Abstract
Adult Ixodes ricinus (Acari: Ixodidae) ticks collected near Ljubljana, Slovenia, were tested for the agent of human granulocytic ehrlichiosis (HGE) by using PCR assays based on the 16S rRNA gene. Three (3.2%) of 93 ticks were found to contain granulocytic ehrlichiae. Nucleotide sequences of portions of the bacterial groESL heat shock operon amplified from these ticks were identical or nearly (99.8%) identical to those previously determined for human patients with HGE from Slovenia, providing additional evidence that the ticks were infected with the HGE agent. This study identified I. ricinus as the likely vector for these ehrlichial pathogens of humans in this part of Europe.
In 1996, four cases of human granulocytic ehrlichiosis (HGE) were identified near Ljubljana, Slovenia (5, 8). All four patients reported exposure to ticks, and none had traveled outside of Slovenia within the putative incubation period. The aim of this study was to collect Ixodes ricinus ticks from the Ljubljana area to determine if they were infected with an ehrlichial species and, if so, to assess the DNA sequence homologies between amplicons derived from ticks with those obtained from three of the patients (one patient developed antibodies to the HGE agent, but no ehrlichial DNA was recovered). I. ricinus is the most abundant tick in central Slovenia, and this species typically comprises more than 98% of the ticks recovered during tick sampling (9, 11). In Slovenia, as in other European countries, I. ricinus is the main vector of the causative agents of Lyme borreliosis and tick-borne encephalitis (9, 11). In Europe, the tick I. ricinus is known as a vector of the agent causing tick-borne fever (Ehrlichia phagocytophila) (14).
In early summer of 1996, 101 unfed adult I. ricinus ticks were collected by flagging vegetation. The collection site was a woodland with clearings in which the major tree species were immature birch (Betula pendula), oak (Quercus cerris), hazel (Corylus avellana), and pine (Pinus silvestris) trees, and the undergrowth was predominantly fern (Pteridium aquilinum) and bilberry (Vaccinium myrtillus) shrubs. All ticks were collected at the same location near Ljubljana, and gender and species were identified by an entomologist. DNA was extracted by first digesting individual ticks overnight in TE buffer supplemented with detergent (1 mM EDTA, 1% Tween, 1% Nonidet P-40) and proteinase K (2,500 μg/ml), followed by phenol-chloroform-isoamyl alcohol extraction and ethanol precipitation. To confirm that the DNA extraction was successful, PCR assays were conducted as previously described to amplify 16S mitochondrial rRNA genes (rDNA) of tick origin (3).
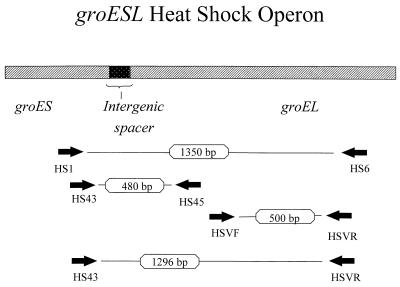
To examine for ehrlichial DNA, each sample was tested with separate primer pairs specific for the 16S rDNAs of E. chaffeensis and the E. phagocytophila genogroup (1, 2, 6). For amplification of the groESL region of the DNA extracted from ticks, primers HS1 and HS6 were used in the primary PCRs, and 1-μl aliquots of the first reactions were used as templates for nested reactions with primers HS43 and HSVR (Fig. 1) (5, 10). The groESL PCR would also amplify the homologous region of the E. chaffeensis groESL operon if it were present in the extracted DNA. Amplification of portions of the groESL operon from the blood of three of the patients with HGE has been previously described (5).
FIG. 1.
Diagram indicating PCR primer positions and amplicon sizes. Primers HS1 and HS6 were used in primary PCRs, and amplicons produced in nested PCRs with the indicated primers were sequenced. The nucleotide sequence of the 1,256-bp region flanked by primers HS43 and HSVR was obtained from five of six samples, two of three human patients, and three I. ricinus ticks. Sequences of the regions flanked by primers HS43 and HS45 (442 bp) and primers HSVF and HSVR (395 bp) were obtained from the remaining human patient. The overapping sequences from five samples were identical in size. The sequence from one of the female ticks contained three single nucleotide substitutions: A to G at position 51, G to A at position 450, and T to C at position 660. The sequences were numbered by designating the A of the groEL translation initiation codon nucleotide 1. The nucleotide substitutions did not alter the deduced amino acid sequence.
Among 101 adult I. ricinus ticks, there were 55 females and 46 males. Mitochondrial 16S rDNA of tick origin was successfully amplified in 92.1% of the samples. Of the 93 ticks from which tick DNA was successfully extracted, 3 (1 male and 2 female ticks) were positive when tested with primers (Ehr 521 and Ehr 790) specific for the 16S rDNA of the E. phagocytophila genogroup. All three of these ticks also yielded amplicons of the expected length (1,293 bp) for granulocytic Ehrlichia spp. when tested by the nested groESL PCR (Fig. 1). Negative PCR results were obtained for all of the ticks with primers (HE1 and HE3) specific for the E. chaffeensis 16S rDNA. In this report, we use the terms granulocytic Ehrlichia spp. and granulocytic ehrlichiosis in reference to E. phagocytophila sensu lato.
