DNA and has a strong associated β,δ-eliminating AP-lyase activity (8, 39). The guanine derivative 8-oxoG has miscoding and mutagenic properties (24, 25), whereas faPy residues represent blocks to DNA replication and are mostly cytotoxic (7). The E. coli fpg (mutM) mutant has a spontaneous mutator phenotype (32).
No eukaryotic sequence counterpart of fpg has been identified, whereas the Nth family of glycosylases are present throughout phylogeny (2, 17). Nth specifically removes radiolysis products of thymine and cytosine, including ring-saturated, -fragmented, or -contracted lesions (14). An important lesion to be removed is thymine glycol (Tg), which strongly inhibits DNA replication in vitro and represents a cytotoxic lesion in vivo (23, 35). The cytosine modification 5-hydroxycytosine (5-OHC) has been shown to be mutagenic in E. coli (18). Like Fpg, Nth has a strong associated AP-lyase activity but one producing only β elimination. Another DNA glycosylase identified in E. coli is Nei (endonuclease VIII), which has substrate specificity similar to that of Nth (31).
Genes encoding homologues of the prokaryotic Nth have been identified in several eukaryotic species, including Saccharomyces cerevisiae (17), Schizosaccharomyces pombe (34), Caenorhabditis elegans (41), and humans (2, 21). Multiple alignment of the Nth gene family reveals two highly conserved domains: a helix-hairpin-helix (HhH) motif that is involved in the proposed catalytic mechanism and a [4Fe-4S] cluster which is assumed to be important for DNA binding (28). The [4Fe-4S] cluster is found in all members of the enzyme family except S. cerevisiae Ntg1 (3, 17). The NTG1 gene was originally identified by the presence of the HhH motif. Expression analysis in E. coli revealed that Ntg1 behaves like its prokaryotic counterpart in removing Tg and producing strand breaks at AP sites (3, 17). However, in contrast to what has been reported for Nth, the S. cerevisiae Ntg1 was found to release faPy residues with high efficiency and therefore shares some of the properties of the Fpg enzyme from E. coli (17).
The NTG1 gene (EMBL accession no. L05146) is contained within a larger sequence fragment from chromosome I analyzed prior to the completion of the yeast genome sequence (4). Completion of the S. cerevisiae genome sequence revealed a second Nth homologue, Ntg2, on chromosome XV (EMBL accession no. Z74785). Ntg2 contains the [4Fe-4S] cluster characteristic of the other members of the Nth family (Fig. 1 shows an alignment and reference to relevant sequence characteristics). In this study, we purified both of these enzymes to near physical homogeneity and found differences in substrate specificity, although both enzymes work on Tg and faPy residues in DNA. Furthermore, we found that each single mutant lacking one or the other of Ntg1 and Ntg2 had a much higher spontaneous and peroxide-induced mutation frequency than wild-type cells. The frequency of mutations is even higher in the double mutant, indicating separate functions of NTG1 and NTG2 in yeast repair.
FIG. 1.
Alignment of the S. cerevisiae Ntg1 and Ntg2 proteins. Highlighted amino acids represent identical residues. Different structural features are underlined above and below the alignment as indicated with reference to Ntg1 and Ntg2, respectively. NLS, nuclear localization signal; MLS, mitochondrial localization signal.
MATERIALS AND METHODS
Yeast and bacterial strains.
E. coli strains used were BH20 (fpg) (6) and BK3004 (fpg::kan) made by T4 transduction of fpg::kan from BH20 into BL21. Wild-type yeast FF18733 (MATa his7-3 leu2-1,112 lys1-1 trp1-289 ura3-52) was obtained from Serge Boiteux, and the NTG1 and NTG2 mutants were made by targeted gene disruption of the wild-type alleles in FF18733. The URA3 gene marker was inserted in the BglII restriction site of NTG1 cloned in pUC19, and the LEU2 marker was inserted in the EcoRI restriction site of NTG2 in pVL1393 (PharMingen). The NTG1 and NTG2 genes with markers were excised, partially degraded with BAL 31, and used for transformation of FF18733 by the lithium acetate method (22). The ntg1 ntg2 double mutant was made by transforming the ntg1 single mutant with the ntg2::LEU2 insert. The genotypes of the resulting transformants, RH101 (ntg1::URA3), RH102 (ntg2::LEU2), and RH103 (ntg1::URA3 ntg2::LEU2), were confirmed by Southern blot analysis.
Reagent enzymes.
E. coli Nth was purified from cells containing an Nth expression plasmid obtained from Richard Cunningham. Endonuclease IV (Nfo) was purified by the His6 tag Ni affinity purification system (Qiagen Ltd.). E. coli Fpg was kindly provided by S. Boiteux. Uracil-DNA glycosylase was purchased from Boehringer Mannheim, and restriction enzymes, T4 DNA ligase, T4 DNA polymerase, and T4 polynucleotide kinase were all from New England Biolabs.
Ntg1 and Ntg2 expression and purification.
The NTG1 coding region (1,200 bp) was cloned into pUC19 (17) to yield pUC-NTG1. E. coli BH20 was transformed by pUC-NTG1 and grown in 10 liters of LB medium at 37°C to an optical density of 0.8. Isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) was added, and incubation continued for 2 h. Cell extract was made by a combination of plasmolysis and lysozyme treatment as previously described previously (36). Assay for faPy activity was used to monitor Ntg1 purification. Cell extract was applied to an Affi-Gel Blue (Bio-Rad) column (2 by 8 cm) equilibrated with buffer A (0.1 M Tris [pH 8.0], 1 mM EDTA, 20% glycerol, 10 mM β-mercaptoethanol). After washing, active fractions were eluted by a two-step salt gradient (1 and 2 M KCl in buffer A). Fractions with faPy activity were pooled, dialyzed against buffer B (50 mM morpholinoethanesulfonic acid [pH 6.0], 1 mM EDTA, 20% glycerol, 10 mM β-mercaptoethanol), and applied to a MonoS column (HR 5/5; Pharmacia). The column was eluted with a 0 to 1.0 M NaCl linear gradient, and peak fractions eluting between 0.3 and 0.4 M NaCl were pooled and dialyzed against buffer A. The desalted fraction was applied to a calf thymus DNA cellulose column (HR 5/5; Pharmacia) and eluted by a 0 to 2.0 M KCl linear gradient in buffer A. Active fractions eluting between 0.25 and 0.4 M KCl were collected, desalted on Sephadex G-25M (PD-10 column; Pharmacia) equilibrated with buffer A, and applied to a MonoQ column (HR 5/5; Pharmacia). The column was eluted with a 0 to 1.0 M NaCl linear gradient and purified Ntg1 eluted at 0.2 M NaCl.
