Abstract
Mungbeans and lentils are relatively easily grown and cheaper sources of microgreens, but their phytonutrient diversity is not yet deeply explored. In this study, 20 diverse genotypes each of mungbean and lentil were grown as microgreens under plain-altitude (Delhi) and high-altitude (Leh) conditions, which showed significant genotypic variations for ascorbic acid, tocopherol, carotenoids, flavonoid, total phenolics, DPPH (1, 1-diphenyl-2-picrylhydrazyl), FRAP (ferric-reducing antioxidant power), peroxide activity, proteins, enzymes (peroxidase and catalase), micronutrients, and macronutrients contents. The lentil and mungbean genotypes L830 and MH810, respectively, were found superior for most of the studied parameters over other studied genotypes. Interestingly, for most of the studied parameters, Leh-grown microgreens were found superior to the Delhi-grown microgreens, which could be due to unique environmental conditions of Leh, especially wide temperature amplitude, photosynthetically active radiation (PAR), and UV-B content. In mungbean microgreens, total phenolics content (TPC) was found positively correlated with FRAP and DPPH, while in lentil microgreens, total flavonoid content (TFC) was found positively correlated with DPPH. The most abundant elements recorded were in the order of K, P, and Ca in mungbean microgreens; and K, Ca, and P in the lentil microgreens. In addition, these Fabaceae microgreens may help in the nutritional security of the population residing in the high-altitude regions of Ladakh, especially during winter months when this region remains landlocked due to heavy snowfall.
Keywords: Vigna microgreens, Lens microgreens, Fabaceae microgreens, antioxidants, mineral composition
Introduction
Microgreens are 7 to 21-day-old, 3- to 8-cm-long seedlings mostly produced from the seeds of vegetables, herbs, and pulses and harvested at the first true leaf stage of plants (Xiao et al., 2012). Microgreens are gaining popularity during the last few years as they provide an array of textures, colors, flavors, and aromas to the food. They have outstanding nutritional and antioxidant properties and are also considered “functional foods” (Kyriacou et al., 2016). Microgreens are reported four to six times nutrient-rich than their mature counterparts (Xiao et al., 2012), as, during germination, there is an extensive breakdown of seed-storage compounds and simultaneous synthesis of structural proteins and other cell components (Danilcenko et al., 2017). Additionally, microgreens generate little or no food wastage during consumption as no biomass (except roots) gets wasted as trimming (Weber, 2017).
They differ from “sprouts” as they need light and a growing medium and have a longer growth cycle which may vary depending upon the species used, with an edible portion being stem and a pair of first true leaves (Xiao et al., 2012). Depending on the growth stage of the plant, the phytonutrient levels differ, and often a reduction is recorded from the seedling (sprout, microgreen) to the fully developed stage (Nakamura et al., 2001; Barillari et al., 2005).
In recent years, the microgreens market is growing rapidly (Charlebois, 2018; Riggio et al., 2019) and is also sold as a “living product” with the growing media. This helps consumers use them fresh as per their convenience (Renna et al., 2017). Till now, it has gained the market mostly in the western countries. However, in India, it is gaining popularity typically in the metro cities. The US is the major microgreens contributor in the year 2019 and was followed by Canada and Mexico. The global microgreens market is expected to grow at a CAGR of 7.5% from 2020 to 2025 (https://www.mordorintelligence.com/industry-reports/microgreens-market). Broccoli, lettuce, arugula, and basil are key microgreens grown across the regions under hydroponics and vertical farming.
Diet-related diseases like diabetes, obesity, hypertension, cancer, etc., are on the rise in both developing and developed countries, and are partly due to the imbalanced intake of the food and are mostly below recommended levels [World Health Organization and Food and Agriculture Organization of the United Nations (WHO/FAO), 2005; Choe et al., 2018]. Food supplementation through microgreens is known to modulate weight gain, cholesterol metabolism and protects against cardiovascular diseases (Pinto et al., 2015; Huang et al., 2016). Eradication of hidden global hunger and food insecurity is the key component of the sustainable development goals, and we should aim to achieve Zero Hunger and better nutrition by 2030 (www.fao.org; White and Broadley, 2009). For food diversification, microgreens are considered a novel product rich in several phytochemicals, including vitamins, antioxidants, and minerals. Increasing culinary demand and the ease of microgreens cultivation have generated a lot of interest in both growers and the consumers (Weber, 2017).
In South Asia, mungbean is consumed mainly as porridge/dhal, while in the rest of Asia, it is consumed as sprouts or noodles. Moreover, pulse sprouts have long been an essential, year-round component of Asian and vegan diets (Ebert et al., 2015a). Mungbean sprouts are used in different combination as a dietary supplement in healthcare; while lentil is also rich in fiber, protein, and complex carbohydrates (Gan et al., 2017). In addition, pulses are low in glycemic index, which makes them a good food choice for any diabetic person on a controlled diet (http://www.urbancultivator.net/herbguide/lentils/).
Rapid growth cycle, limited space requirement, and high economic produce make microgreens a nutrient alternative that may contribute to the nutritional security in the plain regions and the high-altitude areas of Ladakh (India) (Angmo et al., 2019). Ladakh is situated at 3,500 m AMSL and remains landlocked for 6 months due to heavy snowfall. Thus, this region needs novel and economically sustainable technology like microgreens (using cheap and abundant sources such as lentil and mungbean) in a big way to assure the nutritional security of the population residing in that region, especially during landlocked conditions of the winter months (Cohen and Garrett, 2010). Microgreens have the potential to contribute to food and nutritional security in the Ladakh sector as they can be easily grown at any altitude where land and low temperature is often a limiting factor, either under the greenhouse or even in the houses (near the glass window), independent of seasonal growth cycles (Ebert et al., 2015b, 2017). Light conditions are highly influential on the morpho-physiology of microgreens and the biosynthesis and accumulation of phytochemicals (Delian et al., 2015). However, there is scarce information about the nutritional properties of mungbean and lentil microgreens when grown under different environmental conditions. Against this backdrop, the present study has been carried out to find the comparative phytonutrient composition of a set of lentils and mungbean microgreens when grown under plain-altitude (Delhi) and high-altitude conditions of Leh-Ladakh (India).
Materials and Methods
Genotypes Used for Lentil and Mungbean Microgreens and Growing Conditions
To identify the genotypic differences for various biochemical parameters, a set of 20 diverse genotypes of mungbean and lentils were used for the analysis (Figure 1; Supplementary File 1). These were grown under partially controlled conditions in the National Phytotron Facility, IARI, New Delhi, located at the latitude and longitude of 28.6412°N, 77.1627°E, respectively, and an elevation of 228.61 m AMSL. The desired temperature was maintained for mungbean (28/26°C) and lentil (21/18°C) along with natural day and night cycles. Same genotypes were also grown under the greenhouse at Leh-Ladakh, which is situated at 3500 m AMSL at the latitude and longitude of 34.1383°N, 77.5727°E, respectively. The day length in terms of sunshine hours (h) was recorded as 10.4 ± 0.007 h and 10.3 ± 0.005 h at Leh and Delhi, respectively, during the microgreens growing period (Supplementary File 1).
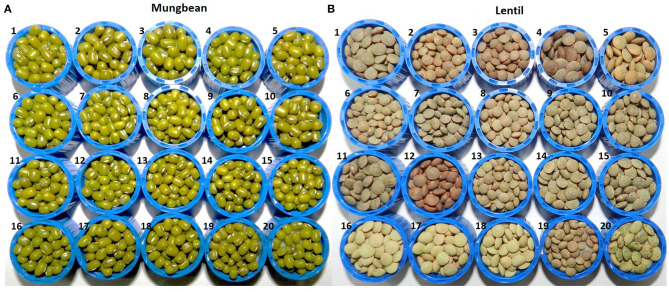
Figure 1.
Seed morphology details of 20 genotypes each of (A) mungbean and (B) lentils used in the study, where (A) mungbean genotypes are (1) Pusa Baisakhi, (2) Pusa Ratna, (3) Pusa Vishal, (4) Pusa105, (5) Pusa0672, (6) Pusa9072, (7) Pusa9531, (8) MH96-1, (9) MH318, (10) MH421, (11) MH521, (12) MH810, (13) ML512, (14) ML818, (15) PS16, (16) TM 96-2, (17) IPM02-3, (18) IPM02-14, (19) IPM409-4, (20) PMR1; while (B) lentil genotypes are (1) L4076, (2) L4147, (3) L4594, (4) L7903, (5) HM1, (6) BM4, (7) JL1, (8) Sehore74-3, (9) NDL1, (10) IPL81, (11) IPL321, (12) K75, (13) KLS218, (14) DPL58, (15) DPL62, (16) PL1, (17) PL2, (18) PL6, (19) L830, (20) L4602.
The sample under study was sown during the first week of November 2019 at both Delhi and Leh conditions. Seeds were sown in three replicates in the seedling trays, having 50 cells per tray of cell-size 4.5 ×4.5 ×5.7 cm. The growing media consisted of coco peat: vermiculite: sand in the ratio of 2:1:1 for both mungbean and lentil. Harvesting of the microgreens was performed once it reached the optimum stage. Mungbean and lentil microgreens were harvested on the 7th and 9th days after sowing, respectively. Microgreens were manually collected using ethanol-cleaned scissors by cutting the stem ~1.0 cm above the growing medium. Harvested microgreens were immediately weighed using analytical balance to determine the total fresh weight (FW) and are used for various analyses. In addition, a set of microgreens were also dried in hot air GenLab vertical oven at 40.0°C for 72.0 h and are kept in airtight containers. Before analysis, the samples were kept in a vacuum desiccator for 24 h.
Total Phenolics Content (TPC)
Total phenolics content was analyzed by modified Folin–Ciocalteu colorimetric method (Singleton et al., 1999) using regression equation of the calibration curve of Gallic acid (conc. from 50 to 500 μg/mL) and expressed as mg Gallic acid equivalents per g FW (mg GAE/g FW).
Total Flavonoids Content (TFC)
Ethanolic extract was prepared by crushing the 0.1 g of sample in 80% ethanol, and to 0.5 mL sample, 0.5 mL of 2% AlCl3 ethanolic solution was added, which was then incubated for 1.0 h at room temperature, absorbance was measured at 420 nm. TFC was estimated as quercetin equivalent from a calibration curve (Woisky and Salatino, 1998).
DPPH Scavenging Activity
The methanolic extract was prepared by crushing the 0.1 g sample in 1 mL of methanol, and this was used to determine the DPPH scavenging activity by measuring the absorbance of the mixture spectrophotometrically at 517 nm (Brand-Williams and Berset, 1995) and the ability to scavenge DPPH radical was calculated using the following equation:
| (1) |
where AbsControl is the absorbance of DPPH radical + methanol; AbsSample is the absorbance of DPPH radical + sample extract.
FRAP Assay
For extract preparation, a 0.1 g sample was crushed using liquid N2, then 1.0 mL ethanol (80%) was added, and the homogenate was centrifuged (12,000 rpm; 15.0 min). The supernatant was collected, and 100 μL extract per genotype was used for FRAP reaction (Benzie and Strain, 1999). To this 3.0 mL pre-warmed freshly prepared FRAP reagent (acetate buffer: TPTZ: FeCl3.6H2O in 10:1:1 ratio) was added, and the mixture was incubated (37°C for 10.0 min) and increase in absorbance was measured using a spectrophotometer at 593 nm, and was compared with that of the standard calibration curve (20 mM FeSO4.7H2O), and the final concentration was expressed as mM/g of FW.
Peroxide Quantification
Briefly, 0.1 g leaf samples were crushed in liquid N2, homogenized in 3.0 mL trichloroacetic acid (TCA; 1.0% w/v), centrifuged (10,000 rpm; 15 min at 4.0°C), and the supernatant was collected. Subsequently, 0.75 mL supernatant was mixed with 0.75 mL potassium phosphate buffer (10 mM; pH 7.0) and 1.5 mL freshly prepared potassium iodide (KI; 1.0 M) solution. The peroxide content was quantified in the supernatant by comparing the absorbance at 390 nm with that of the standard calibration curve ranging from 10 to 200 μmol/mL of H2O2. The final concentration was expressed as μmol/g of FW (Loreto and Velikova, 2001).
Total Tocopherol Content (TTC)
Tocopherol content was determined by the bathophenanthroline method (Tsen, 1961), which utilizes iron (Fe) (III)-bathophenanthroline complex as the oxidizing agent. The sample extract was exposed to an ethanolic solution comprising 4,7-diphenyl-1,10-phenanthroline (Bathophenanthroline) and FeCl3, and to this H3PO4 was added after 15 s, and its stability was observed for 60 min. The absorbance of the solution was measured at 534 nm, and the tocopherol concentration was calculated from a standard curve and expressed in μg/g of FW.
Total Carotenoids Content (TCC)
Briefly, 0.34 g of sample was crushed in liquid N2, then 6.0 mL ethyl alcohol:BHT (1.0 mg of BHT/mL of ethanol) was added and incubated at 85°C for 6 min with continuous vortexing. To this, 120 μL KOH was added and incubated for 5.0 min (at 85°C), then cooled on ice and then 4.0 mL distilled water and 3.0 mL PE:DE (2:1, v/v) was added. This was centrifuged for 10 min at room temperature, and the upper phase containing carotenoids was collected, and the solution was diluted to 10 mL (Lichtenthaler and Wellburn, 1983). The increase in absorbance was measured at 470 nm, and total carotenoids content was calculated using the formula:
| (2) |
where Atotal= absorbance at 450 nm; Vol (mL) = total volume of extract; A1% = absorbance coefficient for carotenoid by column mixture.
Ascorbic Acid
Ascorbic acid estimation was done through titration method using 2,6 dichloroindophenols dye solution and 4% oxalic acid as a stabilizing medium (Sadasivam and Balasubraminan, 1987; Nielsen, 2017). The dye solution was prepared in NaHCO3 hot solution, while dye standardization was done by titrating the standard ascorbic acid (1.0 mg/mL) to pale pink color, which persisted for 15.0 s, and calculation was done using the following formula:
| (3) |
where Xmg = mg of standard ascorbic acid; V1 = Titer value of Standard ascorbic acid against dye; V2 = Titer value of sample against dye; YmL = Amount of aliquot taken (mL) for estimation; ZmL = total amount (mL) of the extracted sample.
Protein and Enzyme Extract Preparation
Briefly, 0.1 g fresh sample of each genotype was ground in liquid N2, then 3.0 mL of 0.1 M potassium phosphate buffer (pH 7.0) was added, and the homogenate was centrifuged at 13,000 rpm for 20 min at 4.0°C. The supernatant was used for the estimation of enzymatic activity and total soluble protein.
Total Soluble Protein
The Bradford assay was used to determine the total soluble protein in the fresh lentil and mungbean samples (Bradford, 1976). The binding of protein molecules to Coomassie dye under acidic conditions results in a change in color from brown to blue, measured at 420 nm, and a calibration graph was prepared using 2, 4, 6, 8, 10 mg/mL BSA.
Peroxidase (POD) Activity
For the estimation of POD activity (Lin and Kao, 1999), 976 μL potassium phosphate buffer (50.0 mM; pH 7.0) was mixed with 3.0 μL supernatant. To this, 10.0 μL guaiacol (900 mM) and 20.0 μL H2O2 (500 mM) were added, and guaiacol oxidation was determined by measuring the increase in absorbance at 470 nm. Enzyme activity was calculated using the extinction coefficient of 26.60 mM−1cm−1 for guaiacol and expressed as U min−1mg−1 protein of oxidized guaiacol.
Catalase (CAT) Enzymatic Activity
CAT activity was estimated in the supernatant by measuring the decline in absorbance (decomposition of H2O2) at 240 nm (Aebi, 1984).
Determination of Mineral Composition
Sample Preparation
The thoroughly dried samples were first homogenized using Agate mortar and pestle, and 100 mg powder was put in a cup having 23.9 mm aperture. The sample powder was gently pressed using an acrylic piston to prevent any void in the sample. The sample thickness was kept as 7.0 mm and was put in a vacuum desiccator for 24 h to make it completely moisture-free.
