Abstract
Irinotecan, a topoisomerase inhibitor, is a common cytotoxic agent prescribed for metastatic colorectal cancer (mCRC) patients. Diarrhea is the most common adverse event (AE). The underlying mechanism of irinotecan-induced diarrhea is intestinal mucosal damage caused by SN-38 (active metabolite of irinotecan) hydrolyzed from SN-38G (inactive metabolite) by bacterial β-glucuronidase (βG). According to an animal study, silymarin reduces the activity of bacterial βG without impairing antitumor efficacy. We conducted a prospective open-label pilot study to evaluate the effect of silymarin as supplementation in reducing toxicities of mCRC patients undergoing irinotecan-based chemotherapy. We enrolled and randomized 70 mCRC patients receiving first-line FOLFIRI (5-fluorouracil/leucovorin/irinotecan) plus bevacizumab. In each treatment cycle, the study group was administered silymarin capsules (150 mg) three times daily for 7 days. The study group experienced less AEs in diarrhea (5.7% vs. 14.6%, p = 0.002) and nausea (27.0% vs. 40.2%, p = 0.005) in comparison with the control group, but no significant differences in hepatic toxicities were observed. In conclusion, simultaneous administration of silymarin is a potential effective supplementation for reducing toxicities in mCRC patients undergoing first-line FOLFIRI plus bevacizumab, especially in diarrhea and nausea.
Key words: Silymarin, FOLFIRI plus bevacizumab, Metastatic colorectal cancer, Toxicity
INTRODUCTION
Irinotecan, a topoisomerase inhibitor that interrupts deoxyribonucleic acid (DNA) replication in cancer cells, is a cytotoxic agent commonly prescribed for metastatic colorectal cancer (mCRC) patients. The most common adverse reaction to irinotecan is bone marrow suppression through anemia (60% to 97%), leukopenia (63% to 96%), thrombocytopenia (96%), and neutropenia (30% to 96%), which is followed by diarrhea (late: 83% to 88%; early: 43% to 51%)1. Such adverse events (AEs) may interfere with a patient’s treatment course and quality of life. Irinotecan-induced diarrhea is of two types: early onset (beginning within 24 h), which is mild, transient, and part of a broader cholinergic syndrome that may be prevented by intravenous administration of atropine, and delayed onset diarrhea (beginning after more than 24 h of infusion), which appears to be multifactorial and includes dysmotility and secretory factors2,3. The underlying mechanism of irinotecan-induced delayed diarrhea is intestinal mucosal damage caused by SN-38 (the active metabolite of irinotecan), which is hydrolyzed from SN-38G (the inactive metabolite) by bacterial β-glucuronidase (βG)4–8. At the present time, the intestinal bacterial microflora is one of the reasons causing damage to the intestinal mucosa because of their capacity of transforming the SN-38G in SN-38 in the intestinal lumen. Thus, the methods to eradicate intestinal microflora or reduce the activity of intestinal bacterial βG seem to be a reasonable mechanism to reduce such transforming capacity. Takasuna et al. reported using penicillin plus streptomycin to decrease accumulation of SN-38 in the large intestine of a rat9. Likewise, Kehrer et al. reported reduced irinotecan-induced intestinal toxicity after prescribing the oral form neomycin in seven colorectal patients10. Cheng et al. suggested that TCH-3562, another inhibitor of βG, had protective effects against irinotecan-induced diarrhea without interfering with the therapeutic efficacy of irinotecan in tumor-bearing mice11.
Silymarin is a bioflavonoid complex extract from Silybum marianum Gaertneri (common name: milk thistle) composed of various flavonolignans and discovered in 195212. Standardized silymarin products must have 30%–65% silymarin content, which is composed of 20%–45% silychristin and silydianin, 40%–65% silybins A and B, and 10%–20% isosilybins A and B13. This compound has been used for more than 2,000 years to treat cirrhosis and hepatitis and to protect the liver against toxins. Various studies performed in animals and humans have confirmed that silymarin and particularly its active ingredient, silybin, exert prominent antioxidant effects through free radical scavenging and inhibition of lipid peroxidation14,15. Silymarin inhibits lipid peroxidation and exerts antioxidant, anti-inflammatory, antifibrotic, immunomodulatory, and membrane-stabilizing effects; it is also able to regenerate the liver in experimental models of hepatic diseases16.
Kim et al. showed that silybin, a compound of silymarin, inhibited βG activity in rat intestinal bacteria, HGU-1 and HGU-2, and Escherichia coli HB101 noncompetitively17. Moreover, βG expression in the feces of a healthy individual and of an individual with colon cancer was also inhibited by silybin and silymarin. Silymarin has been used as an antioxidant to treat liver disease for many years and is well tolerated and safe to prescribe14. To the best of our knowledge, there is no case report or clinical trial working on the effect of silymarin as supplementation in mCRC patients treated with irinotecan-based therapy. Herein, we conducted a prospective open-label pilot study to evaluate the effect of supplemental silymarin in mCRC patients treated with irinotecan-based chemotherapy.