The NTG2 gene coding region (1,143 bp) was amplified by PCR using Pfu polymerase and primers 5′-cgggatccATGAGAGAGGAAAGTAGGTCTAGG (linker sequence in lowercase letters) and 5′-aactgcagCCACAATGAATGGTGGTTCTAT. The NTG2 fragment was inserted into the BamHI and PstI restriction sites of pT7-SCII (Stratagene). The NdeI-BamHI fragment was removed from the polylinker to shorten the distance between the ribosomal binding site and the translation start of NTG2, producing pT7-NTG2. E. coli BK3004 was transformed by pT7-NTG2 and grown in 10 liters of K medium (M9 buffer supplemented with 1% glucose, 1% Casamino Acids, and 0.0001% thiamine) to an optical density of 0.8. IPTG (0.1 mM) was added, incubation continued for 2 h, and extract was prepared as for Ntg1. Ntg2 was purified by a protocol similar to that used for Ntg1. The extract was applied to an Affi-Gel Blue column equilibrated with 0.1 M KCl in buffer A and eluted by a step gradient of 1 and 2 M KCl in buffer A. Active fractions were pooled, dialyzed against buffer A containing 0.1 M KCl, and applied to a MonoQ column. Ntg2 was collected in the flowthrough and applied to a DNA-cellulose column. The chromatography was as for Ntg1 except that an NaCl gradient was used. Active fractions eluting between 0.2 and 0.4 M NaCl were pooled, dialyzed against buffer B, and applied to a MonoS column. Peak fractions eluting at 0.4 M NaCl were applied to a Sephadex-75 gel filtration column (HR 5/5; Pharmacia) equilibrated with buffer B. The column was eluted with 50 mM NaCl in buffer B.
Assays for faPy-DNA glycosylase activity.
All enzyme activities were assayed in a reaction buffer containing 70 mM morpholinopropanesulfonic acid (pH 7.5), 1 mM dithiothreitol, 1 mM EDTA, and 5% glycerol for 30 min at 37°C. N-[3H]methyl-N′-nitrosourea (18 Ci/mmol) was used to prepare poly(dG-dC) DNA containing faPy residues (5,000 dpm/μg of DNA) as described elsewhere (8). FaPy-containingDNA (faPy-DNA) glycosylase activity was measured in a total volume of 50 μl containing 0.4 μg of faPy-DNA substrate as described previously (17).
Assays for cleavage of Tg- and 5-OHC-containing DNA.
The Tg-containing DNA (Tg-DNA) was constructed by hybridization of three oligonucleotides, I (5′-TTGACATTGCCCT), II (5′-CGCGA[Tg]ACGCC), and III (5′-TAGACGAATTCCG), to a complementary 37-mer oligonucleotide. Oligonucleotide II contains the Tg at position 6 (kindly provided by Philip Bolton, Department of Chemistry, Wesleyan University, Middletown, Conn.). Oligonucleotide I was 5′-end labeled with [γ-32P]ATP (5,000 Ci/mmol; Amersham) and T4 polynucleotide kinase prior to ligation by T4 DNA ligase (8 U) at 16°C for 3 h. Nfo (2.5 ng) was added to remove AP sites in the substrate prior to purification by 15% denaturing polyacrylamide gel electrophoresis (PAGE) (Long Ranger gel; FMC BioProducts). The full-length Tg-containing single strand was extracted by electroelution and rehybridized to the 37-mer complementary oligonucleotide. Duplex DNA containing a single 5-OHC residue was made by annealing a 5′ 32P-end-labeled 12-mer (5′-GCTCATGCGCAG-3) to a 26-mer complementary oligonucleotide (5′-GACCTCCCTTTCCGCTGCGCATGAGC-3′) and extending the primer by T4 DNA polymerase (0.02 U) with 50 μM 5-d[OH]CTP (kindly provided by Lawrence Loeb, Department of Pathology, University of Washington, Seattle), 50 μM dGTP, and 50 μM dATP at 37°C for 30 min to form a complete double-stranded molecule. The duplex DNA was purified by nondenaturing PAGE and isolated by electroelution. Each reaction mixture contained 10 fmol of substrate and enzyme as indicated in a total volume of 10 μl. After incubation at 37°C for 30 min, the reaction products were separated on 20% denaturing polyacrylamide gel (Long Ranger; FMC BioProducts) with 1× Tris-borate-EDTA. The radiolabeled fragments was visualized with a PhosphorImager (Molecular Dynamics model 445 SI).
Assays for cleavage of 8-oxoG- and hypoxanthine-containing DNA.
Duplex DNA contained a single 8-oxoG residue at position 10 (5′-ATCACCGGC[8-oxoG]CCACACGAGCTG-3′) opposite A, G, C, or T as previously described (5). The hypoxanthine-containing DNA was made by labeling, annealing, and extension of a 25-mer oligonucleotide containing hypoxanthine (5′-GCTCATGCGCAG[hypoxanthine]CAGCCGTACTCG-3′) to a complementary 18-mer oligonucleotide (5′-CGAGTACGGCTGTCTGCG-3′). For both substrates, the 5′ ends were labeled by T4 polynucleotide kinase and [γ-32P]ATP (3,000 Ci/mmol; Amersham). The reaction mixtures contained 10 fmol of substrates and enzymes as indicated in a total volume of 5 to 10 μl, and conditions were as described for the Tg-DNA cleavage analysis.
Assays for glycosylase activity on UV-irradiated [3H]C-DNA.
To generate a [3H]cytosine-DNA ([3H]C-DNA) substrate, a 285-bp fragment was amplified by PCR from the nthsp gene in Schizosaccharomyces pombe, using upstream primer 5′-ATATCACACATGAATGAACC-3′ and downstream primer 5′-CCCAAGCTTAAAGACGGAGTTGTAA-3′. To a reaction volume of 100 μl, 30 μl of [5-3H]dCTP (30 Ci/mmol; Amersham) and 0.2 μM concentrations of dATP, dTTP, and dGTP were added in 1× Taq buffer (20 mM Tris-HCl [pH 9.2], 60 mM KCl, 2 mM MgCl2). Amplification was for 30 cycles at 93°C for 1 min, 52°C for 1 min, and 72°C for 30 s with Taq polymerase. The PCR product was separated from any contaminating DNA by 10% nondenaturing PAGE and extracted by electroelution. The DNA was UV irradiated (254 nm) at 10,000 J/m2. Each sample contained 0.02 μg of UV-irradiated [3H]C-DNA (750,000 dpm/mg) in a total volume of 50 μl, and assay conditions were as for the faPy-DNA.
Sequence analysis of enzyme cleavage of UV-irradiated DNA.
The same 285-bp PCR fragment was amplified for the sequence analysis of the cleavage of UV-irradiated DNA. The upstream primer was end labeled with [γ-33P]ATP (2,500 Ci/mmol; Amersham) prior to amplification, while the downstream primer was unlabeled. PCR was as for the [3H]C-DNA except that unlabeled dCTP and Vent polymerase were used. After purification by PAGE, the DNA was exposed to UV (254 nm) at 10,000 J/m2. Assays for sequence analysis contained 50 ng of DNA (625,000 dpm/μg) and 20 ng of enzyme in a total volume of 8 μl. Cleavage products were analyzed on 7 M urea–8% polyacrylamide gels alongside Sanger dideoxy sequencing reactions (Thermo Sequenase radiolabeled terminator cycle sequencing; Amersham).
