Abstract
Background
Tenofovir monoester is a relatively lipophilic intermediate formed during the hydrolysis of tenofovir disoproxil to tenofovir. Its clinical pharmacokinetic profile and influence on the cellular pharmacology of tenofovir diphosphate have not been reported.
Methods
Plasma, PBMC and dried blood spots (DBS) were obtained from HIV-uninfected adults participating in a randomized, cross-over bioequivalence study of single-dose tenofovir disoproxil fumarate (TDF)/emtricitabine unencapsulated or encapsulated with a Proteus® ingestible sensor. Plasma pharmacokinetics of tenofovir monoester and tenofovir were characterized using non-compartmental methods. Relationships with tenofovir diphosphate in DBS and PBMC were examined using mixed-effects models.
Results
Samples were available from 24 participants (13 female; 19 white, 3 black, 2 Hispanic). Tenofovir monoester appeared rapidly with a median (range) Tmax of 0.5 h (0.25–2) followed by a rapid monophasic decline with a geometric mean (coefficient of variation) t½ of 26 min (31.0%). Tenofovir monoester Cmax was 131.6 ng/mL (69.8%) and AUC0–4 was 93.3 ng·h/mL (47.9%). The corresponding values for plasma tenofovir were 222.2 ng/mL (37.1%) and 448.1 ng·h/mL (30.0%). Tenofovir monoester AUC0–∞ (but not tenofovir AUC0–∞) was a significant predictor of tenofovir diphosphate in both PBMC (P = 0.015) and DBS (P = 0.005), increasing by 3.8% (95% CI 0.8%–6.8%) and 4.3% (95% CI 1.5%–7.2%), respectively, for every 10 ng·h/mL increase in tenofovir monoester.
Conclusions
Tenofovir monoester Cmax and AUC0–4 were 59.2% and 20.6% of corresponding plasma tenofovir concentrations. Tenofovir monoester was significantly associated with intracellular tenofovir diphosphate concentrations in PBMC and DBS, whereas tenofovir concentrations were not. Tenofovir monoester likely facilitates cell loading, thereby increasing tenofovir diphosphate exposures in vivo.
Introduction
Tenofovir is an NRTI used in the treatment and prevention of HIV infection. Tenofovir exerts its mechanism of action following conversion to its active diphosphate form within cells, competing with endogenous deoxyadenosine triphosphate and ultimately terminating chain elongation during viral replication.1 Tenofovir is hydrophilic and permeates poorly across the intestinal tract and other cell membranes,2,3 and thus was formulated as a prodrug to improve its oral absorption and cell permeability, first as tenofovir disoproxil fumarate (TDF) and more recently as tenofovir alafenamide fumarate (TAF). Entry of tenofovir into target lymphoid cells is critical to its efficacy.
Multiple studies have demonstrated the enhanced ability of the prodrug form to load cells in comparison with tenofovir. In monkeys, oral administration of TDF resulted in ∼8-fold higher tenofovir diphosphate concentrations in lymphoid cells as compared with an equivalent subcutaneous tenofovir dose.4 That study suggested that some tenofovir disoproxil (or monoester) reached the systemic circulation to facilitate cell loading. In comparison with TDF, and despite lower tenofovir dose equivalents, TAF results in ∼2.4- to 7-fold higher tenofovir diphosphate concentrations.5–7 This demonstrates more efficient cell loading for TAF, due in part to its superior stability and longer half-life in blood of 25 min5 versus <5 min for TDF.8 The pathways for conversion of the prodrug moieties have been described in the literature. However, the role and pharmacokinetics (PK) of intermediates formed during the conversion of these prodrugs remain unknown.
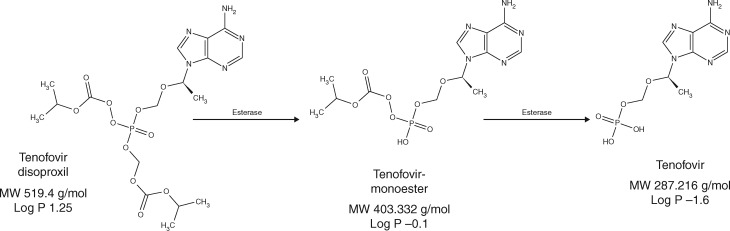
Following oral administration, tenofovir disoproxil is hydrolysed by esterases in the intestinal tract, liver and blood to tenofovir monoester and then tenofovir.9 When tenofovir is present in cells, it undergoes phosphorylation by intracellular kinases to its active moiety, tenofovir diphosphate.1 Previous literature has reported that the conversion from TDF to tenofovir occurs rapidly, resulting in tenofovir as the only circulating moiety in blood.1,10,11 However, more recently, Nye et al.12 reported the detection of tenofovir monoester in plasma during the course of assay development, which circulated at low levels following TDF administration. The plasma PK of tenofovir monoester has not been characterized in vivo, but physiochemical characteristics of tenofovir monoester and in vitro studies suggest that this moiety can penetrate cells more efficiently than tenofovir. Predicted octanol–water partition (log P) coefficients for tenofovir monoester and tenofovir are −0.1 and −1.6, respectively (Figure 1).13,14 Though the disoproxil form is the most lipophilic of the three,15 tenofovir monoester is more lipophilic than tenofovir and thus could influence intracellular loading and tenofovir diphosphate concentrations.