MATERIALS AND METHODS
Study Design and Ethics
The present study, a prospective open-label pilot study, was approved by the institutional review board of our hospital [KMUHIRB-F(II)-20160038] and registered on ClinicalTrials.gov (identifier: NCT03130634) before participants were enrolled. The Declaration of Helsinki and International Council for Harmonisation-Clinical Research Practice (ICH-GCP) were followed. All evaluations were conducted at our hospital from September 2016 to July 2019. Written informed consent was obtained from each patient before screening.
Sample Size Estimation
Each patient had six cycles of therapy, and we took AE in each cycle as one event (per-cycle AEs). Based on our unpublished preliminary data, null [H(0)] and alternative [H(A)] hypothesis were following: H(0)—absence of any grade diarrhea in 85% of patients; H(A)—absence of any grade diarrhea in 94% of patients. Three hundred sixty-two samples (181 cycles in each group) were needed to achieve 80% power to detect a difference between the group proportions (for any grade diarrhea) of 9% at the significance level of 0.0518. The proportion of patients without any grade diarrhea in the study group was assumed to be 85% under the null hypothesis and 94% under the alternative hypothesis. The proportion of patients without any grade diarrhea in the control group was 85%. The test statistic used was the two-sided Z-test with pooled variance. Because of the expected 15% ineligibility, the proposed number of samples was 416. In the end, we enrolled 70 patients, which we assumed provided 420 cycles of treatment for statistical analysis. All analyses were performed on an intention-to-treat basis.
Study Participants
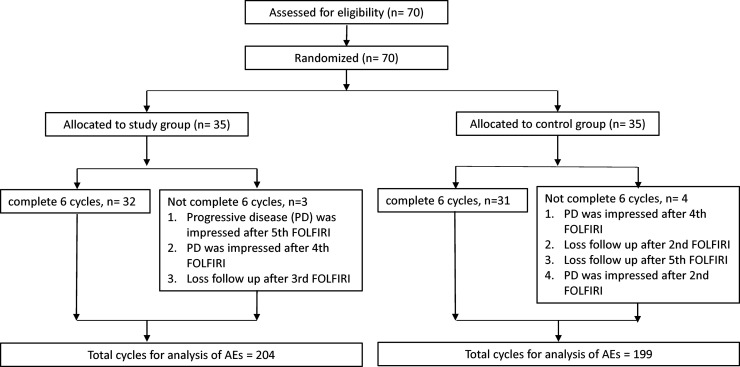
A study flow diagram is presented in Figure 1. A total of 70 mCRC patients were evenly assigned into study and control groups between September 2016 and July 2019. The participants were randomized using sealed, opaque, individually numbered envelopes. The envelopes contained data sheets with information on group allocation and a randomization number generated by a statistician with SAS 9.4 (SAS Institute, Cary, NC, USA).
Figure 1.
Study flow diagram of mCRC patients.
Eligibility Criteria
Inclusion criteria were defined as follows: (1) age between 20 and 80 years, and (2) confirmed mCRC patients scheduled to receive first-line systemic therapy with FOLFIRI plus bevacizumab. Patients with the following criteria were excluded: (1) pregnant or lactating; (2) allergy, sensitivity, or contraindication to irinotecan, silymarin, or any ingredient of the medications used in the study; (3) viral hepatitis or a carrier or impaired liver function with unknown etiology; (4) a severe comorbidity; or (5) Eastern Cooperative Oncology Group (ECOG) performance status equal to or greater than 319.
Investigational Medications
According to the treatment guideline in our hospital, the recommended first-line chemotherapy regimen is FOLFIRI. In this study, all the patients also received bevacizumab as biological therapy. In our treatment setting, all mCRC patients were hospitalized every 14 days and received six cycles of biological therapy with bevacizumab (5 mg/kg) followed by the standard FOLFIRI regimen at a dose of 180 mg/m2 irinotecan and 200 mg/m2 leucovorin as intravenous infusion over 2 h followed by fluorouracil (2,800 mg/m2 as intravenous infusion over a 46-h period). Prophylactic atropine 0.25 mg was prescribed just before infusion of irinotecan to prevent acute cholinergic syndrome for every patient. According to our clinical observations, most delayed onset toxicities will develop within 7 days after FOLFIRI infusion. Therefore, we assumed to prescribe silymarin for a duration of 7 days, and the dose of silymarin was 150 mg three times daily according to package insert. At the initiation of each cycle (i.e., at the beginning of chemotherapy), one capsule of silymarin (150 mg) was administered orally three times a day for 7 days to the study group, whereas the control group received FOLFIRI plus bevacizumab only. The NutriMate silymarin capsules (300 mg Extr. Fructus Cardui Mariae extract equivalent to 150 mg silymarin) were produced by Taiwan Biotech Co. Ltd. (Taoyuan City, Taiwan).