Sample Analysis Using Energy Dispersive X-ray Fluorescence Spectrometer (EDXRF)
Analyses of metals were carried out using EDXRF (Epsilon5 Spectrometer, PANalytical, United Kingdom) fitted with PAN-32 Ge detector. The 3-D polarized optics and low power improved the detection limit by lowering the spectral background for which samples were repeatedly used in the Epsilon 5 spectrometer. The resolution of the instrument was >150 eV. As the EDXRF scans the whole periodic table, the individual elements were detected at certain specific sites. During sampling, the instrument was run at a flow rate of 30.0 L/min for 24 h at all the sites. The EDXRF spectrometer was pre-calibrated before running, which has performed a semi-quantitative analysis of elements with 5–10% error. One blank was included for every 20 samples analyzed for quality assurance, and all the samples were run in triplets for accuracy.
Photosynthetically Active Radiation (PAR), UV-B, and Photoperiod
The PAR and UV-B were recorded with a radiometer (PMA2100, Solar Light, USA), and the details of photoperiod were obtained from the Indian Agricultural Research Institute, New Delhi (India), and Defence Institute of High Altitude Research, Leh-Ladakh (India).
Statistical Analysis
The experiments were conducted in three replications, and the results were presented as mean ± SD. One-way ANOVA was performed using SPSS11.5 to compare the groups, and Pearson's correlation test was used to assess the correlation between means. The mean comparison was performed using Tukey's test. Dendrograms were constructed using the Ward method, and distance is expressed as Euclid distance, and P <0.05 was regarded as significant. Principal component analysis (PCA) was performed on various antioxidant activities [e.g., DPPH, FRAP, peroxide, TTC, TCC, total ascorbic acid (TAA), POD, and CAT], protein content, phenolics [total phenolics content (TPC)], and flavonoids contents (TFC) in the mungbean and lentil microgreens when grown at normal altitude (Delhi) and high altitude (Leh) conditions. Analysis was done using STAR ver. 2.0.1 software and PCA plot were generated based on the first and second principal components (PC1 and PC2).
Results and Discussion
Total Phenolics Content (TPC)
The nutraceutical potential of microgreens is usually determined by their phenolics content which exhibits antioxidant, anti-inflammatory, and anticancer properties (Chon, 2013). TPC in the mungbean and lentil microgreens ranged from 190.92 to 240.81 and 212.60 to 276.98 mg GAE/100 g FW, respectively when grown at Delhi; while the same ranged from 200.34 to 241.75 and 218.23 to 321.35 mgGAE/100 g FW, respectively when grown under Leh conditions (Table 1). In mungbean, the genotype MH810 and in lentil the genotype DPL62 showed maximum TPC. Overall, TPC was found relatively more in the lentil microgreens (212.60 to 321.35 mgGAE/100 g FW) over mungbean microgreens (190.92–241.75 mgGAE/100 g FW). Similarly, a wide range of TPC ranging from 88.6 mgGAE/100 g FW in Upland cress to 811.2 mg GAE/100 g FW in Radish ruby has been reported (Xiao et al., 2019). However, no significant differences were recorded for either TPC or overall antioxidant capacity in broccoli microgreens when grown on either soil media or under hydroponic conditions (Tan et al., 2020).
Table 1.
Mean concentration of total phenolics, flavonoids, carotenoids, tocopherols, and ascorbic acid in mungbean microgreens grown under Delhi and Leh conditions.
| S. No. | Genotype | TPC (mg GAE/100 g FW) | TFC (mg/100 g FW) | TTC (mg/100 g FW) | TCC (mg/100 g FW) | TAA (mg/100 g FW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1. | Pusa Baisakhi | 230.34 ± 5.78ab | 234.09 ± 7.44abc | 3.27 ± 0.20ab | 4.41 ± 0.08fg | 1.72 ± 0.058a | 1.74 ± 0.176ab | 7.03 ± 0.24ef | 7.77 ± 0.33de | 34.55 ± 1.28b | 65.04 ± 2.99efg |
| 2. | PusaRatna | 234.67 ± 8.85ab | 235.19 ± 13.26ab | 2.92 ± 0.36b | 5.12 ± 0.11ab | 1.73 ± 0.035a | 1.68 ± 0.055abc | 7.43 ± 0.47cdef | 7.92 ± 0.21de | 32.37 ± 1.45c | 63.53 ± 4.28efg |
| 3. | Pusa Vishal | 209.15 ± 5.21defg | 203.63 ± 1.62de | 3.44 ± 0.11ab | 4.07 ± 0.09h | 1.70 ± 0.057ab | 1.82 ± 0.065a | 7.90 ± 0.33cde | 8.72 ± 0.35cd | 34.67 ± 1.45ab | 73.81 ± 4.28bcd |
| 4. | Pusa105 | 224.46 ± 5.21abcd | 232.38 ± 9.58abc | 3.29 ± 0.23ab | 4.60 ± 0.17ef | 1.59 ± 0.015cd | 1.62 ± 0.102abcde | 8.27 ± 0.28c | 9.05 ± 0.35bc | 34.30 ± 2.48bc | 88.63 ± 2.99a |
| 5. | Pusa0672 | 240.71 ± 14.06a | 233.94 ± 16.20abc | 2.93 ± 0.03b | 4.57 ± 0.14ef | 1.17 ± 0.027k | 1.14 ± 0.051g | 6.98 ± 0.26f | 7.37 ± 0.47e | 33.94 ± 2.31bc | 67.46 ± 7.27def |
| 6. | Pusa9072 | 220.71 ± 5.21bcde | 232.90 ± 7.37abc | 2.99 ± 0.77b | 4.25 ± 0.11gh | 1.24 ± 0.009jk | 1.28 ± 0.032fg | 7.25 ± 0.59def | 8.03 ± 0.19de | 33.15 ± 1.37bc | 76.23 ± 3.42bc |
| 7. | Pusa9531 | 199.25 ± 4.17fg | 207.90 ± 8.84de | 2.81 ± 0.12b | 4.06 ± 0.10h | 1.29 ± 0.052ij | 1.42 ± 0.079def | 7.97 ± 0.14cd | 8.27 ± 0.33cde | 34.24 ± 2.22ab | 82.58 ± 2.99ab |
| 8. | MH96-1 | 194.88 ± 6.77fg | 219.35 ± 1.47bcd | 2.95 ± 0.29b | 5.25 ± 0.07a | 1.52 ± 0.045de | 1.56 ± 0.048abcde | 7.72 ± 0.31cdef | 7.78 ± 0.54de | 35.51 ± 1.11ab | 71.69 ± 2.14cde |
| 9. | MH318 | 202.69 ± 2.08efg | 203.21 ± 2.21de | 3.13 ± 0.25ab | 5.13 ± 0.13ab | 1.48 ± 0.017ef | 1.51 ± 0.090bcdef | 10.67 ± 0.38b | 9.97 ± 0.14b | 35.88 ± 1.45ab | 76.84 ± 2.57bc |
| 10. | MH421 | 207.06 ± 8.33defg | 236.02 ± 10.31ab | 2.95 ± 0.89b | 5.17 ± 0.12a | 1.47 ± 0.051ef | 1.49 ± 0.044bcdef | 11.43 ± 0.47ab | 12.00 ± 0.38a | 33.58 ± 1.28bc | 65.04 ± 2.99efg |
| 11. | MH521 | 196.96 ± 6.77fg | 218.31 ± 8.84bcde | 3.23 ± 0.14ab | 4.73 ± 0.08cde | 1.48 ± 0.085ef | 1.42 ± 0.079bcdef | 12.22 ± 0.59a | 12.07 ± 0.33a | 34.36 ± 1.03bc | 67.16 ± 4.28def |
| 12. | MH810 | 240.81±4.17a | 241.75±2.21a | 3.71±0.18a | 4.84 ± 0.15bcde | 1.32 ± 0.034hij | 1.40 ± 0.061def | 12.10±0.38a | 12.10±0.80a | 35.33±1.20ab | 87.73±4.28a |
| 13. | ML512 | 194.25 ± 5.21fg | 208.26 ± 4.94de | 3.35 ± 0.23ab | 4.64 ± 0.21def | 1.62 ± 0.031bc | 1.67 ± 0.163abcd | 7.20 ± 0.47def | 7.58 ± 0.59e | 33.82 ± 0.94bc | 65.46 ± 3.59efg |
| 14. | ML818 | 190.92 ± 1.56g | 213.42 ± 10.46de | 2.99 ± 0.22b | 4.13 ± 0.12gh | 1.70 ± 0.040ab | 1.71 ± 0.103ab | 7.27 ± 0.42def | 7.67 ± 0.38e | 34.55 ± 1.63b | 65.34 ± 3.42efg |
| 15. | PS16 | 192.27 ± 5.73fg | 209.20 ± 1.10de | 2.89 ± 0.11b | 4.41 ± 0.33fg | 1.59 ± 0.020cd | 1.63 ± 0.052abcde | 7.32 ± 0.54def | 7.60 ± 0.66e | 34.30 ± 1.11bc | 57.78 ± 3.85g |
| 16. | TM96-2 | 192.79 ± 4.17fg | 200.34 ± 5.52e | 3.09 ± 0.14ab | 5.22 ± 0.14a | 1.57 ± 0.015cd | 1.69 ± 0.056ab | 7.12 ± 0.16def | 7.32 ± 0.35e | 35.03 ± 1.97ab | 63.83 ± 3.85efg |
| 17. | IPM02-3 | 210.66 ± 5.36cdef | 206.80 ± 7.29de | 3.21 ± 0.12ab | 5.07 ± 0.12ab | 1.18 ± 0.027k | 1.56 ± 0.371bcde | 7.42 ± 0.31cdef | 8.05 ± 0.35cde | 36.00 ± 2.48a | 64.43 ± 2.99efg |
| 18. | IPM02-14 | 219.56 ± 3.96bcde | 216.75 ± 9.58cde | 3.41 ± 0.15ab | 4.95 ± 0.13abcd | 1.44 ± 0.008efg | 1.55 ± 0.047bcde | 7.32 ± 0.49def | 7.50 ± 0.61e | 34.91 ± 1.11ab | 61.11 ± 4.28fg |
| 19. | IPM409-4 | 198.16 ± 5.78fg | 220.45 ± 10.24bcd | 3.37 ± 0.24ab | 5.03 ± 0.14abc | 1.36 ± 0.025ghi | 1.43 ± 0.040cdef | 7.28 ± 0.54def | 7.43 ± 0.66e | 34.73 ± 1.54ab | 66.85 ± 2.14def |
| 20. | PMR-1 | 229.56 ± 5.21abc | 241.75 ± 5.16a | 2.85 ± 0.29b | 5.11 ± 0.08ab | 1.40 ± 0.08gfh | 1.41 ± 0.077ef | 7.50 ± 0.24cdef | 7.67 ± 0.57e | 35.21 ± 2.40ab | 62.62 ± 2.99fg |
TPC, total phenolics content; TFC, total flavonoids content; TCC, total carotenoids content; TTC, total tocopherols content; TAA, total ascorbic acid. Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Total Flavonoids Content (TFC)
Flavonoids generally have more antioxidant activity (AoA) than phenolics (Heim et al., 2002), and they have anticancer properties due to their role in the signal transduction pathways of cell division (Aron and Kennedy, 2008). When grown at Leh, TFC was found significantly more for mungbean microgreens (4.06–5.25 mg/100 g FW) than the lentil microgreens (1.06–2.84 mg/100 g FW) and was also higher than those of the Delhi-grown mungbean (2.81–3.71 mg/100 g FW) and lentil (0.98–1.86 mg/100 g FW) microgreens (Tables 1, 2). Similarly, the flavonoids in other culinary microgreens like mustard (1.1 mg/100 g FW) and roselle (6.5 mg/100 g FW) were recorded in a similar range (Ghoora et al., 2020). In addition, Swieca and Gwalik-Dziki (2015) recorded TFC in the 6-day sprouts of mungbean (0.57 mg/g DW) and lentil (0.57 mg/g DW); whereas, Ebert et al. (2017) reported flavonoids (as Kaempferol) in the sprouts of different mungbean genotypes in the range of 0.32 to 0.40 μmol/100 g.
Table 2.
Mean concentration of total phenolics, flavonoids, carotenoids, tocopherols, and ascorbic acid in lentil microgreens grown under Delhi and Leh conditions.