Figure 1.
Structures of tenofovir disoproxil, tenofovir monoester, and tenofovir. Figure adapted from Murphy RA, Valentovic MA. Factors contributing to the antiviral effectiveness of tenofovir. J Pharmacol Exp Ther 2017; 363: 156–163, by permission of The American Society for Pharmacology and Experimental Therapeutics.
Tenofovir diphosphate is the active form of tenofovir and is quantifiable in multiple cell and tissue types. Several studies have focused on the concentrations of this moiety in PBMC, given that HIV infects these cell types (notably CD4 T lymphocytes). Tenofovir diphosphate is also quantifiable in red blood cells, which can be assayed using dried blood spots (DBS). Tenofovir diphosphate has a long t½ in DBS (17 days), which has been used to examine cumulative medication adherence to TDF in persons living with HIV16–18 and HIV-uninfected individuals on pre-exposure prophylaxis.19–22 Tenofovir diphosphate exhibits coefficients of variation (CVs) of ∼50% and ∼30% in PBMC and DBS at steady-state, but sources of variability have been difficult to identify.23,24 If tenofovir monoester contributes to cell loading in vivo, then variability in its PK could be a source of variability for intracellular tenofovir diphosphate.
In support of this possibility, we recently found that tenofovir diphosphate concentrations were elevated in persons on TDF in combination with sofosbuvir-containing therapies,25 and this occurred concurrently with increases in tenofovir monoester concentrations in individuals on TDF in combination with ledipasvir/sofosbuvir.26 However, the full PK profile and potential influence of tenofovir monoester on cell loading have not been adequately studied. Thus, the aims of the present study were to characterize the plasma PK of tenofovir monoester in humans following single-dose administration of TDF 300 mg/emtricitabine, and to examine relationships between tenofovir monoester and intracellular tenofovir diphosphate in PBMC and DBS.
Patients and methods
Study design
Samples were obtained from a previously conducted intensive PK study in HIV-uninfected individuals at low risk of HIV designed to determine the bioequivalence of TDF/emtricitabine at 300/200 mg when co-encapsulated with the Proteus® Sensor System (Proteus Digital Health®, Redwood City, CA, USA) (ClinicalTrials.gov NCT02968576).27 Enrolment began in December 2016, with the last follow-up visit in May 2017. Secondary endpoints of the study included examining sources of PK variability in drug and anabolite concentrations in DBS and PBMC. Briefly, participants were randomized to receive single oral doses of unencapsulated or co-encapsulated TDF/emtricitabine, separated by a 14 day washout between study visits. PK assessments were performed following an overnight fast of at least 10 h at both study visits, with serial PK sampling at time 0 (pre-dose), 0.25, 0.5, 1, 2, 4, 6, 10, 24, 48 and 72 h post-dose.
Ethics
This study was approved by the Colorado Multiple Institutional Review Board (16–1478) and all participants provided written informed consent.
Sample preparation
Samples of interest included plasma, PBMC and DBS. Blood samples for plasma separation were collected in EDTA tubes at all specified timepoints above. Whole blood was spun down at 1200 g for 10 min at 4°C, and the plasma was transferred to aliquots and stored at −80°C until analysis. PBMC and DBS were isolated at the 24 h post-dose timepoint at both study visits. Sample processing procedures followed previously described methods23,28 and samples were stored at −80°C until analysis.