Primary and Secondary Endpoint
The primary endpoint was the incidence of gastrointestinal (GI) toxicities. The AEs were monitored and graded according to the Common Terminology Criteria for Adverse Events version 4.03 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm) of the National Cancer Institute. The secondary endpoints were median progression-free survival (PFS) and overall survival (OS) of these patients. All the analyzed data were contributed from the medical chart record. Patient-reported outcomes were collected by medical records through outpatient visit or telephone contact. The dosage of additional antidiarrheal drugs was also recorded. Patient diary and compliance of study drug were not evaluated.
Safety Assessment
The following safety-related parameters and events were recorded and evaluated for both groups among the hospitalization in each cycle during the study period: vital signs, concomitant medications, and AEs occurring after the administration of the investigated medications. If any severe AEs occurred (of grade equal to or greater than 3), the chemotherapy and concomitant medications were to be postponed until the AE grade was relieved to equal to or less than 2. Patients received full supportive care during the study including antidiarrheal drugs (loperamid, diosmectite, mepenzolate, and dicyclomine), antiemetics, and analgesics when appropriate as a standard of care.
Statistical Analysis
PFS was defined as the time elapsed between the first treatment and documented disease progression or death of a patient. OS was defined as the time elapsed between the first treatment and death of a patient due to any cause. Continuous variables were represented as means ± standard deviations, and dichotomous variables as numbers and percentage values. All statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Patient profiles and AE results were compared using the Pearson chi-square test, survival rates were estimated using the Kaplan–Meier method, and the log rank test was used to compare time-to-event distributions. A value of p < 0.05 was considered statistically significant.
RESULTS
Seventy mCRC patients were enrolled and randomized evenly to each group. The clinical profiles of seven mCRC patients are summarized in Table 1. The patients’ median age was 60.5 years (range from 24 to 83), and 62.9% were female. There were 72.9% of mCRC patients with left side colon cancer, and the most frequent metastatic site was the liver (38.6%), followed by the lung (12.9%) and distant lymph nodes (11.4%), and 22.9% of patients presented at least two metastatic sites. The other metastatic sites included the adrenal gland, ovary, spleen, prostate, and urinary bladder. ECOG performance status was better in the control group (p = 0.003), but all mCRC patients were suitable for receiving chemotherapy and 90% of them went through the six-cycle therapy completely. The collectible somatic, germline, and tumor genetic profiles revealed nonsignificant differences between the two groups in BRAF mutation (2.9% vs. 11.8%, p = 0.163), mutant UGT1A1 genotype (28.6% vs. 22.9%, p = 0.314), and positive expression of epidermal growth factor receptor (100.0% vs. 97.1%, p = 0.484), but the study group had higher frequency of KRAS mutation than did the control group (74.3% vs. 35.3%, p = 0.001).
Table 1.