NaCNBH3-mediated trapping of enzyme on AP-DNA.
Duplex DNA containing a single AP site at position 13 (5′-GCTCATGCGCAG[AP site]CAGCCGTACTCG-3′) opposite A, G, C, or T (AP-DNA) was prepared as described elsewhere (17). 32P-5′-end-labeled AP-DNA (80 fmol) was incubated with enzymes as indicated in the presence of 40 mM sodium cyanoborhydride (NaCNBH3), 20 mM HEPES (pH 7.5), 50 mM KCl, and 5 mM EDTA in a total volume of 12.5 μl. After incubation at room temperature for 2 h, 3 μl of 5× sodium dodecyl sulfate (SDS)-PAGE loading buffer was added. Samples were boiled for 5 min, separated on 10% Tricine-SDS gels, and analyzed by phosphorimaging.
DNA binding assays.
DNA binding of Ntg1 and Ntg2 was determined by electrophoretic mobility shift analysis using 32P-5′-end-labeled duplex DNA substrates containing a Tg or a tetrahydrofuran (THF) residue (5-GCTGTTGAGATCCAGTTCG[THF]AGTAACCCACTCGTGC-3; kindly provided by Elmar Weinhold, Max-Planck-Institut für Molekulare Physiologie, Dortmund, Germany). DNA (10 to 80 fmol) and enzyme as indicated were incubated on ice for 15 min in a total volume of 10 μl. The samples were analyzed by 10% nondenaturing PAGE at 4°C. Conditions for competition experiments were as above except that 0.1 to 10 pmol of nondamaged duplex oligonucleotide was added.
NTG1 and NTG2 promoter-lacZ fusion analysis.
A 270-bp segment of the NTG1 promoter region (including start codon and 30 bp of downstream sequence) was amplified by PCR using primers 5′-tttggtaccTTTAACAATTCTAGGTT-3′ and 5′-tttggatccATAGATGAGTATTTACTG-3′, and 160 bp of the NTG2 promoter region (including start codon and 18 bp of downstream sequence) was amplified by using primers 5′-tttggtaccTCAGCTGGTGAAGCAGTT-3′ and 5′-tttggatcccTACTTTCCTCTCTCATTAT-3′ (lowercase letters indicate the linker regions containing restriction sites). The amplified promoter fragments were cloned between the KpnI and BamHI restriction sites of the centromeric yeast-E. coli shuttle plasmid pHQ107 (provided by Louise Prakash, University of Texas Medical Branch, Galveston) in front of and in frame with the lacZ gene of E. coli. Exponentially growing FF18733 cells in YPD (1% yeast extract, 2% peptone, 2% dextrose) harboring the NTG1- or NTG2-lacZ fusion were exposed to methylmethanesulfonate (MMS; 0.05%) or menadione (0.3 mM) for 2.5 h. Aliquots (1.5 ml) were pelleted and incubated in 250 μl of 1 M sorbitol–0.1% Triton–100 U of lyticase for 15 min at 30°C. The β-galactosidase reaction was carried out in Z buffer (0.75 ml) with chlorophenol red β-d-galactopyranoside (0.8 mg; Boehringer Mannheim), and the activity was monitored by measuring absorbance at 574 nm. The reaction mixture was incubated for 60 min at 25°C, and the reaction was stopped by addition of 0.4 ml of 1 M Na2CO3. Values are corrected for variations in protein concentrations of the extracts.
Northern blot analysis.
RNA extraction and Northern blot analysis were performed as previously described (17). Briefly, total RNA was isolated from FF18733 cells exponentially growing in YPD medium and exposed to H2O2 (1 mM), menadione (5 mM), MMS (0.05%), or 4-nitroquinoline-N-oxide (NQO; 2 μg/ml) for 30 min at 30°C. The RNA (10 μg) was size separated by electrophoresis in a formaldehyde–1% agarose gel, blotted onto a nylon membrane, and probed with the appropriate 32P-labeled DNA probes. Hybridization was quantified by phosphorimaging.
Yeast survival.
Yeast cells were grown in YPD to 2 × 106 cells/ml and exposed to DNA-damaging agents (MMS, NQO, H2O2, menadione, UVC at 254 nm, and UVA at 360 nm). At various dose intervals, cells were diluted and plated for survival measurements.
Mutagenesis.
Exponentially growing cells (2 × 106 cells/ml) in YPD were treated with 0.5, 1, 2, and 3 mM H2O2 for 30 min at 30°C. Cells were diluted and spread on YPD plates to measure survival. After collection by centrifugation, cells were plated on synthetic medium lacking arginine (Difco YNB without amino acids and supplemented with 150 μg each of leucine, adenine, uracil, tryptophan, and histidine per ml and 2% glucose) and containing 100 μg of canavanine per ml and were incubated at 30°C for 3 days. Mutation frequency was calculated as the number of canavanine-resistant colonies per 107 surviving cells.
Intracellular localization of NTG1 and NTG2 fused to GFP.
For the N-terminal fusions of green fluorescent protein (GFP) to Ntg1 and Ntg2 (Ntg1-GFPn and Ntg2-GFPn, respectively), the NTG1 and NTG2 coding sequences were first introduced into the yeast-E. coli shuttle vector pCUP1-Lux vector (obtained from Odd Stokke Gabrielsen, University of Oslo, Oslo, Norway). The pCUP1-Lux vector contains the strong CUP1 gene promoter responding to addition of CuSO4 by its cis-acting metal-responsive elements (30). The GFP sequence was PCR amplified from the pEGFP-N1 vector (Clontech), using upstream primer 5′-aactgcagATGGTGAGCAAGGGCGAGGAGC-3′ (PstI) and downstream primers 5′-cgggatccgcCTTGTACAGCTCGTCCATGCC-3′ (BamHI) (excluding GFP stop codon and including linker for in-frame fusion to Ntg1) or 5′-cgggatccCTTGTACAGCTCGTCCATGCC-3′ (BamHI) (excluding GFP stop codon and including linker for in-frame fusion to Ntg2). For the C-terminal fusions (Ntg1-GFPc and Ntg2-GFPc), the stop codons of NTG1 and NTG2 were removed by PCR using downstream primers 5′-aactgcagGTCCTCTACTTTAACAGAAATATC-3′ (PstI) and 5′-aactgcagTTTTTTCTTGTGTCTTTCTGTTG-3′ (PstI), respectively, and upstream primers 5-aactgcagATGCAAAAGATCAGTAAATA-3′ (PstI) for NTG1 and 5-cgggatccATGAGAGAGGAAAGTAGGTCTAGG-3′ (BamHI) for NTG2. The PCR products were cloned in front of GFP already inserted into the pCUP1-Lux vector (amplified by the upstream primer as described above for GFP and a downstream primer including the stop codon [5′-aactgcagATGCAAAAGATCAGTAAATA-3′]; PstI). All constructs were checked and verified by DNA sequencing. Yeast FF18733 was transformed via the lithium acetate method (22) by the various fusion constructs, and a construct harboring only GFP was included as a control. Exponentially growing cells were induced for fusion construct expression by 100 μM CuSO4 at 30°C for 2 h. Cells were fixed in 4% paraformaldehyde at room temperature for 30 min and permeabilized with lyticase in sorbitol buffer (1.2 M sorbitol, 0.1 M KH2PO4 [pH 6.5]) at 30°C for 30 min. After incubation in 0.05% Triton X-100 in phosphate-buffered saline at room temperature for 5 min, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence by GFP and/or DAPI was visualized in a Leica DMIRB/E microscope equipped with a Leica MPS 60 camera. The emission filters were S13832 for fluorescein isothiocyanate and S13824 for DAPI.
