Abstract
Objective:
Standard cervical cancer screening is seen as the most efficient way of preventing cases of cervical cancer. This study aimed to test indirect husband support pathways and the use of self-efficacy and Visual Inspection with Acetic Acid (VIA) testing among women in Indonesian rural areas.
Methods:
The research implemented a cross-sectional design carried out in East Java, Indonesia, a remote county. The inclusion criteria were women between the ages of 30 and 50 years, married or women having experienced of having sexual intercourse, have been utilized VIA test at least 3 years ago. The Self-Efficacy Scale and the Husband Help Survey were used to test the interest variable. A structural equation modeling was used to assess the relationship between husband help and VIA test self-efficacy.
Results:
The study was followed by a total of 219 respondents. Women's mean age was 33.03 years (standard deviation [SD]: 6.44), and the mean age for the husband was 37.51 (SD: 7.45). Just 7.31% had a year ago VIA test, and most (65.75%) had a VIA test within 4 years. A husband's help had the greatest direct impact on the use of Papanicolaou tests, with a 0.312 direction coefficient (P < 0.001). The mediator between the husband's help and the use of VIA tests was self-efficacy (standardized coefficients of the path: 0.123, P < 0.001).
Conclusions:
Our analysis revealed a route through which husband help influences the use of VIA tests among women in Indonesian rural areas. Providers must consider the effect of husband support on VIA testing in promoting the use of VIA tests among females in Indonesia. One potential communication approach is that providers make improvements to improve the use of VIA tests in supporting self-efficacy.
Keywords: Cancer, husband, screening, self-efficacy, support
Introduction
Cervical cancer is the fourth leading cause of death from cancer among women worldwide. Globally, about 570,000 women were reported to have been diagnosed with cervical cancer in 2018 and around 311,000 died from this disease.[1] In developed countries, about 87% of cervical cancer occurs.[2] Eighty to 90% of cases of cervical cancer occur within women who have rarely or never been checked for cervical cancer, and another 10%–20% of cases of cervical cancer occur among women who have been screened but have not received sufficient follow-up treatment.[3] In Indonesia, cervix cancer is the second-highest incidence of cancer among women, with approximately 23.4 per 100,000 women diagnosed with cervical cancer, and the mortality rate was 13.9 per 100,000 people (Ministry of Health, 2018).
Cervical cancer screening is the most effective way of reducing cervical cancer cases. In many developing countries, the incidence of invasive cervical cancer has decreased, primarily due to early detection efforts.[4] National procedure guidelines recommend visual infection with acetic acid (VIA) monitoring for average-risk women aged 20–65 years or married women at least every year for 3 years.[5] VIA is a screening check for cervical cancer through direct adjustments in the cervix after applied with 3%–5% acetic acid that is considered cheaper than Papanicolaou (Pap) smear tests.[6] However, only around 2.45% of Indonesian women done a VIA screening test, which is still far away from the Indonesian goal of around 50% in 2019.[7] Many factors influencing women's involvement in cervical cancer screening include education, behaviors, access to information, and husband support.[8]
Husband support is considered the most important factor associated with women's involvement in the early detection of cervical cancer. Husband support can provide emotional benefits and provide individuals with a sense of security and motivation and take health action, while lack of husband support may be a barrier to cervical screening for women.[9] Husband support is composed of four types of support: emotional support, information, tangible assistance, and appreciation.[10] Emotional support involves support in the form of love, faith, focus and listening, and being heard. Information support is the husband's providing information that is used to convey the issue. Tangible assistance is a direct source of assistance in terms of both resources, labor, and means. Appreciation includes giving feedback, advice, and problem resolution.[11] A previous study stated that lack of emotional and husband support for cervical screening is a major factor associated with low use of VIA screening test.[12,13,14] However, other studies found that cervical cancer screening had no connection with emotional or informative support.[15] Because of the previous research reported conflicting results and a few reports, the relationship between husband support and cervical cancer screening needs to be better understood.
Moreover, self-efficacy is characterized as one's ability to regulate one's health behavior that has correlated with involvement in certain cancer screening behaviors.[16] A previous study reported that strong self-efficacy has increased women's involvement in cervical cancer screening by 4.3 times, which indicates that women with high self-efficacy should prepare their own for early cervical cancer detection.[17,18] Understanding the process that connects husband support and self-efficacy with the use of VIA screening tests may provide valuable information to establish and change interventions. However, to the best of our knowledge, few studies have studied an indirect association between husband support, self-efficacy, and use of VIA screening test. The aim of this study was to test the indirect and direct effect of husband support on self-efficacy and the use of VIA screening testing among women in rural areas, Indonesia.