Patient Profiles
| Study [n (%)] | Control [n (%)] | All [n (%)] | p Value | |
|---|---|---|---|---|
| Patients | 35 | 35 | 70 | |
| Age | 0.918 | |||
| Mean (SD) | 61.5 (9.8) | 61.2 (13.0) | 60.9 (11.6) | |
| Median (range) | 61 (40–79) | 61 (24–83) | 60.5 (24–83) | |
| Gender | 1.000 | |||
| Male | 13 (37.1%) | 13 (37.1%) | 26 (27.1%) | |
| Female | 22 (62.9%) | 22 (62.9%) | 44 (62.9%) | |
| Primary tumor site | 0.788 | |||
| Right | 10 (28.6%) | 9 (25.7%) | 19 (27.1%) | |
| Left | 25 (71.4%) | 26 (74.3%) | 51 (72.9%) | |
| Metastatic site | 0.812 | |||
| Liver | 13 (37.1%) | 14 (40.0%) | 27 (38.6%) | |
| Lung | 6 (17.1%) | 3 (8.6%) | 9 (12.9%) | |
| Distant lymph nodes | 3 (8.6%) | 5 (14.3%) | 8 (11.4%) | |
| Bone | 1 (2.9%) | 2 (5.7%) | 3 (4.3%) | |
| Other | 3 (8.6%) | 4 (11.4%) | 7 (10.0%) | |
| Multiple | 9 (25.7%) | 7 (20.0%) | 16 (22.9%) | |
| ECOG performance status | 0.003 | |||
| 0 | 4 (11.4%) | 15 (42.9%) | 19 (27.1%) | |
| 1 | 31 (88.6%) | 20 (57.1%) | 51 (72.9%) | |
| KRAS status | 0.001 | |||
| Wild | 9 (25.7%) | 22 (64.7%) | 31 (44.9%) | |
| Mutant | 26 (74.3%) | 12 (35.3%) | 38 (55.1%) | |
| BRAF status | 0.163 | |||
| Wild | 33 (97.1%) | 30 (88.2%) | 63 (92.6%) | |
| Mutant | 1 (2.9%) | 4 (11.8%) | 5 (7.4%) | |
| No data | 1 | 1 | 2 | |
| UGT1A1 status | 0.314 | |||
| Wild (6/6) | 25 (71.4%) | 27 (77.1%) | 52 (74.3%) | |
| Mutant (6/7) | 10 (28.6%) | 8 (22.9%) | 18 (25.7%) | |
| EGFR status | 0.484 | |||
| Positive | 35 (100.0%) | 34 (7.1%) | 69 (98.6%) | |
| Negative | 0 (0.0) | 1 (2.9%) | 1 (1.4%) |
Abbreviations: SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Thirty-two patients in the study group completed the six cycles of treatment, which counted for 192 samples. Thirty-one patients in the control group completed the six cycles, which counted for 186 samples. Three patients in the study group and four patients in the control group were unable to complete six cycles of treatment. According to the basis of intention to treat, we also counted the completed treatment cycles of these seven patients for further analysis. In the result, the study group had 204 samples, and the control group had 199 samples for further statistical analysis.
Because the occurrence of severe AEs (grade equal to or greater than 3) was limited (Table 2), the severity of AEs could be compared between two groups. The incidences of any grade AEs were compared and analyzed (Table 3). Among GI toxicities, the study group had less diarrhea (5.4% vs. 14.6%, p = 0.002) and nausea (27.0% vs. 40.2%, p = 0.005) than the control group. The study group had less leukopenia (25.5% vs. 36.7%, p = 0.015) but more anemia (78.4% vs. 65.8%, p = 0.005) than control group among hematologic toxicities. No statistical differences were noted in the two groups with regard to the symptom of vomiting and hepatic toxicities (all p > 0.005).
Table 2.
Adverse Events of 70 mCRC Patients
| Adverse Events | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study [n (%)] |
Control [n (%)] |
Study [n (%)] |
Control [n (%)] |
Study [n (%)] |
Control [n (%)] |
Study [n (%)] |
Control [n (%)] |
Study [n (%)] |
Control [n (%)] |
|
| Gastrointestinal toxicities | ||||||||||
| Diarrhea | 193 (94.6) | 170 (85.4) | 7 (3.4) | 17 (8.5) | 4 (2.0) | 8 (4.0) | 0 | 4 (2.0) | 0 | 0 |
| Nausea | 149 (73.0) | 119 (66.5) | 48 (23.5) | 62 (31.2) | 7 (3.4) | 16 (8.0) | 0 | 2 (1.0) | 0 | 0 |
| Vomiting | 181 (88.7) | 168 (48.1) | 15 (7.4) | 15 (7.5) | 8 (3.9) | 13 (6.5) | 0 | 3 (1.5) | 0 | 0 |
| Hepatic toxicities | ||||||||||
| Increased SGOT level | 168 (82.4) | 173 (86.9) | 31 (15.2) | 21 (10.6) | 5 (2.5) | 3 (1.5) | 0 | 1 (0.5) | 0 | 1 (0.5) |
| Increased SGPT level | 175 (85.8) | 173 (86.9) | 27 (13.2) | 24 (12.1) | 2 (1.0) | 0 | 0 | 1 (0.5) | 0 | 1 (0.5) |
| Hematologic toxicities | ||||||||||
| Leukopenia | 152 (74.5) | 126 (63.3) | 41 (20.1) | 47 (23.6) | 11 (5.4) | 25 (12.6) | 0 | 1 (0.5) | 0 | 0 |
| Anemia | 44 (21.6) | 68 (34.2) | 115 (56.4) | 98 (49.2) | 45 (22.1) | 33 (16.6) | 0 | 0 | 0 | 0 |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Table 3.
