Implications.
Livestock are a critical part of worldwide communities and do emit greenhouse gases from these activities.
There are several methods to measure enteric methane emissions from livestock and there are limitations and benefits with these methods.
There are several methods including diet additives/modification as well as genetic selection methods that show promise for mitigation of enteric emissions from livestock.
There are growing methods of how emissions are modeled that may further expand the understanding of the role of livestock in greenhouse gas emissions that include life-cycle assessments.
Introduction
Livestock is an integral part of societies worldwide and contributes to a host of human activities beyond food production, including income, heritage, insurance, labor, and culture. Livestock’s positive contributions to society are contrasted by environmental impacts, which include greenhouse gas (GHG) emissions, biodiversity loss, and natural resource depletion, among others. Though environmental impacts of ruminant livestock production extend beyond GHG emissions (Rotz, 2020), considerable effort has been dedicated specifically to quantifying and mitigating enteric methane (CH4) emissions from beef and dairy cattle, which is the focus of this review.
The primary sources of GHGs in livestock systems are enteric CH4, CH4 and nitrous oxide (N2O) from manure handling and management, and N2O from feed production. Total GHG emissions are reported on a CO2-equivalent (CO2e) basis and represent the sum of all GHGs standardized to a common unit by weighting each gas to a global warming potential (GWP). GWPs weighting factors were defined by the Intergovernmental Panel on Climate Change (IPCC). For example, the GWP of carbon dioxide (CO2), CH4, and N2O have been computed as 1, 28, and 265, respectively, to represent their relative warming potential for a 100-yr period relative to CO2 (EPA, 2020).
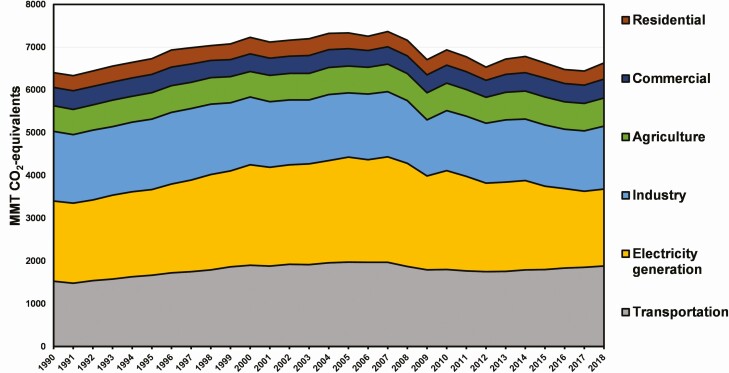
Agriculture contributes about 10% of total U.S. GHG emissions (Figure 1). Livestock contributes about 4% of total U.S. GHG emissions, excluding emissions from feed production and fuel use (IPCC, 2014).
Figure 1.
U.S. GHG emissions by economic sector (EPA, 2020).
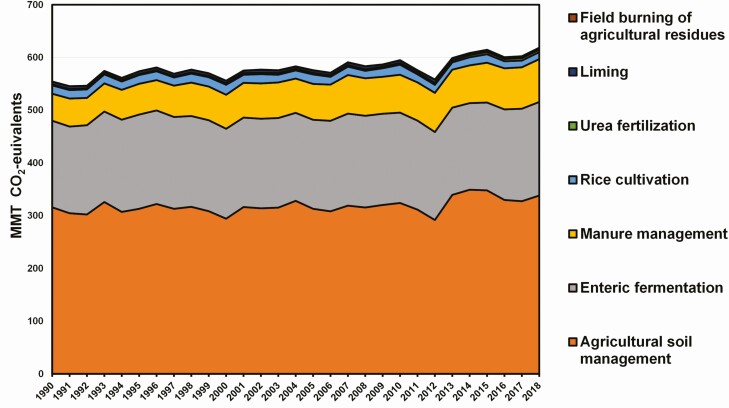
Emissions from enteric fermentation and manure management (direct emissions) represent about 41% of agriculture’s total GHG emissions, measured in CO2 equivalents, which means the aggregation of all emissions (Figure 2). Thus, while direct livestock contributions to the U.S. total GHG emissions are relatively small, they are directly responsible for 38% of U.S. CH4 emissions and 4% of U.S. N2O emissions.
Figure 2.
U.S. agricultural GHG emissions by activity (EPA, 2020).
In this review, we provide a high-level overview of the current state of enteric CH4 research in beef and dairy systems. First, we discuss common methods of measuring enteric CH4 emissions. Second, we discuss modeling individual enteric CH4 emissions. Third, we highlight current trends in feed additive mitigation research, with a brief discussion of the potential for soil carbon sequestration to offset carbon emissions from ruminant livestock. Last, we offer comments on how models and life-cycle assessments (LCA) can be used to extrapolate from animal emissions to broader farm, regional, and supply chain contexts.
Measuring Enteric Methane Emissions from Ruminant Livestock
There are many methods for directly measuring enteric CH4 from ruminant livestock, each with its strengths and weaknesses (Hammond et al., 2016; Jonker and Waghorn, 2020). Currently, widely accepted techniques for measuring enteric CH4 emissions are respiration chambers (i.e., the “gold standard”), the sulfur hexafluoride (SF6) tracer method (Johnson et al., 1994), and an automated head-chamber system (GreenFeed System; C-Lock Inc., Rapid City, SD). Irrespective of the method, calibration, and recovery, tests are required for method development and routine operations (Hammond et al., 2016). All three methods can measure enteric CH4 emissions from individual animals (or “point-source” measurements) and require an acclimation and/or training period. Deciding which technique to use depends on the experimental objectives, available resources, the research team’s experience, and the experimental environment.
When researchers are interested in collecting highly accurate measures of enteric including hindgut CH4 emissions from a single to a few animals in a confinement environment, and where ample resources (highly skilled operators, time, and funds) are available, they may find respiration chambers well suited. However, while chambers provide highly accurate measures under these conditions, they are also more disruptive to animal behavior, have decrease feed consumption, and are not representative of open-air environments (Gunter and Cole, 2016).
Both the SF6 and GreenFeed systems are suitable for measuring emissions in open-air environments (e.g., feedlots, barns, or pastures) and for a larger number of animals (Gunter and Cole, 2016). However, neither of these methods capture hindgut emissions, and only the GreenFeed system can capture diel variation through spot sampling, as the SF6 air-collection method is integrative and diel variations of emissions are not divisible.
When a project calls for a greater number of samples at a lower cost than respiration chambers or sampling in an open-air environment, the SF6 method provides a suitable alternative. This method still requires a skilled operator to ensure precision and can be highly variable, as measurements are influenced by background gas concentrations (which may be of concern if used inside barns; Hammond et al., 2016), sample collection rate (Deighton et al., 2014a), reticulo-rumen environment (Deighton et al., 2014b), and cannulation (Beauchemin et al., 2012). Following the modified SF6 protocol (Deighton et al., 2014a) and avoiding the use of cannulated animals if possible, or increasing their number if unavoidable, can help to reduce some of the experimental error associated with this method. Characterization of the influence of the reticulo-rumen environment, which may vary with diet and genetics, on CH4 sampling using the modified SF6 method is the next step in further refining the SF6 method (Deighton et al., 2014b).