Methods
Study design and sample
This study was used a cross-sectional design conducted on July 8–August 1, 2019, in the rural area of East Java province, Indonesia. According to the Indonesian Guideline for the Prevention of Cervical Cancer (2015), women between the ages of 30 and 50 years old, married or women having having experienced of sexual intercourse, should have VIA tests every year. However, only 1.2% of married women used VIA tests in rural East Java in Indonesia, while more women (10%) done VIA tests in the urban area. Furthermore, approximately 1350 women were diagnosed with cervical cancer in East Java province.
The inclusion criteria were women aged between 20 and 65 years, married or having experienced of sexual intercourse, who were able to read and talk [Table 1]. Women with a prior history of noncancerous radical hysterectomy were excluded from this study. The sample size was determined using a power analysis (G*Power software version 3.1, Heinrich-Heine-Universität Düsseldor, Düsseldorf, Germany), effect size = 0.15, power level = 0.80, resulting in a sample size of 116. A convenience sampling technique was used to select participants due to resource constraints.
Table 1.
Characteristics of studied respondent (n=219)
| Characteristics | n | % |
|---|---|---|
| Wife’ age (mean±SD) | 33.03 | 6.44 |
| Wife’ occupancy | ||
| Government employees | 13 | 5.94 |
| Housewife | 161 | 73.50 |
| Entrepreneur | 45 | 20.51 |
| Wife’s level of education | ||
| No education | 32 | 14.61 |
| Elementary school | 21 | 9.58 |
| Primary school | 34 | 15.52 |
| High school | 107 | 48.85 |
| Diploma level | 14 | 6.39 |
| Bachelor degree | 11 | 5.05 |
| Year of VIA screening | ||
| 2016 | 144 | 66 |
| 2017 | 30 | 14 |
| 2018 | 29 | 13 |
| 2019 | 16 | 7 |
| Husband’s level of education | ||
| No education | 15 | 6.85 |
| Elementary school | 36 | 16.44 |
| Primary school | 42 | 19.20 |
| High school | 102 | 46.05 |
| Diploma level | 10 | 4.57 |
| Bachelor degree | 14 | 6.39 |
| Husband’s age (mean±SD) | 37.51 | 7.45 |
| Family income | ||
| <Basic regional minimum salary | 67 | 30.59 |
| >Basic regional minimum salary | 152 | 69.41 |
SD: Standard deviation; VIA: Visual inspection with acetic acid
Instrument
Demographic data
Demographic data included age, level of education, and job status for both wife and husband, years of VIA research, and family income.
Self-efficacy scale
Self-efficacy was measure using a seven-item Self-Efficacy Scale.[19] This is a Likert scale with a score ranging from 0 (cannot do) to 100 (can certainly do). A higher score indicates greater self-efficacy for conducting VIA screening test. In the current study, the alpha coefficient of the Cronbach was 0.77.
Husband support
Husband support was assessed using 12 husband support-related items including emotional support, supporting gratitude, supporting information, and instrumental support.[20] This questionnaire used a set of Likert scales from 1 (strongly disagree) to 5 (strong agreement). Score <22 indicated low support for the husband, score from 22 to 30 indicated moderate support, score from 31 to 41 indicated high support for the husband, and score above 41 indicated very high support. Using the alpha coefficient of Cronbach, the reliability rate was 0.76.[20]
Data collection
The study had been approved by the institutional review board (Approval No. 7871-12). Agreement to use instruments has been obtained from the authors. The participants were recruited by a volunteer from three public health centers in East Java province, Indonesia. Preliminary analysis was obtained before written consent to notify. Questions about the survey were established in Bahasa Indonesia. To ensure the accuracy of the material, the research teams were translation and back translation of the questionnaire. The time to complete all questions were listed at 10–15 minutes.