Nucleotide sequence accession numbers.
EMBL accession numbers for the sequences for Ntg1 and Ntg2 are L05146 and Z74785, respectively.
RESULTS
S. cerevisiae ntg1 and ntg2 mutant analysis.
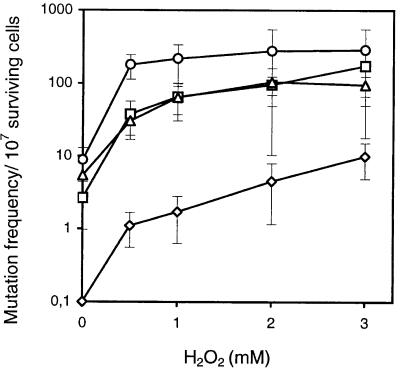
The presence of two E. coli Nth homologues in yeast, Ntg1 and Ntg2, raised the question of whether both functions are essential for protection against oxidative DNA damage or if these genes serve as mutual backup functions. ntg1 and ntg2 single mutants, as well as the ntg1 ntg2 double mutant, were constructed by targeted gene disruption to analyze the in vivo functions of the glycosylase genes. Both single mutants had a considerable increase in the spontaneous mutation frequency relative to wild-type cells (40- to 60-fold), and the mutation frequency was even further increased in the double mutant (Fig. 2). It thus appears that both Ntg1 and Ntg2 are essential for repairing spontaneous DNA damage. Upon exposure to H2O2, 10- to 15-fold increases in mutation frequency were observed for the double as well as single mutants, supporting independent roles of NTG1 and NTG2 in preventing mutations induced by oxidative stress. However, neither of the single mutants nor the double mutant showed any hypersensitivity to H2O2 or other DNA-damaging agents (UV, MMS, NQO, or menadione [data not shown]), in contrast to what we have reported previously for the ntg1 mutant derived from the wild-type strain FL200 (17). We have reinvestigated and confirmed the hypersensitivity of the original ntg1 mutant to menadione and H2O2; the apparent discrepancy must be ascribed to different genetic backgrounds of the two strains.
FIG. 2.
Spontaneous and H2O2-induced mutation frequencies of S. cerevisiae mutants lacking Ntg1 and/or Ntg2. The frequency of mutations to canavanine resistance in S. cerevisiae FF18733 (wild type; ◊), RH101 (ntg1::URA3; □), RH102 (ntg2::LEU2; ▵), and RH103 (ntg1::URA3, ntg2::LEU2; ○) was measured in nontreated cells and cells exposed to H2O2 at the doses indicated. The level of spontaenous mutations plotted for wild-type cells is an upper estimate of the mutation frequency, as no mutants were detected when 108 cells were plated on the canavanine plates.
Purification of Ntg1 and Ntg2.
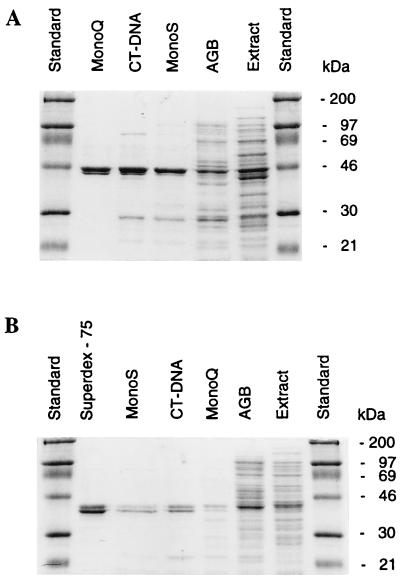
Separate roles of NTG1 and NTG2 as indicated by the mutagenesis studies could possibly be correlated to differences in biochemical properties, e.g., substrate specificity. For biochemical analysis, the NTG1 and NTG2 functions were expressed in E. coli, and the enzymes were purified on the basis of their ability to release faPy residues from DNA. It was initially observed by whole-extract analysis that Ntg2, similar to what was previously observed for Ntg1 but unlike the case for E. coli Nth, was active in the removal of faPy residues from DNA. The fpg mutant background was chosen to avoid any interference of the E. coli formamidopyrimidine DNA glycosylase activity during purification. SDS-PAGE of the purified fractions showed that both enzymes were recovered as two tightly linked bands on the gel with molecular masses of 45.5 and 42 kDa for Ntg1 and Ntg2, respectively, in good agreement with the predicted calculated molecular masses of 45.6 and 43.8 kDa (Fig. 3). We do not know the reason for the appearance of the double band, but such a migration pattern is observed throughout the purification procedure and appears to be associated with the expression and/or the conformation of the enzymes or the electrophoretic separation.
FIG. 3.
SDS-PAGE of different protein fractions obtained during purification of Ntg1 and Ntg2. (A) Ntg1. Samples include molecular weight standards (lanes 1 and 7), MonoQ fraction (lane 2), DNA-cellulose fraction (lane 3), MonoS fraction (lane 4), Affi-Gel Blue (AGB) fraction (lane 5), and cell extract (lane 6). (B) Ntg2. Samples include molecular weight standards (lanes 1 and 8), Superdex-75 fraction (lane 2), MonoS fraction (lane 3), DNA-cellulose fraction (lane 4), MonoQ fraction (lane 5), Affi-Gel Blue fraction (lane 6), and cell extract (lane 7).
Excision of faPy but not 8-oxoG by Ntg1 and Ntg2.