Assay methods
Concentrations of tenofovir in plasma and tenofovir diphosphate in PBMC and DBS were quantified using validated LC-MS/MS methods as previously described.29–31 Tenofovir diphosphate concentrations in PBMC were normalized to 106 cells and in DBS were normalized to a 3 mm punch. The lower limit of quantification (LLOQ) for tenofovir in plasma was 10 ng/mL and for tenofovir diphosphate in PBMC and DBS it was 5.0 and 25 fmol/sample, respectively. A validated punch stacking procedure was used when necessary to ensure quantifiable concentrations in DBS. A method for tenofovir disoproxil in plasma was not developed due to instability of the analyte in this matrix. A UPLC-MS-MS assay was developed for the determination of tenofovir monoester (Toronto Research Chemicals, Toronto, Ontario, Canada) in human plasma, and was validated for use with EDTA plasma matrix. Plasma proteins were precipitated with trifluoroacetic acid followed by chromatographic separation performed on a Waters Acquity H-Class UPLC. Separation was achieved with a Waters Acquity HSS T3 (2.1 × 100 mm, 1.8 μm) reversed-phase UPLC column with gradient elution at 0.5 mL/min. The gradient went from 2% acetonitrile to 50% acetonitrile (0.1% formic acid) over 3.0 min. Detection was achieved by electrospray ionization (ESI) positive ionization tandem MS on a Waters Xevo TQ-S Micro detector. Precursor/product transitions (m/z) in the positive ion mode were 404/176. Linearity of the method was in the range 0.1–500 ng/mL using a 1/concentration2 weighted calibration curve. The assay had a minimum quantifiable limit of 0.1 ng/mL when 0.25 mL of plasma was analysed. Intra-day accuracy was within ±10.6% and precision ≤10.3%. The inter-assay accuracy was within ±6.8% and precision ≤7.9%. Carryover and matrix effects were insignificant for the EDTA plasma matrix. Conditional stability was shown for two freeze/thaw cycles, 6 h in matrix at ambient conditions, and extracted sample stability for 8 days.
PK analysis
PK parameters for tenofovir monoester and tenofovir in plasma were calculated via non-compartmental methods (Phoenix WinNonlin, v8.0). Cmax and last measurable concentrations (Clast) were determined based on direct visualization of the data. The elimination rate constant (ke) was estimated as the value of the linear regression slope of data points during the elimination phase. Half-life was calculated as ln(2)/ke. AUC0–4 and AUC0–24 were estimated using the linear up-log down trapezoidal rule. AUC0–∞ was determined by adding AUC through the last measurable timepoint to the value of the last measurable concentration divided by ke. Results below the LLOQ were imputed as half the LLOQ for the primary analysis and as missing for the sensitivity analysis.
Statistical analysis
Statistical analyses were performed using SAS Enterprise Guide v7.1 (SAS Institute, Inc., Cary, NC, USA). Demographic variables were summarized as mean (SD). Plasma PK parameters were natural log (ln)-transformed prior to analysis and summarized as geometric mean (GM) and CV. Ratio comparisons between tenofovir monoester and tenofovir for key PK parameters were compared directly and following molar conversion of these moieties from ng/mL to nM to compare tenofovir equivalents using molecular weights of 403.332 and 287.216 g/mol, respectively.13,14 Associations between tenofovir monoester and tenofovir in plasma were examined by linear regression.
The associations of tenofovir monoester and tenofovir with intracellular tenofovir diphosphate concentrations in PBMC and DBS were examined using mixed-effects models to account for repeated measures, arising from the cross-over design. Tenofovir monoester AUC0–∞ and tenofovir AUC0–∞ were analysed separately as fixed effects and participants as random effects. For the mixed-effects models, the primary outcome of interest (tenofovir diphosphate) was ln-transformed, and predictors of interest remained on the original scale. Clinical and demographic factors, including baseline BMI, estimated glomerular filtration rate (eGFR) and sex, in addition to potential confounding effects of formulation (unencapsulated versus encapsulated), randomization sequence (unencapsulated to encapsulated, or encapsulated to unencapsulated) and visit number, were also examined as predictors of tenofovir diphosphate concentrations. These factors were then examined as covariates in base models of tenofovir monoester AUC0–∞ and tenofovir AUC0–∞, and final model selection was based on improvement in the Akaike information criterion by at least 2 points from the base models. Model results are reported as percentage change (95% CI) for predictors of interest.
For illustrative purposes, raw geometric mean tenofovir monoester AUC0–∞ results from visit 1 were divided into quartiles and comparisons were made between the lowest and highest quartiles versus corresponding tenofovir diphosphate concentrations in PBMC and DBS using the first study visit. P values for these comparisons reflected two-tailed unpaired t-tests. These results were back-transformed to the original scale and presented as geometric mean (CV) for interpretability of study findings.
Results
Study population
A total of 24 participants were enrolled (13 female; 19 white, 3 black, 2 Hispanic) with data available from two study visits, resulting in 48 total observations for analysis. Mean (SD) age, weight and BMI were 28.1 (3.5) years, 74.5 (14.2) kg and 25.2 (4.0) kg/m2, respectively. Mean (SD) eGFR was 118.7 (24.2) mL/min/1.73 m2.