Statistical analysis
Descriptive statistical analysis was used to define variables of interest by providing means and standard deviations (SDs) for continuous variables and frequency and percentage for categorical variables. A structural equation modeling was used to assess the husband support relationship and self-efficacy with the use of VIA screening test. Two-step analysis was conducted, namely measurement and testing of structural models (Anderson and Gerbing, 1988). We used the confirmatory factor analysis in the first step to create a latent husband support variable [Table 2]. A indicators to test the model fit was used comparative fit index [CFI] = 0.991, root mean square error of approximation [RMSEA] = 0.087 [90% confidence interval [CI]: 0.056–0.121], Tucker–Lewis index [TLI] = 0.981, and π[2] /df = 26/5), which suggests generally acceptable.[21] The second stage to measure the impact of a husband support on self-efficacy and use of VIA screening test using a fit index model goodness, the CFI >0.95, the RMSEA = 0.06, and the TLI >0.95 suggested an appropriate fit model.[21] A ratio of the Chi-square test (χ2/df) between 2.0 and 5.0 was used to indicate the acceptability.[22]
Table 2.
Measurement model of husband support
| Indicators | Factor loading | SE | P |
|---|---|---|---|
| Emotional support | 0.711 | 0.021 | <0.001 |
| Informational support | 0.734 | 0.018 | <0.001 |
| Instrumental support | 0.810 | 0.041 | <0.001 |
| Appreciation support | 0.652 | 0.052 | <0.001 |
The measurement model was constructed using weighted least squares (WLSMV). Fit indices were comparative fit index=0.992, root mean square error of approximation=0.083 (90%CI 0.051, 0.136), Tucker-Lewis index=0.980, and χ2/df (24/4)=6. CI: Confidence interval; WLSMV: Weighted least squares; SE: Standard error
Results
The mean age of participants was 33.03 years (SD: 6.44), and the mean age for the husband was 37.51 (SD: 7.45). Both wife and husband had at least a high school diploma, 48.85% and 46.05%, respectively. Only 7.31% of them had VIA screening test on a year ago, and most (65.75%) had a VIA test within 4 years. Most of them (69.41%) had monthly family income above the regional universal minimum wage [Table 1].
This model generated the goodness-of-fit indices: CFI = 0.953, RMSEA = 0.054 (90% CI: 0.042–0.069), TLI = 0.912, and χ2/df (151/54) = 2.796. Although all other pathways were significant (P < 0.05), the direct pathway between age, self-efficacy, and VIA test use was not significant (path coefficient = 0.05, P = 0.159, and path coefficient = 0.03, P = 0.423, respectively).
Figure 1 shows the direct impact of the husband support on self-efficacy, and VIA screening test use. The husband support was positively correlated with self-efficacy and predicts the use of VIA screening tests. A husband support had the greatest direct impact on the use of VIA screening tests, with a 0.312 direction coefficient (P < 0.001). The mediator between the husband support and the use of VIA screening tests was self-efficacy (standardized coefficients of the path: 0.123, P < 0.001).
Figure 1.
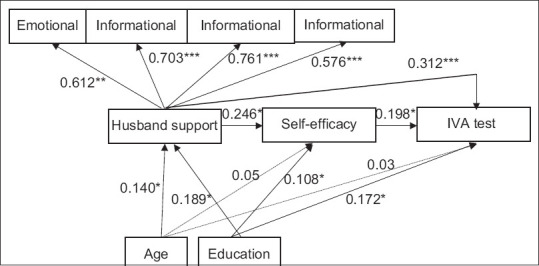
Structure model of husband support and Visual Inspection with Acetic Acid (VIA) test use. Note: Structural equation modeling was performed using weighted least squares. Path coefficients are standardized. Fit indices were as follows: Comparative fit index = 0.953, root mean square error of approximation = 0.054 (90% confidence interval: 0.042–0.069), Tucker–Lewis index = 0.912, and χ2/df (151/54) = 2.796. *P < 0.05, **P < 0.001. Note: Structural equation modeling was performed using weighted least squares. Path coefficients are standardized. Fit indices were as follows: CFI = 0.953, RMSEA = 0.054 (90%CI: 0.042–0.069), TLI = 0.912, and χ2/df (151/54) = 2.796. *P < 0.05, **P < 0.001.  Significant
Significant  Non-significant
Non-significant
Discussion
Our analysis revealed a path through which husband support influences self-efficacy and cervical cancer screening among women in the rural area of Indonesia. A previous study also reported the same result regarding the association between social support with Pap smear test.[15,23,24] Gaining significant husband support has led to a higher self-efficacy concerning VIA test use and a higher rate for VIA testing. Earlier studies have shown that husband support may influence cancer screening by increasing self-efficacy, providing women with a more accurate sense of personal risk, and helping them resolve screening barriers.[25] Another study describes that husband support can influence screening behavior through social norms and religious beliefs.[26] As most of our sample were Muslims; in Muslims, support for husbands is mandatory for wives, which can have a more direct effect on health behaviors.[26] In addition, according to Indonesian culture, women are very dependent on husbands, wives must obey their husbands, and wives follow what their husbands say. This means that the education of husbands by health workers should be strengthened so that husbands can learn about cervical cancer and enable their wives to conduct VIA tests on a regular basis every year. A further research needs to be carried out regarding culturally dependent on the encouragement of husbands for different cancer screening behaviors.