Occurrence Rate of Toxicities Between Two Groups
| Adverse Events | Study | Control | p Value |
|---|---|---|---|
| Gastrointestinal toxicities | |||
| Diarrhea | 0.002 | ||
| Yes | 11 (5.4%) | 29 (14.6%) | |
| No | 193 (94.6%) | 170 (85.4%) | |
| Nausea | 0.005 | ||
| Yes | 55 (27.0%) | 80 (40.2%) | |
| No | 149 (73.0%) | 119 (59.8%) | |
| Vomiting | 0.205 | ||
| Yes | 23 (11.3%) | 31 (15.6%) | |
| No | 181 (88.7%) | 168 (84.4%) | |
| Hepatic toxicities | |||
| Increased SGOT level | 0.202 | ||
| Yes | 36 (17.6%) | 26 (13.1%) | |
| No | 168 (82.4%) | 173 (86.9%) | |
| Increased SGPT level | 0.737 | ||
| Yes | 29 (14.2%) | 26 (13.1%) | |
| No | 175 (85.8%) | 173 (86.9%) | |
| Hematologic toxicities | |||
| Leukopenia | 0.015 | ||
| Yes | 52 (25.5%) | 73 (36.7%) | |
| No | 152 (74.5%) | 126 (63.3%) | |
| Anemia | 0.005 | ||
| Yes | 160 (78.4%) | 131 (65.8%) | |
| No | 44 (21.6%) | 68 (34.2%) | |
Abbreviations: SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
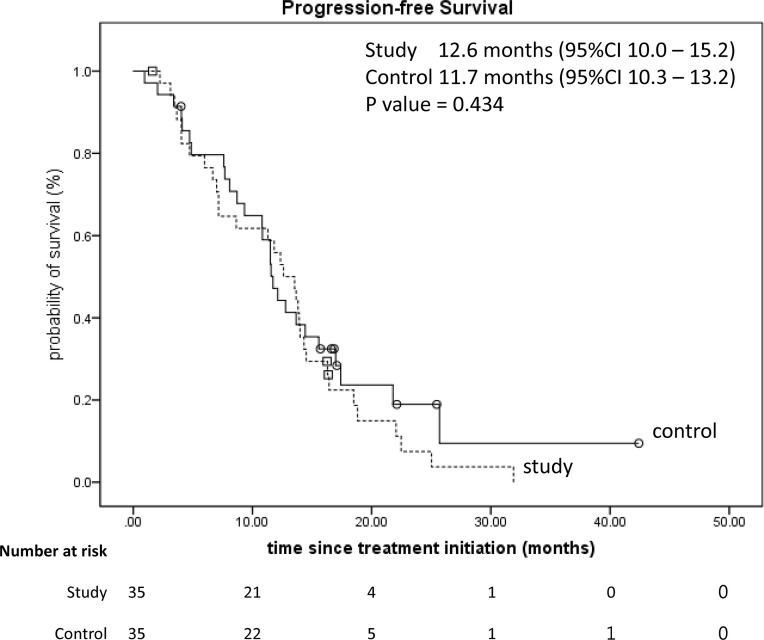
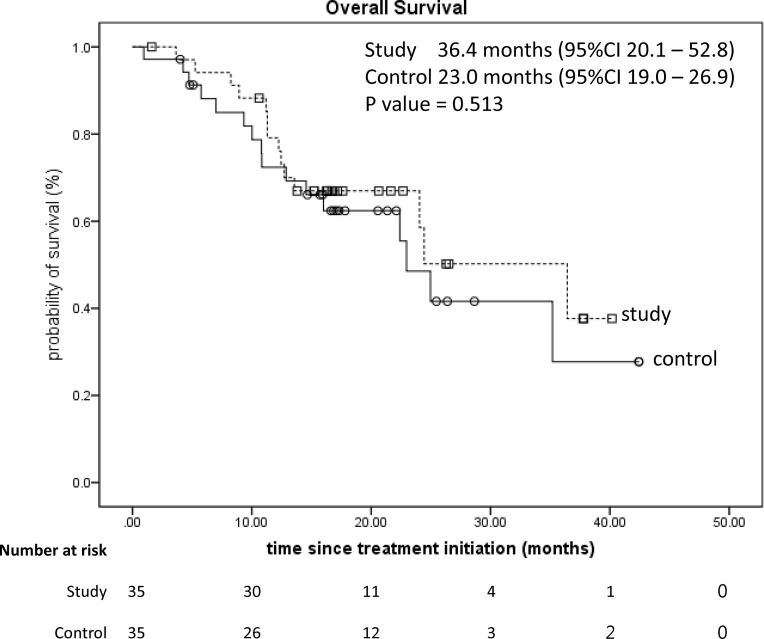
The median PFS between two groups were 12.6 months [95% confidence interval (CI): 10.0–15.2] versus 11.7 months (95% CI: 10.3–13.2, p = 0.434) (Fig. 2); the OS was 36.4 months (95% CI: 20.1–52.8) versus 23.0 months (95% CI: 19.0–26.9, p = 0.513) (Fig. 3). Not only had the numbers of patients who ever used antidiarrheal medications but also the consumption of medication dosage had no statistical differences (all p > 0.005) (Table 4).