| S. No. | Genotype | TPC (mg GAE/100 g FW) | TFC (mg/100 g FW) | TTC (mg/100 g FW) | TCC (mg/100 g FW) | TAA (mg/100 g FW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | L4076 | 259.35 ± 12.20abcde | 290.10 ± 6.19bcde | 1.66 ± 0.023c | 2.14 ± 0.023d | 1.10 ± 0.014e | 1.11 ± 0.006efgh | 14.18 ± 0.08h | 14.70 ± 0.368i | 16.94 ± 0.86fghi | 24.50 ± 0.43efg |
| 2 | L4147 | 235.85 ± 8.66efghi | 245.73 ± 7.07hij | 1.23 ± 0.068g | 1.39 ± 0.068f | 1.01 ± 0.070g | 1.10 ± 0.024fgh | 14.48 ± 0.17gh | 16.10 ± 0.198h | 17.12 ± 0.26fghi | 25.53 ± 0.17cde |
| 3 | L4594 | 260.73 ± 14.14abcd | 302.60 ± 6.19abc | 1.83 ± 0.034a | 2.11 ± 0.091d | 1.08 ± 0.004ef | 1.20 ± 0.232defg | 20.4 ± 0.06a | 20.70 ± 0.085bc | 17.91 ± 0.51ef | 26.74 ± 0.17ab |
| 4 | L7903 | 248.23 ± 8.84bcdefg | 276.98 ± 10.61def | 1.72 ± 0.011bc | 2.14 ± 0.113d | 1.07 ± 0.007efg | 1.05 ± 0.007h | 18.52 ± 0.23b | 18.56 ± 0.113e | 17.00 ± 0.94fghi | 24.64 ± 0.60efg |
| 5 | HM1 | 222.54 ± 5.04hi | 228.85 ± 16.79ijk | 1.86 ± 0.034a | 2.58 ± 0.079b | 1.24 ± 0.013ab | 1.09 ± 0.014gh | 16.82 ± 0.48cd | 17.02 ± 0.198g | 16.15 ± 0.26i | 25.17 ± 0.34cdef |
| 6 | BM4 | 266.35 ± 4.42ab | 288.23 ± 5.30bcde | 0.98 ± 0.102h | 1.22 ± 0.011gh | 1.12 ± 0.014cde | 1.12 ± 0.008efgh | 17.16 ± 0.17c | 17.82 ± 0.311f | 16.64 ± 0.43hi | 25.71 ± 0.43bcd |
| 7 | JL1 | 241.98 ± 3.54cdefgh | 292.60 ± 4.42bcd | 1.23 ± 0.045g | 1.39 ± 0.045f | 1.22 ± 0.029ab | 1.21 ± 0.021cdefg | 15.34 ± 0.82fg | 14.40 ± 0.453i | 16.76 ± 0.26ghi | 25.77 ± 0.51bc |
| 8 | Sehore74-3 | 218.48 ± 18.56hi | 224.48 ± 12.37jk | 1.39 ± 0.023f | 1.94 ± 0.068e | 1.02 ± 0.025fg | 1.11 ± 0.022efgh | 15.5 ± 0.08ef | 15.76 ± 0.283h | 17.48 ± 0.09fgh | 24.50 ± 0.43efg |
| 9 | NDL-1 | 252.23 ± 11.49bcdef | 281.35 ± 7.95cde | 1.54 ± 0.091d | 2.66 ± 0.091b | 1.20 ± 0.023ab | 1.22 ± 0.024cdefg | 14.74 ± 0.54fgh | 15.68 ± 0.280h | 17.30 ± 0.51fgh | 24.68 ± 1.03defg |
| 10 | IPL81 | 212.60 ± 6.19i | 225.73 ± 17.68jk | 1.42 ± 0.045ef | 2.32 ± 0.181c | 1.25 ± 0.008a | 1.24 ± 0.018cde | 16.44 ± 0.34cd | 16.68 ± 0.283g | 17.18 ± 0.34fghi | 25.53 ± 0.17cde |
| 11 | IPL321 | 221.35 ± 11.49hi | 224.48 ± 1.77jk | 1.53 ± 0.057de | 2.61 ± 0.113b | 1.22 ± 0.019ab | 1.23 ± 0.005cdef | 16.66 ± 0.48cd | 20.48 ± 0.170bc | 17.79 ± 0.34efg | 26.02 ± 0.17abc |
| 12 | K75 | 251.98 ± 11.25bcdef | 270.73 ± 15.91efg | 1.82 ± 0.045ab | 2.62 ± 0.045b | 1.21 ± 0.007ab | 1.40 ± 0.010a | 20.58 ± 0.20a | 20.96 ± 0.171b | 17.91 ± 0.34ef | 26.86 ± 0.34a |
| 13 | KLS218 | 271.98 ± 15.91ab | 307.60 ± 9.72ab | 1.22 ± 0.034g | 1.06 ± 0.034h | 1.12 ± 0.004cde | 1.34 ± 0.132abc | 19.14 ± 0.59b | 19.22 ± 0.141d | 21.42 ± 0.51a | 24.26 ± 0.09fg |
| 14 | DPL58 | 261.91 ± 5.21abc | 287.60 ± 6.19bcde | 1.24 ± 0.011g | 1.24 ± 0.011fg | 1.21 ± 0.036ab | 1.24 ± 0.013cde | 19.32 ± 0.34b | 19.4 ± 0.226d | 21.30 ± 0.68a | 23.96 ± 0.34g |
| 15 | DPL62 | 276.98 ± 15.91a | 321.35 ± 7.95a | 1.25 ± 0.045g | 1.23 ± 0.068fg | 1.10 ± 0.024de | 1.19 ± 0.030defg | 19.36 ± 0.57b | 20.32 ± 0.226c | 18.57 ± 0.60de | 23.84 ± 0.51g |
| 16 | PL1 | 234.48 ± 8.84fghi | 218.23 ± 5.30k | 1.23 ± 0.068g | 1.13 ± 0.011gh | 1.21 ± 0.005ab | 1.26 ± 0.010bcd | 17.1 ± 0.54cd | 17.98 ± 0.424f | 19.84 ± 0.17bc | 24.02 ± 0.60g |
| 17 | PL2 | 224.16 ± 10.16ghi | 235.73 ± 8.84hijk | 1.62 ± 0.011cd | 2.50 ± 0.091b | 1.18 ± 0.034bc | 1.16 ± 0.059defgh | 16.24 ± 0.28de | 16.66 ± 0.255g | 19.66 ± 0.43c | 24.02 ± 0.0.60g |
| 18 | PL6 | 231.29 ± 7.87fghi | 250.10 ± 7.95ghi | 1.23 ± 0.068g | 2.23 ± 0.011cd | 1.17 ± 0.036bcd | 1.17 ± 0.018defgh | 16.76 ± 0.34cd | 18.08 ± 0.170ef | 19.00 ± 0.34cd | 24.50 ± 0.43efg |
| 19 | L830 | 234.23 ± 10.78fghi | 255.85 ± 12.20fgh | 1.86±0.023a | 2.84±0.057a | 1.21±0.005ab | 1.38±0.019ab | 20.62±0.20a | 22.22±0.198a | 20.75±0.26ab | 25.71 ± 0.43bcd |
| 20 | L4602 | 236.85 ± 8.84defgh | 285.10 ± 9.72cde | 1.37 ± 0.034f | 2.09 ± 0.079de | 1.00 ± 0.089g | 1.04 ± 0.009h | 17.02 ± 0.37cd | 18.08 ± 0.057ef | 18.57 ± 0.60de | 26.14 ± 0.68abc |
TPC, total phenolics content; TFC, total flavonoids content; TCC, total carotenoids content; TTC, total tocopherols content; TAA, total ascorbic acid. Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Total Tocopherol Content (TTC)
Among lipophilic antioxidants, the TTC in the mungbean and lentil microgreens were recorded ranging from 1.17–1.73 and 1.0–1.25 mg/100 g FW, respectively, when grown at Delhi; while the same ranged from 1.14–1.82 and 1.04–1.4 mg/100 g FW when grown under Leh conditions (Tables 1, 2). Overall, the mungbean (Pusa Ratna and Pusa Vishal) and lentil (L830 and K75) genotypes expressed higher TTC (Tables 1, 2). However, relatively higher TTC was recorded in brassica-based microgreens, ranging from 1.8 mg/100 g FW in watercress to 5.1 mg/100 g FW in red radish (Xiao et al., 2019).
Total Carotenoids Content (TCC)
The antioxidant capacity of the carotenoid is due to the presence of conjugated double bonds, which gives them the free radical scavenging property (Yang et al., 2018). When grown at Leh, the TCC was found significantly less for mungbean microgreens (7.32–12.10 mg/100 g FW) than the lentil microgreens (14.40–22.22 mg/100 g FW) but was higher than those of the Delhi-grown mungbean (6.98–12.22 mg/100 g FW) and lentil (14.18–20.62 mg/100 g FW) microgreens (Tables 1, 2). The mungbean genotypes, MH521 and MH810, and lentil genotypes L830 and L4594 were better for TCC. Nearly similar TCC was also recorded among 30 brassica microgreens which ranged from 8.2 (Pak Choy) to 18.8 mg/100 g FW (Upland cress) (Xiao et al., 2019). However, Brazaityte et al. (2015b) recorded relatively more TCC in mustard (17.06), red Pak Choi (40.43), and tatsoi (36.03 mg 100/g FM) microgreens under normal illumination.
Total Ascorbic Acid (TAA)
The TAA in mungbean and lentil microgreens was recorded from 32.37 to 36.00 and 16.15 to 21.42 mg/100 g FW, respectively, when grown in Delhi, and the same ranged from 61.11 to 88.63 and 23.84 to 26.86 mg/100 g FW, respectively, when grown under Leh conditions. Significantly more TCC were recorded for the mungbean genotypes IPM02-3, MH310, and Pusa105, while lentil genotypes L830, KLS218, and K78 showed relatively higher TAA content (Tables 1, 2). Similarly, in various brassica-derived microgreens like pepper cress and cauliflower, the TAA was recorded to the tune of 32.9 and 120.8 mg/100 g FW, respectively (Xiao et al., 2019). Similarly, significantly higher vitamin C content was recorded for the soil-grown broccoli microgreens over hydroponically grown samples (Tan et al., 2020).
Non-enzymatic AoA (DPPH, FRAP, and H2O2 Assay)
Since different mechanisms are associated with the AoA of any plant; thus, this can be determined through various ways capturing varied modes of action like reducing abilities (FRAP), antiradical ability (DPPH), preventive effect against lipid (H2O2), and ability to chelate transition metals ions (Prior et al., 2005). Significantly more total AoA was recorded when mungbean microgreens were grown at Leh (FRAP: 11.73–20.22 μmol TE/g FW; DPPH: 3.2–3.79 μmol TE/g FW; H2O2: 5.08–6.14 nmol/g FW) than when grown at Delhi (FRAP: 5.7–11.30 μmol TE/g FW; DPPH: 0.69–1.585 μmol TE/g FW; H2O2: 2.61–4.74 nmol/g FW). In lentil too, the total AoA have recorded more for the Leh-grown microgreens (FRAP: 21.09–55.00 μmol TE/g FW; DPPH: 2.87–3.38 μmol TE/g FW; H2O2: 4.55–5.92 nmol/g FW) than the Delhi-grown samples (FRAP: 14.7–37.94 μmol TE/g FW; DPPH: 0.84–1.55 μmol TE/g FW; H2O2: 1.63–2.24 nmol/g FW). Overall, the mungbean genotype MH810 and lentil genotype L830 recorded higher total non-enzymatic AoA (Tables 3, 4).
Table 3.
Total antioxidant activity, crude protein, and antioxidant enzymatic activities in the mungbean microgreens grown under Delhi and Leh conditions.
| S. No. | Genotypes | FRAP (μmol TE/g FW) | DPPH (μmol TE/g FW) | H 2 O 2 (nmol/g FW) | Crude protein (g/100 g FW) | Peroxidase Activity (U/g FW) | Catalase Activity (U/g FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | Pusa Baisakhi | 9.91 ± 0.515abc | 18.44 ± 0.980abcd | 1.139 ± 0.131bcd | 3.33 ± 0.039cd | 3.75 ± 0.059fg | 5.54 ± 0.124abcdegh | 3.40 ± 0.01e | 2.35 ± 0.04gh | 292.71 ± 3.51bcd | 307.37 ± 5.32e | 383.76 ± 43.07bcdefgh | 456.85 ± 43.07abc |
| 2 | Pusa Ratna | 9.90 ± 0.707abc | 19.29 ± 1.263abc | 1.176 ± 0.105bc | 3.55 ± 0.380abc | 2.91 ± 0.041l | 5.47 ± 0.041abcdefg | 3.35 ± 0.06ef | 2.83 ± 0.08a | 329.77 ± 50.40abc | 458.01 ± 31.74abcd | 398.98 ± 25.84abcdef | 347.21 ± 8.61ghi |
| 3 | Pusa Vishal | 9.63 ± 0.874bc | 16.15 ± 0.990efg | 1.069 ± 0.111bcde | 3.79 ± 0.334a | 3.13 ± 0.024jk | 5.31 ± 0.065defg | 3.58 ± 0.24abcde | 2.46 ± 0.14fgh | 336.92 ± 19.25ab | 452.14 ± 39.40bcd | 370.05 ± 28.00bcdefgh | 345.69 ± 32.30ghi |
| 4 | Pusa105 | 9.49 ± 0.510bc | 18.35 ± 0.505bcd | 1.116 ± 0.137bcd | 3.36 ± 0.144cd | 3.22 ± 0.059ij | 5.86 ± 0.253abcde | 3.39 ± 0.04e | 2.56 ± 0.11cdefgh | 302.33 ± 10.10bcd | 445.60 ± 34.29cd | 321.32 ± 10.77h | 350.25 ± 17.23gh |
| 5 | Pusa0672 | 10.44 ± 2.015ab | 20.25 ± 0.970ab | 1.315 ± 0.144ab | 3.32 ± 0.065cd | 3.41 ± 0.058hi | 6.09 ± 0.194ab | 3.45 ± 0.01de | 2.78 ± 0.10abc | 281.95 ± 52.10cd | 427.33 ± 17.39cd | 421.83 ± 19.38abc | 470.56 ± 23.69a |
| 6 | Pusa9072 | 9.46 ± 0.495bcd | 17.31 ± 1.035cdef | 1.102 ± 0.132bcd | 3.49 ± 0.033bcd | 2.92 ± 0.041kl | 5.90 ± 0.919abcd | 3.57 ± 0.01abcde | 2.81 ± 0.07ab | 312.93 ± 11.06abcd | 460.83 ± 49.44abcd | 397.46 ± 23.69abcdef | 391.37 ± 36.61bcdefgh |
| 7 | Pusa9531 | 8.63 ± 0.450cde | 15.03 ± 0.490gh | 1.046 ± 0.105bcdef | 3.44 ± 0.052bcd | 2.61 ± 0.024m | 5.56 ± 0.613abcdefg | 3.52 ± 0.05abcde | 2.53 ± 0.12defgh | 355.41 ± 49.23a | 466.58 ± 20.26abcd | 423.35 ± 21.54abcde | 371.57 ± 38.77defgh |
| 8 | MH96-1 | 7.74 ± 0.495e | 13.88 ± 0.909hi | 0.889 ± 0.039cdefg | 3.33 ± 0.039cd | 3.45 ± 0.018h | 5.82 ± 0.206abcdef | 3.52 ± 0.34abcde | 2.87 ± 0.15a | 325.04 ± 5.42abc | 446.28 ± 8.77cd | 426.40 ± 43.07abcd | 360.91 ± 40.92fgh |
| 9 | MH318 | 8.79 ± 0.455cde | 14.94 ± 1.364gh | 1.051 ± 0.098bcdef | 3.69 ± 0.046ab | 3.44 ± 0.006h | 5.33 ± 0.324cdefg | 3.51 ± 0.15abcde | 2.37 ± 0.06gh | 325.19 ± 7.12abc | 473.01 ± 34.77abcd | 342.64 ± 28.00fgh | 434.01 ± 49.53abcde |
| 10 | MH421 | 9.40 ± 0.303bcd | 16.04 ± 1.010efg | 1.065 ± 0.105bcde | 3.72 ± 0.105ab | 4.74 ± 0.065b | 6.04 ± 0.583abc | 3.70 ± 0.02ab | 2.72 ± 0.16abcde | 307.52 ± 5.32abcd | 474.47 ± 21.21abcd | 383.76 ± 64.61bcdefgh | 441.62 ± 68.92abcd |
| 11 | MH521 | 8.00 ± 0.343de | 15.32 ± 0.919fgh | 0.949 ± 0.007cdefg | 3.20 ± 0.144d | 4.60 ± 0.035bc | 5.95 ± 0.483abcd | 3.38 ± 0.08ef | 2.69 ± 0.12abcdef | 338.12 ± 30.09ab | 458.68 ± 39.71abcd | 395.94 ± 30.15abcdefgh | 362.44 ± 30.15efgh |
| 12 | MH810 | 11.30±0.510a | 20.44±1.100a | 1.585±0.421a | 3.55±0.111abc | 3.91 ± 0.171f | 6.14±0.065a | 3.75±0.02a | 2.74±0.15abcde | 326.92±10.63abc | 513.50±33.65ab | 467.51±36.61a | 462.94±12.92ab |
| 13 | ML512 | 7.81 ± 0.596e | 13.81 ± 1.010hi | 0.843 ± 0.065defg | 3.52 ± 0.039abc | 3.57 ± 0.301gh | 5.08 ± 0.088g | 3.45 ± 0.01cde | 2.58 ± 0.05bcdefg | 321.20 ± 5.53abc | 482.03 ± 7.97abc | 322.84 ± 55.99gh | 429.44 ± 17.23abcdef |
| 14 | ML818 | 5.70 ± 0.500f | 11.73 ± 0.465j | 0.690 ± 0.020g | 3.56 ± 0.052abc | 2.96 ± 0.200kl | 5.35 ± 0.047cdef | 3.42 ± 0.02e | 2.34 ± 0.13h | 322.48 ± 12.65abc | 452.93 ± 8.29bcd | 239.09 ± 10.79i | 368.53 ± 25.84efgh |
| 15 | PS16 | 5.72 ± 0.551f | 12.40 ± 0.101ij | 0.759 ± 0.079fg | 3.58 ± 0.033abc | 5.14 ± 0.053a | 5.13 ± 0.035fg | 3.38 ± 0.04e | 2.51 ± 0.06efgh | 317.29 ± 14.89abcd | 419.21 ± 38.76d | 351.78 ± 40.92defgh | 275.63 ± 58.15i |
| 16 | TM96-2 | 6.07 ± 0.460f | 12.65 ± 0.247ij | 0.796 ± 0.118efg | 3.60 ± 0.013abc | 3.76 ± 0.483fg | 5.39 ± 0.053bcdefg | 3.15 ± 0.28f | 2.45 ± 0.08gh | 325.56 ± 12.76abc | 518.80 ± 33.49a | 365.48 ± 47.38cdefgh | 385.28 ± 6.46cdefgh |
| 17 | IPM02-3 | 9.40 ± 0.455bcd | 16.74 ± 0.379defg | 1.074 ± 0. 118bcde | 3.48 ± 0.046bcd | 2.78 ± 0.012lm | 5.36 ± 0.106cdefg | 3.68 ± 0.08abc | 2.34 ± 0.05h | 268.35 ± 5.42d | 457.22 ± 28.39abcd | 441.62 ± 21.54ab | 405.08 ± 21.54abcdefg |
| 18 | IPM02-14 | 9.39 ± 0.505bcd | 16.65 ± 0.854defg | 1.088 ± 0.0.111bcde | 3.50 ± 0.072abc | 4.18 ± 0.053e | 5.18 ± 0.171efg | 3.44 ± 0.04cde | 2.75 ± 0.15abcd | 266.17 ± 9.57d | 511.80 ±± 15.95ab | 465.99 ± 43.07a | 367.01 ± 10.77efgh |
| 19 | IPM409-4 | 7.84 ± 0.434e | 13.82 ± 1.212hi | 1.019 ± 0.092bcdef | 3.51 ± 0.092abc | 4.37 ± 0.041de | 5.15 ± 0.029efg | 3.47 ± 0.02bcde | 2.54 ± 0.13defgh | 321.65 ± 25.52abc | 459.70 ± 6.70abcd | 420.30 ± 8.61abcde | 446.19 ± 19.38abc |
| 20 | PMR-1 | 9.51 ± 0.505bc | 17.87 ± 1.515cde | 1.125 ± 0.124bcd | 3.44 ± 0.105bcd | 4.41 ± 0.047cd | 6.10 ± 0.183ab | 3.67 ± 0.01abcd | 2.55 ± 0.04cdefgh | 304.96 ± 4.04abccd | 476.84 ± 31.26abcd | 347.21 ± 25.84efgh | 319.80 ± 17.23hi |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Similarly, a wide range of DPPH activity was recorded in the upland cress (1.57 μmol TE/g FW) and radish ruby-based microgreens (8.06 μmol TE/g FW) (Xiao et al., 2019). However, relatively higher DPPH activity was also recorded in basil (9.60 μmol/g), beet (7.91 μmol/g), and pak choi (7.65 μmol/g) (Brazaityte et al., 2015a). Additionally, the mustard microgreens produced from cold plasma (at 21 and 23 kV) treated seeds showed significantly more DPPH activity over control (Saengha et al., 2021). FRAP activity in onion microgreens was recorded as 7.0 μmol Fe2+/g, while this was 36.3 μmol Fe2+/g in roselle (Ghoora et al., 2020). H2O2 in leaf tissue is known to respond to environmental stimuli (Cheeseman, 2006). Hydrogen peroxide acts as a signaling molecule in the plant system and imparts a second line of defense, and its increase is associated with oxidative damage (Ozden et al., 2009). There are only very few reports on peroxide content estimation in microgreens. In Bruguiera parviflora leaf tissue, the concentrations of H2O2 were reported increasing from 0.067 to 0.089 μmol (gFW)−1 when salinization treatment was given (Parida and Das, 2005). A similar increasing trend of H2O2 content was also recorded for Leh-grown microgreens over Delhi-grown microgreens.