Ntg2 has an activity similar to that of Ntg1 for the excision of faPy residues but fourfold less efficient than that of E. coli Fpg (Fig. 4). However, with excess amounts of enzymes, faPy excision by Ntg1 and Ntg2 reached the level obtained with Fpg, suggesting no qualitative difference in the removal of faPy by Ntg1, Ntg2, and Fpg (data not shown). We also examined the potential activities of purified Ntg1 and Ntg2 toward both hypoxanthine and 8-oxoG in DNA. However, no excision of 8-oxoG or hypoxanthine was observed for DNA substrates containing all combinations of normal bases in the opposite strand (data not shown). This finding agrees with our previous results obtained with Ntg1 expression in E. coli extracts but is apparently inconsistent with the recent report of Bruner et al. (9) that Ntg1/Ogg2 has some activity for 8-oxoG removal, especially opposite A or G in the complementary strand. We found no such activity associated with our enzyme preparations even when as much as 2 μg of Ntg1 or Ntg2 was used in the assays (data not shown). For comparison, 1 ng of enzyme is sufficient for significant cleavage of an oligonucleotide containing Tg (see below). The apparent discrepancy could possibly be ascribed to differences in the sequence contexts of the oligonucleotides used.
FIG. 4.
Release of faPy residues by Ntg1 and Ntg2. Excision of faPy from [3H]faPy-poly(dG-dC)DNA (0.4 μg) was measured after enzyme incubation for 30 min at 37°C with increasing amounts of purified Ntg1(□), Ntg2 (▵), Fpg (◊), and Nth (○).
Removal of UV-induced DNA damage.
In addition to cyclobutyl pyrimidine dimers and pyrimidine (6-4) pyrimidone adducts, UV irradiation (254 nm) of DNA produces faPy residues and single-pyrimidine photoproducts that are mainly cytosine derivatives saturated at the C-5–C-6 double bond (10, 16). Two approaches were used to study the activities of Ntg1 and Ntg2 on UV-irradiated DNA. In one set of experiments, 33P-end-labeled DNA was UV irradiated and incubated with saturating concentrations of Ntg1 and Ntg2, and the formation of enzyme-induced strand breaks was analyzed on DNA sequencing gels (Fig. 5A). Quantification of DNA at each band position indicates that Ntg1 excises cytosine hydrates in all sequence positions more efficiently than Ntg2, whereas purine photoproducts appear to be slightly better substrates for Ntg2. No significant strand breakage was produced by the UV irradiation alone or by incubation with an AP-endonuclease (E. coli Nfo [not shown]). In another approach, UV (254-nm)-irradiated DNA containing 3H-labeled cytosines was used as substrate for glycosylase assays monitoring the release of radiolabeled base residues (Fig. 5B). An excess of Ntg1 and E. coli Nth released equal amounts of cytosine photoproducts, whereas Ntg2 was able to remove only about 50% of the C lesions under conditions of saturating enzyme concentrations. These results show that Ntg1 has a broader substrate range specificity for UV-induced cytosine photoproducts than Ntg2 and appears more similar to E. coli Nth in this respect. It may be that Ntg2 is capable of detecting only one of two different stereo isoforms of cytosine hydrates formed in DNA, but other interpretations are also possible.
FIG. 5.
Activities of Ntg1 and Ntg2 on UV-irradiated DNA. (A) Enzyme cleavage of a 33P-end-labeled UV-irradiated DNA fragment (250 fmol) incubated with an excess amount of Ntg1 and Ntg2 for 30 min at 37°C. The cleavage products were separated on a DNA sequencing gel alongside DNA sequencing reactions. The type of base acted upon for each band position was identified as indicated, and band intensities were quantified by phosphorimaging (Ntg1 [open bars] and Ntg2 [filled bars]). (B) Release of radiolabeled cytosines from UV-irradiated [3H]C-DNA by increasing amounts of purified Ntg1 (□), Ntg2 (▵), and Nth (○) after incubation for 30 min at 37°C.
Enzyme activities on DNA containing Tg and 5-OHC.
Tg and 5-OHC are two major pyrimidine oxidation products in DNA. These lesions have quite different biological effects, since Tg is considered to be primarily cytotoxic (23) and 5-OHC thought to have miscoding properties (18). The activities of Ntg1 and Ntg2 were tested on duplex oligonucleotides containing a single Tg or 5-OHC residue (Fig. 6). Ntg2 cleaved both products efficiently and at a ratio similar to that of E. coli Nth. Ntg1 also efficiently cleaved the oligonucleotide containing the Tg; however, no significant release of 5-OHC by Ntg1 was detected with the enzyme concentrations used. It thus appears that Ntg1 and Ntg2 have separate roles in the removal of cytosine lesions: Ntg1 is more efficient in the excision of UV-induced cytosine hydrates, whereas Ntg2 is required for efficient removal of 5-OHC induced by oxidizing agents.
FIG. 6.
Cleavage of Tg- and 5-OHC-containing DNA. (A) Duplex 37-mer oligodeoxyribonucleotide containing a single Tg at position 19 (10 fmol) was incubated for 30 min at 37°C with 1 and 5 ng of Nth (lanes 1 and 2), Ntg1 (lanes 3 and 4), Ntg2 (lanes 5 and 6), or Nfo (lanes 7 and 8) or no enzyme (lane 9). Cleaved DNA was separated from intact DNA by 20% denaturing PAGE and visualized by phosphorimaging. (B) A duplex 26-mer containing 5-OHC at position 13 (10 fmol) was incubated for 30 min at 37°C with 5 and 20 ng each of Nth, Ntg1, Ntg2, or Nfo or no enzyme as indicated. Cleavage products were analyzed by 20% denaturing PAGE and phosphoimaging. nt, nucleotides.
DNA binding analysis and borhydride trapping of covalent DNA-enzyme intermediates.
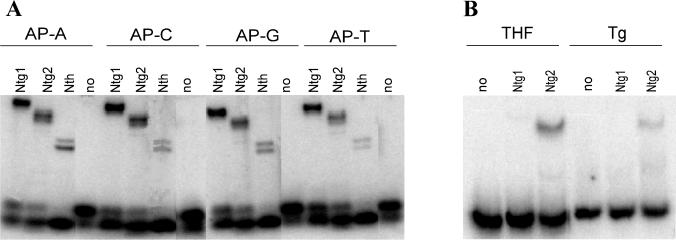
As previously reported, Ntg1 and Ntg2 possess an AP-lyase activity cleaving duplex DNA containing abasic sites (15). DNA glycosylases with associated AP-lyase activities release damaged bases via a nucleophilic attack at the C′-1 carbon and subsequent β elimination for cleavage of the phosphodiester chain. The intermediate covalent imino enzyme-DNA complex can be trapped with NaCNBH3 and detected by SDS-PAGE analysis (15). Trapping experiments with AP-DNA demonstrated complex formation with Ntg1 and Ntg2 independent of the base positioned opposite the abasic site (Fig. 7A). This is in contrast to the 8-oxoG DNA glycosylases from human and yeast, which have a clear preference for acting at abasic sites opposite C (5, 19). However, trapping of Ntg1 was found to be more efficient than that of Ntg2 and E. coli Nth, suggesting a longer lifetime of the enzyme DNA intermediate for Ntg1. In another set of experiments, band shift assays with Tg- or THF-containing DNA produced shifts with Ntg2, whereas no shift could be detected with Ntg1 (Fig. 7B). Further analysis of Ntg2 binding showed shifts with 100-fold excess nondamaged DNA (data not shown), thus demonstrating that Ntg2 has a strong specificity for damaged DNA. There thus appear to be both quantitative and qualitative differences in the reaction mechanisms utilized by Ntg1 and Ntg2 for substrate recognition and interactions with DNA. Further experiments on the kinetics of the protein-DNA interactions are required to elucidate these differences in more detail.