Figure 2.
Kaplan–Meier survival analysis of the progression-free survival of two groups (p = 0.434).
Figure 3.
Kaplan–Meier survival analysis of the overall survival of two groups (p = 0.513).
Table 4.
Additional Consumption of Antidiarrheal Medications Between Two Groups
| Study (n = 35) | Control (n = 35) | p Value | |
|---|---|---|---|
| Use of antidiarrheal drugs | 0.615 | ||
| Yes | 24 (68.6%) | 22 (62.9%) | |
| No | 11 (31.4%) | 13 (37.1%) | |
| Loperamide (mg) [mean (SD)] | 15.1 (48.8) | 27.1 (58.3) | 0.354 |
| Diosmectite (g) [mean (SD)] | 68.2 (117.9) | 82.0 (130.3) | 0.644 |
| Mepenzolate (mg) [mean (SD)] | 13.5 (44.7) | 80.6 (222.8) | 0.089 |
| Dicyclomin (mg) [mean (SD)] | 0 | 8.6 (38.2) | 0.193 |
DISCUSSION
The FOLFIRI regimen revealed its benefit as first-line treatment for mCRC patients20; however, irinotecan frequently induces neutropenia and diarrhea, affecting the treatment course. A previous study reported late onset diarrhea occurred in 87% advanced colorectal cancer (CRC) patients who received fluorouracil-based chemotherapy plus irinotecan (350 mg/m2) and 39% patients had grades 3–4 diarrhea21. In recent decade, the use of FOLFIRI plus bevacizumab as first-line therapy in advanced CRC patients disclosed the incidences of any grade and grades 3–4 diarrhea being 35.8%–62.0% and 5.0%–15.0%, respectively, in Western countries22–28, and 17.8%–54.0% and 2.6%–9.0%, respectively, in Asian countries29–33. In this study, the incidences of any grade and grades 3–4 diarrhea in the control group were 14.4% and 2.2%, respectively. The evolution and modification of chemotherapy and the racial difference may be an explanation to such difference in diarrhea incidence, but it still needs further investigation to prove it.
Many potential approaches to reducing incidence of irinotecan-induced late onset diarrhea have been tested, including schedule/dose modification, intestinal alkalization, structural/chemical modification, genetic testing, antidiarrheal therapies, transporter (ABCB1, ABCC2, and BCRP2) inhibitors, enzyme (βG, UGT1A1, CYP3A4, carboxylesterase, and COX-2) inducers and inhibitors, probiotics, antibiotics, adsorbing agents, cytokine and growth factor activators and inhibitors, and other miscellaneous agents3. However, these approaches may cause other problems, such as constipation, drug resistance, a high economic burden, or other drug-related side effects.
In our study, the mCRC patients who received silymarin as supplementation experienced a significant reduction in the occurrence of diarrhea; moreover, the occurrence of nausea was also markedly decreased. The study group had longer survival periods than the control group in either PFS (12.6 months vs. 11.7 months) or OS (36.4 months vs. 23.0 months). Besides, the patients of the study group have more KARS mutations (75%) and worse ECOG performance status (>0: 90.6%) than the control group. Such differences may affect the interval to disease progression in the study group. However, PFS and OS revealed no significant statistical difference between the two groups, which may imply such differences in KRAS mutation, ECOG status, and silymarin supplementation may not interfere with the survival of mCRC patients undergoing first-line FOLFIRI plus bevacizumab regimen.
In the present study, no severe liver function impairment occurred in both groups, but the study group has a higher proportion of grade 1 liver toxicities without statistical difference. Therefore, silymarin revealed little hepatic function protective effect in our study. The relatively low incidence of liver dysfunction in both groups might explain this result.
Leukopenia and anemia are two common hematologic toxicities caused by irinotecan. In our study, the study group experienced less leukopenia but more anemia. Currently, no studies focus on the influence of silymarin in bone marrow suppression caused by irinotecan. The effect of silymarin on hematologic toxicities in our study is ambiguous, and it might need a large-scale study for further investigation.
The limitations of the study are threefold. First, it was not double blind, and a placebo effect cannot be eliminated. Second, the study was limited with only 70 mCRC patients in a single institution in an Asian country and should be expanded to include patients in other institutions (or even Caucasian mCRC patients), and some of the 70 mCRC patients were not completely evaluated for six cycles of the treatment course. Third, we may need a patient diary to assess patients’ compliance and patient-reported outcomes to make our assessment of the primary endpoint more complete. Fourth, no animal model investigation was performed to explore underlying mechanisms.