Total Crude (Soluble) Protein Content
Microgreens showed large variations for total crude protein content between genotypes and also between mungbean and lentil species. When grown in Delhi, the mungbean microgreens showed more crude protein content (3.15–3.75 mg/100 g FW) than when grown at Leh conditions (2.34–2.87 mg/100 g FW). Also, in the lentil microgreens, the protein content was recorded more for the Delhi samples (2.18–2.67 mg/100 g FW) over Leh-grown samples (1.84–2.16 mg/100 g FW). The lentil genotype L830 and mungbean genotype MH810 showed maximum protein content (Tables 3, 4). Similarly, in Cichorium intybus and Brassica oleracea derived microgreens, the protein contents were estimated as 1.9 and 3.0 mg/100 FW, respectively (Paradiso et al., 2018). More protein content was recorded for the mungbean microgreens among lentil and mungbean microgreens when grown at either Delhi or Leh conditions.
Table 4.
Total antioxidant activity, crude protein, and enzymatic antioxidant activities of lentil microgreens grown under Delhi and Leh environmental conditions.
| S. No. | Genotypes | FRAP (μmol TE/g FW) | DPPH (μmol TE/g FW) | H 2 O 2 (nmol/g FW) | Protein (g/100 g FW) | Peroxidase Activity (U/g FW) | Catalase Activity (U/g FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | L4076 | 25.24 ± 1.49de | 36.36 ± 0.13efg | 1.08 ± 0.013c | 3.15 ± 0.026cde | 2.347 ± 0.059ab | 4.551 ± 0.018b | 2.22 ± 0.02de | 1.91 ± 0.04bc | 341.97 ± 6.93fg | 466.41 ± 17.23abc | 193.40 ± 2.15efg | 370.05 ± 49.53abcde |
| 2 | L4147 | 30.76 ± 0.37b | 44.61 ± 3.21bc | 0.92 ± 0.039c | 3.06 ± 0.065efg | 2.138 ± 0.177abc | 5.176 ± 0.041ab | 2.28 ± 0.17cde | 1.99 ± 0.13abc | 437.59 ± 19.57a | 478.52 ± 67.64ab | 159.90 ± 36.61g | 367.01 ± 88.30abcde |
| 3 | L4594 | 19.34 ± 0.30gh | 27.88 ± 1.21ij | 1.19 ± 0.065bc | 3.14 ± 0.020cde | 1.959 ± 0.242bcdef | 4.784 ± 0.194b | 2.56 ± 0.13abcd | 1.88 ± 0.02bc | 418.94 ± 4.36ab | 495.10 ± 16.64a | 190.36 ± 40.92efg | 261.93 ± 17.23ghi |
| 4 | L7903 | 18.11 ± 1.60h | 26.03 ± 0.80j | 1.09 ± 0.020c | 3.17 ± 0.026cd | 2.355 ± 0.024ab | 4.601 ± 0.289b | 2.24 ± 0.06cde | 2.04 ± 0.22abc | 369.67 ± 13.97defg | 459.18 ± 57.78abc | 292.39 ± 30.15abc | 333.50 ± 62.46bcdefg |
| 5 | HM1 | 14.70 ± 0.42i | 21.09 ± 0.62k | 1.55 ± 0.483a | 3.21 ± 0.039c | 1.780 ± 0.012cdef | 4.872 ± 0.059b | 2.18 ± 0.02e | 2.01 ± 0.06abc | 380.14 ± 40.84bcdef | 491.87 ± 20.44a | 216.24 ± 4.31defg | 287.82 ± 58.15efghi |
| 6 | BM4 | 23.26 ± 0.36ef | 33.61 ± 1.48gh | 0.84 ± 0.039c | 3.00 ± 0.052g | 1.705 ± 0.035def | 4.713 ± 0.047b | 2.34 ± 0.06abcde | 1.91 ± 0.08bc | 394.48 ± 16.97abcde | 437.63 ± 10.54abc | 289.34 ± 8.61abc | 321.32 ± 23.69bcdefgh |
| 7 | JL1 | 24.68 ± 0.12de | 35.69 ± 1.95efg | 1.00 ± 0.046c | 3.04 ± 0.072fg | 1.659 ± 0.041ef | 4.772 ± 0.200b | 2.39 ± 0.16abcde | 1.98 ± 0.07abc | 414.62 ± 10.75abc | 401.53 ± 10.49c | 289.34 ± 21.54abc | 300.00 ± 32.30defghi |
| 8 | Sehore74-3 | 27.06 ± 1.57cd | 39.11 ± 0.04de | 1.05 ± 0.033c | 3.11 ± 0.026def | 1.638 ± 0.035f | 4.988 ± 0.189ab | 2.40 ± 0.12abcde | 1.96 ± 0.13abc | 427.32 ± 4.07a | 437.53 ± 46.88abc | 309.14 ± 19.38ab | 389.85 ± 47.38abcd |
| 9 | NDL-1 | 19.42 ± 1.68gh | 27.93 ± 0.80ij | 1.08 ± 0.007c | 3.38 ± 0.013a | 1.672 ± 0.047ef | 4.808 ± 0.487b | 2.34 ± 0.17abcde | 1.93 ± 0.14bc | 346.87 ± 17.62fg | 460.29 ± 36.80abc | 313.71 ± 43.07ab | 402.03 ± 21.54ab |
| 10 | IPL-81 | 23.36 ± 1.41ef | 33.70 ± 0.05fgh | 1.06 ± 0.026c | 3.18 ± 0.007cd | 2.384 ± 0.065a | 4.904 ± 0.308b | 2.29 ± 0.02bcde | 1.88 ± 0.07bc | 335.33 ± 10.30g | 410.47 ± 9.13bc | 190.36 ± 28.00efg | 347.21 ± 21.54abcdef |
| 11 | IPL321 | 26.81 ± 0.04cd | 38.81 ± 2.25de | 1.06 ± 0.026c | 3.31 ± 0.020ab | 2.309 ± 0.065ab | 5.279 ± 0.579ab | 2.25 ± 0.14cde | 1.87 ± 0.02c | 346.37 ± 11.04fg | 449.87 ± 78.51abc | 257.36 ± 36.61abcd | 274.11 ± 12.92fghi |
| 12 | K75 | 37.94 ± 2.04a | 55.00 ± 0.33a | 1.11 ± 0.013c | 3.34 ± 0.046a | 2.243 ± 0.147ab | 4.902 ± 0.676b | 2.33 ± 0.07abcde | 1.84 ± 0.04c | 380.71 ± 17.36bcdef | 485.73 ± 17.71ab | 251.27 ± 19.38bcde | 365.48 ± 30.15abcde |
| 13 | KLS218 | 30.26 ± 0.95b | 43.90 ± 4.01bc | 0.92 ± 0.007c | 2.87 ± 0.065h | 2.251 ± 0.053ab | 5.435 ± 0.335ab | 2.58 ± 0.36abc | 1.94 ± 0.11abc | 406.95 ± 2.26abcd | 479.59 ± 18.48ab | 184.26 ± 40.92fg | 237.56 ± 38.77hi |
| 14 | DPL58 | 21.44 ± 0.47fg | 30.94 ± 1.16hi | 1.01 ± 0.059c | 3.03 ± 0.052fg | 2.026 ± 0.572abcdef | 5.239 ± 0.494ab | 2.24 ± 0.27cde | 2.09 ± 0.08ab | 401.83 ± 22.09abcd | 502.80 ± 24.64a | 251.27 ± 2.15bcde | 345.69 ± 75.38abcdefh |
| 15 | DPL62 | 26.12 ± 0.54cde | 37.78 ± 1.46de | 1.02 ± 0.052c | 2.99 ± 0.013g | 2.122 ± 0.059abcd | 4.813 ± 0.648b | 2.55 ± 0.34abcd | 1.93 ± 0.09bc | 367.11 ± 28.68defg | 436.77 ± 32.57abc | 319.80 ± 38.77a | 217.77 ± 2.15i |
| 16 | PL1 | 25.66 ± 0.23cde | 37.12 ± 1.88efg | 0.96 ± 0.013c | 2.98 ± 0.092g | 2.063 ± 0.519abcde | 5.128 ± 0.356ab | 2.22 ± 0.13de | 2.03 ± 0.12abc | 371.76 ± 10.74cdefg | 496.95 ± 31.90a | 239.09 ± 15.08cdef | 363.96 ± 2.15abcdef |
| 17 | PL2 | 28.53 ± 3.20bc | 41.18 ± 2.20cd | 1.03 ± 0.072c | 3.23 ± 0.059bc | 2.138 ± 0.047abc | 5.075 ± 0.820ab | 2.55 ± 0.08abcd | 1.88 ± 0.06bc | 428.89 ± 22.38a | 426.66 ± 19.99abc | 280.20 ± 34.46abc | 335.03 ± 43.07bcdefg |
| 18 | PL6 | 25.95 ± 2.46cde | 37.45 ± 1.35def | 0.97 ± 0.013c | 3.14 ± 0.020cde | 2.180 ± 0.035abc | 5.925 ± 0.735a | 2.57 ± 0.09abcd | 1.92 ± 0.05bc | 348.19 ± 26.80fg | 472.14 ± 23.63abc | 278.68 ± 40.92abcd | 391.37 ± 10.77abc |
| 19 | L830 | 31.28 ± 0.73b | 45.31 ± 1.65b | 1.53±0.509ab | 3.37±0.033a | 2.247±0.047ab | 4.684 ± 0.666b | 2.67±0.16a | 2.16±0.15a | 395.51±35.79abcd | 495.84±22.94a | 237.56 ± 25.84cdef | 435.53±38.77a |
| 20 | L4602 | 26.00 ± 0.33cde | 37.60 ± 1.77de | 1.04 ± 0.034c | 3.11 ± 0.013def | 2.305 ± 0.071ab | 4.949 ± 0.551ab | 2.65 ± 0.06ab | 1.97 ± 0.05abc | 352.28 ± 17.61efg | 492.06 ± 23.40a | 286.29 ± 12.92abc | 303.05 ± 6.46cdefghi |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Antioxidant Enzyme Activity (POD and CAT)
The peroxidase activity in the mungbean microgreens, when grown at Delhi (266.17–355.41 U/g of FW), was significantly less than those of Leh-grown microgreens (307.37–518.8 U/g of FW). Similarly, peroxidase activity was recorded more for the Leh-grown lentil microgreens (401.53–502.80 U/g of FW) than the Delhi samples (335.33–437.59 U/g of FW) (Tables 3, 4). The POD activity in the lentil microgreens was found much higher than that reported in 5-day-old lentil sprouts (139.17 U/g of FW) (Swieca and Gwalik-Dziki, 2015). However, POD activity to the tune of 596.67 (U/g FW) has been recorded in the 2-day-old lentil sprouts when given continuous elicitation with 150 mM H2O2 (Swieca and Gwalik-Dziki, 2015).
CAT is the most efficient enzyme, which neutralizes H2O2 into H2O and O2, and its Kcat was the highest among all the antioxidant enzymes (Singh et al., 2015). Large variations were observed for the CAT activities of both mungbean and lentil microgreens when grown either at Leh or Delhi conditions. No specific trend has been recorded for the mungbean microgreens when grown at Delhi (239.09–467.51 U/g FW) or Leh conditions (275.63–470.56 U/g FW). Similar observations were also recorded for the lentil microgreens when grown at Delhi (159.9–319.80 U/g FW) or Leh conditions (217.77–435.53 U/g FW) (Tables 3, 4). Nearly similar CAT activity (444.00 U/g of FW) was reported in the 5-day-old lentil sprouts (Swieca and Gwalik-Dziki, 2015).
Correlation Studies and Cluster Analysis
The correlation of measured values between total AoA (H2O2, FRAP, DPPH activity), CAT, POD, TPC, TFC, TTC, TCC, and TAA was studied using Pearson's correlation method (Table 5). In mungbean, TPC was found positively correlated with FRAP (P <0.01) and DPPH (P <0.05), while in lentil, TFC was found positively correlated with DPPH (P <0.01). Interestingly, FRAP and DPPH activity was also found positively correlated (P <0.01) in mungbean, while TCC and TAA in lentil microgreens (P <0.01). Also, a significant (P <0.05) negative correlation has been observed between CAT and TCC in mungbean microgreens. DPPH having a significantly positive correlation with FRAP (r = 0.966) was also reported by Fidrianny et al. (2018) in sweet potatoes. This means the AoA of samples is linearly correlated in DPPH and FRAP methods. Thus, these assays indicate that phenolic compounds (in mungbean) and flavonoid content (in lentils) are mainly contributing to the antioxidant activities, which is in tune with the previous studies (Bhoyar et al., 2011, 2018; Fidrianny et al., 2018). Interestingly, TAA was also found significantly correlated with FRAP (r = 0.501; P <0.05) and total carotenoid content (r = 0.704; P <0.01) in lentil microgreens, indicating their role in the total AoA of lentil microgreens. Such correlation studies can give a more precise idea of the parameter(s) contributing to the AoA (Patel et al., 2016; Bhalani et al., 2019; Devi et al., 2019).
Table 5.