Finally, the GreenFeed system presents an alternative to the SF6 method for sampling in open-air environments (Gunter and Beck, 2018). This system has also been successfully used in pen-feeding situations (Huhtanen et al., 2019). However, as sampling requires voluntary visitation to the head chamber by the animal and animals may choose not to visit, sampling with the GreenFeed system requires more animals sampled over longer time periods and need to be carefully timed throughout the day to collect enough samples to accurately quantify and account for daily patterns in enteric CH4 emissions (Hammond et al., 2015, 2016).
Modeling Enteric Methane Emissions from Ruminant Livestock
While direct measurements of enteric CH4 emissions are ideal, collecting these data can be expensive and time-consuming. Mathematical models can be used as a complement to experimental data to predict enteric CH4 emissions or mitigation potential of emerging innovations or to extend the analysis beyond the animal or farm boundaries (Rotz, 2018; Tedeschi, 2019). Models can be classified in the following ways:
Empirical: based on statistical correlations between variables
Mechanistic: based on underlying causal relationships
Static: represents a single point in time
Dynamic: represents change over time
Deterministic: represents all variables as constants
Stochastic: includes variability in model parameters
Although models can be used to extrapolate findings or reduce the cost of research, like experimental methods, mathematical models vary in their suitability for a particular application, the accuracy and precision of their estimates, and their ease of use. A number of models have been developed to predict enteric CH4 emissions, among other variables, each with varying specificity and accuracy across species and production environments (e.g., Mills et al., 2003; Kebreab et al., 2008, 2019; Dougherty et al., 2017, 2019; Niu et al., 2018; Benaouda et al., 2019; Van Amburgh et al., 2019; Tedeschi and Fox, 2020; Hansen et al., 2021). Enteric CH4 prediction equations range from simple correlations with nutrient intake to a mechanistic and dynamic representation of carbohydrate and protein digestion and absorption over time.
Empirical models are well suited for use in conditions similar to those in which they were developed, as their results are specific to those contexts. They are also useful when input data or resources are limited. Predictions from these models outside of the conditions in which they were developed should be interpreted with caution. While convenient, these models will not provide the same level of nuance offered by mechanistic models. For practical applications or where more detailed input data and resources are available, mechanistic models may be a more appropriate choice. However, due to their complexity, engagement with an expert user is recommended to ensure the model is correctly parameterized and applied. Scaling results beyond the animal to the farm or region can be completed using process-based, whole-farm models, which represent all operations within the boundary of a farm or ranch (e.g., the Integrated Farm System Model [IFSM]; Rotz et al., 2018), the Ruminant Farm Systems Model (Hansen et al., 2021).
Nutritional and Genetic Opportunities for Mitigating Enteric Methane Emissions from Ruminant Livestock
Across diets, dry matter intake drives ruminal methanogenesis, but diet composition is also critically important. As such, much of the mitigation literature has focused on nutritional interventions (Beauchemin et al., 2008, 2020; Caro et al., 2016), though some reviews have also covered reproductive, genetic, and management interventions (Hristov et al., 2013a, 2013b; Wattiaux et al., 2019; Uddin et al., 2020), including grazing beef systems (Thompson and Rowntree, 2020). Therefore, this section provides a high-level highlight of emerging, promising mitigation approaches from nutrition perspectives.
Feed additives
Many novel feed additives designed to reduce ruminal methanogenesis are currently being tested (Honan et al., 2021); however, mostly in vitro and, therefore, still require in vivo and system-scale evaluation. In addition to requiring validation in vivo, questions about the practicality and safety of some products may prevent widespread commercial use. One novel CH4 inhibitor that has gained recognition in recent years for successful short-term mitigation is 3-nitroxypropanol (3-NOP). 3-NOP has been shown to reduce CH4 emissions in dairy cattle by 20% to 40% (Lopes et al., 2016; Melgar et al., 2020a, 2020b, 2021), with greater reductions in dairy than beef cattle (Dijkstra et al., 2018). Studies in dairy cattle suggest that this decrease is achieved with no change in milk yield and little to no effects on milk composition (Lopes et al., 2016; Melgar et al., 2021). While variability exists across studies, generally, increasing 3-NOP dose decreases CH4 emissions, though the effect is mitigated by dietary factors, including dietary fiber content (Dijkstra et al., 2018).
Plant-based products (e.g., condensed tannins, saponins, and essential oils) can also serve as CH4 inhibitors (Tedeschi et al., 2021). Most phytochemicals also have beneficial functions in the gastrointestinal tract of ruminants beyond reducing CH4 production (e.g., anthelmintic and antioxidant properties) that may increase productive efficiency (Provenza and Villalba, 2010; Tedeschi et al., 2021) and play important ecological roles in wild and working lands (Villalba et al., 2019). Essential oils such as oregano and thyme have received attention in the past decade with demonstrated in vitro methane mitigation potential at high concentrations, but the translation to in vivo effects has proven difficult due to inhibition of rumen function and animal productivity at high feeding levels (Benchaar and Greathead, 2011). One novel plant-based product that has recently received special attention is Asparagopsis taxiformis (seaweed), which was shown to reduce emissions by as much as 98% (Kinley et al., 2020). However, additional research regarding the feasibility and sustainability of seaweed as a feed additive is needed to answer critical questions related to the production of required quantities and bromoform stability and its long-term effects on productivity, reproduction, animal health, and welfare (Stefenoni et al., 2021).
Advancing the potential for 3-NOP, seaweed, and phytochemical feed additives to serve as CH4 mitigators at the commercial scale requires additional research addressing the practicality, scalability, and safety of their widespread use. For phytochemicals that have demonstrated in vitro CH4 mitigation potential and have documented ecological and antimicrobial benefits, additional in vivo and systems-level research quantifying potential benefits, co-benefits, synergisms among different plant-based products and tradeoffs of their use for enteric CH4 mitigation is needed.
Genetic selection
Perhaps less studied, genetic selection may play direct and indirect roles in reducing enteric CH4 emissions. Methane emissions from livestock have been indicated as moderately heritable, with heritability estimates ranging from 0.12 to 0.45 (Basarab et al., 2013; Beauchemin et al., 2020). Selection can occur through breed choice, parent selection for trait improvement, or heterosis. While direct selection for enteric CH4 mitigation is unlikely, reductions in enteric CH4 emissions are more likely to come from indirect selection and management decisions, for example, through combinations between genetic selection for nutrient utilization and longevity, forage characteristics, and management practices (Knapp et al., 2014). Selection programs to improve feed utilization and efficiency in livestock are attractive options for potentially mitigating enteric CH4 emissions but must be balanced with other important outcomes (e.g., longevity). Other promising opportunities include epigenetic control mechanisms or the possibility of integrating desirable genetic material into individuals using gene editing. Despite acquiring enormous quantities of genomic information and associated knowledge to date, we are only approaching the beginnings of understanding these data, which may be used to inform genetic selection approaches that directly or indirectly mitigate enteric methane emissions (Pickering et al., 2015; Koltes et al., 2019).
An undervalued approach is a management decision to match breed type to local conditions (Provenza, 2008). Especially in beef and dairy production, many breeds are used in regions for which they are clearly not adapted, potentially reducing productive efficiency. Matching breed with environment and management has positive implications for productive efficiency, potentially reducing enteric CH4 emissions per unit of product (Knapp et al., 2014). However, one barrier to the implementation of this management strategy is that producers are rewarded economic incentive based on animal performance and carcass quality attributes rather than the animal’s effect on or interaction with the ecosystem which can limit utilization of more adapted breeds.