PK of tenofovir monoester versus tenofovir
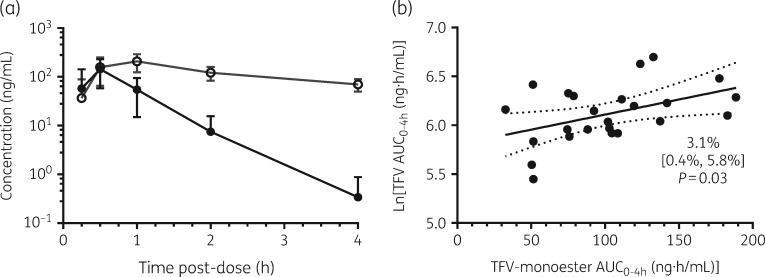
This study was not designed to assess tenofovir monoester bioequivalence. Therefore, summary PK focused on the unencapsulated formulation. Tenofovir monoester concentrations were rapidly quantifiable following oral administration of TDF/emtricitabine and reached peak concentrations of 131.6 ng/mL at a median of 0.5 h post-dose (Figure 2a and Table 1). The concentration–time profile for this moiety exhibited monophasic decline, with a geometric mean t½ of 0.44 h (26 min). By 4 h post-dose, 7/24 samples were below the LLOQ of 0.1 ng/mL for tenofovir monoester. Tenofovir monoester AUC0–4 was 93.3 ng·h/mL, which was >99% of the AUC0–∞ for this moiety. Tenofovir monoester Cmax and AUC0–4 were 59.2% and 20.6% of tenofovir on a ng/mL basis (42.2% and 14.7% on a nM basis). Tenofovir monoester AUC0–4 demonstrated a significant relationship with tenofovir AUC0–4 [3.1% increase for every 10 ng·h/mL increase (95% CI 0.4%–5.8%), R2 = 0.20, P = 0.03] (Figure 2b), though this relationship weakened with tenofovir versus tenofovir monoester AUC0–∞ comparisons [2.3% increase for every 10 ng·h/mL increase (95% CI −0.5% to 5.1%), R2 = 0.12, P = 0.10] (data not shown).
Figure 2.
(a) Mean ± SD plasma concentrations of tenofovir monoester (filled circles) and tenofovir (TFV; open circles) until 4 h post-dose. (b) Tenofovir versus tenofovir monoester AUC0–4 in plasma. Tenofovir AUC0–4 was ln transformed prior to analysis for comparison with tenofovir monoester (original scale). Data are presented as line of best fit with 95% CI indicated by the dotted lines.
Table 1.
Pharmacokinetics of tenofovir monoester and tenofovir (TFV) in plasma
| Moiety | AUC0–4 (ng·h/mL) | AUC0–∞ (ng·h/mL) | Tmax (h) | Cmax (ng/mL) | C4 (ng/mL) | t½ (h) |
|---|---|---|---|---|---|---|
| TFV monoestera | 93.3 (47.9%) | 93.9 (46.8%) | 0.5 (0.25–2.0) | 131.6 (69.8%) | 0.17 (152.4%) | 0.44 (31.0%) |
| TFVa | 448.1 (30.0%) | 1986.0 (26.9%) | 1.0 (0.5–2.0) | 222.2 (37.1%) | 67.4 (26.9%) | 20.5 (24.6%) |
| TFV monoester versus TFVb | 20.6% (17.2%–24.6%) | 4.8% (3.9%–5.7%) | −0.5 (−1.5 to 0.0) | 59.2% (49.0%–71.5%) | 0.3% (0.2%–0.4%) | 2.1% (1.8%–2.5%) |
Data are presented as geometric mean (CV), except Tmax, which is reported as median (range).
Comparisons between tenofovir monoester and tenofovir are presented as geometric mean ratio (95% CI), except Tmax, which is reported as the median (range) difference.
Influence on tenofovir diphosphate concentrations
Results from both the encapsulated and unencapsulated forms were used for analyses throughout this section. As expected for a 2 week washout, visit was a significant predictor of tenofovir diphosphate concentrations in DBS but not PBMC. Geometric mean (CV) tenofovir diphosphate concentrations in PBMC at 24 h post-dose were 10.8 (42.4%) and 10.3 (54.7%) fmol/106 cells at visits 1 and 2, respectively. In DBS, geometric mean tenofovir diphosphate concentrations were 45.3 (48.2%) and 88.3 (51.9%) fmol/punch at visits 1 and 2, respectively (P < 0.0001) (Table 2). Formulation, sequence and clinical covariates of sex, BMI and eGFR were not significantly associated with tenofovir diphosphate concentrations in PBMC or DBS in univariate models (Table 2).
Table 2.