Correlation between various antioxidant and enzymatic parameters in (a) Mungbean and (b) Lentil microgreens.
| (a) Mungbean | DPPH | FRAP | H2O2 | CAT | POD | TPC | TFC | TTC | TCC | TAA |
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 1 | 0.709** | 0.036 | 0.473* | 0.178 | 0.532* | 0.28 | −0.202 | 0.353 | 0.371 |
| FRAP | 1 | −0.052 | 0.574** | −0.195 | 0.888** | 0.206 | −0.391 | 0.273 | 0.388 | |
| H2O2 | 1 | −0.188 | 0.178 | 0.128 | 0.383 | 0.269 | 0.262 | −0.374 | ||
| CAT | 1 | −0.085 | 0.367 | 0.436 | −0.563** | 0.244 | 0.200 | |||
| POD | 1 | −0.308 | 0.219 | −0.098 | 0.334 | 0.272 | ||||
| TPC | 1 | 0.124 | −0.321 | 0.135 | 0.184 | |||||
| TFC | 1 | −0.02 | 0.312 | −0.014 | ||||||
| TTC | 1 | −0.159 | −0.231 | |||||||
| TCC | 1 | 0.397 | ||||||||
| TAA | 1 | |||||||||
| (b) Lentil | ||||||||||
| DPPH | 1 | −0.115 | −0.213 | 0.231 | 0.029 | −0.342 | 0.909** | 0.309 | 0.233 | 0.066 |
| FRAP | 1 | 0.385 | 0.079 | 0.169 | −0.033 | −0.045 | 0.304 | 0.236 | 0.501* | |
| H2O2 | 1 | −0.203 | 0.007 | −0.206 | −0.076 | 0.158 | 0.229 | 0.445* | ||
| CAT | 1 | −0.182 | −0.258 | 0.224 | 0.004 | −0.201 | −0.141 | |||
| POD | 1 | 0.117 | −0.115 | −0.049 | 0.354 | 0.486* | ||||
| TPC | 1 | −0.363 | −0.085 | 0.295 | 0.137 | |||||
| TFC | 1 | 0.21 | 0.146 | 0.011 | ||||||
| TTC | 1 | 0.414 | 0.489* | |||||||
| TCC | 1 | 0.704** | ||||||||
| TAA | 1 |
(**) Correlation is significant at P ≤ 0.01; (*) at P <0.05 level. TPC, total phenolics content; TFC, total flavonoids content; TCC, total carotenoids content; TTC, total tocopherols content; TAA, total ascorbic acid.
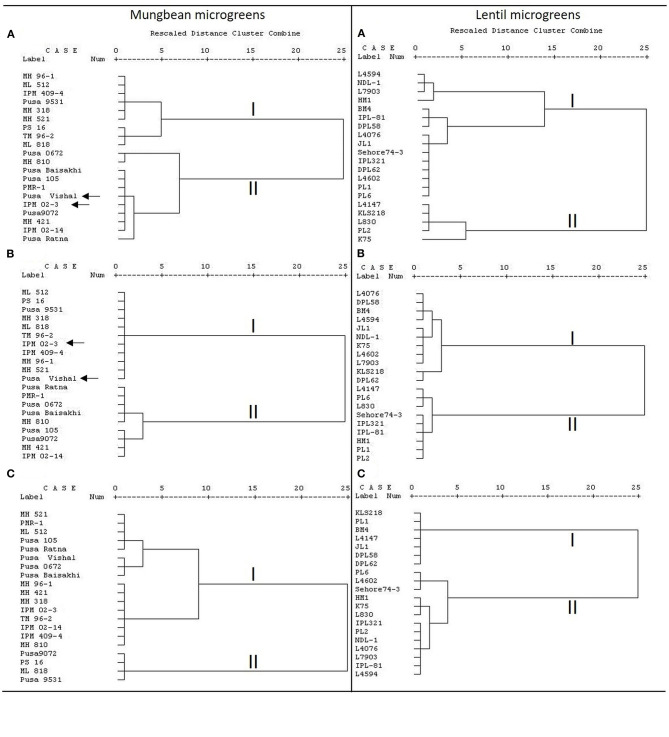
Furthermore, the clusters based on total AoA and TPC are found very similar for mungbean microgreens (Figures 2A,B); and of two major clusters except for Pusa Vishal and IPM02-3, all other genotypes grouped similarly. However, for the lentil microgreens, the clusters based on total AoA, TFC, and TPC did not show any similarity in the grouping of genotypes (Figures 2A–C). In lentils, several parameters contribute to the total AoA; while in mungbean, TPC is primarily involved in imparting the total AoA. A highly positive relationship between total phenols and AoA appears to be the trend in many plant species (Oktay et al., 2003). Phenolics play a crucial role in total bioactive activities (Pereira et al., 2009). A similar trend was recorded by Bhoyar et al. (2011) for the antioxidant activities of caper leaves which were collected from different altitudes of Ladakh (India).
Figure 2.
Cluster analysis based on (A) total AoA as measured by DPPH, ferric-reducing antioxidant power (FRAP), and peroxide assay; (B) total phenolics content (TPC); (C) total flavonoids content (TFC) in mungbean and lentil microgreens, where dendrogram was prepared using the Ward method.
PCA of Functional Characteristics of Lentil and Mungbean Microgreens
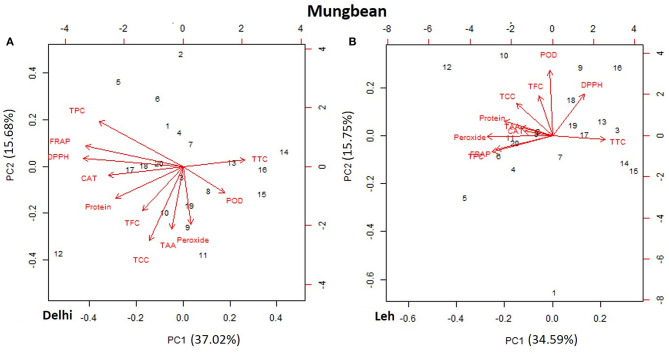
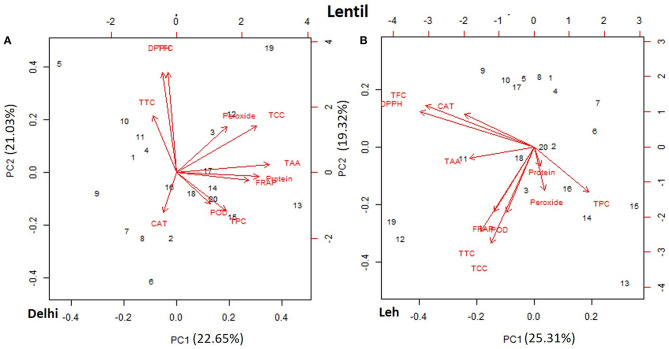
The PCA of various AoA, protein, and polyphenols of mungbean and lentil microgreens, when grown at Delhi and Leh conditions, is presented in Figures 3, 4. For mungbean microgreens when grown at Delhi and Leh, the first two principal components (PCs) explained 52.7 and 50.34% of the total variance, with PC1 and PC2 accounting for 37.02 and 15.68% and 34.59 and 15.75% of the variance, respectively (Figures 3A,B). However, for lentil microgreens when grown at Delhi and Leh, the first two PCs explained 43.68 and 44.63% of the total variance, with PC1 and PC2 accounting for 22.65 and 21.03%, and 25.31 and 19.32% of the variance, respectively (Figures 4A,B). Similarly, El-Nakhel et al. (2020) reported that the first two PCs explained 99.1% of the total variance for lettuce microgreens. The usefulness of PCA in understanding the variations at the species level across various attributes in response to varied factors like genetic makeup, maturity duration, grow-conditions, etc., have been reported by various workers (El-Nakhel et al., 2019a,b; Kyriacou et al., 2019). This study was conducted under partially controlled conditions, where growth parameters were relatively stable for Delhi conditions, while for Leh conditions, a bit harsh conditions prevailed. In addition, species-level variations are also observed in this study for lentil and mungbean species.
Figure 3.
Principal component plot derived from various antioxidant activities [1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), peroxide, total carotenoids content (TCC), total tocopherol content (TTC), total ascorbic acid (TAA), peroxidase (POD), and catalase (CAT)], protein, phenolics TPC, and TFC in the mungbean microgreens when grown at (A) normal altitude (Delhi) and (B) high altitude (Leh) conditions.
Figure 4.
Principal component plot derived from various antioxidant activities [1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP), peroxide, total tocopherols content (TTC), total carotenoids content (TCC), total ascorbic acid (TAA), peroxidase (POD), and catalase (CAT)], TPC, and TFC contents in the lentil microgreens when grown at (A) normal altitude (Delhi) and (B) high altitude (Leh) conditions.
Macro and Micronutrient Analysis
The macro (Ca, K, Mg, P, Na) and micro-nutrient contents (Fe, Zn, Cu, Mn) of 20 genotypes each of mungbean and lentil microgreens (expressed on a FW basis), when grown under Delhi and Leh conditions are presented in Tables 6–9. The most abundant elements in all the samples were, in the order, K, P, and Ca (mungbean) and K, Ca, and P (in lentil). Other studies also report that K is the main element accumulated in several microgreens (Wang et al., 2016; Paradiso et al., 2018). Except for K (239–369 mg/100 g FW), most of the other macro-nutrients (Ca: 47–87; Mg: 36–56; P: 66–87; Na: 30–60 mg/100 g FW) were recoded significantly more in Delhi-grown mungbean microgreens than the Leh-grown microgreens (K: 323–475; Ca: 42–57; Mg: 36–56; P: 39–67; Na: 20–36 mg/100 g FW). However, in lentil microgreens all the macronutrient contents were more for the Delhi (Ca: 45–70; K: 299–389; Mg: 29–66; P: 32–46; Na: 27–45 mg/100 g FW) over Leh-grown microgreens (Ca: 32–58; K: 217–369; Mg: 25–38; P: 63–88; Na: 31–42 mg/100 g FW). Interestingly, Ca and Mg were recorded more in lettuce microgreens over mature lettuce leaves (El-Nakhel et al., 2020). Also, an increase in K and Na content was recorded in various microgreens species like amaranth, cress, mizuna, and purslane when monochromatic red and blue lights were applied over normal light conditions (Kyriacou et al., 2019).
Table 6.
Micro-nutrient content in various mungbean microgreens grown at Delhi and Leh conditions.
| S. No. | Genotype (Mungbean) | Fe (mg/100 g FW) | Zn (mg/100 g FW) | Cu (mg/100 g FW) | Mn (mg/100 g FW) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | Pusa Baisakhi | 0.50 ± 0.01de | 0.76 ± 0.07abc | 0.24 ± 0.014abcd | 0.29 ± 0.015abcd | 0.06 ± 0.0011d | 0.09 ± 0.0015ab | 0.15 ± 0.003defg | 0.14 ± 0.008bcd |
| 2 | Pusa Ratna | 0.56 ± 0.012b | 0.77 ± 0.04abc | 0.23 ± 0.01abcde | 0.25 ± 0.009efgh | 0.06 ± 0.001d | 0.08 ± 0.002c | 0.17 ± 0.004abcd | 0.15 ± 0.003ab |
| 3 | Pusa Vishal | 0.46 ± 0.012fgh | 0.71 ± 0.06abcdefg | 0.21 ± 0.01cde | 0.25 ± 0.012defgh | 0.07 ± 0.0011c | 0.05 ± 0.0016e | 0.14 ± 0.005efg | 0.09 ± 0.005g |
| 4 | Pusa105 | 0.50 ± 0.01de | 0.74 ± 0.04abcde | 0.25 ± 0.012ab | 0.24 ± 0.01efghj | 0.08 ± 0.0012c | 0.08 ± 0.008c | 0.14 ± 0.005defg | 0.18 ± 0.008a |
| 5 | Pusa0672 | 0.56 ± 0.015b | 0.70 ± 0.01abcdefg | 0.23 ± 0.015abcde | 0.26 ± 0.009cdef | 0.05 ± 0.0006ef | 0.09 ± 0.0014b | 0.09 ± 0.005j | 0.16 ± 0.005ab |
| 6 | Pusa9072 | 0.49 ± 0.006def | 0.70 ± 0.02abcdefg | 0.26 ± 0.01a | 0.25 ± 0.012efgh | 0.07 ± 0.0008c | 0.08 ± 0.008c | 0.16 ± 0.006bcde | 0.16 ± 0.006ab |
| 7 | Pusa9531 | 0.48 ± 0.01efg | 0.64 ± 0.03efg | 0.21 ± 0.011cde | 0.21 ± 0.006h | 0.08 ± 0.0022c | 0.05 ± 0.0016e | 0.18 ± 0.008ab | 0.10 ± 0.011fg |
| 8 | MH96-1 | 0.52 ± 0.015cd | 0.78 ± 0.02abc | 0.26 ± 0.01a | 0.30 ± 0.017ab | 0.05 ± 0.0007e | 0.10 ± 0.009a | 0.18 ± 0.008ab | 0.13 ± 0.009cde |
| 9 | MH318 | 0.42 ± 0.009ij | 0.65 ± 0.02efg | 0.24 ± 0.01abc | 0.31 ± 0.009ab | 0.06 ± 0.0004d | 0.06 ± 0.001d | 0.10 ± 0.007ij | 0.13 ± 0.002cde |
| 10 | MH421 | 0.53 ± 0.015bc | 0.72 ± 0.02abcdefg | 0.21 ± 0.016cde | 0.22 ± 0.008gh | 0.03 ± 0.0006g | 0.04 ± 0.0017f | 0.10 ± 0.003ij | 0.18 ± 0.008a |
| 11 | MH521 | 0.59 ± 0.011a | 0.68 ± 0.02cdefg | 0.21 ± 0.004de | 0.29 ± 0.015abcd | 0.04 ± 0.0017f | 0.09 ± 0.0058ab | 0.11 ± 0.002hij | 0.16 ± 0.006ab |
| 12 | MH810 | 0.52 ± 0.015cd | 0.76±0.01abcd | 0.22 ± 0.008bcde | 0.25 ± 0.013efgh | 0.05 ± 0.001e | 0.08 ± 0.0022c | 0.14 ± 0.008efg | 0.15±0.003ab |
| 13 | ML512 | 0.50 ± 0.01de | 0.63 ± 0.03g | 0.22 ± 0.1bcde | 0.28 ± 0.007bcde | 0.09 ± 0.0004b | 0.09 ± 0.009cb | 0.16 ± 0.005bcde | 0.16 ± 0.005ab |
| 14 | ML818 | 0.40 ± 0.011j | 0.74 ± 0.03abcdef | 0.21 ± 0.015de | 0.24 ± 0.014fgh | 0.09 ± 0.001b | 0.08 ± 0.0022c | 0.18 ± 0.007ab | 0.13 ± 0.002cde |
| 15 | PS16 | 0.48 ± 0.008efg | 0.63 ± 0.01fg | 0.20 ± 0.010e | 0.32 ± 0.019a | 0.05 ± 0.0016e | 0.09 ± 0.0016b | 0.10 ± 0.011ij | 0.12 ± 0.005cdef |
| 16 | TM96-2 | 0.51 ± 0.004cde | 0.69 ± 0.02bcdefg | 0.26 ± 0.009a | 0.29 ± 0.013abc | 0.03 ± 0.0014g | 0.06 ± 0.0012d | 0.16 ± 0.007bcde | 0.11 ± 0.002efg |
| 17 | IPM02-3 | 0.44 ± 0.01hi | 0.79 ± 0.02a | 0.22 ± 0.012bcde | 0.25 ± 0.015cdefg | 0.08 ± 0.0008c | 0.10 ± 0.009a | 0.13 ± 0.002fgh | 0.12 ± 0.004def |
| 18 | IPM02-14 | 0.52 ± 0.011cd | 0.62 ± 0.02g | 0.21 ± 0.006cde | 0.28 ± 0.007bcde | 0.06 ± 0.0012d | 0.09 ± 0.001b | 0.13 ± 0.009fgh | 0.13 ± 0.007cde |
| 19 | IPM409-4 | 0.43 ± 0.011ij | 0.68 ± 0.01cdefg | 0.23 ± 0.006bcde | 0.24 ± 0.01efgh | 0.08 ± 0.0002c | 0.06 ± 0.004d | 0.17 ± 0.014abc | 0.12 ± 0.009cdef |
| 20 | PMR-1 | 0.45 ± 0.011ghi | 0.65 ± 0.01defg | 0.22 ± 0.008bcde | 0.29 ± 0.008abc | 0.10 ± 0.0009a | 0.09 ± 0.0027ab | 0.19 ± 0.013a | 0.14 ± 0.003bc |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Table 9.