Different social support factors have been identified as being correlated with screening behaviors. A previous study in the US found a significant between annual mammogram screening and emotional/informative support and positive social interaction.[27] Moreover, having a lower degree of positive social interaction has been correlated with lower chances of getting a repeat mammogram working women.[27] Another study conducted in Brazil has also found that Pap smear test screening was correlated with social support.[15] The explanations for the possible beneficial impact of social support on women's cancer screening remain unknown, however, it was proposed that social support could serve as a buffer and help to reduce the negative effect of stressful events.[26]
Self-efficacy was a mediator of relationship between husband support and VIA test use. These results indicate that self-efficacy toward VIA screening is crucial in pushing women to the cervical cancer screening test. Taiwan's Pap screening research suggested to apply transtheoretical model stages of change in the planning strategies in order to increase a Pap screening test,[28] which is consistent with our findings. Other research in Iran found that the intervention to increase Pap smear test in women with poor self-efficacy was limited, thus only 30.8% of women in Iran had Pap smears test in the last 3 years, and only 69.1% of women with strong self-efficacy would do Pap smears test.[29] In all the cases listed, it is shown that, if the individual has confidence in his or her capacity, he or she may have more adequate output to preserve his or her health, including an effort to perform VIA or other cervical cancer screening.
There is some limitation of this study. First, considering the existence of cross-sectional analysis, it is difficult to discuss the causal effects. Second, the use of VIA tests was evaluated using self-reporting questionnaire, which could be produced a bias affected to the overestimated or underestimated the prevalence of VIA test screening in this study. The two instruments measuring husband and self-efficacy were not validated with the Indonesian sample; however, both measurements had strong reliability of internal consistency in the current study. Finally, participants were recruited from three public health centers in East Java, Indonesia; therefore, it may not reflect all women's condition in Indonesia which has 34 provinces.
Conclusions
Husband support has a significant indirect correlation with VIA test use among women in the rural area of Indonesia. In particular, we were able to identify possible pathways through which husband support affected VIA test use with critical psychosocial determinants. Husband support influenced VIA test use both directly and indirectly. Future interventions should consider optimizing husband support to promote VIA screening test use. Husband support had the strongest direct effect on VIA test use, yet only a few women received support from the husband to have a VIA test. Thus, providers need to acknowledge the impact of husband support on VIA tests in promoting VIA test use among women in Indonesia. To close disparities in VIA test use among women in the rural area of Indonesia, our findings have implications for health care providers to provide comprehensive and continue education to enable his wife to do VIA screening test.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawaya GF, Kulasingam S, Denberg TD, Qaseem A Clinical Guidelines Committee of American College of Physicians. Cervical Cancer Screening in Average-Risk Women: Best Practice Advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;162:851–9. doi: 10.7326/M14-2426. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health, Republic of Indonesia. Baseline of Health Research (Riset Kesehatan Dasar; RISKESDAS) Jakarta: Research and Development Agency of the Indonesian Ministry of Health; 2015. [Google Scholar]
- 6.Andrews G. Women's Sexual Health. Jakarta: EGC; 2009. [Google Scholar]
- 7.Ministry of Health, Republic of Indonesia. Performance Report of the Directorate General of Pharmaceuticals and Medical Devices. Jakarta: Research and Development Agency of the Indonesian Ministry of Health; 2018. [Google Scholar]
- 8.Fauza M, Aprianti A, Azrimaidalisa A. Factors associated with the early detection of cervical cancer by the IVA method at Padang Primary Health Care. Indonesian Promot Health J. 2018;14:68–80. [Google Scholar]