Univariate associations of tenofovir monoester, tenofovir and clinical covariates with tenofovir diphosphate concentrations in PBMC and DBS
| Variable | TFV-DP in PBMC |
TFV-DP in DBSa |
|||||
|---|---|---|---|---|---|---|---|
| change in TFV-DP | 95% CI | P valueb | change in TFV-DP | 95% CI | P valueb | ||
| Visit (2 versus 1) | −5.0% | −24.7% to 20.0% | 0.66 | 95.1% | 59.2%–139.0% | <0.0001 | |
| Randomization sequence | 5.1% | −23.8% to 44.9% | 0.75 | 25.0% | −11.0% to 75.6% | 0.19 | |
| Formulation (encapsulated versus not) | −10.0% | −28.5% to 13.1% | 0.35 | −4.8% | −22.7% to 17.2% | 0.63 | |
| Sex (female versus male) | −9.3% | −34.1% to 24.9% | 0.53 | −5.9% | −33.9% to 33.9% | 0.73 | |
| BMI (kg/m2) | −2.2% | −6.0% to 1.7% | 0.25 | −1.0% | −5.3% to 3.5% | 0.63 | |
| eGFR (mL/min/1.73 m2) | 0.1% | −0.6% to 0.8% | 0.84 | −0.0% | −0.8% to 0.7% | 0.97 | |
| Tenofovir monoester AUC0–∞ (per 10 ng·h/mL)c | 3.8% | 0.8%–6.8% | 0.015 | 4.3% | 1.5%–7.2% | 0.005 | |
| Tenofovir AUC0–∞ (per 10 ng·h/mL)c | 0.2% | −0.1% to 0.5% | 0.11 | 0.0% | −0.3% to 0.3% | >0.99 | |
TFV-DP, tenofovir diphosphate.
Reported point estimates for TFV-DP in DBS were controlled for study visit.
P values <0.05 are shown in bold.
Percentage changes reported per 10 ng·h/mL increase in tenofovir monoester or tenofovir.
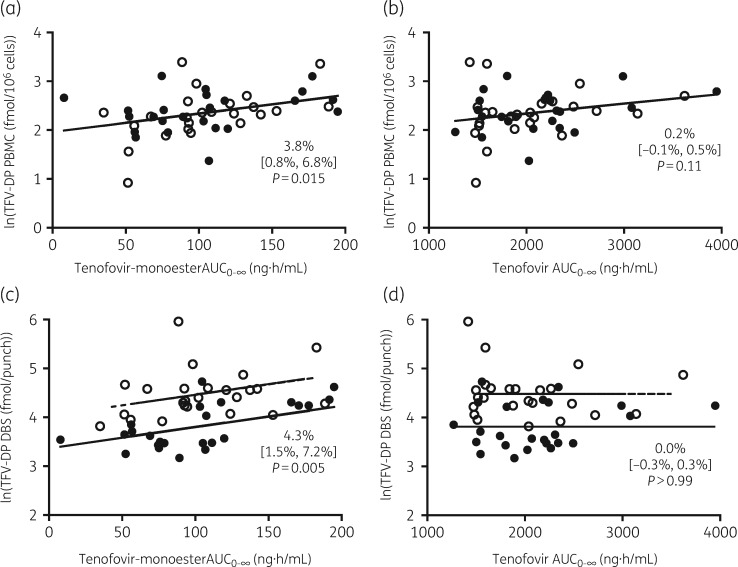
Tenofovir monoester was a significant predictor of tenofovir diphosphate concentrations in both PBMC (P = 0.015) and DBS (P = 0.005), the latter of which was controlled for study visit. For every 10 ng·h/mL increase in the AUC0–∞ of tenofovir monoester, concentrations of tenofovir diphosphate in PBMC increased by 3.8% (95% CI 0.8%–6.8%) (Table 2 and Figure 3) and 4.3% (95% CI 1.5%–7.2%) in DBS. For illustration, at visit 1 participants in the lowest quartile for tenofovir monoester AUC0–∞ had a geometric mean of 40.9 ng·h/mL, with corresponding tenofovir diphosphate concentrations of 9.4 fmol/106 cells in PBMC and 36.8 fmol/punch in DBS. In comparison, participants in the highest quartile for tenofovir monoester AUC0–∞ had a geometric mean of 167.9 ng·h/mL, and corresponding tenofovir diphosphate concentrations of 13.3 fmol/106 cells in PBMC (P = 0.10) and 68.3 fmol/punch in DBS (P = 0.004). In contrast to tenofovir-monoester, plasma tenofovir AUC0–∞ was not a significant predictor of tenofovir diphosphate concentrations in PBMC (95% CI −0.05% to 0.45%; P = 0.11) or DBS (95% CI −0.27% to 0.27%; P > 0.99). Point estimates, 95% CIs and P values were similar for the sensitivity analysis with LLOQ values entered as missing for tenofovir monoester and tenofovir AUC0–∞.
Figure 3.
Observed versus predicted plots of tenofovir diphosphate concentrations in PBMC with (a) tenofovir monoester and (b) tenofovir, and tenofovir diphosphate concentrations in DBS with (c) tenofovir monoester and (d) tenofovir. Data are presented as percentage change (95% CI). Filled circles indicate data points from visit 1. Open circles indicate data points from visit 2. Solid lines indicate predictions from all visits in PBMC and visit 1 in DBS, and dashed lines indicate predictions from visit 2.