Macro-nutrient content in various lentil microgreens grown at Delhi and Leh conditions.
| S. No. | Genotype (Lentil) | Ca (mg/100 g FW) | K (mg/100 g FW) | Mg (mg/100 g FW) | P (mg/100 g FW) | Na (mg/100 g FW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | L4076 | 50 ± 3.61de | 40 ± 3.61defg | 389 ± 12.77a | 272 ± 8.89cd | 35 ± 1.00fg | 33 ± 1.00abc | 34 ± 3.00ab | 64 ± 2.65fg | 33 ± 4.36bcd | 32 ± 1.89ab |
| 2 | L4147 | 62 ± 3.46abc | 56 ± 3.61ab | 374 ± 13.23ab | 364 ± 7.94a | 47 ± 4.58bcde | 31 ± 3.46abc | 35 ± 3.60ab | 69 ± 4.36defg | 32 ± 1.76bcd | 40 ± 4.58ab |
| 3 | L4594 | 57 ± 5.00bcd | 58 ± 2.65a | 317 ± 10.00def | 234 ± 6.56fg | 32 ± 2.00g | 28 ± 2.00bc | 45 ± 3.61ab | 70 ± 5.29defg | 32 ± 1.70bcd | 35 ± 3.46ab |
| 4 | L7903 | 51 ± 1.73de | 55 ± 4.36abc | 374 ± 17.32ab | 230 ± 6.56fg | 51 ± 4.36b | 31 ± 4.00abc | 37 ± 2.00ab | 71 ± 2.00defg | 33 ± 4.36bcd | 32 ± 1.72ab |
| 5 | HM1 | 48 ± 2.00de | 57 ± 4.00a | 348 ± 13.45bcde | 235 ± 6.08fg | 47 ± 3.00bcde | 36 ± 3.00ab | 38 ± 8.72ab | 69 ± 3.61defg | 29 ± 2.00cd | 41 ± 1.73ab |
| 6 | BM4 | 49 ± 4.36de | 49 ± 2.00abcd | 373 ± 25.51ab | 292 ± 7.21c | 37 ± 2.00efg | 30 ± 2.00abc | 37 ± 4.58ab | 68 ± 5.00defg | 25 ± 1.00d | 42 ± 3.00a |
| 7 | JL1 | 70 ± 5.29a | 36 ± 4.36g | 319 ± 8.91cdef | 254 ± 5.00def | 51 ± 1.73b | 26 ± 3.00c | 33 ± 4.36ab | 87 ± 6.24ab | 33 ± 4.36bcd | 32 ± 1.56ab |
| 8 | Sehore74-3 | 53 ± 3.00cde | 36 ± 3.61g | 364 ± 9.71ab | 288 ± 10.00c | 49 ± 4.00bc | 36 ± 2.65ab | 36 ± 3.61ab | 66 ± 4.58efg | 28 ± 4.58cd | 38 ± 3.61ab |
| 9 | NDL-1 | 50 ± 2.65de | 49 ± 2.00abcd | 353 ± 11.00abcd | 369 ± 11.79a | 43 ± 2.65bcdef | 28 ± 2.65bc | 33 ± 1.00ab | 76 ± 2.00bcde | 29 ± 2.65cd | 36 ± 1.11ab |
| 10 | IPL81 | 52 ± 2.65cde | 35 ± 1.00g | 299 ± 16.46f | 239 ± 7.55efg | 36 ± 2.00fg | 33 ± 4.58abc | 37 ± 6.56ab | 75 ± 2.65cdef | 30 ± 2.65bcd | 39 ± 4.58ab |
| 11 | IPL321 | 67 ± 6.24ab | 46 ± 2.65cdef | 361 ± 9.00ab | 255 ± 12.29def | 38 ± 3.61defg | 25 ± 3.61c | 43 ± 5.25ab | 77 ± 1.73abcde | 34 ± 5.20bcd | 39 ± 5.24ab |
| 12 | K75 | 58 ± 1.73bcd | 32 ± 1.73g | 363 ± 5.28ab | 254 ± 10.00def | 48 ± 4.58bcd | 29 ± 2.05bc | 37 ± 5.22ab | 74 ± 3.61cdefg | 34 ± 2.00bcd | 42±3.00a |
| 13 | KLS218 | 66 ± 2.00ab | 47 ± 5.20bcde | 367 ± 10.00ab | 253 ± 7.94def | 66 ± 4.58a | 29 ± 1.76bc | 36 ± 2.00ab | 78 ± 3.00abcd | 29 ± 1.00cd | 38 ± 3.61ab |
| 14 | DPL58 | 53 ± 1.73cde | 46 ± 2.65cdef | 359 ± 12.49abc | 271 ± 8.00cd | 50 ± 2.65b | 27 ± 1.00c | 38 ± 5.00ab | 77 ± 1.00abcde | 27 ± 3.61d | 37 ± 5.21ab |
| 15 | DPL62 | 51 ± 3.61de | 47 ± 2.65bcde | 312 ± 7.21ef | 247 ± 1.54defg | 34 ± 3.00fg | 30 ± 2.65abc | 42 ± 4.36ab | 71 ± 2.65defg | 45 ± 3.61a | 37 ± 5.29ab |
| 16 | PL1 | 57 ± 4.00bcd | 38 ± 3.61efg | 351 ± 10.00abcde | 269 ± 7.55cde | 29 ± 2.00g | 31 ± 1.74abc | 46 ± 4.58a | 63 ± 2.65g | 39 ± 2.00ab | 36 ± 0.50ab |
| 17 | PL2 | 45 ± 2.00e | 36 ± 1.00g | 352 ± 14.00abcde | 217 ± 10.00g | 31 ± 5.25g | 29 ± 1.95bc | 32 ± 1.73b | 88 ± 4.58a | 34 ± 1.00bcd | 39 ± 3.00ab |
| 18 | PL6 | 69 ± 4.58a | 37 ± 1.73fg | 355 ± 12.5abcd | 296 ± 22.11c | 44 ± 4.00bcdef | 27 ± 1.00c | 36 ± 3.59ab | 69 ± 1.00defg | 37 ± 5.26abc | 31 ± 1.00b |
| 19 | L830 | 52 ± 2.65cde | 56±4.00ab | 355±20.22abcd | 295 ± 6.24c | 39 ± 2.00cdefg | 29 ± 1.00bc | 38±2.00ab | 85±5.29abc | 32 ± 1.72bcd | 32±1.86ab |
| 20 | L4602 | 50 ± 1.73de | 52 ± 2.00abc | 345 ± 2.65bcde | 329 ± 11.14b | 48 ± 2.65bcd | 38 ± 3.00a | 36 ± 2.00ab | 75 ± 2.00cdef | 33 ± 1.00bcd | 37 ± 5.27ab |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Table 7.
Micro-nutrient content in various lentil microgreens grown at Delhi and Leh conditions.
| S. No. | Genotype (Lentil) | Fe (mg/100 g FW) | Zn (mg/100 g FW) | Cu (mg/100 g FW) | Mn (mg/100 g FW) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| 1 | L4076 | 0.66 ± 0.01abc | 0.66 ± 0.031defg | 0.34 ± 0.007b | 0.39 ± 0.009abcdefg | 0.18 ± 0.007a | 0.20 ± 0.027ab | 0.15 ± 0.003a | 0.13 ± 0.01abcde |
| 2 | L4147 | 0.53 ± 0.095efgh | 0.61 ± 0.02fgh | 0.40 ± 0.008a | 0.26 ± 0.017i | 0.06 ± 0.002efg | 0.14 ± 0.036bcdef | 0.10 ± 0.009c | 0.18 ± 0.036a |
| 3 | L4594 | 0.61 ± 0.02cde | 0.65 ± 0.01efgh | 0.39 ± 0.009ab | 0.37 ± 0.024defgh | 0.12 ± 0.002bc | 0.13 ± 0.031bcdef | 0.10 ± 0.009c | 0.13 ± 0.031abcde |
| 4 | L7903 | 0.57 ± 0.025cdef | 0.75 ± 0.032ab | 0.40 ± 0.013ab | 0.40 ± 0.034abcdefg | 0.05 ± 0.002g | 0.09 ± 0.011ef | 0.08 ± 0.004d | 0.09 ± 0.011de |
| 5 | HM1 | 0.55 ± 0.032defg | 0.72 ± 0.018bcd | 0.39 ± 0.016ab | 0.28 ± 0.041hi | 0.05 ± 0.002g | 0.12 ± 0.017cdef | 0.08 ± 0.004d | 0.14 ± 0.036abcde |
| 6 | BM4 | 0.64 ± 0.042bcd | 0.79 ± 0.01a | 0.38 ± 0.006ab | 0.32 ± 0.02ghi | 0.07 ± 0.010ef | 0.16 ± 0.010abcd | 0.10 ± 0.009c | 0.18 ± 0.035a |
| 7 | JL1 | 0.51 ± 0.010efgh | 0.69 ± 0.015cde | 0.38 ± 0.015ab | 0.46 ± 0.028abcd | 0.06 ± 0.001fg | 0.19 ± 0.020ab | 0.11 ± 0.003bc | 0.17 ± 0.010abc |
| 8 | Sehore74-3 | 0.50 ± 0.04fgh | 0.64 ± 0.031efgh | 0.39 ± 0.056ab | 0.49 ± 0.015ab | 0.06 ± 0.001fg | 0.18 ± 0.032abc | 0.08 ± 0.004d | 0.17 ± 0.023ab |
| 9 | NDL-1 | 0.46 ± 0.025gh | 0.74 ± 0.036abc | 0.37 ± 0.010ab | 0.39 ± 0.057bcdefg | 0.08 ± 0.005e | 0.22 ± 0.028a | 0.10 ± 0.001c | 0.13 ± 0.031abcde |
| 10 | IPL81 | 0.56 ± 0.029cdefg | 0.68 ± 0.017de | 0.39 ± 0.009ab | 0.47 ± 0.058abc | 0.13 ± 0.004b | 0.10 ± 0.001def | 0.12 ± 0.02b | 0.13 ± 0.030abcde |
| 11 | IPL321 | 0.59 ± 0.040cdef | 0.59 ± 0.020h | 0.40 ± 0.025a | 0.33 ± 0.037fghi | 0.06 ± 0.006efg | 0.09 ± 0.001f | 0.10 ± 0.004c | 0.09 ± 0.016de |
| 12 | K75 | 0.74 ± 0.041ab | 0.79 ± 0.015a | 0.23 ± 0.020c | 0.49 ± 0.010a | 0.10 ± 0.001d | 0.15 ± 0.030bcdef | 0.12 ± 0.006b | 0.12 ± 0.017abcde |
| 13 | KLS218 | 0.55 ± 0.039defgh | 0.65 ± 0.001efgh | 0.39 ± 0.009ab | 0.44 ± 0.038abcde | 0.11 ± 0.009cd | 0.10 ± 0.009def | 0.15 ± 0.004a | 0.11 ± 0.003bcde |
| 14 | DPL58 | 0.59 ± 0.010cdef | 0.60 ± 0.023gh | 0.38 ± 0.029ab | 0.34 ± 0.037efghi | 0.05 ± 0.002g | 0.10 ± 0.009def | 0.08 ± 0.005de | 0.08 ± 0.003e |
| 15 | DPL62 | 0.45 ± 0.006h | 0.73 ± 0.021abcd | 0.26 ± 0.033c | 0.42 ± 0.068abcdef | 0.11 ± 0.003cd | 0.09 ± 0.002ef | 0.10 ± 0.009c | 0.08 ± 0.006e |
| 16 | PL1 | 0.55 ± 0.001defgh | 0.68 ± 0.017cde | 0.24 ± 0.019c | 0.37 ± 0.010cdefgh | 0.12 ± 0.004b | 0.16 ± 0.019abcde | 0.08 ± 0.005de | 0.17 ± 0.010abc |
| 17 | PL2 | 0.58 ± 0.020cdef | 0.67 ± 0.001bef | 0.25 ± 0.013c | 0.48 ± 0.030abc | 0.13 ± 0.009b | 0.13 ± 0.010bcdef | 0.06 ± 0.001e | 0.15 ± 0.030abcd |
| 18 | PL6 | 0.59 ± 0.020cdef | 0.52 ± 0.017i | 0.40 ± 0.015ab | 0.36 ± 0.007defghi | 0.06 ± 0.001fg | 0.18 ± 0.035abc | 0.10 ± 0.009c | 0.15 ± 0.030abcd |
| 19 | L830 | 0.59 ± 0.004cdef | 0.76±0.010ab | 0.23 ± 0.004c | 0.49±0.015a | 0.11 ± 0.002cd | 0.19±0.024abc | 0.12 ± 0.006b | 0.10 ± 0.021cde |
| 20 | L4602 | 0.75 ± 0.032a | 0.79 ± 0.035ab | 0.37 ± 0.001ab | 0.45 ± 0.010abcd | 0.07 ± 0.010ef | 0.17 ± 0.003abc | 0.10 ± 0.009c | 0.17 ± 0.010abc |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
Table 8.