Due to its influence on improving animal performance, heterosis may be a more immediate genetic approach to reducing enteric CH4 emissions per unit of product and potentially broader environmental impact concerns for an industry comprised of fewer (with respect to numbers of animals) production units, for example, the U.S. beef production system. More concentrated industries, such as dairy, poultry, or pork, may be able to utilize genetic selection programs with indirect impacts on reducing enteric CH4 emissions per unit product to a greater extent, even for traits with low heritability, due to the vertically integrated nature of the industry and faster genetic turnover.
Broadening the Scope: From Enteric Methane Emissions to Carbon Footprints
A role for carbon sequestration?
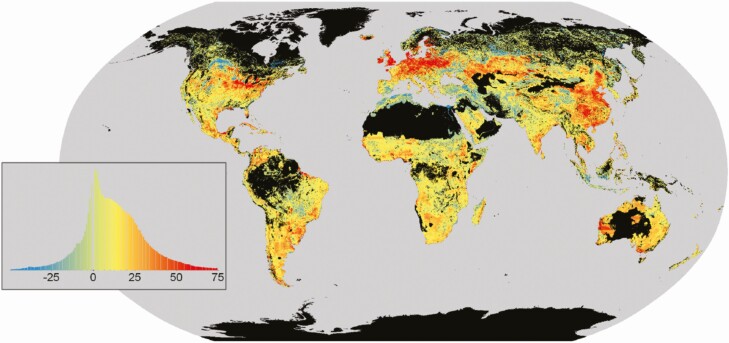
While not a direct enteric CH4 mitigation strategy, soil carbon sequestration has received increasing attention as a potential climate change mitigator, with ruminant livestock playing a role as graziers of grasslands worldwide (Teague et al., 2016; Fargione et al., 2018; Bossio et al., 2020). Globally, we have lost an estimated 133 Pg of soil carbon due to agricultural activity (Figure 3; Sanderman et al., 2017). With great loss comes great opportunity; as grasslands cover approximately half of the terrestrial surface, they remain an enormous soil carbon reservoir with the potential for sequestering additional carbon (Sanderman et al., 2017).
Figure 3.
Global distribution of modeled soil organic carbon (SOC; Mg C ha−1) change in the top 2 m. The legend is a histogram of SOC loss (Mg C ha−1), with positive values indicating loss and negative values depicting gains in SOC. Figure adapted from Sanderman et al. (2017).
Soil organic carbon sequestration potential is highly context-specific and varies across ecoregions (McSherry and Ritchie, 2013). Drivers of soil carbon content and sequestration are climate, soil texture, and management history. Grazing management changes may also alter the productive capability and direct CH4 emission of a grassland, potentially with implications for decreased CH4 emissions per unit of product but not in all cases (Savian et al., 2018; Thompson and Rowntree, 2020). In some cases, improved herbage utilization efficiency has resulted in increases in absolute CH4 emission by the grazing system (Savian et al., 2018).
It has been posited that grasslands that are sequestering carbon eventually reach a new soil carbon equilibrium, this convention has been contested recently in some regions, with a long-term grazing experiment in appropriately grazed vs. non-grazed grasslands (Liebig et al., 2010; Rowntree et al., 2020) and an on-farm chronosequence study of a multi-species grazing livestock operation showing continual soil carbon accrual (Rowntree et al., 2020). As drivers of long-term soil carbon accrual continue to be identified, at least in the short term, soil carbon sequestration may reduce the carbon footprint of livestock production, though some change in management is required to stimulate this process (Stanley et al., 2018). The long-term permanence of sequestered carbon varies across soil types and has implications for soil carbon sequestration as a potential long-term mitigation opportunity for livestock production systems (Cotrufo et al., 2019). Accurately measuring soil carbon sequestration, permanence, and change over time, however, is difficult and has significant uncertainty (Jandl et al., 2014). Achieving a greater understanding of soil organic matter dynamics and carbon sequestration across ecological regions, management, and soil depths is critical to understanding the potential long-term contribution of carbon sequestration to reducing the carbon footprint of livestock production systems (Cotrufo et al., 2019).
While soil carbon sequestration may offset carbon emissions from livestock production, from another perspective, land use for agriculture necessarily incurs tradeoffs with sustaining biodiversity in natural ecosystems as well as the carbon sequestration and other ecosystem services provided by those ecosystems. The potential for carbon sequestration to offset emissions from livestock production systems, therefore, must be matched with considerations for the “carbon opportunity cost” of land use—or the opportunity for land to store carbon if not used for agriculture (Hayek et al., 2021).
LCA and carbon footprints
Carbon footprints (or the sum of all GHG emissions weighted by their relative radiative forcing, per unit of product) are often used to evaluate the potential climate impact of products and have increasingly been applied to livestock production systems in the last couple of decades (de Vries and de Boer, 2010; de Vries et al., 2015; Mcclelland et al., 2018). Carbon footprints put the contribution of enteric CH4 emissions from the animal (and thus, potential mitigation strategies) in the context of a farm, region, or supply chain. They are calculated using the LCA methodology.
LCA is an accounting methodology for quantifying the impacts of goods and services over their full life cycle: from raw material extraction through production, processing, transport/distribution, consumption, and disposal. Environmental impacts related to human health, resource use, and ecosystem damage can be assessed with LCA (e.g., global warming, water consumption, or ecotoxicity, among others). As LCA was designed to evaluate industrial processes, applying it to agricultural systems presents some challenges: the necessary data required to complete an LCA are often unavailable from a single farm or ranch, and uncertainty in environmental flows in agroecological systems can complicate the collation of required “inventory” data. In addition, many of the standard impact assessment frameworks available only provide spatially and temporally integrated characterization (e.g., eutrophication factors are only readily available at continental scale) and cannot provide accurate, locally relevant environmental impact estimates. The ability of LCA to quantify biodiversity and ecosystem impacts is also limited despite ongoing research in the area (Teillard et al., 2016). Process-based models are sometimes used to fill gaps in life cycle inventory data and address these spatiotemporal limitations (e.g., Kim et al. (2019) used inventory data partially supplied by IFSM to conduct an LCA of changes in dairy management practices in the northeastern United States). Despite these limitations, LCA remains the best available approach to calculating product life cycle environmental impacts. Methodologies to overcome the aforementioned challenges are rapidly evolving; for example, there are new spatially and temporally specific characterization factors for water scarcity (Boulay et al., 2020).
Global warming potential
Integrating enteric CH4 emissions with total GHG produced by livestock systems requires standardizing emissions for multiple gases across multiple sources. To accomplish this, IPCC developed the aforementioned GWP, or “carbon footprint” metric. The GWP characterizes the heat absorbed by a GHG relative to the amount of heat that would be absorbed by CO2 over a pre-specified time horizon, divided by the system’s total output. Thus, GWP converts the climate contribution of different GHGs such as CH4 or N2O into a common scale referred to as CO2-equivalents (CO2e) and is used to evaluate potential climate impacts from various sources. When applied to an evaluation of U.S. milk production, this method enabled the estimation of a national carbon footprint of about 2.1 kg CO2e per kilogram of milk consumed, wherein 25% of the footprint was attributed to enteric CH4, 23% to manure CH4 and N2O, and 19% to fuel and fertilizer emissions from the feed production phase (Thoma et al., 2013). Transportation represented about 8% of the total footprint.