FIG. 7.
Borhydride trapping and DNA binding analysis of Ntg1 and Ntg2. (A) Probing for covalent enzyme–AP-DNA intermediates by NaCNBH3 reduction. Duplex 32P-labeled oligodeoxyribonucleotide (80 fmol) containing a single AP site opposite A (lanes 1 to 4), C (lanes 5 to 8), G (lanes 9 to 12), and T (lanes (13 to 16) was incubated with 40 ng of Ntg1 (lanes 1, 5, 9, and 13), Ntg2 (lanes 2, 6, 10, and 14), or Nth (lanes 3, 7, 11, and 15) or without enzyme (lane 4, 8, 12, and 16). Protein-DNA complexes were separated from DNA by 10% Tricine-SDS-PAGE. (B) Duplex DNA containing a single THF (10 fmol; lanes 1 to 3) or Tg (50 fmol; lanes 4 to 6) residue was incubated with 5 ng of Ntg1 (lanes 2 and 5), 5 ng of Ntg2 (lanes 3 and 6), or no enzyme (lanes 1 and 4) for 15 min at 4°C and analyzed by 10% nondenaturing PAGE.
Cellular localization.
To investigate the subcellular localization and intracellular sorting mechanisms of Ntg1 and Ntg2, yeast cells were transformed by gene constructs of NTG1 or NTG2 fused to GFP at the C- or N-terminal end. Ntg2-GFPc (Fig. 8) and Ntg2-GFPn (data not shown) both produced very strong signals of GFP in the nucleus (yellow staining) and only a weak, even background staining of the cytoplasm. In contrast, Ntg1-GFPc (Fig. 8) was found to accumulate both in the nucleus and in the DAPI-stained cytoplasmic bodies representing the mitochondria. In a separate experiment, the DAPI-stained bodies in the cytoplasm were found to colocalize with a mitochondrion-specific marker (Mitotracker Red; Molecular Probes) (data not shown). Control experiments with an expression vector containing only the GFP protein showed no compartment-specific staining and produced an even background color throughout the cells (Fig. 8). These observations demonstrated that the cellular distributions of the Ntg enzymes are different, Ntg2 being sorted to the nucleus and Ntg1 targeted to both the nucleus and the mitochondria. However, we cannot exclude the possibility that a small percentage of Ntg2, below the detection level of GFP, is sorted to other cellular compartments. Mitochondrial localization of Ntg1 is suggested by a putative N-terminal mitochondrial targeting sequence (Fig. 1; residues 15 to 20) as first predicted by Barton and Kaback (4). Fusion of GFP to the N-terminal part of Ntg1 prevented the mitochondrial sorting (Ntg1-GFPn [Fig. 8]) probably by interfering with the putative leader sequence for mitochondrial membrane transport. Both Ntg1 and Ntg2 contain regions with a high density of positively charged amino acids (residues 14 to 17 and 31 to 37 of Ntg1 and residues 8 to 12 and 376 to 380 of Ntg2 [Fig. 1]), with a strong prediction for nuclear localization signals as indicated by the PSORT algorithm (33).
FIG. 8.
Fluorescence microscopy of S. cerevisiae expressing fusions of Ntg1- and Ntg2 to GFP. S. cerevisiae FF18733 was transformed by DNA constructs expressing GFP alone, NTG1-GFPn, NTG1-GFPc, or NTG2-GFPc. Cells were also stained with DAPI to visualize the DNA in the nucleus and the mitochondria.
S. cerevisiae NTG2 is not DNA damage inducible.
In vivo expression of NTG gene functions was measured by Northern blot analysis (Fig. 9A) and by analysis of gene promoter fusions to the bacterial lacZ reporter gene (Fig. 9B). As previously reported (17), mRNA levels and promoter induction confirm that NTG1 is DNA damage inducible. In contrast, NTG2 is not induced to any significant extent, as indicated by both types of experiments. Moreover, relative basal expression as monitored by the lacZ gene fusion analysis suggested that NTG2 is expressed at a significantly lower level than NTG1 even under noninducible conditions.
FIG. 9.
Expression of NTG1 and NTG2 in S. cerevisiae exposed to DNA-damaging agents. (A) Northern blot analysis. Total RNA isolated from untreated cells and cells exposed to H2O2 (1 mM), menadione (5 mM), MMS (0.05%) and NQO (2 μg/ml) was size separated on a formaldehyde–1% agarose gel and blotted onto a nylon membrane. The blot was hybridized with a 1.2-kb NTG1 probe, stripped, rehybridized with a 1.1-kb NTG2 probe, stripped again, and finally hybridized with a 2.1-kb β-actin probe. Quantification and calculation of the hybridization signals of NTG1/NTG2 relative to β-actin (normalized to 1 for the nontreated samples) were 1.2/0.8, 2.3/0.7, 1.9/1.2, and 1.9/1.3 for H2O2, menadione, MMS, and NQO, respectively. (B) Promoter fusion analysis. Yeast FF18733 carrying the NTG1::lacZ or the NTG2::lacZ fusion on a centromeric plasmid was assayed for β-galactosidase activity expressed in nontreated cells (open bars) or cells exposed to menadione (0.3 mM; grey bars) or MMS (0.05%; filled bars).
DISCUSSION
DNA sequence analysis has revealed the presence of two genes in S. cerevisiae with similarity to E. coli endonuclease III, as has also been indicated by the biochemical purification of two distinct endonuclease III activities (termed ScrI and ScrII) from yeast cell extracts by Augeri et al. (3). We have previously characterized the NTG1 function in yeast by mutant analysis and enzyme expression in E. coli (17). In this work, we purified both Ntg1 and Ntg2 to apparent physical homogeneity and compared their enzyme properties. Furthermore, we constructed S. cerevisiae mutants with single or double defects in NTG1 and NTG2. The two enzymes are similar with respect to the efficiency for the removal of Tg and faPy residues from DNA. However, Ntg1 appears rather inefficient in removal of 5-OHC, which is a good substrate for Ntg2 and E. coli Nth (20). Conversely, Ntg1 was shown to have a broader specificity for UV-induced cytosine-derived DNA damage (Fig. 5). The elevated levels of spontaneous and hydrogen peroxide-induced mutations for both the ntg1 and ntg2 mutants demonstrate that both enzymes are required to repair endogenous as well as induced oxidative DNA damage. It thus appears that despite considerable overlap in enzyme properties, both Ntg1 and Ntg2 seem indispensable for maintaining the integrity of the DNA during normal conditions and genetic stress situations. This observation can possibly be explained by the differences observed in substrate specificity. Furthermore, Ntg1 may also be required to cope with genetic stress conditions because of its inducibility and also have a unique role in the repair of mitochondrial DNA. Damage to the mitochondria has been assumed to affect an increased production of ROS (12), which in turn may damage the cells to an extent that overloads the capacity of the noninducible Ntg2 enzyme in the ntg1 mutant. However, NTG1 and NTG2 may have other, separate roles in repair caused by differences in intracellular complex formation or compartments, as discussed below.