- 9.Hassani L, Dehdari T, Hajizadeh E, Shojaeizadeh D, Abedini M, Nedjat S. Barriers to Pap smear test for the second time in women referring to health care centers in the south of Tehran: A qualitative approach. Int J Community Based Nurs Midwifery. 2017;5:376–85. [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman Marily M. Jakarta: EGC; 1998. Family Nursing: Theory and Practice. [Google Scholar]
- 11.Harnilawati. Family Nursing Concepts and Processes. Pustaka As Salam. 2013 [Google Scholar]
- 12.Anggraeni, Holy. Self Efficacy of Women of Fertile Age to Do Pap Smear in Terms of Knowledge and Husband's Support. Surya Mitra Husada Kediri School of Health Science. Viva Medika. 2017:10. [Google Scholar]
- 13.Marlina E, Kurniawati T. Relationship between Husband's Support and Wife's Behavior Conducting Pap Smear Examination at Umbulharjo II Primary Health Care Yogyakarta City in 2014. PhD Thesis. School of Health Science Aisyiyah Yogyakarta. 2014 [Google Scholar]
- 14.Mouttapa M, Park Tanjasiri S, Wu Weiss J, Sablan-Santos L, DeGuzman Lacsamana J, Quitugua L, et al. Associations between women's perception of their husbands'/partners' social support and Pap screening in Pacific Islander Communities. Asia Pac J Public Health. 2016;28:61–71. doi: 10.1177/1010539515613412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva IT, Griep RH, Rotenberg L. Social support and cervical and breast cancer screening practices among nurses. Rev Lat Am Enfermagem. 2009;17:514–21. doi: 10.1590/s0104-11692009000400013. [DOI] [PubMed] [Google Scholar]
- 16.Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1982;37:122–47. [Google Scholar]
- 17.Fauza M, Aprianti A, Azrimaidalisa A. Factors associated with the early detection of cervical cancer by the IVA method at Puskesmas Kota Padang. Indonesian J Health Promot. 2018;14:68–80. [Google Scholar]
- 18.Armadhani R, Mudigdo A, Budihastuti UR. Path analysis on the determinants of Pap smear uptake in women of reproductive age in Tegal, Central Java. J Matern Child Health. 2019;4:77–86. [Google Scholar]
- 19.Lechner L, Brug J, de Vries H. Misconceptions of fruit and vegetable consumption: Differences between objective and subjective estimation of intake. J Nutr Educ. 1997;29:313–20. [Google Scholar]
- 20.Alisjahbana AS, Yusuf AA, Anna Z, Hadisoemarto PF, Kadarisman A, Maulana N, et al. Bandung: UNPAD Press; 2017. Welcoming the SDGs Readiness of Regions in Indonesia. [Google Scholar]
- 21.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling Multidiscip J. 1999;6:1–55. [Google Scholar]
- 22.Wheaton B, Muthen B, Alwin DF, Summers GF. Assessing reliability and stability in panel models. Sociol Methodol. 1977;8:84–136. [Google Scholar]
- 23.Kim K, Xue QL, Walton-Moss B, Nolan MT, Han HR. Decisional balance and self-efficacy mediate the association among provider advice, health literacy and cervical cancer screening. Eur J Oncol Nurs. 2018;32:55–62. doi: 10.1016/j.ejon.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamarra CJ, Paz EP, Griep RH. Social support and cervical and breast cancer screening in Argentinean women from a rural population. Public Health Nurs. 2009;26:269–76. doi: 10.1111/j.1525-1446.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 25.Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol Nurs Forum. 2002;29:845–52. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Janicki-Deverts D. Can we improve our physical health by altering our social networks? Perspect Psychol Sci. 2009;4:375–8. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messina CR, Lane DS, Glanz K, West DS, Taylor V, Frishman W, et al. Relationship of social support and social burden to repeated breast cancer screening in the women's health initiative. Health Psychol. 2004;23:582. doi: 10.1037/0278-6133.23.6.582. [DOI] [PubMed] [Google Scholar]
- 28.Tung WC, Lu M, Cook D. Papanicolaou screening in Taiwan: Perceived barriers and self-efficacy. Health Care Women Int. 2010;31:421–34. doi: 10.1080/07399330903349699. [DOI] [PubMed] [Google Scholar]
- 29.Majdfar Z, Khodadost M, Majlesi F, Rahimi A, Shams M, Mohammadi G. Relationships between self-efficacy and pap smear screening in Iranian women. Asian Pac J Cancer Prev. 2016;17:263–8. doi: 10.7314/apjcp.2016.17.s3.263. [DOI] [PubMed] [Google Scholar]