In summary, silymarin supplementation can reduce the occurrence of diarrhea and nausea in mCRC patients undergoing first-line FOLFIRI plus bevacizumab. Silymarin (150 mg) three times daily from the beginning of chemotherapy for 7 days is an effective and well-tolerated supplementation that does not interfere with antitumor efficacy for mCRC patients.
ACKNOWLEDGMENTS
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 109-2314-B-037-035, MOST 109-2314-B-037-040, and MOST 109-2314-B-037-046-MY3) and the Ministry of Health and Welfare (MOHW107-TDU-B-212-123006, MOHW109-TDU-B-212-134026, MOHW109-TDU-B-212-114006, and MOHW110-TDU-B-212-1140026) and funded by the health and welfare surcharge of tobacco products, the Kaohsiung Medical University (KMU) Hospital (KMUH109-9R32, KMUH109-9R33, KMUH109-9R34, KMUH109-9M30, KMUH109-9M31, KMUH109-9M32, KMUH109-9M33, KMUHS10903, KMUH-DK(C)110010, KMUH-DK(B)110004-3, and KMUHSA10903), the KMU Center for Cancer Research (KMU-TC109A04-1), as well as a KMU Center for Liquid Biopsy and Cohort Research Center Grant (KMU-TC109B05), Kaohsiung Medical University. In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, R.O.C. This manuscript was edited by Wallace Academic Editing.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Product Information: CAMPTOSAR (R) intravenous injection, irinotecan intravenous injection. Pharmacia & Upjohn Co (per Manufacturer), New York. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf [accessed January 30, 2021] [Google Scholar]
- 2.Hecht JR. Gastrointestinal toxicity or irinotecan. Oncology (Williston Park) 1998;12(8 Suppl 6):72–8. [PubMed] [Google Scholar]
- 3.Swami U, Goel S, Mani S. Therapeutic targeting of CPT-11 induced diarrhea: A case for prophylaxis. Curr Drug Targets 2013;14(7):777–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saliba F, Hagipantelli R, Misset JL, Bastian G, Vassal G, Bonnay M, Herait P, Cote C, Mahjoubi M, Mignard D, Cvitkovic E. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: A prospective assessment. J Clin Oncol. 1998;16(8):2745–51. [DOI] [PubMed] [Google Scholar]
- 5.Lin XB, Farhangfar A, Valcheva R, Sawyer MB, Dieleman L, Schieber A, Gänzle MG, Baracos V. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One 2014;9(1):e83644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother Pharmacol. 2008;62(1):33–41. [DOI] [PubMed] [Google Scholar]
- 7.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Laurence J, Keefe DM. Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol. 2009;64(1):123–32. [DOI] [PubMed] [Google Scholar]
- 8.Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh AS, Al-Dasooqi N, Keefe DM. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol. 2009;90(5):489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T. Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol. 1998;42(4):280–6. [DOI] [PubMed] [Google Scholar]
- 10.Kehrer DF, Sparreboom A, Verweij J, de Bruijn P, Nierop CA, van de Schraaf J, Ruijgrok EJ, de Jonge MJ. Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res. 2001;7(5):1136–41. [PubMed] [Google Scholar]
- 11.Cheng KW, Tseng CH, Tzeng CC, Leu YL, Cheng TC, Wang JY, Chang JM, Lu YC, Cheng CM, Chen IJ, Cheng YA, Chen YL, Cheng TL. Pharmacological inhibition of bacterial beta-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol Res. 2019;139:41–9. [DOI] [PubMed] [Google Scholar]
- 12.Schindler H. [Active substances in pharmaceutical plants; methods to determine plant tinctures; contributions to a supplementary issue of the homoiopathic pharmacopeia]. Arzneimittelforschung 1952;2(6):291–6. [PubMed] [Google Scholar]
- 13.Csupor D, Csorba A, Hohmann J. Recent advances in the analysis of flavonolignans of Silybum marianum. J Pharm Biomed Anal. 2016;130:301–17. [DOI] [PubMed] [Google Scholar]
- 14.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs 2001;61(14):2035–63. [DOI] [PubMed] [Google Scholar]
- 15.Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: A systematic review. J Viral Hepat. 2005;12(6):559–67. [DOI] [PubMed] [Google Scholar]
- 16.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum). Integr Cancer Ther. 2007;6(2):104–9. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Jin YH, Park JB, Kobashi K. Silymarin and its components are inhibitors of beta-glucuronidase. Biol Pharm Bull. 1994;17(3):443–5. [DOI] [PubMed] [Google Scholar]
