Abstract
The body composition phenotype of an athlete displays the complex interaction among genotype, physiological and metabolic demands of a sport, diet, and physical training. Observational studies dominate the literature and describe the sport-specific physique characteristics (size, shape, and composition) of adult athletes by gender and levels of competition. Limited data reveal how body composition measurements can benefit an athlete. Thus, the objective is to identify purposeful measurements of body composition, notably fat and lean muscle masses, and determine their impact on the health and performance of athletes. Areas of interest include relationships among total and regional body composition measurements, muscle function, sport-specific performance, risk of injury, return to sport after injury, and identification of activity-induced fluid shifts. Discussion includes the application of specific uses of dual x-ray absorptiometry and bioelectrical impedance including an emphasis on the need to minimize measurement errors and standardize protocols, and highlights opportunities for future research. This focus on functional body composition can benefit the health and optimize the performance of an athlete.
Keywords: body fat, fat-free mass, lean soft tissue mass, muscle, performance, injury, muscle asymmetry, hydration, dual x-ray absorptiometry, bioimpedance, return to sport
Introduction
Interest in athletes’ body composition has flourished for at least 70 years with peer-reviewed publications exceeding 12,000 in the multi-disciplinary field of exercise science [1]. These reports catalog diverse phenotypes of athletes, including body weight, size, shape, body fat percentage (%fat), fat mass (FM), fat-free mass (FFM), lean soft tissue mass (LSTM), and muscle mass, by sport, gender, and competitive level. The initial focus of these studies was %fat [2], but the research scope has broadened to stress evaluating athletes’ total and regional skeletal muscle mass, measured historically as FFM and more recently as LSTM, particularly as it relates to improving athletes’ sport performance and minimizing injury risk [3].
Purposeful body composition assessment necessitates a focused and solution-oriented application to benefit the individual athlete [4]. This innovative approach transcends the basic description of compositional measurements and emphasizes the identification of total and regional (e.g., arms, legs, trunk) body compositional characteristics associated with sport-specific performance, increased injury risk, return to sport (RTS) after injury, and training progression [5,6]. When evaluated in conjunction with strength and force measurements and sport-specific outcomes, functional body composition assessment enables the applied use of compositional measurements to aid an athlete in preparation for competition. This review summarizes newer and novel body composition assessment techniques more commonly employed in the sport performance setting, the role of body composition on physical performance, risk of injury and safe RTS after injury, classification of sport performance level, and practical monitoring of fluid balance in response to training and competition. It also highlights limitations in body composition assessment across the literature and opportunities for future research on body composition assessment in sport.
Applications of Body Composition Assessment in Sport
Body composition
Athletes and coaches are aware that skeletal muscle mass and body fat are related to competitive performance [3]. Skeletal muscle mass, historically measured as FFM and more recently measured as LSTM, represents functional mass and positively contributes to strength and force production, thus improving sport performance [7]. Conversely, FM is considered non-functional mass, with increasing amounts of FM mechanically and metabolically hindering sport performance [8] and adversely affecting thermoregulation [9].
The terms FFM and LSTM are often used interchangeably, but their meaning is different [10]. Fat-free mass refers to total body mass minus FM and therefore includes quantification of bone and skeletal muscle, organs, and connective tissue [10]. FFM is measured using assessment methods which divide the body into two components, namely FM and FFM. These methods include anthropometry (circumferences and skinfold thickness), total-body densitometry (underwater weighing, air displacement plethysmography), isotope dilution, and bioelectrical impedance analysis (BIA). Recent advancements in body composition technology allow for dividing the body into three components, namely LSTM, FM, and bone. Simply stated, LSTM is FFM minus bone mineral content, with total body water, total body protein, carbohydrates, non-fat lipids, and soft tissue minerals also included in LSTM measurements [10]. As such, LSTM is considered a proxy for skeletal muscle mass. Three-component body composition models include dual x-ray absorptiometry (DXA), magnetic resonance imaging (MRI), computerized tomography (CT), and ultrasound (US) imaging. Although CT, MRI, and ultrasound provide information pertaining to muscle cross-sectional area (CSA) and muscle volume (MV), these methods are costly, require high investigator expertise, and results may be difficult to analyze; thus, these methods do not offer practicality in the sport performance setting [11]. As such, the most widely used three-component model for measuring athletes’ body composition is DXA due to its quick and non-invasive assessments and, perhaps more importantly, its accuracy and reliability [11,12] particularly when compared to measurements obtained using the gold standard four-component body composition model [4,13]. The more recent use of DXA in the sport performance setting [14] provides sport practitioners immediate information regarding athletes’ total and regional body composition measurements, useful for tracking changes over time in LSTM and FM, particularly in association with strength and sport performance measures.
Total and regional lean soft tissue mass (LSTM)
Widespread availability and utility of DXA in the sport performance environment stimulated the growth of body composition assessment, notably LSTM, among various groups of athletes (14). One key outcome is the progress in understanding the importance of regional body composition in sport performance and the potential to identify injury risk.