The GWP metric is time-integrated and relates the radiative forcing of one pulse emission of GHG to a pulse emission of CO2 over a chosen time horizon: 100 or 20 yr in the case of GWP100 or GWP20, respectively (Myhre et al., 2013). The GWP100 is calculated based on a time horizon of 100 yr and is the current universal standard GHG metric. The GWP100 metric is under criticism for the use of short-lived climate pollutants (SLCP) like CH4, because it does not completely account for the fact that CH4 is both produced and destroyed (Pierrehumbert, 2014; Lynch et al., 2020).
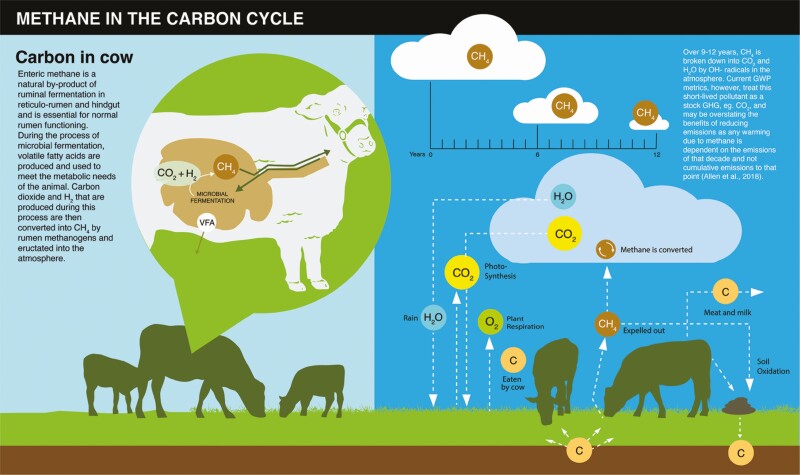
Methane is constantly being removed from the atmosphere by a process called hydroxyl oxidation (Figure 4). If CH4 emissions exceed the amount being oxidized, global warming will occur. However, if CH4 emissions are less than oxidization, then temporary cooling should occur. Therefore, it is the rate of change in CH4 emissions over time that determines its climate impact through its effect on atmospheric concentration. A related metric that accounts for this dynamic is the GWP* (Allen et al., 2018). The GWP* better accounts for SLCPs like CH4. Instead of converting GHG emissions to CO2e, which is always positive, it equates the climate impacts from a one-step permanent change of an SLCP emission to that caused by a one-off “pulse” change of CO2 (CO2 warming-equivalent; CO2we). Therefore, CO2we can be either positive or negative to indicate the “warming” or “cooling” of the temperature compared with 20 yrs ago, related to an increase or decrease of CH4, respectively. Lynch et al. (2020) compared GWP100 and GWP* across emission scenarios and showed that GWP* accounts for the influence of atmospheric CH4 dynamics on climate impacts, whereas GWP100 assumes all CH4 emissions contribute to warming, thus overestimating climate impacts when emissions are constant or decreasing. This is of great importance to the U.S. livestock sector, where national beef and dairy inventories are either constant or shrinking.
Figure 4.
Methane in the carbon cycle. Figure reproduced from Thompson and Rowntree (2020) with permission.
Summary
Technological interventions for reducing enteric CH4 from beef and dairy systems abound. Respiration chambers enable researchers to obtain highly accurate enteric CH4 measurements from controlled environments, whereas SF6 and GreenFeed systems present opportunities for measuring emissions in open-air environments. Several resources are available to aid researchers in method selection, depending upon the intended application. Currently, 3-NOP appears to be a promising inhibitor for enteric CH4 production, with seaweed garnering additional interest. Evaluation of the practicality, feasibility, long-term mitigation potential, and long-term effects on productivity, reproduction, and animal health of feed additives is critical to identifying commercially relevant CH4 mitigation options. As plant phytochemicals have potential animal health and ecological co-benefits in addition to being potential CH4 mitigators, they should be studied from interdisciplinary, system approaches. Beyond the animal, soil carbon sequestration presents a potential opportunity for reducing the carbon footprint of ruminant livestock production systems, at least in the short term.
About the Authors

Jasmine A. Dillon, PhD, has been an Assistant Professor of Beef & Dairy Agroecosystems at Colorado State University since 2019. Her current work includes evaluation of the environmental footprints of livestock production systems using life cycle assessment (LCA) and simulation modeling. Current projects include assessing climate vulnerabilities, potential for net-zero, and impacts of longevity on environmental footprints of dairy production systems, and developing methods for incorporating ecosystem services and social impacts into LCA of beef production systems. She completed her BS in Animal Science from Texas A&M in 2011 and MS in Animal Breeding from Texas A&M in 2013. She earned her PhD in Animal Science from The Pennsylvania State University in 2019, where she worked closely with the USDA Agricultural Research Service’s Pasture Systems & Watershed Management Research Unit to evaluate environmental footprints and eco-efficiency of northeastern grass-fed beef production systems.

Kim R. Stackhouse-Lawson is the Director of the Sustainable Livestock Systems Collaborative (SLSC) and a Professor of Animal Science at Colorado State University. The SLSC was launched to find solutions to feed 12.3 billion people by 2100 by promoting animal health and addressing environmental challenges, all in an economically sustainable way. Prior to her time at Colorado State University, Kim was the Director of Sustainability for JBS USA, where she is responsible for coordinating the North American sustainability program, inclusive of the Company’s beef, pork, poultry, case ready, transportation, and branded product business. Prior to that, she was the Executive Director of Global Sustainability at the National Cattlemen’s Beef Association, where she developed the industry’s sustainability program. Kim received her PhD in Animal Science from the University of California, Davis, and was a postdoctoral fellow at Kansas State University College of Veterinary Medicine Beef Cattle Institute.

Greg J. Thoma, PhD, is the Bates Teaching Professor of Chemical Engineering at the University of Arkansas, where he has been on the faculty since 1994 after receiving his PhD from Louisiana State University in Chemical Engineering. He served as inaugural Director for Research of The Sustainability Consortium. He has led numerous food and agriculture life cycle assessment projects: milk, cheese, milk delivery systems, yogurt, swine, poultry, corn, and beef. He serves on the steering committee for the Swiss National Research Program, “Healthy Nutrition and Sustainable Food Production.” He is the North American subject editor for Agriculture for the International Journal of Lifecycle Assessment and has served as Technical Advisory Group Lead/Co-lead for development of the poultry, swine, and large ruminants’ guidelines for the UN Food and Agriculture Organization’s Livestock Environmental Assessment and Performance (LEAP) Partnership since its inception; most recently, he is serving on the LEAP greenhouse gas technical advisory group.