Sequencing of the PCR products obtained by the HS43 and HSVR primers permitted us to examine a 1,256-bp region (when primer sequences were removed) of the groESL operon that has been known to exhibit variation among strains of granulocytic ehrlichiae (10). Two of the tick sequences were identical to each other and to the corresponding 1,256-bp sequences previously amplified from one of the Slovenian HGE patients (GenBank accession no. AF033101) from which ehrlichial DNA was obtained. The sequence from the third tick (female) differed from the other groESL sequences at three nucleotide positions (99.8% homology). These differences occurred in the groEL coding sequence and did not alter the deduced amino acid sequence. Two smaller regions of the groESL operon that overlap the 1,256-bp region defined by primers HS43 and HSVR were previously amplified from the other two Slovenian HGE patients (5) from which ehrlichial DNA was obtained. These regions are flanked by primers HS43 and HS45 (442 bp) and primers HSVF and HSVR (395 bp) (Fig. 1) (5, 10). The full 1,256-bp sequence was amplified and sequenced for one of these patients during this study and was found to be identical to the 1,256-bp sequence from the first patient (GenBank accession no. AF033101). We did not have a sufficient sample to obtain the full 1,256-bp sequence from the remaining patient. However, the sequences previously amplified from this patient were identical to the overlapping regions of the 1,256-bp sequence (GenBank accession no. AF033101) from the other patients. The groESL sequences from the Slovenian patients and ticks were more similar to sequences previously obtained from European strains of E. phagocytophila than to groESL sequences amplified from E. equi and North American strains of the HGE agent (5, 10).
Researchers from Scotland, Italy, Sweden, and France have reported detection of granulocytic Ehrlichia spp. in I. ricinus ticks by means of electron microscopy and PCR assays (4, 7, 12, 13). The prevalence of infection of I. ricinus ticks by granulocytic Ehrlichia detected by our methods in Slovenia was much lower (3.2%) than the 24.4% prevalence found in ticks collected from Italy (4) but indistinguishable from the prevalence (3.1%) reported from the eastern coast of Sweden (12). It should be noted that we tested only adult stages of I. ricinus, while the cited studies reported results from nymphal ticks (except reference 7).
To our knowledge, this is the first study in which ehrlichial DNA sequences derived from ticks and HGE patients from the same location in Europe were compared. The identity of these ehrlichial sequences implicate I. ricinus as a vector of E. phagocytophila, or closely related agents, in Slovenia. The public health and biologic significance of variation among ehrlichial groESL sequences amplified from ticks and patients cannot be determined at this time. Further studies in Slovenia will assess the prevalence of ehrlichial infection among I. ricinus ticks in various life stages and identify the maintenance cycle in reservoir and vector species that may ultimately lead to human infection.
Acknowledgments
Financial support for this research was provided in part by grant L3-7916-381-95 from the Ministry of Science and Technology, Republic of Slovenia.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sumner J W, Dawson J E, Tzianabos T, Greene C R, Olson J G, Fishbein D B, Olsen-Rasmussen M, Holloway B P, George E H, Azad A F. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black W C, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodidae) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinco M, Padovan D, Murgia R, Maroli M, Frusteri L, Heldtaner M, Johansson K E, Olsson-Engvall E. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J Clin Microbiol. 1997;35:3365–3366. doi: 10.1128/jcm.35.12.3365-3366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotric Furlan S, Petrovec M, Avsic Zupanc T, Nicholson W L, Sumner J W, Childs J E, Strle F. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings in four patients from Slovenia. Clin Infect Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- 6.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Jr, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 7.Parola P, Beati L, Cambon M, Brouqui P, Raoult D. Ehrlichial DNA amplified from Ixodes ricinus (Acari: Ixodidae) in France. J Med Entomol. 1998;35:180–183. doi: 10.1093/jmedent/35.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Petrovec M, Lotric Furlan S, Avsic Zupanc T, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strle F, Cheng Y, Nelson J A, Picken M M, Bouseman J K, Picken R N. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto in Slovenia. Eur J Clin Microbiol Infect Dis. 1995;14:994–1001. doi: 10.1007/BF01691382. [DOI] [PubMed] [Google Scholar]
- 10.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovornik D. Natural focuses of tick-borne encephalitis in Slovenia. In: Lešničar J, editor. Symposium of Tick-Borne Encephalitis. Celje, Slovenia: Slovenian Medical Society; 1973. pp. 23–29. [Google Scholar]
- 12.von Stedingk L V, Gurtelschmid M, Hanson H S, Gustafson R, Dotevall L, Engvall E O, Granstrom M. The human granulocytic ehrlichiosis (HGE) agent in Swedish ticks. Clin Microbiol Infect. 1997;3:573–574. doi: 10.1111/j.1469-0691.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 13.Webster K A, Mitchell G B B. An electron microscopic study of Cytoecetes phagocytophila infection in Ixodes ricinus. Res Vet Sci. 1989;47:30–33. [PubMed] [Google Scholar]
- 14.Woldehiwet Z. Tick-borne fever: a review. Vet Res Commun. 1983;6:163–175. doi: 10.1007/BF02214910. [DOI] [PubMed] [Google Scholar]