Discussion
Tenofovir monoester exhibited significant systemic exposures in humans following oral administration of TDF/emtricitabine. Tenofovir monoester concentrations were ∼42% and ∼15% of tenofovir Cmax and AUC0–4, respectively, on an nM equivalent basis, and this moiety was rapidly eliminated with a t½ of 0.44 h. In line with its increased lipophilicity compared with tenofovir, tenofovir monoester was a significant predictor of tenofovir diphosphate concentrations in PBMC and DBS, whereas no significant association was found for tenofovir. For every 10 ng·h/mL increase in tenofovir monoester AUC0–∞, tenofovir diphosphate increased by ∼4% in both PBMC and DBS. These findings challenge previous literature dismissing the existence of tenofovir monoester in human plasma,10,11,32 and reveal the potential clinical impact of this moiety on in vivo cell loading and tenofovir diphosphate concentrations, which are relevant to the achievement of protective and therapeutic concentrations.
The hydrolysis of TDF is primarily catalysed by carboxylesterases (CES),9 but phosphodiesterases9 and lipases have also been implicated in this pathway.33 CES are found in several tissue types and multiple families have been identified, but CES1 and CES2 are the major forms found in humans.34 CES1 is predominantly expressed in the liver, though CES2 is also found in low amounts. CES2 is the only form expressed in the intestinal tract, and is also the primary form expressed in kidneys, vaginal epithelia and PBMC in comparison with other identified families.34,35 Thus, CES enzymes, notably CES2, play a critical role in the initial hydrolysis of TDF following oral administration and the subsequent delivery of the disoproxil and monoester forms to portal and peripheral blood. Tenofovir disoproxil was not quantified in this study as preliminary work showed it was rapidly degraded when spiked into plasma (data not shown). It is unlikely that tenofovir disoproxil circulates measurably in the systemic circulation, based on its susceptibility to hydrolysis by esterases and pH changes, in addition to efflux by P-glycoprotein, all limiting systemic tenofovir disoproxil exposures.2,9,33,36 Tenofovir monoester exposures demonstrated a significant and positive relationship with tenofovir up to 4 h post-dose, but tenofovir monoester only accounted for ∼20% of the variability in tenofovir, suggesting that tenofovir monoester may distribute out of plasma and into other compartments before returning into plasma as tenofovir. Additionally, this relationship weakened with extrapolation to infinity as tenofovir monoester was eliminated from systemic circulation after 4 h, whereas tenofovir undergoes prolonged re-distribution and elimination, which influence its profile.
Previous literature on the cell permeability of tenofovir monoester is limited to in vitro studies. In Caco-2 cells, tenofovir monoester was able to cross from the apical to the basolateral side, whereas tenofovir transfer was not detected.37 Within this same experiment, tenofovir disoproxil crossed cell layers at a permeability rate ∼10-fold higher than tenofovir monoester, demonstrating the enhanced ability of the disoproxil form to permeate cells. In a separate in vitro study, tenofovir monoester accounted for 76% of tenofovir transport across Caco-2 cell monolayers incubated with tenofovir disoproxil.9 However, the link between systemic circulation of this moiety in humans and its potential influence on cell loading had not been explored, to our knowledge.
In a previous study, we found that tenofovir diphosphate concentrations were increased in individuals receiving TDF-based therapy in combination with ledipasvir/sofosbuvir,25 which was attributed in part to CES2 inhibition by sofosbuvir leading to higher tenofovir disoproxil and monoester delivery to the portal blood and systemic circulation.38,39 Consistent with this, a companion paper in this Journal26 shows elevated tenofovir monoester concentrations concurrent with these increases in a smaller follow-up study. The present analysis examined relationships between tenofovir monoester and intracellular tenofovir diphosphate concentrations in a controlled PK study. A significant and positive relationship was identified between total tenofovir monoester exposure with intracellular tenofovir diphosphate concentrations in PBMC and DBS, consistent with enhanced cell loading leading to higher tenofovir diphosphate in vivo. These findings are in contrast to the lack of association between tenofovir exposures and tenofovir diphosphate in DBS and PBMC. Prior research focused on the long persistence of tenofovir in plasma (t½ ∼15 h) and its cellular uptake via endocytosis leading to tenofovir loading of PBMC and subsequent generation of tenofovir diphosphate.4 However, multiple studies have shown poor cellular penetration of tenofovir.35,37,40 Further work is needed to define the relative contributions of tenofovir and tenofovir monoester to intracellular tenofovir diphosphate in vivo.