- 18.Sample Size Calculator. https://clincalc.com/stats/samplesize.aspx [accessed January 1, 2021]
- 19.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 20.Mitry E, Tournigand C, Andre T, Douillard JYY, Louvet C, Cunningham D, Magherini E, Mery-Mignard D, Gramont ADe, Rougier P. Comparison of the tolerance and efficacy of LV5FU2-CPT11 and FOLFIRI regimens in front-line treatment of advanced colorectal cancer—A pooled analysis of 254 patients included in 2 randomised trials. J Clin Oncol. 2004;22(14_suppl):3576. [Google Scholar]
- 21.Rougier P, Bugat R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Semin Oncol. 1996;23(1 Suppl 3):34–41. [PubMed] [Google Scholar]
- 22.López R, Salgado M, Reboredo M, Grande C, Méndez JC, Jorge M, Romero C, Quintero G, de la Cámara J, Candamio S. A retrospective observational study on the safety and efficacy of first-line treatment with bevacizumab combined with FOLFIRI in metastatic colorectal cancer. Br J Cancer 2010;103(10):1536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosati G, Cordio S, Aprile G, Butera A, Avallone A, Di Lucca G, De Pauli F, Parra HS, Reggiardo G, Bordonaro R. Discontinuation of bevacizumab and FOLFIRI administered up to a maximum of 12 cycles as first-line therapy for metastatic colorectal cancer: A retrospective Italian study. Invest New Drugs 2012;30(5):1978–83. [DOI] [PubMed] [Google Scholar]
- 24.Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, Xynogalos S, Christophyllakis Ch, Kouroussis Ch, Vamvakas L, Georgoulias V, Polyzos A. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab versus FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br J Cancer 2012;106(3):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducreux M, Adenis A, Pignon JP, François E, Chauffert B, Ichanté JL, Boucher E, Ychou M, Pierga JY, Montoto-Grillot C, Conroy T. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: Final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer 2013;49(6):1236–45. [DOI] [PubMed] [Google Scholar]
- 26.Bécouarn Y, Cany L, Pulido M, Beyssac R, Texereau P, Le Morvan V, Béchade D, Brunet R, Aitouferoukh S, Lalet C, Mathoulin-Pélissier S, Fonck M, Robert J. FOLFIRI® and bevacizumab in first-line treatment for colorectal cancer patients: Safety, efficacy and genetic polymorphisms. BMC Res Notes 2014;7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75. [DOI] [PubMed] [Google Scholar]
- 28.Romera A, Peredpaya S, Shparyk Y, Bondarenko I, Mendonça Bariani G, Abdalla KC, Roca E, Franke F, Melo Cruz F, Ramesh A, Ostwal V, Shah P, Rahuman SA, Paravisini A, Huerga C, Del Campo García A, Millán S. Bevacizumab biosimilar BEVZ92 versus reference bevacizumab in combination with FOLFOX or FOLFIRI as first-line treatment for metastatic colorectal cancer: A multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(12):845–55. [DOI] [PubMed] [Google Scholar]
- 29.Nishi T, Hamamoto Y, Warita E, Miyamoto J, Akutsu N, Yamanaka Y, Nagase M, Fujii H. Retrospective analysis of the international standard-dose FOLFIRI (plus bevacizumab) regimen in Japanese patients with unresectable advanced or recurrent colorectal carcinoma. Int J Clin Oncol. 2011;16(5):488–93. [DOI] [PubMed] [Google Scholar]
- 30.Kochi M, Akiyama Y, Aoki T, Hagiwara K, Takahashi T, Hironaka K, Teranishi F, Osuka F, Takeuchi M, Fujii M, Nakajima T. FOLFIRI plus bevacizumab as a first-line treatment for Japanese patients with metastatic colorectal cancer: A JACCRO CC-03 multicenter phase II study. Cancer Chemother Pharmacol. 2013;72(5):1097–102. [DOI] [PubMed] [Google Scholar]
- 31.Lu CY, Huang CW, Hu HM, Tsai HL, Huang CM, Yu FJ, Huang MY, Chang SF, Huang ML, Wang JY. Prognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line setting. Transl Res. 2014;164(2):169–76. [DOI] [PubMed] [Google Scholar]
- 32.Suenaga M, Fuse N, Yamaguchi T, Yamanaka Y, Motomura S, Matsumoto H, Hamamoto Y, Mizunuma N, Doi T, Hatake K, Iwasaki J, Ohtsu A. Pharmacokinetics, safety, and efficacy of FOLFIRI plus bevacizumab in Japanese colorectal cancer patients with UGT1A1 gene polymorphisms. J Clin Pharmacol. 2014;54(5):495–502. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. 2016;27(8):1539–46. [DOI] [PubMed] [Google Scholar]