Stacey A. Gunter is the Research Leader and Rangeland Management Specialist for the USDA—Agricultural Research Service at the Southern Plains Range Research Station in Woodward, Oklahoma. During his career, he has taught courses and conducted research in grazing livestock nutrition and management. He received his BS in Animal Science from Oregon State University (1987), an MS in Animal Science from the University of Nevada-Reno (1989), and PhD in Animal Nutrition (1993) from Oklahoma State University. He joined the Cooperative Extension Service faculty at the University of Maine in 1994 and in 1996 moved to the University of Arkansas Southwest Research & Extension Center in Hope as an Assistant Professor. The nine MS and three PhD students who completed their programs under his guidance have gone on to noteworthy careers. Dr. Gunter’s current research is the energy metabolism and the greenhouse gas emission by cattle grazing expansive grasslands.

C. Alan Rotz, PhD, PE, is an Agricultural Engineer with the USDA’s Agricultural Research Service. His work has included development, evaluation, and application of the Integrated Farm System Model (IFSM) used to evaluate and compare the performance, economics, and environmental impacts of farming systems. Recent work includes life cycle assessment and sustainability analysis of beef and dairy production and adaptation of farming systems to climate change. He holds degrees from Elizabethtown College and The Pennsylvania State University with graduate degrees in Agricultural Engineering. He spent 3 yr as an Assistant Professor at Michigan State University before joining the Agricultural Research Service. His employment includes 16 yr with the East Lansing Cluster of the U.S. Dairy Forage Research Center and 24 yr at the Pasture Systems and Watershed Management Research Unit in University Park, Pennsylvania. He is a registered Professional Engineer and a Fellow of the American Society of Agricultural and Biological Engineers.

Ermias Kebreab is an Associate Dean and holds the Sesnon Endowed Chair in Sustainable Animal Agriculture at UC Davis. He conducts research in nutrition modeling of biological systems and conducts experiments to reduce the impact of animal agriculture on the environment, including the sustainable use of feed additives. He is contributing author to the 2019 IPCC update and chairs feed additive committee of Food and Agriculture Organization. He has authored over 200 peer-reviewed articles. He also received several awards including AFIA Ruminant Nutrition Award. He received BSc from the University of Asmara, Eritrea, and MSc and PhD from University of Reading, UK.

David G. Riley is Professor of Animal Breeding in the Department of Animal Science and is a member of the Faculty of Genetics at Texas A&M University. He previously was Research Beef Cattle Geneticist for the U.S. Department of Agriculture and University of Florida from 2000 to 2009. Earlier, he worked in all aspects of swine production, selection, and service for DeKalb Agribusiness (1985 to 1995) and served in the U.S. Army. He earned BS in Agriculture Economics, MS in Animal Breeding, and PhD in Genetics from Texas A&M University. His research is focused on the use of genomics and quantitative genetic tools for the improvement of livestock. Current research is assessment of epigenetic alterations of stress-related genes in Brahman calves after prenatal stress and correspondence with genes expressed as measured by RNA sequencing in a variety of tissues associated with response to stress and reproduction in cattle.

Luis O. Tedeschi is a professor in the Department of Animal Science at Texas A&M University, Texas A&M AgriLife Research Faculty Fellow, and Chancellor EDGES Fellow. He received his bachelor’s degree in Agronomy Engineering from the University of São Paulo (Brazil) in 1991, his master’s degree in Animal and Forage Sciences from the University of São Paulo (Brazil) in 1996, and his doctorate in Animal Science from Cornell University (NY) in 2001. Prior to joining Texas A&M University in 2005, he was a Research Associate at Cornell University (NY) from 2002 to 2005. His scientific expertise is in ruminant nutrition, bioenergetics of growth and development, assessment of feed quality, and modeling and simulation. His research focuses on integrating scientific knowledge in ruminant nutrition into applied mathematical models to solve contemporary problems. He developed the Ruminant Nutrition System (RNS) to formulate and evaluate diets for ruminants under diverse production conditions.

Juan J. Villalba is a Professor in the Department of Wildland Resources, S.J. & Jessie E. Quinney College of Natural Resources at Utah State University. He received his PhD in Range Science from Utah State University and has held positions as Research Assistant at the Universidad Nacional del Sur, Argentina, and as Postdoctoral Fellow, Research Assistant, and Associate Professor at Utah State University. His research focuses on understanding mechanisms influencing food selection and intake by herbivores, in order to create more efficient alternatives for managing animals and the landscapes they inhabit. He has built a unique research program recognized at the national and international levels, and his lab has trained students and visiting scholars from different countries and institutions around the world.

Frank Mitloehner is a Professor and Air Quality Specialist in Cooperative Extension in the Department of Animal Science at the University of California, Davis. He received his MS degree in Animal Science and Agricultural Engineering from the University of Leipzig, Germany, and his PhD degree in Animal Science from Texas Technical University. He is an expert for agricultural air quality, livestock climate impacts, and livestock housing and husbandry. Overall, he conducts research that is directly relevant to understanding and mitigating of air emissions from livestock operations as well as the implications of these emissions for the health and safety of farm workers and neighboring communities.

Alexander N. Hristov is a Distinguished Professor of Dairy Nutrition in the Department of Animal Science at The Pennsylvania State University. He has a PhD in Animal Nutrition from the Bulgarian Academy of Agricultural Sciences and has worked as a research scientist in his native Bulgaria, USDA-ARS Dairy Forage Research Center in Madison, WI, and the Ag Canada Research Center in Lethbridge, AB. He was on the faculty at the Department of Animal and Veterinary Science, University of Idaho, from 1999 to 2008 and is at Penn State since 2008. His main research interests are in the areas of protein/amino acid nutrition of dairy cattle and mitigation of nutrient losses and gaseous emissions from dairy operations.

Shawn L. Archibeque is a professor in the Animal Science Department at Colorado State University, where he has been on the faculty since 2006 with a research emphasis on addressing environmental issues associated with livestock production. His approach has largely used ruminant nutrition techniques to address these challenges.

John P. Ritten is a Professor and Extension Specialist in the Department of Agricultural and Applied Economics at the University of Wyoming. He received a BS in Marketing from Arizona State University, an MBA from New Mexico State University, and a PhD in Natural Resource Economics from Colorado State University. His research interests include the intersection of agricultural production and natural resource management. In his work, he strives to account for how changes in management and policies may impact both ecological and economic sustainability.

Nathaniel D. Mueller is an Assistant Professor in the Department of Ecosystem Science and Sustainability and the Department of Soil and Crop Sciences at Colorado State University. He received his PhD from the University of Minnesota in Natural Resource Science and Management and his BA from St. Olaf College in Biology and Environmental Studies. He was a Postdoctoral Fellow at Harvard University and served on the faculty of UC Irvine before joining CSU. His research examines agricultural sustainability and climate change impacts at regional to global scales.