These findings have clinical relevance because they provide insight into a previously unrecognized factor influencing intracellular tenofovir diphosphate concentrations, which is the pharmacologically active moiety in PBMC and the moiety used to assess adherence in DBS. TDF has long remained a key component of multiple antiretroviral treatment regimens, and it is currently the only FDA-approved therapy for pre-exposure prophylaxis. In addition, TDF recently became available in generic form and will remain a cornerstone of HIV treatment in resource-limited settings. A number of demographic factors have been shown to be associated with tenofovir diphosphate concentrations in both healthy volunteers and persons living with HIV, including race, gender, renal function and BMI,23 and, in those with HIV, the concomitant ART regimen.18 Our findings suggest that tenofovir monoester exposures should also be considered among the factors that influence tenofovir diphosphate concentrations in PBMC and DBS in individuals receiving TDF. Further research should be pursued to examine biological factors that may influence tenofovir monoester formation, and how this moiety may demonstrate relationships with efficacy or DBS concentrations for adherence in persons on TDF-based therapy.
There are limitations to this study. The primary goal was to establish bioequivalence between the encapsulated versus unencapsulated pills through the measurement of tenofovir and emtricitabine concentrations in plasma, and thus the sample processing and sampling timepoints were selected to accurately capture peak concentrations and the decline of tenofovir and emtricitabine. Given the rapid appearance and decline of the monoester form, additional timepoints may have permitted a more accurate assessment of tenofovir monoester PK in plasma, including the potential to identify a multi-phasic decline similar to that of tenofovir. TDF hydrolysis to the monoester form appears to be slowed under fed versus fasted conditions, potentially due to competition for esterases.11 All participants were fasted for both PK assessments, and thus variability from this source was not investigated. PBMC and DBS samples were only available at 24 h post-dose in this study, whereas earlier or multiple timepoints may have provided additional kinetic information relative to cell loading. Tenofovir diphosphate concentrations in PBMC and DBS from our study were similar to those published with other single-dose studies.24,28,41
In conclusion, this study showed that tenofovir monoester reaches concentrations that approach tenofovir concentrations following oral administration of TDF. This moiety appeared rapidly, but was swiftly eliminated, suggesting quick uptake into cells and metabolism in the liver and blood. Tenofovir monoester was a significant predictor of tenofovir diphosphate concentrations in both PBMC and DBS, suggesting that its PK may be an important source of variability for tenofovir diphosphate within cells. This gives rise to potential clinical relevance for tenofovir monoester, a question that deserves further examination.
Acknowledgements
We thank the study participants, clinical staff at the University of Colorado Clinical Translational Research Center (CTRC) and members of the Colorado Antiviral Pharmacology Laboratory. Parts of these data were previously presented at the 18th International Workshop on Clinical Pharmacology in Baltimore, MD (22–24 May 2018) and at the Conference on Retroviruses and Opportunistic Infections (CROI) 2019 in Seattle, WA (4–7 March 2019).
Funding
This work was supported by the National Institutes of Health (NIH) (Colorado CTSA Grant Number UL1TR001082, R01 AI122308 to G. D. H., R01 AI122298 to P. L. A., R01 DA040499 to J. J. K., and K23 AI104315 to J. R. C-M.). Study drug was donated by Gilead Sciences, Inc.
Transparency declarations
J. J. K. and G. D. H. receive research funding (paid to their institutions) from Gilead Sciences and donated study medication from Gilead Sciences for an NIH-sponsored study. P. L. A. receives contract and research funding and donated study medication from Gilead Sciences (paid to his institution). K. M. B., M. E. I., J. R. C-M., S. M., K. A., S. T., B. J. K., L. E., C. M., L. R. B. and S. H.: none to declare.
Disclaimer
The contents of this article are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- 1.Murphy RA, Valentovic MA.. Factors contributing to the antiviral effectiveness of tenofovir. J Pharmacol Exp Ther 2017; 363: 156–63. [DOI] [PubMed] [Google Scholar]
- 2.Cundy KC, Sueoka C, Lynch GR. et al. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob Agents Chemother 1998; 42: 687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JP, Sueoko CM, Oliyai R. et al. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm Res 1997; 14: 1824–9. [DOI] [PubMed] [Google Scholar]
- 4.Durand-Gasselin L, Van Rompay KK, Vela JE. et al. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol Pharm 2009; 6: 1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Custodio J, Garner W, Callebaut C.. The pharmacokinetics of tenofovir and tenofovir diphosphate following administration of tenofovir alafenamide versus tenofovir disoproxil fumarate. In: 16th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, DC, USA, Abstract 6. [Google Scholar]
- 6.Podany AT, Bares SH, Havens J. et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32: 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruane PJ, DeJesus E, Berger D. et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63: 449–55. [DOI] [PubMed] [Google Scholar]
- 8.Lee WA, He GX, Eisenberg E. et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005; 49: 1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naesens L, Bischofberger N, Augustijns P. et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother 1998; 42: 1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearney BP, Flaherty JF, Shah J.. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43: 595–612. [DOI] [PubMed] [Google Scholar]
- 11.Geboers S, Haenen S, Mols R. et al. Intestinal behavior of the ester prodrug tenofovir DF in humans. Int J Pharm 2015; 485: 131–7. [DOI] [PubMed] [Google Scholar]
- 12.Nye LC, Gray N, McClure M. et al. Identification of a novel human circulating metabolite of tenofovir disoproxil fumarate with LC-MS/MS. Bioanalysis 2015; 7: 643–52. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Biotechnology Information. PubChem Compound Database; CID=53468588. https://pubchem.ncbi.nlm.nih.gov/compound/53468588.