E. coli Nth has been shown to depend on residues Lys 120 and Asp 138 for enzyme activity (40). Lys 120 is proposed to act as a primary nucleophile at the C′-1 carbon deoxyribose effecting both base removal and subsequent strand cleavage by β elimination. Both of these residues are also conserved in Ntg1 (Lys 243 and Asp 262) and Ntg2 (Lys 248 and Asp 267) and are likely to be required for the ɛ-α amino intermediate trapping (Fig. 7). The [4Fe-4S] cluster is thought to be involved in DNA binding of Nth, and this motif is conserved in Ntg2 but absent from Ntg1. The stronger DNA damage binding property of Ntg2 versus Ntg1 is consistent with a role of the [4Fe-4S] cluster in DNA binding. It is suggested from structural analysis of Nth that the positively charged cleft between the [4Fe-4S] cluster and the HhH motif can accommodate DNA with a flipped-out base bound within the active site (40). The same mechanism is likely to apply for Ntg2, whereas further structural analysis is required to elucidate the reaction mode of Ntg1. However, differences in substrate specificity are also observed between Nth and Ntg2 and may result from variable binding of extrahelical modified bases within the active-site pocket. Nth appears to be exclusive for the excision of oxidized pyrimidine residues, whereas the substrate range of the Ntg enzymes also includes formamidopyrimidines. Since imidazole-fragmented purines resemble pyrimidines with steric side groups, it thus appears that Ntg2 may contain a more flexible active-site cleft than Nth.
The DNA in mitochondria is particularly vulnerable to DNA damage from its proximity to ROS produced as by-products during oxidative phosphorylation (12). To our knowledge, the mitochondrial localization of Ntg1 demonstrated in this report is the first direct evidence for an excision repair enzyme being sorted to the mitochondria in yeast and suggests an essential role of this enzyme to avoid accumulation of oxidative DNA damage in mitochondria. Studies of petite rates of the ntg1 mutant compared to the wild type might show the extent to which Ntg1 prevents mutations being formed in the mitochondria.
Although the substrate specificity differences can account for the requirement of both Ntg1 and Ntg2 for yeast repair, other factors may also be involved. It could be that these enzymes operate at different levels of the genome structure. Since chromatin structure affects the accessibility of DNA to proteins, DNA processing reactions including transcription and repair must be coupled and regulated by structural and dynamic properties of chromatin. Heterogeneity of repair of pyrimidine dimers by nucleotide excision repair (NER) and photolyase at different levels of genomic organization has been demonstrated in yeast (38). NER works faster on the transcribed than the nontranscribed strand, in a way not correlated to the chromatin structure. Efficient photolyase reactivation was observed in nonnucleosomal regions, whereas pyrimidine dimers protected by nucleosomes are slowly repaired by the photolyase. However, in active genes dimer removal by photolyase is faster on the nontranscribed than the transcribed strand. Previous in vivo studies of S. cerevisiae have shown that Tg damage is more rapidly repaired on the transcribed than the nontranscribed strand. Moreover, removal of Tg appears to be independent of NER functions, except for the role of the RAD2 gene, suggesting a repair pathway initiated by a DNA glycosylase (29). The acidic domain at the C-terminal end of Ntg1 could be involved in protein-protein interactions, suggesting Ntg1 as a possible candidate of transcription-coupled repair of Tg. In contrast to Ntg1, Ntg2 has a strong affinity for DNA, suggesting that Ntg2 may be able to compete with histones for DNA accessibility. Thus, perhaps Ntg2 to some extent could act in the protected regions of the chromatin structure, implicating a potential global repair function of Ntg2 for oxidative DNA damage. Further investigations of the in vivo role of the NTG1 and NTG2 functions should involve characterization of repair in chromosome structures, including nucleosomes, linker regions, and expressed genes.
During preparation of this report, You et al. (42) published a paper describing the characterization of purified Ntg1/Scr1 and Ntg2/Scr2 from yeast. Their results are similar to ours with respect to activity toward Tg and faPy residues in DNA.
ACKNOWLEDGMENTS
We are grateful to Serge Boiteux for the generous gift of reagent enzymes and strains, to Larry Loeb for 5-OH-dCTP, and to Philip Bolton and Elmer Weinhold for the gift of modified oligonucleotides.
L.E. was a fellow of the Norwegian Cancer Society, and I.A., M.P., and T.R. were fellows of the Norwegian Research Council. E.S. acknowledges grant support from the Norwegian Cancer Society, the Norwegian Research Council, and the Anders Jahres Foundation.