Macro-nutrient content in various mungbean microgreens grown at Delhi and Leh conditions.
| S. No. | Genotype (Mungbean) | Ca (mg/100 g FW) | K (mg/100 g FW) | Mg (mg/100 g FW) | P (mg/100 g FW) | Na (mg/100 g FW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | Delhi | Leh | ||
| Pusa Baisakhi | 65 ± 2.0bcd | 50 ± 1.73bcde | 291 ± 4.4fghi | 420 ± 6.60cd | 56 ± 3.0a | 43 ± 3.6abc | 74 ± 1.00ef | 45 ± 2.6cdef | 40 ± 3.00c | 36 ± 0.5a | |
| Pusa Ratna | 51 ± 2.0fg | 45 ± 1.00defg | 303 ± 10.8defgh | 429 ± 2.60cd | 49 ± 1.00b | 30 ± 1.0fg | 87 ± 2.00a | 40 ± 1.0ef | 30 ± 1.00d | 23 ± 1.7efg | |
| Pusa Vishal | 47 ± 2.0g | 44 ± 2.00efg | 239 ± 8.2 l | 354 ± 5.30e | 40 ± 2.00de | 41 ± 2.0abcde | 71 ± 1.73fgh | 51 ± 2.6bc | 30 ± 1.71d | 20 ± 0.06g | |
| Pusa105 | 81 ± 2.6a | 53 ± 2.65abc | 342 ± 9.6abc | 467 ± 2.00ab | 52 ± 2.00ab | 35 ± 1.0cdefg | 81 ± 2.65bc | 41 ± 1.7def | 30 ± 1.00d | 36 ± 1.1a | |
| Pusa0672 | 51 ± 2.0fg | 45 ± 2.00defg | 281 ± 7.5ghij | 445 ± 5.60bc | 48 ± 1.73bc | 33 ± 2.6efg | 75 ± 1.73def | 45 ± 3.5cdef | 30 ± 1.72d | 24 ± 1.8efg | |
| Pusa9072 | 60 ± 2.6de | 45 ± 1.00defg | 325 ± 12.1cde | 438 ± 8.90cd | 44 ± 2.00cd | 42 ± 4.0abcd | 83 ± 2.00ab | 44 ± 3.6cdef | 50 ± 4.58b | 26 ± 1.0def | |
| Pusa9531 | 66 ± 2.2bcd | 42 ± 1.73fg | 320 ± 8.7cde | 323 ± 9.80f | 50 ± 1.73b | 31 ± 1.0fg | 81 ± 2.65bc | 42 ± 3.0def | 60 ± 2.65a | 22 ± 0.5fg | |
| MH96-1 | 60 ± 1.0de | 46 ± 1.00defg | 369 ± 14.9a | 436 ± 4.40cd | 48 ± 1.00bc | 30 ± 4.6fg | 68 ± 1.73gh | 43 ± 1.7def | 30 ± 2.00d | 30 ± 2.0bcd | |
| MH318 | 47 ± 2.0g | 40 ± 1.00g | 251 ± 8.5kl | 423 ± 7.50cd | 42 ± 1.00d | 35 ± 2.0cdefg | 84 ± 1.73ab | 47 ± 3.0cde | 50 ± 3.00b | 31 ± 2.0bc | |
| MH421 | 67 ± 1.0 bc | 54 ± 2.00ab | 309 ± 4.6def | 435 ± 10.60cd | 50 ± 1.00b | 37 ± 2.6bcdef | 77 ± 1.73cde | 45 ± 2.6cdef | 50 ± 4.00b | 33 ± 1.5ab | |
| MH521 | 60 ± 3.0de | 57 ± 3.61a | 250 ± 9.0kl | 430 ± 2.60cd | 50 ± 1.73b | 40 ± 3.5abcde | 77 ± 1.00cde | 46 ± 1.0cde | 30 ± 1.00d | 29 ± 1.0bcd | |
| MH810 | 54 ± 2.0ef | 47 ± 2.65cdef | 258 ± 9.8jkl | 473±8.90a | 44 ± 1.00cd | 34 ± 1.0defg | 75 ± 2.65def | 38 ± 1.7f | 30 ± 1.7d | 21 ± 1.9g | |
| ML512 | 70 ± 1.0b | 51 ± 1.73abcd | 304 ± 11.5defg | 427 ± 10.80cd | 48 ± 1.00bc | 40 ± 2.6abcde | 81 ± 2.00bc | 42 ± 2.0def | 40 ± 2.65c | 21 ± 1.0g | |
| ML818 | 70 ± 2.6b | 53 ± 2.00abc | 330 ± 12.0bcd | 475 ± 6.00a | 42 ± 2.00d | 34 ± 1.0defg | 80 ± 3.00bcd | 41 ± 2.6def | 30 ± 1.00d | 32 ± 1.5ab | |
| PS16 | 68 ± 2.0bc | 56 ± 2.65ab | 267 ± 7.5ijk | 377 ± 9.80e | 51 ± 1.73b | 45 ± 4.4ab | 66 ± 2.00h | 43 ± 3.0def | 60 ± 5.00a | 27 ± 1.2cde | |
| TM96-2 | 86 ± 2.6a | 42 ± 1.00fg | 357 ± 5.2ab | 435 ± 11.10cd | 49 ± 2.60b | 36 ± 2.0cdefg | 73 ± 1.73efg | 56 ± 3.5b | 30 ± 1.74d | 32 ± 0.9ab | |
| IPM02-3 | 69 ± 1.7b | 47 ± 1.00cdef | 276 ± 7.2hijk | 415 ± 12.00d | 36 ± 1.73e | 28 ± 1.7g | 76 ± 1.00cdef | 44 ± 2.6cdef | 60 ± 5.57a | 21 ± 1.0g | |
| IPM02-14 | 62 ± 2.0cd | 50 ± 3.00bcde | 299 ± 6.6efgh | 369 ± 9.20e | 49 ± 1.73b | 45 ± 2.0ab | 72 ± 2.00efg | 67 ± 1.7a | 60 ± 2.00a | 20 ± 0.7g | |
| IPM409-4 | 67 ± 2.6bc | 50 ± 2.00bcde | 256 ± 7.2jkl | 419 ± 8.00d | 56 ± 2.60a | 46 ± 3.0a | 72 ± 2.65efg | 48 ± 2.0cd | 40 ± 2.65c | 31 ± 1.0bc | |
| PMR-1 | 67 ± 1.0bc | 42 ± 1.00fg | 259 ± 6.2jkl | 428 ± 10.00cd | 41 ± 2.00b | 42 ± 1.7abcd | 72 ± 1.00efg | 45 ± 3.6cdef | 60 ± 4.58a | 31 ± 1.7bc | |
Values are expressed as mean ± SD (n = 3), and different letters indicate a significant difference (p ≤ 0.05).
For micronutrients, the Delhi-grown mungbean microgreens showed significantly less Fe (0.4–0.59 mg/100 g FW) and Zn (0.2–0.26 mg/100 g FW) content than the Leh-grown microgreens (Fe: 0.62–0.79; Zn: 0.21–0.32 mg/100 g FW). However, no such trend was recorded for Cu and Mn content in mungbean microgreens when grown at Delhi (Cu: 0.03–0.1; Mn: 0.09–0.19 mg/100 g FW) or Leh conditions (Cu: 0.04–0.1; Mn: 0.09–0.18 mg/100 g FW). In lentil, the Fe and Cu content was found more for Leh-grown microgreens (Fe: 0.52–0.79; Cu: 0.09–0.22; mg/100 g FW) than the Delhi-grown microgreens (Fe: 0.45–0.75 Cu: 0.05–0.18; mg/100 g FW). However, no such trend was recorded for other micronutrients in the lentil microgreens when grown at Delhi (Zn: 0.23–0.4; Mn: 0.06–0.15 mg/100 g FW) or Leh conditions (Zn: 0.26–0.49; Mn: 0.08–0.18 mg/100 g FW). Overall, of the 20 mungbean and lentil genotypes studied, the majority of them showed relatively more micronutrient content at Leh over Delhi-grown microgreens (Tables 6–9).
The range of various macro-nutrients in the Cichorium, Lactuca, and Brassica derived microgreens for P (536–1,039 μg/g FW), K (2,498–4,735 μg/g FW), Ca (1,008–2,027 μg/g FW), Mg (200–252 μg/g FW), and Na (73–474 μg/g FW) (Paradiso et al., 2018) were found nearly similar to that of mungbean and lentil microgreens. Similarly, the micronutrients such as Fe (4.3–16.6 μg/g FW), Zn (3.0–5.2 μg/g FW), Mn (5.3–13.2 μg/g FW), and Cu (0.29–1.18 μg/g FW) (Paradiso et al., 2018) were also in the similar range in different microgreens as that of mungbean, and lentil derived microgreens.
Among micronutrients, Fe content was always higher than Zn, as also reported for several microgreens like Cichorium intybus (Fe: 14.1; Zn: 4.4 μg/g FW) and Lactuca sativa (Fe: 16.6; Zn 5.2 μg/g FW) (Paradiso et al., 2018). In addition, the lettuce cultivar “Trocadero” showed much higher micro-and macro-nutrient contents (P: 872; K: 4,735; Mg: 248; Fe: 16.6 μg/g FW) when compared with “Bionda da taglio” cultivar (P: 536; K: 2,865; Mg: 200; Fe: 4.3 μg/g FW) (Paradiso et al., 2018). This again reiterated the need to do a wide genotypic study to find the best genotype having more mineral contents. Moreover, wide variations in the biomolecule composition of the same genotype under Delhi and Leh conditions signify the differential expression of several genes under different environmental conditions.
Effect of Weather Parameters
The samples under study were grown during the first week of November 2019, under Leh and Delhi conditions. Relatively larger temperature amplitude (14–30°C), total photoperiod (10.4 h), UV-B (0.2–0.3 μW/cm2), and PAR (600 to 800 μmol/m2/s) were recorded under Leh growing conditions. Whereas, under Delhi conditions, the temperature was kept in the range of 18 to 26°C (mungbean; 28/26°C and lentil 21/18°C), along with natural day and night cycles (with mean day length of nearly 10.30 h) and PAR (350–500 μmol/m2/s); while UV-B could not be detected during the microgreens growth period in the glasshouse (Supplementary File 1). The differential growing conditions might have created more oxidative stress at Leh, and in response, the tiny plants have over-activated their antioxidant defense mechanism at Leh over Delhi conditions. Higher AoA of the same mungbean or lentil genotype(s) at Leh over Delhi conditions seems associated with differential gene expression.
Light conditions are known to influence the morpho-physiology of microgreens significantly, and also the biosynthesis and accumulation of phytochemicals (Delian et al., 2015). A lower dose of UV-B radiation is reported causing alterations in antioxidant status, e.g., regulation of glutathione pathways, phenylpropanoids, cinnamates, or flavonoids pathways, and pyridoxine biosynthesis pathways (Hideg et al., 2013). Thus, the presence of even very little UV-B radiation seems to have triggered some defense response which is reflected in terms of higher antioxidant levels (e.g., phenols and flavonoids) in the Leh-grown microgreens (Olsson et al., 1998; Hideg et al., 2013). Similarly, Brazaityte et al. (2015a) have also shown the improved antioxidant characteristics of various microgreens like basil, beet, and red Pak Choi by applying UV irradiation. A higher level of PAR has also shown higher TPC and TFC in capsicum fruits and also more flavonoids in bean leaves than a low level of PAR (Cen and Bornman, 1990). Additionally, Kamal et al. (2020) have also shown the effect of supplemental lighting causing improved vegetative growth, mineral and vitamin contents in various brassica microgreens like Kohlrabi purple, Cabbage red, Broccoli, Kale Tucsan, Komatsuna red, Tatsoi, and Cabbage green.
Stress response in the plant is associated with the enhanced production of phenolics which act as either signaling compounds, antioxidants, and/or cell wall precursors (Swieca and Gwalik-Dziki, 2015). Furthermore, phenolics synthesis activation in plants through various elicitation mechanisms means an increased antioxidant capacity. Nearly 65% increase in the phenolics content in lentil sprouts (over control) was recorded when treated with 15 mM H2O2 (Swieca and Gwalik-Dziki, 2015). Similarly, Swieca and Baraniak (2013) reported a 1.6- and 1.9-fold increase in total antioxidant capacity of 4-day-old lentil sprouts when treated with 20 and 200 mM H2O2 at 2-day-old sprouts stage. In addition, the imposition of temperature stress at 4 and 40°C for 1 h also enhanced the phenolic content and antioxidant capacity of lentil sprouts (Swieca et al., 2014b). In lentil sprouts, the UV-B treatment has resulted in enhanced phenolic content and AoA (Swieca et al., 2014a).
In general, the mean content of total phenolics, flavonoids, carotenoids, tocopherols, and ascorbic acid was recorded more in the microgreens grown under Leh than when grown under partially controlled growing conditions of Delhi. When mungbean and lentil microgreens were compared, TPC and TCC were relatively more in the lentil-based microgreens while TFC and TAA were more in the mungbean microgreens (Tables 1, 2). Except for the crude protein content, other parameters such as FRAP, DPPH, peroxide activities, and enzymatic antioxidant activities of mungbean and lentil microgreens also recorded more for the Leh-grown microgreens than the microgreens grown under partially controlled Delhi conditions. Also, relatively more FRAP and peroxidase activities were recorded for the lentil microgreens, while protein, peroxidase, and catalase were relatively more for the mungbean microgreens (Tables 3, 4). Genetic factors and growing conditions also play an important role in the formation of secondary metabolites, including various compounds imparting AoA (Islam et al., 2003). Variation in the AoA between Leh and Delhi samples could be attributed to the variation in the growing conditions. The higher accumulation of various antioxidants in the microgreens grown at Leh could be due to the relatively harsh growing conditions at Leh than that of Delhi, which may have provided better tolerance to the microgreens by either scavenging the free oxygen radicals or by protecting the innate protein from denaturation. It seems that under Leh conditions, the tiny plants preferred to synthesize more antioxidants at the cost of protein synthesis. This needs further detailed analysis.
Conclusion
This study has identified a wide diversity in the phytochemical composition, antioxidant capacities, and nutrient contents among the microgreens of 20 diverse genotypes, each of mungbean and lentils when grown at plain-altitude and high-altitude regions. Similarly, significant variations were also recorded for mineral, phytochemical and antioxidant capacity traits among the microgreens of two lettuce cultivars (green and red Salanova®) (El-Nakhel et al., 2020). Paradiso et al. (2018) have also studied and compared the diverse nutritional profile of two varieties each of three species viz. chicory (i.e., Molfetta and Italico a costa rossa), lettuce (i.e., Bionda da taglio and Trocadero), and broccoli (i.e., Mugnuli and Natalino).
As microgreens can be grown easily at home or under very harsh conditions of Leh–Ladakh (under greenhouse conditions), they can be considered an alternative for the nutritional security of the population living in those remote areas, especially during land-locked conditions. Among 20 lentil genotypes studied, the genotype L830 was superior for several parameters like TFC, TCC, and TAA. Even for other antioxidant parameters like TPC and TTC, L830 has expressed higher content over many genotypes. Similarly, for mungbean genotypes, MH810 was found significantly superior for TPC, TFC, TCC, and TAA. In addition, microgreens cultivation should be explored as a means for providing larger quantities of nutrients (including antioxidants) per gram plant biomass over cost-intensive air-transported mature vegetables in the high-altitude areas of Ladakh, especially during winter months (Weber, 2017). Wide variations in the microgreens phytochemical compositions of the same genotype of mungbean and lentil, when grown under Delhi and Leh conditions, suggests the need to identify the genes/pathways which change its expression under different environmental conditions. This, in the long term, will help in increasing the various phytochemical compositions of microgreens by manipulating the microgreens growing conditions. This study reiterates the need to develop a deeper understanding of the intricate regulation of various antioxidant responses of the microgreens under varied environmental conditions, including temperature amplitude, UV-B, and PAR. The nutrient composition of mungbean and lentil microgreens obtained is of considerable importance to the nutritionist, consumers, and growers to select nutrient-rich, easily available, and cost-effective microgreens.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Priti, MT, and AS conducted formal analysis, methodology, data curation, and writing of the original draft. GM, HD, and TS conducted conceptualization, funding acquisition, project administration, resources, writing, review and editing of the manuscript. VT, SS, MA, RK, and KT conducted methodology, writing, review and editing of the manuscript. SK, RMN, and SP conducted conceptualization, resources, writing, review and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The technical support received from Mr. Dilip Kumar is duly acknowledged. Authors also acknowledge the support from the long-term strategic donors to the World Vegetable Center, Taiwan, United States Agency for International Development (USAID), UK Government's Foreign, Commonwealth & Development Office (FCDO), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan. RMN acknowledges funding from ACIAR Project on International Mungbean Improvement Network (CIM-2014-079).