- 14.National Center for Biotechnology Information. PubChem Compound Database; CID=464205. https://pubchem.ncbi.nlm.nih.gov/compound/464205.
- 15.Viread (R) [Package insert]. Foster City, CA, USA: Gilead Sciences, Inc., 2018.
- 16.Seifert SM, Castillo-Mancilla JR, Erlandson K. et al. Brief Report: adherence biomarker measurements in older and younger HIV-infected adults receiving tenofovir-based therapy. J Acquir Immune Defic Syndr 2018; 77: 295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Mancilla JR, Searls K, Caraway P. et al. Short Communication: tenofovir diphosphate in dried blood spots as an objective measure of adherence in HIV-infected women. AIDS Res Hum Retroviruses 2015; 31: 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo-Mancilla JR, Morrow M, Coyle RP. et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with HIV infection. Clin Infect Dis 2019; 68: 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant RM, Lama JR, Anderson PL. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant RM, Anderson PL, McMahan V. et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14: 820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu AY, Cohen SE, Vittinghoff E. et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosek SG, Rudy B, Landovitz R. et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr 2017; 74: 21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson PL, Liu AY, Castillo-Mancilla JR. et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62: e01710-01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louissaint NA, Cao YJ, Skipper PL. et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29: 1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacBrayne CE, Marks KM, Fierer DS. et al. Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate. J Antimicrob Chemother 2018; 73: 2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks KM, Castillo-Mancilla JR, Blum J. et al. Increased tenofovir-monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir. J Antimicrob Chemother 2019; 74: 2360–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim ME, Brooks KM, Castillo-Mancilla JR. et al. Short Communication: bioequivalence of tenofovir and emtricitabine after coencapsulation with the Proteus ingestible sensor. AIDS Res Hum Retroviruses 2018; 34: 835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifert SM, Chen X, Meditz AL. et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses 2016; 32: 981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng JH, Rower C, McAllister K. et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delahunty T, Bushman L, Fletcher CV.. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2006; 830: 6–12. [DOI] [PubMed] [Google Scholar]
- 31.Bushman LR, Kiser JJ, Rower JE. et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011; 56: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gelder J, Deferme S, Annaert P. et al. Increased absorption of the antiviral ester prodrug tenofovir disoproxil in rat ileum by inhibiting its intestinal metabolism. Drug Metab Dispos 2000; 28: 1394–6. [PubMed] [Google Scholar]
- 33.Moss DM, Domanico P, Watkins M. et al. Simulating intestinal transporter and enzyme activity in a physiologically based pharmacokinetic model for tenofovir disoproxil fumarate. Antimicrob Agents Chemother 2017; 61: e00105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laizure SC, Herring V, Hu Z. et al. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy 2013; 33: 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taneva E, Crooker K, Park SH. et al. Differential mechanisms of tenofovir and tenofovir disoproxil fumarate cellular transport and implications for topical preexposure prophylaxis. Antimicrob Agents Chemother 2015; 60: 1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan LC, Dahl TC, Oliyai R.. Degradation kinetics of oxycarbonyloxymethyl prodrugs of phosphonates in solution. Pharm Res 2001; 18: 234–7. [DOI] [PubMed] [Google Scholar]
- 37.Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Tenofovir Disoproxil Fumarate. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-356_Viread_biopharmr.pdf.
- 38.Shen Y, Yan B.. Inhibition of carboxylesterase 2 (CES2) by sofosbuvir: metabolism-reduced potency, in vivo inhibition and reduced activation of the anti-HIV drug tenofovir disoproxil. Drug Metab Pharmacokinet 2018; 33: S53. [Google Scholar]
- 39.Shen Y, Yan B.. Covalent inhibition of carboxylesterase-2 by sofosbuvir and its effect on the hydrolytic activation of tenofovir disoproxil. J Hepatol 2017; 66: 660–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins BL, Srinivas RV, Kim C. et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 1998; 42: 612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castillo-Mancilla JR, Zheng JH, Rower JE. et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29: 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]