Literature Cited
- Allen, M.R., Shine K.P., Fuglestvedt J.S., Millar R.J., Cain M., and Frame D.J.. 2018. A solution to the misrepresentations of CO2-equivalent emissions of short-lived climate pollutants under ambitious mitigation. Npj Clim. Atmospheric Sci. 1:16. doi: 10.1038/s41612-018-0026-8 [DOI] [Google Scholar]
- Basarab, J.A., Beauchemin K.A., Baron V.S., Ominski K.H., Guan L.L., Miller S.P., and Crowley J.J.. 2013. Reducing GHG emissions through genetic improvement for feed efficiency: effects on economically important traits and enteric methane production. Animal 7 (Suppl 2):303–315. doi: 10.1017/S1751731113000888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin, K.A., Coates T., Farr B., and McGinn S.M.. 2012. Technical Note: Can the sulfur hexafluoride tracer gas technique be used to accurately measure enteric methane production from ruminally cannulated cattle? J. Anim. Sci. 90:2727–2732. doi: 10.2527/jas.2011-4681 [DOI] [PubMed] [Google Scholar]
- Beauchemin, K.A., Kreuzer M., O′Mara F., and McAllister T.A.. 2008. Nutritional management for enteric methane abatement: a review. Aust. J. Exp. Agric. 48:21. doi: 10.1071/EA07199 [DOI] [Google Scholar]
- Beauchemin, K.A., Ungerfeld E.M., Eckard R.J., and Wang M.. 2020. Review: Fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation. Animal 14(S1):s2–s16. doi: 10.1017/S1751731119003100 [DOI] [PubMed] [Google Scholar]
- Benaouda, M., Martin C., Li X., Kebreab E., Hristov A.N., and Yu Z.. 2019. Evaluation of the performance of existing mathematical models predicting enteric methane emissions from ruminants: animal categories and dietary mitigation strategies. Anim. Feed Sci. Technol. 255:114207. doi: 10.1016/j.anifeedsci.2019.114207 [DOI] [Google Scholar]
- Benchaar, C., and Greathead H.. 2011. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 166–167:338–355. doi: 10.1016/j.anifeedsci.2011.04.024 [DOI] [Google Scholar]
- Bossio, D.A., Cook-Patton S.C., Ellis P.W., Fargione J., Sanderman J., Smith P., . et al. 2020. The role of soil carbon in natural climate solutions. Nat. Sustain. 3:391–398. doi: 10.1038/s41893-020-0491-z [DOI] [Google Scholar]
- Boulay, A.-M., Benini L., and Sala S.. 2020. Marginal and non-marginal approaches in characterization: how context and scale affect the selection of an adequate characterization model. The AWARE model example. Int. J. Life Cycle Assess. 25:2380–2392. doi: 10.1007/s11367-019-01680-0 [DOI] [Google Scholar]
- Caro, D., Kebreab E., and Mitloehner F.M.. 2016. Mitigation of enteric methane emissions from global livestock systems through nutrition strategies. Clim. Change 137:467–480. doi: 10.1007/s10584-016-1686-1 [DOI] [Google Scholar]
- Cotrufo, M.F., Ranalli M.G., Haddix M.L., Six J., and Lugato E.. 2019. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 12:989–994. doi: 10.1038/s41561-019-0484-6 [DOI] [Google Scholar]
- de Vries, M., and de Boer I.J.M.. 2010. Comparing environmental impacts for livestock products: a review of life cycle assessments. Livest. Sci. 128:1–11. doi: 10.1016/j.livsci.2009.11.007 [DOI] [Google Scholar]
- de Vries, M., van Middelaar C.E., and de Boer I.J.M.. 2015. Comparing environmental impacts of beef production systems: a review of life cycle assessments. Livest. Sci. 178:279–288. doi: 10.1016/j.livsci.2015.06.020 [DOI] [Google Scholar]
- Deighton, M.H., Williams S.R.O., Hannah M.C., Eckard R.J., Boland T.M., and Wales W.J.. 2014a. A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 197:47–63. doi: 10.1016/j.anifeedsci.2014.08.003 [DOI] [Google Scholar]
- Deighton, M.H., Williams S.R.O., Lassey K.R., Hannah M.C., Boland T.M., and Eckard R.J.. 2014b. Temperature, but not submersion or orientation, influences the rate of sulphur hexafluoride release from permeation tubes used for estimation of ruminant methane emissions. Anim. Feed Sci. Technol. 194:71–80. doi: 10.1016/j.anifeedsci.2014.05.006 [DOI] [Google Scholar]
- Dijkstra, J., Bannink A., France J., Kebreab E., and van Gastelen S.. 2018. Short Communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 101:9041–9047. doi: 10.3168/jds.2018-14456 [DOI] [PubMed] [Google Scholar]
- Dougherty, H.C., Ahmadi A., Oltjen J.W., Mitloehner F.M., and Kebreab E.. 2019. Review: Modeling production and environmental impacts of small ruminants—incorporation of existing ruminant modeling techniques, and future directions for research and extension. Appl. Anim. Sci. 35:114–129. doi: 10.15232/aas.2018-01753 [DOI] [Google Scholar]
- Dougherty, H.C., Kebreab E., Evered M., Little B.A., Ingham A.B., and Hegarty R.S.. 2017. The AusBeef model for beef production: I. Description and evaluation. J. Agric. Sci. 155:1442–1458. doi: 10.1017/S0021859617000429 [DOI] [Google Scholar]
- EPA. 2020. Inventory of U.S. Greenhouse Gas Emissions and Sinks.U.S. Environmental Protection Agency. Available from https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks [accessed May 3, 2021]. [Google Scholar]
- Fargione, J.E., Bassett S., Boucher T., Bridgham S.D., Conant R.T., Cook-Patton S.C., Ellis P.W., Falcucci A., Fourqurean J. W., Gopalakrishna T., et al. 2018. Natural climate solutions for the United States. Sci. Adv. 4:eaat1869. doi: 10.1126/sciadv.aat1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter, S.A., and Beck M.R.. 2018. Measuring the respiratory gas exchange by grazing cattle using an automated, open-circuit gas quantification system1. Transl. Anim. Sci. 2:11–18. doi: 10.1093/tas/txx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter, S.A., and Cole N.A.. 2016. Invited Review: Getting more information from your grazing research beyond cattle performance. Prof. Anim. Sci. 32:31–41. doi: 10.15232/pas.2015-01488 [DOI] [Google Scholar]
- Hammond, K.J., Crompton L.A., Bannink A., Dijkstra J., Yáñez-Ruiz D.R., and O′Kiely P.. 2016. Review of current in vivo measurement techniques for quantifying enteric methane emission from ruminants. Anim. Feed Sci. Technol. 219:13–30. doi: 10.1016/j.anifeedsci.2016.05.018 [DOI] [Google Scholar]
- Hammond, K.J., Humphries D.J., Crompton L.A., Green C., and Reynolds C.K.. 2015. Methane emissions from cattle: estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim. Feed Sci. Technol. doi: 10.1016/j.anifeedsci.2015.02.008 [DOI] [Google Scholar]
- Hansen, T.L., Li M., Li J., Vankerhove C.J., Sotirova M.A., and Tricarico J.M.. 2021. The ruminant farm systems animal module: a biophysical description of animal management. Animals 11:1373. doi: 10.3390/ani11051373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek, M.N., Harwatt H., Ripple W.J., and Mueller N.D.. 2021. The carbon opportunity cost of animal-sourced food production on land. Nat. Sustain. 4:21–24. doi: 10.1038/s41893-020-00603-4 [DOI] [Google Scholar]