Footnotes
Funding. This research was funded by the Life Science Research Board, Defence Research and Development Organization (DRDO), New Delhi (LSRB-333), and the Indian Council of Agricultural Research (ICAR), IARI New Delhi. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.710812/full#supplementary-material
References
- Aebi H. (1984). Catalase in vitro. Meth. Enzymol. 105, 121–126. 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- Angmo P., Chorol S., Namgail D., Chaurasia O. P., Stobdan T. (2019). Effect of maturation on phenolics and flavonoids content of greenhouse-grown beet leaf. Pharmacogn J. 11, 1010–1013. 10.5530/pj.2019.11.159 [DOI] [Google Scholar]
- Aron P. M., Kennedy J. A. (2008). Flavan-3-ols: nature, occurrence and biological activity. Mol. Nutr. Food Res. 52, 79–104. 10.1002/mnfr.200700137 [DOI] [PubMed] [Google Scholar]
- Barillari J., Canistro D., Paolini M., Ferroni F., Pedulli G. F., Iori R., et al. (2005). Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J. Agric. Food Chem. 53, 2475–2482. 10.1021/jf047945a [DOI] [PubMed] [Google Scholar]
- Benzie F. F., Strain J. J. (1999). Ferric Reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 299, 15–23. 10.1016/S0076-6879(99)99005-5 [DOI] [PubMed] [Google Scholar]
- Bhalani H., Mishra G. P., Sarkar T., Radhakrishnan T. (2019). Regulation of antioxidant mechanisms by AtDREB1A improves soil-moisture deficit stress tolerance in transgenic peanut (Arachis hypogaea L.). PLoS ONE 14:e0216706. 10.1371/journal.pone.0216706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoyar M. S., Mishra G. P., Naik P. K., Singh S. B. (2018). Evaluation of antioxidant capacities and total polyphenols in various edible parts of Capparis spinosa L. collected from trans-Himalayas. Def. Life Sci J. 3, 30–36. 10.14429/dlsj.3.12570 [DOI] [Google Scholar]
- Bhoyar M. S., Mishra G. P., Naik P. K., Srivastava R. B. (2011). Estimation of antioxidant activity and total phenolics among natural populations of Caper (Capparis spinosa) leaves collected from cold arid desert of trans-Himalayas. Aust. J. Crop Sci. 5, 912–919. Available online at: http://www.cropj.com/mishra_5_7_2011_912_919.pd
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brand-Williams C. M. E., Berset C. (1995). Use of a free radical method. LWT Food Sci. Technol. 28, 25–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- Brazaityte A., Sakalauskiene S., Samuoliene G., Jankauskiene J., Viršile A., Novic?kovas A., et al. (2015b). The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 173, 600–606. 10.1016/j.foodchem.2014.10.077 [DOI] [PubMed] [Google Scholar]
- Brazaityte A., Viršile A., Jankauskiene J., Sakalauskiene S., Samuoliene G., Sirtautas R., et al. (2015a). Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 29, 13–22. 10.1515/intag-2015-0004 [DOI] [Google Scholar]
- Cen Y.-P., Bornman J. F. (1990). The response of bean plants to UV-B radiation under different irradiances of background visible light. J. Exp. Bot. 41, 1489–1495. 10.1093/jxb/41.11.1489 [DOI] [Google Scholar]
- Charlebois S. (2018). Can greenbelt microgreens expand its model, A discussion on the future of microgreens. J. Agric. Stud. 6, 17–34. 10.5296/jas.v6i2.12885 [DOI] [Google Scholar]
- Cheeseman J. M. (2006). Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 57, 2435–2444. 10.1093/jxb/erl004 [DOI] [PubMed] [Google Scholar]
- Choe U., Yu L. L., Wang T. T. Y. (2018). The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 66, 11519–11530. 10.1021/acs.jafc.8b03096 [DOI] [PubMed] [Google Scholar]
- Chon S. (2013). Total polyphenols and bioactivity of seeds and sprouts in several legumes. Curr. Pharmaceut. Design 19, 6112–6124. 10.2174/1381612811319340005 [DOI] [PubMed] [Google Scholar]
- Cohen M. J., Garrett J. L. (2010). The food price crisis and urban food (in) security. Environ. Urban. 22, 467–482. 10.1177/095624781038037522833656 [DOI] [Google Scholar]
- Danilcenko H., Dabkevicius Z., Jariene E., Taraseviciene Z., Televiciute T., Tamošiunas A., et al. (2017). The effect of stinging nettle and field horsetail extracts on the synthesis of biologically active compounds in germinated leguminous and quinoa seed. Zemdirbyste Agric. 104, 337–344. 10.13080/z-a.2017.104.043 [DOI] [Google Scholar]
- Delian E., Chira A., Bădulescu L., Chira L. (2015). Insights into microgreens physiology. Scientific Papers. Series B, Horticulture, Vol. LIX. 2015, ISSN-L 2285-5653. Available online at: http://horticulturejournal.usamv.ro/pdf/2015/art69.pdf
- Devi J., Sanwal S. K., Koley T. K., Mishra G. P., Karmakar P., Singh P. M., et al. (2019). Variations in the total phenolics and antioxidant activities among garden pea (Pisum sativum L.) genotypes differing for maturity duration, seed and flower traits and their association with the yield. Sci. Hortic. 244, 141–150. 10.1016/j.scienta.2018.09.048 [DOI] [Google Scholar]
- Ebert A., Wu D., Yang R.-Y. (2015b). Amaranth sprouts and microgreens - a homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum. 10.13140/2.1.2722.6404 [DOI] [Google Scholar]
- Ebert A. W., Chang C. H., Yan M. R., Yang R. Y. (2017). Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. Food Chem. 237, 15–22. 10.1016/j.foodchem.2017.05.073 [DOI] [PubMed] [Google Scholar]
- Ebert A. W., Wu T. H., Yang R. Y. (2015a). “Amaranth sprouts and microgreens–a homestead vegetable production option to enhance food and nutrition security in the rural-urban continuum,” in Proceedings of the Regional Symposium on Sustaining Small-Scale Vegetable Production and Marketing Systems for Food and Nutrition Security (SEAVEG2014). (Bangkok: ), 233–244. [Google Scholar]
- El-Nakhel C., Giordano M., Pannico A., Carillo P., Fusco G. M., De Pascale S., et al. (2019b). Cultivar-specific performance and qualitative descriptors for butterhead Salanova lettuce produced in closed soilless cultivation as a candidate salad crop for human life support in space. Life 9:61. 10.3390/life9030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nakhel C., Pannico A., Graziani G., Kyriacou M. C., Giordano M., Ritieni A., et al. (2020). Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 9:300. 10.3390/antiox9040300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nakhel C., Pannico A., Kyriacou M. C., Giordano M., De Pascale S., Rouphael Y. (2019a). Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 99, 6962–6972. 10.1002/jsfa.9985 [DOI] [PubMed] [Google Scholar]
- Fidrianny I., Suhendy H., Insanu M. (2018). Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia. Asian Pac. J. Trop. Biomed. 8, 25–30. 10.4103/2221-1691.221131 [DOI] [Google Scholar]
- Gan R. Y., Lui W. Y., Wu K., Chan C. L., Dai S. H., Sui Z. Q., et al. (2017). Bioactive compounds and bioactivities of germinated edible seeds and sprouts: an updated review. Trends Food Sci. Technol. 59, 1–14. 10.1016/j.tifs.2016.11.010 [DOI] [Google Scholar]
- Ghoora M. D., Haldipur A. C., Srividya N. (2020). Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res. 2:100046. 10.1016/j.jafr.2020.100046 [DOI] [Google Scholar]
- Heim K. E., Tagliaferro A. R., Bobilya D. J. (2002). Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 13, 572–584. 10.1016/S0955-2863(02)00208-5 [DOI] [PubMed] [Google Scholar]
- Hideg E., Jansen M. A., Strid A. (2013). UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates. Trends Plant Sci. 18, 107–115. 10.1016/j.tplants.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Huang H., Jiang X., Xiao Z., Yu L., Pham Q., Sun J., et al. (2016). Red cabbage microgreen lower circulating LDL, liver cholesterol and inflammatory cytokines in mice fed a high fat diet. J. Agric. Food Chem. 64, 9161–9171. 10.1021/acs.jafc.6b03805 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Yoshimoto M., Ishigure K., Okuno S., Yamakawa O. (2003). Effect of artificial shading and temperature on radical scavenging activity and polyphenolic composition in sweet potato (Ipomoea batatas L.) leaves. J. Am. Soc. Hort. Sci. 128, 182–187. 10.21273/JASHS.128.2.0182 [DOI] [Google Scholar]
- Kamal K. Y., Khodaeiaminjan M., El-Tantawy A. A., Moneim D. A., Salam A. A., Ash-shormillesy S. M. A. I., et al. (2020). Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol. Plant. 169, 625–638. 10.1111/ppl.13083 [DOI] [PubMed] [Google Scholar]
- Kyriacou M. C., El-Nakhel C., Pannico A., Graziani G., Soteriou G. A., Giordano M., et al. (2019). Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 10:1501. 10.3389/fpls.2019.01501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou M. C., Rouphael Y., Di Gioia F., Kyratzis A., Serio F., Renna M., et al. (2016). Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 57, 103–115. 10.1016/j.tifs.2016.09.005 [DOI] [Google Scholar]
- Lichtenthaler H. K., Wellburn A. R. (1983). Determination of total carotenoids and chlorophylls A and B of leaf in different solvents. Biol. Soc. Trans. 11, 591–592. 10.1042/bst0110591 [DOI] [Google Scholar]
- Lin C. C., Kao C. H. (1999). NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 216, 147–155. 10.1023/A:1004714506156 [DOI] [Google Scholar]
- Loreto F., Velikova V. (2001). Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 127, 1781–1787. 10.1104/pp.010497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Kaihara A., Yoshii K., Tsumura Y., Ishimitsu S., Tonogai Y. (2001). Content and composition of isoflavonoids in mature or immature beans and bean sprouts consumed in Japan. J. Health Sci. 47, 394–396. 10.1248/jhs.47.394 [DOI] [Google Scholar]
- Nielsen S. S. (2017). “Vitamin C determination by indophenol method,” in Food Analysis Laboratory Manual. Food Science Text Series (Cham: Springer; ), 143–146. 10.1007/978-3-319-44127-6_15 [DOI] [Google Scholar]
- Oktay M., Gulcin I., Kufrevioglu O. I. (2003). Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss Techol. 36, 263–271. 10.1016/S0023-6438(02)00226-8 [DOI] [Google Scholar]
- Olsson L. C., Veit M., Weissenböck G., Bornman J. F. (1998). Differential flavonoid response to enhanced UV-B radiation in Brassica napus. Phytochemistry 49, 1021–1028. 10.1016/S0031-9422(98)00062-4 [DOI] [Google Scholar]
- Ozden M., Demirel U., Kahraman A. (2009). Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 119, 163–168. 10.1016/j.scienta.2008.07.031 [DOI] [Google Scholar]
- Paradiso V. M., Castellino M., Renna M., Gattullo C. E., Calasso M., Terzano R., et al. (2018). Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 9, 5629–5640. 10.1039/C8FO01182F [DOI] [PubMed] [Google Scholar]
- Parida A. K., Das A. B. (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf. 60, 324–349. 10.1016/j.ecoenv.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Patel K. G., Mandaliya V. B., Mishra G. P., Dobaria J. R., Radhakrishnan T. (2016). Transgenic peanut overexpressing mtlD gene confers enhanced salinity-stress tolerance via mannitol accumulation and differential antioxidative responses. Acta Physiol. Plant 38:181. 10.1007/s11738-016-2200-0 [DOI] [Google Scholar]
- Pereira D. M., Valentao P., Pereira J. A., Andrade P. B. (2009). Phenolics: from chemistry to biology. Molecules 14, 2202–2211. 10.3390/molecules14062202 [DOI] [Google Scholar]
- Pinto E., Almeida A. A., Aguiar A. A., Ferreira (2015). Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Comp. Anal. 37, 38–43. 10.1016/j.jfca.2014.06.018 [DOI] [Google Scholar]
- Prior R. L., Wu X., Schaich K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53, 4290–4302. 10.1021/jf0502698 [DOI] [PubMed] [Google Scholar]
- Renna M., Di Gioia F., Leoni B., Mininni C., Santamaria P. (2017). Culinary assessment of self-produced microgreens as basic ingredients in sweet and savory dishes. J. Culin. Sci. Technol. 15, 126–142. 10.1080/15428052.2016.1225534 [DOI] [Google Scholar]
- Riggio G. M., Wang Q., Kniel K. E., Gibson K. E. (2019). Microgreens-A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 290, 76–85. 10.1016/j.ijfoodmicro.2018.09.027 [DOI] [PubMed] [Google Scholar]
- Sadasivam S., Balasubraminan T. (1987). Practical Manual in Biochemistry. Coimbatore: Tamil Nadu Agricultural University, 14. [Google Scholar]
- Saengha W., Karirat T., Buranrat B., Matra K., Deeseenthum S., Katisart T., et al. (2021). Cold plasma treatment on mustard green seeds and its effect on growth, isothiocyanates, antioxidant activity and anticancer activity of microgreens. Int. J. Agric. Biol. 25, 667–676. 10.17957/IJAB/15.1715 [DOI] [Google Scholar]
- Singh R., Haukka M., McKenzie C. J., Nordlander E. (2015). High turnover catalase activity of a mixed-valence mn II mn III complex with terminal carboxylate donors. Eur. J. Inorg. Chem. 2015, 3485–3492. 10.1002/ejic.201500468 [DOI] [Google Scholar]
- Singleton V. L., Orthofer R., Lamuela-Raventos R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 299, 152–178. 10.1016/S0076-6879(99)99017-1 [DOI] [Google Scholar]
- Swieca M., Baraniak B. (2013). Influence of elicitation with hydrogen peroxide on health promoting phytochemicals and nutritional quality of dark germinated Lens culinaris sprouts. J. Sci. Food Agric. 94, 489–496. 10.1002/jsfa.6274 [DOI] [PubMed] [Google Scholar]
- Swieca M., Gwalik-Dziki U. (2015). Effects of sprouting and postharvest storage under cool temperature conditions on starch content and antioxidant capacity of green pea, lentil and young mung bean sprouts. Food Chem. 185, 99–105. 10.1016/j.foodchem.2015.03.108 [DOI] [PubMed] [Google Scholar]
- Swieca M., Seczyk Ł., Gawlik-Dziki U. (2014a). Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts. Food Chem. 161, 288–295. 10.1016/j.foodchem.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Swieca M., Surdyka M., Gawlik-Dziki U., Złotek U., Baraniak B. (2014b). Antioxidant potential of fresh and stored lentil sprouts affected by elicitation with temperature stresses. Int. J. Food Sci. Technol. 49, 1811–1817. 10.1111/ijfs.12489 [DOI] [Google Scholar]
- Tan L., Nuffer H., Feng J., Kwan S. H., Chena H., Tong X., et al. (2020). Antioxidant properties and sensory evaluation of microgreens from commercial and local farms. Food Sci. Human Wellness 9, 45–51. 10.1016/j.fshw.2019.12.002 [DOI] [Google Scholar]
- Tsen C. C. (1961). An improved spectrophotometric method for the determination of tocopherols using 4,7-Diphenyl-1,10-phenanthroline. Anal. Chem. 7, 849–851. 10.1021/ac60175a010 [DOI] [Google Scholar]
- Wang Q., Xiao Z., Codling E. E., Lester G. E., Nou X., Luo Y. (2016). Microgreens of Brassicaceae: mineral composition and content of 30 varieties. J. Food Compos. Anal. 49, 87–93. 10.1016/j.jfca.2016.04.006 [DOI] [Google Scholar]
- Weber C. F. (2017). Broccoli Microgreens: a mineral-rich crop that can diversify food systems. Front Nutr. 4:7. 10.3389/fnut.2017.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Broadley M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 18, 49–84. 10.1111/j.1469-8137.2008.02738.x [DOI] [PubMed] [Google Scholar]
- Woisky R. G., Salatino A. (1998). Analysis of propolis: some parameters and procedure for chemical quality control. J. Apic. Res. 37, 99–105. 10.1080/00218839.1998.11100961 [DOI] [Google Scholar]
- World Health Organization and Food and Agriculture Organization of the United Nations (WHO/FAO) (2005) Fruit and Vegetables for Health: Report of a Joint FAO/WHO Workshop (Kobe) . [Google Scholar]
- Xiao Z., Lester G. E., Luo Y., Wang Q. (2012). Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J. Agric. Food Chem. 60, 7644–7651. 10.1021/jf300459b [DOI] [PubMed] [Google Scholar]
- Xiao Z., Rausch S. R., Luo Y., Sun J., Yu L., Wang Q., et al. (2019). Microgreens of Brassicaceae: genetic diversity of phytochemical concentrations and antioxidant capacity. Food Sci. Technol. 101, 731–737. 10.1016/j.lwt.2018.10.076 [DOI] [Google Scholar]
- Yang C., Shahidi F., Tsao R. (2018). “Biomarkers of oxidative stress and cellular-based assays of indirect antioxidant measurement,” in Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, eds R. Apak, E. Capanoglu, and F. Shahidi (Hoboken, NJ: John Wiley and Sons Ltd; ), 165–186. 10.1002/9781119135388.ch9 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.