REFERENCES
- 1.Ames B N, Gold L S, Willett W C. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspinwall R, Rothwell D G, Roldan-Arjona T, Anselmino C, Ward C J, Cheadle J P, Sampson J R, Lindahl T, Harris P C, Hickson I D. Cloning and characterization of a functional human homolog of Escherichia coli endonuclease III. Proc Natl Acad Sci USA. 1997;94:109–114. doi: 10.1073/pnas.94.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augeri L, Lee L, Barton A B, Doetsch P W. Purification, characterization, gene cloning, and expression of Saccharomyces cerevisiae redoxyendonuclease, a homolog of Escherichia coli endonuclease III. Biochemistry. 1997;36:721–729. doi: 10.1021/bi9625511. [DOI] [PubMed] [Google Scholar]
- 4.Barton A B, Kaback D B. Molecular cloning of chromosome I DNA from Saccharomyces cerevisiae: analysis of the genes in the FUN38-MAK16-SPO7 region. J Bacteriol. 1994;176:1872–1880. doi: 10.1128/jb.176.7.1872-1880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjørås M, Luna L, Johnsen B, Hoff E, Haug T, Rognes T, Seeberg B. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997;16:6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boiteux S, Huisman O. Isolation of a formamidopyrimidine-DNA glycosylase (fpg) mutant of Escherichia coli K12. Mol Gen Genet. 1989;215:300–305. doi: 10.1007/BF00339732. [DOI] [PubMed] [Google Scholar]
- 7.Boiteux S, Laval J. Imidazole open ring 7-methylguanine: an inhibitor of DNA synthesis. Biochem Biophys Res Commun. 1983;110:552–558. doi: 10.1016/0006-291x(83)91185-3. [DOI] [PubMed] [Google Scholar]
- 8.Boiteux S, O’Connor T R, Laval J. Formamidopyrimidine-DNA glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein. EMBO J. 1987;6:3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruner S D, Nash H M, Lane W S, Verdine G L. Repair of oxidatively damaged guanine in Saccharomyces cerevisiae by an alternative pathway. Curr Biol. 1998;8:393–403. doi: 10.1016/s0960-9822(98)70158-7. [DOI] [PubMed] [Google Scholar]
- 10.Cadet J, Vigny P. Photochemistry of nucleic acids. In: Morrison H, editor. Bioorganic photochemistry. New York, N.Y: John Wiley & Sons; 1990. pp. 1–272. [Google Scholar]
- 11.Caldecott K W, McKeown C K, Tucker J D, Ljungquist S, Thompson L H. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croteau D L, Bohr V A. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272:25409–25412. doi: 10.1074/jbc.272.41.25409. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham R P. DNA glycosylases. Mutat Res. 1997;383:189–196. doi: 10.1016/s0921-8777(97)00008-6. [DOI] [PubMed] [Google Scholar]
- 14.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 15.Dodson M L, Schrock III R D, Lloyd R S. Evidence for an imino intermediate in the T4 endonuclease V reaction. Biochemistry. 1993;32:8284–8290. doi: 10.1021/bi00083a032. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch P W, Zasatawny T H, Martin A M, Dizdaroglu M. Monomeric base damage products from adenine, guanine, and thymine induced by exposure of DNA to ultraviolet radiation. Biochemistry. 1995;34:737–742. doi: 10.1021/bi00003a005. [DOI] [PubMed] [Google Scholar]
- 17.Eide L, Bjørås M, Pirovano M, Alseth I, Berdal K G, Seeberg E. Base excision of oxidative purine and pyrimidine DNA damage in Saccharomyces cerevisiae by a DNA glycosylase with sequence similarity to endonuclease III from Escherichia coli. Proc Natl Acad Sci USA. 1996;93:10735–10740. doi: 10.1073/pnas.93.20.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig D I, Sowers L C, Loeb L A. Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci USA. 1994;91:6609–6613. doi: 10.1073/pnas.91.14.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard P M, Guibourt P M, Boiteux S. The Ogg1 protein of Saccharomyces cerevisiae: a 7,8-dihydro-8-oxoguanine DNA glycosylase/AP-lyase whose lysine 241 is a critical residue for catalytic activity. Nucleic Acids Res. 1997;25:3204–3211. doi: 10.1093/nar/25.16.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatahet Z, Kow Y W, Purmal A A, Cunningham R P, Wallace S S. New substrates for old enzymes. 5-Hydroxy-2′-deoxycytidine and 5-hydroxy-2′-deoxyuridine are substrates for Escherichia coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2′-deoxyuridine is a substrate for uracil DNA N-glycosylase. J Biol Chem. 1994;269:18814–18820. [PubMed] [Google Scholar]
- 21.Hilbert T P, Chaung W, Boorstein R J, Cunningham R P, Teebor G W. Cloning and expression of the cDNA encoding the human homologue of the DNA repair enzyme, Escherichia coli endonuclease III. J Biol Chem. 1997;272:6733–6740. doi: 10.1074/jbc.272.10.6733. [DOI] [PubMed] [Google Scholar]
- 22.Hill J, Donald K A, Griffiths D E. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ide H, Kow Y W, Wallace S S. Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res. 1985;13:8035–8052. doi: 10.1093/nar/13.22.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasai H, Nishimura S. Formation of 8-hydroxydeoxyguaninosine in DNA by oxygen radicals and its biological significance. In: Sies H, editor. Oxidative stress: oxidants and antioxidants. London, England: Academic Press; 1991. pp. 99–116. [Google Scholar]
- 25.Kasai H, Crain P F, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 26.Krokan H E, Standal R, Slupphaug G. DNA glycosylases in the base excision repair of DNA. Biochem J. 1997;325:1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota Y, Nash R A, Klungland A, Schär P, Barnes D, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase β and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo C F, McRee D E, Fisher C L, O’Handley S F, Cunningham R P, Tainer J A. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science. 1992;258:434–440. doi: 10.1126/science.1411536. [DOI] [PubMed] [Google Scholar]
- 29.Leadon S A, Barbee S L, Dunn A-B. The yeast RAD2, but not RAD1, gene is involved in the transcription-coupled repair of thymine glycols. Mutat Res. 1995;337:169–178. doi: 10.1016/0921-8777(95)00021-b. [DOI] [PubMed] [Google Scholar]
- 30.Macreadie I G, Jagadish M N, Azad A A, Vaughan P R. Versatile cassettes designed for the copper inducible expression of protein in yeast. Plasmid. 1989;21:147–150. doi: 10.1016/0147-619x(89)90059-0. [DOI] [PubMed] [Google Scholar]
- 31.Melamede R J, Hatahet Z, Kow Y W, Ide H, Wallace S S. Isolation and characterization of endonuclease VIII from Escherichia coli. Biochemistry. 1994;33:1255–1264. doi: 10.1021/bi00171a028. [DOI] [PubMed] [Google Scholar]
- 32.Michaels M L, Pham L, Cruz C, Miller J H. MutM, a protein that prevents G.C----T.A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1991;19:3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roldan-Arjona T, Anselmino C, Lindahl T. Molecular cloning and functional analysis of a Schizosaccharomyces pombe homologue of Escherichia coli endonuclease III. Nucleic Acids Res. 1996;24:3307–3312. doi: 10.1093/nar/24.17.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouet P, Essigmann J M. Possible role for thymine glycol in the selective inhibition of DNA synthesis on oxidized DNA templates. Cancer Res. 1985;45:6113–6118. [PubMed] [Google Scholar]
- 36.Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci USA. 1978;75:2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeberg E, Eide L, Bjørås M. The base excision repair pathway. Trends Biochem Sci. 1995;20:391–397. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 38.Suter B, Livingstone-Zatchej M, Thoma F. Chromatin structure modulates DNA repair by photolyase in vivo. EMBO J. 1997;16:2150–2160. doi: 10.1093/emboj/16.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tchou J, Kasai H, Shibutani S, Chung M H, Laval J, Grollman A P, Nishimura S. 8-Oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci USA. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thayer M M, Ahern H, Xing D, Cunningham R P, Tainer J A. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. EMBO J. 1995;14:4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, Coulson A, Craxton M, Dear S, Du Z, Durbin R, Favello A, Fraser A, Fulton L, Gardner A. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- 42.You H J, Swanson R L, Doetsch P W. Saccharomyces cerevisiae possesses two functional homologues of Escherichia coli endonuclease III. Biochemistry. 1998;37:6033–6040. doi: 10.1021/bi973042h. [DOI] [PubMed] [Google Scholar]