- Honan, M., Feng X., Tricarico J.M., Kebreab E., Honan M., and Feng X.. 2021. Feed additives as a strategic approach to reduce enteric methane production in cattle: modes of action, effectiveness and safety. Anim. Prod. Sci. In press. doi: 10.1071/AN20295 [DOI] [Google Scholar]
- Hristov, A.N., Oh J., Firkins J.L., Dijkstra J., Kebreab E., and Waghorn G.. 2013a. SPECIAL TOPICS–Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 91:5045–5069. doi: 10.2527/jas.2013-6583 [DOI] [PubMed] [Google Scholar]
- Hristov, A. N., Ott T., Tricarico J., Rotz A., Waghorn G., and Adesogan A.. 2013b. SPECIAL TOPICS–Mitigation of methane and nitrous oxide emissions from animal operations: III. A review of animal management mitigation options. J. Anim. Sci. 91:5095–5113. doi: 10.2527/jas.2013-6585 [DOI] [PubMed] [Google Scholar]
- Huhtanen, P., Ramin M., and Hristov A.N.. 2019. Enteric methane emission can be reliably measured by the GreenFeed monitoring unit. Livest. Sci. 222:31–40. doi: 10.1016/j.livsci.2019.01.017 [DOI] [Google Scholar]
- IPCC (International Panel for Climate Change). 2014. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the IPCC. Geneva (Switzerland):IPCC. [Google Scholar]
- Jandl, R., Rodeghiero M., Martinez C., Cotrufo M.F., Bampa F., van Wesemael B., Harrison R.B., Guerrini I.A., D. D.Richter, Jr, Rustad L., et al. 2014. Current status, uncertainty and future needs in soil organic carbon monitoring. Sci. Total Environ. 468-469:376–383. doi: 10.1016/j.scitotenv.2013.08.026 [DOI] [PubMed] [Google Scholar]
- Johnson, K., Huyler M., Westberg H., Lamb B., and Zimmerman P.. 1994. Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ. Sci. Technol. 28:359–362. doi: 10.1021/es00051a025 [DOI] [PubMed] [Google Scholar]
- Jonker, A., and Waghorn G.C.. 2020. Guidelines for estimating methane emissions from individual ruminants using: GreenFeed, “sniffers”, hand-held laser detector and portable accumulation chambers. New Zealand. Wellington: New Zealand Agricultural Greenhouse Gas Research Centre. Available from http://www.mpi.govt.nz/news-and-resources/publications/ [accessed August 12, 2021]. [Google Scholar]
- Kebreab, E., Johnson K.A., Archibeque S.L., Pape D., and Wirth T.. 2008. Model for estimating enteric methane emissions from United States dairy and feedlot cattle. J. Anim. Sci. 86:2738–2748. doi: 10.2527/jas.2008-0960 [DOI] [PubMed] [Google Scholar]
- Kebreab, E., Reed K.F., Cabrera V.E., Vadas P.A., Thoma G., and Tricarico J.M.. 2019. A new modeling environment for integrated dairy system management. Anim. Front. 9:25–32. doi: 10.1093/af/vfz004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D., Stoddart N., Rotz C.A., Veltman K., Chase L., and Cooper J.. 2019. Analysis of beneficial management practices to mitigate environmental impacts in dairy production systems around the Great Lakes. Agric. Syst. 176:102660. doi: 10.1016/j.agsy.2019.102660 [DOI] [Google Scholar]
- Kinley, R.D., Martinez-Fernandez G., Matthews M.K., de Nys R., Magnusson M., and Tomkins N.W.. 2020. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 259:120836. doi: 10.1016/j.jclepro.2020.120836 [DOI] [Google Scholar]
- Knapp, J.R., Laur G.L., Vadas P.A., Weiss W.P., and Tricarico J.M.. 2014. Invited Review: Enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 97:3231–3261. doi: 10.3168/jds.2013-7234 [DOI] [PubMed] [Google Scholar]
- Koltes, J.E., Cole J.B., Clemmens R., Dilger R.N., Kramer L.M., Lunney J.K., McCue M.E., McKay S.D., Mateescu R.G., Murdoch B.M., et al. 2019. A vision for development and utilization of high-throughput phenotyping and big data analytics in livestock. Front. Genet. 10:1197. doi: 10.3389/fgene.2019.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebig, M.A., Gross J.R., Kronberg S.L., Phillips R.L., and Hanson J.D.. 2010. Grazing management contributions to net global warming potential: a long-term evaluation in the Northern Great Plains. J. Environ. Qual. 39:799–809. doi: 10.2134/jeq2009.0272 [DOI] [PubMed] [Google Scholar]
- Lopes, J.C., de Matos L.F., Harper M.T., Giallongo F., Oh J., Gruen D., Ono S., Kindermann M., Duval S., and Hristov A. N.. 2016. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 99:5335–5344. doi: 10.3168/jds.2015-10832 [DOI] [PubMed] [Google Scholar]
- Lynch, J., Cain M., Pierrehumbert R., and Allen M.. 2020. Demonstrating GWP*: a means of reporting warming-equivalent emissions that captures the contrasting impacts of short- and long-lived climate pollutants. Environ. Res. Lett. 15:044023. doi: 10.1088/1748-9326/ab6d7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclelland, S.C., Arndt C., Gordon D.R., and Thoma G.. 2018. Type and number of environmental impact categories used in livestock life cycle assessment: a systematic review. Livestock Sci. 209:39–45. doi: 10.1016/j.livsci.2018.01.008 [DOI]
- McSherry, M.E., and Ritchie M.E.. 2013. Effects of grazing on grassland soil carbon: a global review. Glob. Chang. Biol. 19:1347–1357. doi: 10.1111/gcb.12144 [DOI] [PubMed] [Google Scholar]
- Melgar, A., Harper M.T., Oh J., Giallongo F., Young M.E., Ott T.L., Duval S., and Hristov A.N.. 2020a. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 103:410–432. doi: 10.3168/jds.2019-17085 [DOI] [PubMed] [Google Scholar]
- Melgar, A., Lage C.F.A., Nedelkov K., Räisänen S.E., Stefenoni H., Fetter M.E., Chen X., Oh J., Duval S., Kindermann M., et al. 2021. Enteric methane emission, milk production, and composition of dairy cows fed 3-nitrooxypropanol. J. Dairy Sci. 104:357–366. doi: 10.3168/jds.2020-18908 [DOI] [PubMed] [Google Scholar]
- Melgar, A., Welter K.C., Nedelkov K., Martins C.M.M.R., Harper M.T., Oh J., Räisänen S.E., Chen X., Cueva S.F., Duval S., et al. 2020b. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 103:6145–6156. doi: 10.3168/jds.2019-17840 [DOI] [PubMed] [Google Scholar]
- Mills, J.A.N., Kebreab E., Yates C.M., Crompton L.A., Cammell S.B., and Dhanoa M.S.. 2003. Alternative approaches to predicting methane emissions from dairy cows1. J. Anim. Sci. 81:3141–3150. doi: 10.2527/2003.81123141x [DOI] [PubMed] [Google Scholar]
- Myhre, G., Shindell D., Bréon F.M., Collins W., Fuglestvedt J.S., and Huang J.. 2013. Anthropogenic and natural radiative forcing. In: Stocker, T.F., Qin D., Plattner G.-K., Tignor M., Allen S.K., Boschung J., et al. editors. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge (UK) and New York (NY, USA): Cambridge University Press. Available from https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter08_FINAL.pdf [accessed March 21, 2021]. [Google Scholar]
- Niu, M., Kebreab E., Hristov A.N., Oh J., Arndt C., Bannink A., Bayat A.R., Brito A.F., Boland T., Casper D., et al. 2018. Prediction of enteric methane production, yield, and intensity in dairy cattle using an intercontinental database. Glob. Chang. Biol. 24:3368–3389. doi: 10.1111/gcb.14094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering, N.K., Oddy V.H., Basarab J., Cammack K., Hayes B., Hegarty R.S., Lassen J., McEwan J.C., Miller S., Pinares-Patiño C.S., et al. 2015. Animal Board Invited Review: Genetic possibilities to reduce enteric methane emissions from ruminants. Animal 9:1431–1440. doi: 10.1017/S1751731115000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrehumbert, R.T. 2014. Short-lived climate pollution. Annu. Rev. Earth Planet. Sci. 42:341–379. doi: 10.1146/annurev-earth-060313-054843 [DOI] [Google Scholar]
- Provenza, F.D. 2008. What does it mean to be locally adapted and who cares anyway? J. Anim. Sci. 86(14 Suppl):E271–E284. doi: 10.2527/jas.2007-0468 [DOI] [PubMed] [Google Scholar]
- Provenza, F.D., and Villalba J.J.. 2010. The role of natural plant products in modulating the immune system: an adaptable approach for combating disease in grazing animals. Small Ruminant Res. 89:131–139. doi: 10.1016/j.smallrumres.2009.12.035 [DOI] [Google Scholar]
- Rotz, C.A. 2018. Modeling greenhouse gas emissions from dairy farms. J. Dairy Sci. 101:6675–6690. doi: 10.3168/jds.2017-13272 [DOI] [PubMed] [Google Scholar]
- Rotz, A. 2020. Environmental sustainability of livestock production. Meat Muscle Biol. 4:1–18. doi: 10.22175/mmb.11103. [DOI] [Google Scholar]
- Rotz, C.A., Corson M.S., Chianese D.S., Hafner S.D., Bonifacio H.F., and Coiner U.. 2018. Integrated farm system model version 4.5 reference manual; p. 254. https://www.ars.usda.gov/ARSUserFiles/80700500/Reference%20Manual.pdf [accessed August 12, 2021]. [Google Scholar]
- Rowntree, J.E., Stanley P.L., Maciel I.C.F., Thorbecke M., Rosenzweig S.T., and Hancock D.W.. 2020. Ecosystem impacts and productive capacity of a multi-species pastured livestock system. Front. Sustain. Food Syst. 4:1–13. doi: 10.3389/fsufs.2020.544984 [DOI] [Google Scholar]
- Sanderman, J., Hengl T., and Fiske G.J.. 2017. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. U. S. A. 114:9575–9580. doi: 10.1073/pnas.1706103114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savian, J.V., Schons R.M.T., Marchi D.E., Freitas de T.S., da Silva Neto G.F., and Mezzalira J.C.. 2018. Rotatinuous stocking: a grazing management innovation that has high potential to mitigate methane emissions by sheep. J. Clean. Prod. 186:602–608. doi: 10.1016/j.jclepro.2018.03.162 [DOI] [Google Scholar]
- Stanley, P.L., Rowntree J.E., Beede D.K., DeLonge M.S., and Hamm M.W.. 2018. Impacts of soil carbon sequestration on life cycle greenhouse gas emissions in Midwestern USA beef finishing systems. Agric. Syst. 162:249–258. doi: 10.1016/j.agsy.2018.02.003 [DOI] [Google Scholar]
- Stefenoni, H.A., Räisänen S.E., Cueva S.F., Wasson D.E., Lage C.F.A., Melgar A., Fetter M.E., Smith P., Hennessy M., Vecchiarelli B., et al. 2021. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 104:4157–4173. doi: 10.3168/jds.2020-19686 [DOI] [PubMed] [Google Scholar]
- Teague, W.R., Apfelbaum S., Lal R., Kreuter U.P., Rowntree J., and Davies C.A.. 2016. The role of ruminants in reducing agriculture’s carbon footprint in North America. J. Soil Water Conserv. 71:156–164. doi: 10.2489/jswc.71.2.156 [DOI] [Google Scholar]
- Tedeschi, L.O. 2019. ASN-ASAS SYMPOSIUM: FUTURE OF DATA ANALYTICS IN NUTRITION: mathematical modeling in ruminant nutrition: approaches and paradigms, extant models, and thoughts for upcoming predictive analytics. J. Anim. Sci. 97:1921–1944. doi: 10.1093/jas/skz092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L.O., and Fox D.G.. 2020. The Ruminant Nutrition System Volume I. An applied model for predicting nutrient requirement and feed utilization in ruminants. 3rd ed.Ann Arbor (MI):XanEdu. Available from http://www.xanedu.com/catalog-product-details/ruminant-nutrition-system-volume-1 [accessed June 17, 2021]. [Google Scholar]
- Tedeschi, L.O., Muir J.P., Naumann H.D., Norris A.B., Ramírez-Restrepo C.A., and Mertens-Talcott S.U.. 2021. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 8:628445. doi: 10.3389/fvets.2021.628445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillard, F., Maia de Souza D., Thoma G., Gerber P.J., and Finn J.A.. 2016. What does Life-Cycle Assessment of agricultural products need for more meaningful inclusion of biodiversity? J. Appl. Ecol. 53:1422–1429. doi: 10.1111/1365-2664.12683 [DOI] [Google Scholar]
- Thoma, G., J. Popp, D. Shonnard, D. Nutter, M. Matlock, R. Ulrich, W. Kellogg, D. S. Kim, Z. Neiderman, N. Kemper, et al. 2013. Regional analysis of greenhouse gas emissions from USA dairy farms: A cradle to farm-gate assessment of the American Dairy industry circa 2008. Int. Dairy J. 31:S29–S40. doi: 10.1016/j.idairyj.2012.09.010 [DOI] [Google Scholar]
- Thompson, L.R., and Rowntree J.E.. 2020. INVITED REVIEW: Methane sources, quantification, and mitigation in grazing beef systems. Appl. Anim. Sci. 36:556–573. doi: 10.15232/aas.2019-01951 [DOI] [Google Scholar]
- Uddin, M.E., Larson R.A., and Wattiaux M.A.. 2020. Effects of dairy cow breed and dietary forage on greenhouse gas emissions from manure during storage and after field application. J. Clean. Prod. 270:122461. doi: 10.1016/j.jclepro.2020.122461 [DOI] [Google Scholar]
- Van Amburgh, M.E., Russomanno K.L., Higgs R.A., and Chase L.E.. 2019. Invited Review: Modifications to the Cornell Net Carbohydrate and Protein System related to environmental issues—capability to evaluate nitrogen and phosphorus excretion and enteric carbon dioxide and methane emissions at the animal level. Appl. Anim. Sci. 35:101–113. doi: 10.15232/aas.2018-01783 [DOI] [Google Scholar]
- Villalba, J.J., Beauchemin K.A., Gregorini P., and MacAdam J.W.. 2019. Pasture chemoscapes and their ecological services. Transl. Anim. Sci. 3:829–841. doi: 10.1093/tas/txz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiaux, M.A., Uddin M.E., Letelier P., Jackson R.D., and Larson R.A.. 2019. Invited Review: Emission and mitigation of greenhouse gases from dairy farms: the cow, the manure, and the field. Appl. Anim. Sci. 35:238–254. doi: 10.15232/aas.2018-01803 [DOI] [Google Scholar]