Numerous reports describe the body composition of professional and collegiate male and female athletes by position within the same sport [15–26]. They indicate minimal within-sport variability in total and regional body composition in some sports such as men’s hockey [18], women’s softball [20], and soccer [27] with marked differences by position in other sports, including American football and basketball [7,15–17,21,22,25]. Some important findings emerge from the preceding investigations. In American football, “mirroring” positions, such as opposing offensive and defensive positions, demonstrate similar total and regional LSTM measurements, while football athletes in other “non-mirroring” positions have significant regional LSTM and FM differences [15,16]. In collegiate male basketball athletes, centers demonstrate the highest arm and leg LSTM and FM compared to all other positions except power forwards [21]. These observations suggest there may be a relationship between measures of total and regional body composition and sport- and position- specific demands (i.e., kicking, shooting, speed). Future study is warranted to investigate this potential relationship.
Few reports, however, describe longitudinal DXA changes in athletes’ total and regional body composition during training over one or more competitive seasons [27–35]. The trend is a decrease in fat with a related increase in total body and regional LSTM in sports such as hockey and soccer, among others. For example, Prokop et al. [36] found a decrease in total %fat from pre- to mid- season with an increase in leg LSTM among elite hockey players. Additionally, elite soccer players have been reported to display increased body mass (BM) with 60% of this increase attributable to a LSTM gain in the trunk and legs [30,33]. These studies suggest that although total body composition changes are important to monitor over time, the region of the body in which these changes occur may be more informative, particularly for developing effective sport- and position- specific training programs. Future investigation is warranted to evaluate how changes over time in total and regional body composition affect sport- and position- specific performance outcomes.
Growing awareness of sport- and position- specific regional body composition augments interest in the distribution of LSTM and its significance in sports [7]. Lean mass distribution can be expressed as: (a) upper- to lower- body (U/L) ratios, compared across positions and athletes in the same position; and (b) contralateral asymmetries, informative for optimizing performance and minimizing injury [11]. Some examples of regional composition ratios, calculated from LSTM measurements obtained using DXA, are available for a handful of sports, including American football [15,16], baseball and softball [19,20], men’s and women’s basketball [21], men’s and women’s track and field [22], and equestrian [24]. Among professional American football players, the U/L of total body mass is consistent across positions, but the U/L LSTM differs by position [16]. Specifically, offensive and defensive linemen have demonstrated the lowest U/L LSTM ratios, indicating higher relative lower-body LSTM, while quarterbacks and punters/kickers have exhibited the highest U/L LSTM ratio [16]. Collegiate female basketball athletes also demonstrate ratio differences across positions [21]. The U/L LSTM ratio is highest in point guards and lowest in power forwards and centers. Therefore, LSTM distribution may be more critical than total LSTM evaluation for performance prediction. These data are also aligned with biomechanical principles that involve jumping and change of direction. In most sports, the lower-body supports the upper-body during movement. Thus, upper-body mass affects the amount of lower-body force production (e.g., higher leg LSTM) needed to counteract upper-body momentum to change direction [11], with lower LSTM amounts and increased body fat detrimental to athletes in weight-supported sports. Ratios can therefore provide insight into modifying training programs to optimize athletes’ biomechanics, and thus performance, according to positional needs. Research is warranted to examine how regional composition ratios impact sport- and position- specific performance.
In summary, these findings suggest total-body assessments of LSTM are related to sport- and position- specific performance. Importantly, however, these observations also highlight that regional body composition estimates can play specific beneficial roles in biomechanical aspects and power characteristics that differentiate performance levels in many sports.
Bioelectrical impedance vector analysis (BIVA): Qualitative body composition and performance assessment
Bioelectrical impedance analysis [BIA] is a safe and practical method for body composition assessment of athletes [37]. BIA provides direct measurements that allow non-invasive assessment of soft tissue hydration and cell membrane integrity. It measures the impedance (Z) or hindrance of an applied, 50 kHz alternating current that is related to water and electrolytes in body fluids and tissues, in addition to the delay of current entry into cells and across adjacent tissues. Impedance has two components: resistance (R) and reactance (Xc). Resistance is the opposition of the flow of alternating current by intra- and extra-cellular (ICF and ECF) fluid. Reactance refers to the delay in current flow by cell membranes and tissue interfaces. Phase angle (PhA), which is the arc tan of Xc/R, is a composite indicator of ICF and ECF distribution and cell mass. A common application of BIA in sport is the estimation of total body water (TBW), ECF, and FFM using multiple regression equations that include BIA measurements, BM, height (H), age, and gender [37]. The assumptions of constant hydration of FFM, constant body geometry, and inter-correlation among the independent variables contribute to the high variability in estimation of TBW and FFM (3 to 8%) for an individual that precludes the identification of important changes in sport [38–41].
Bioelectrical impedance spectroscopy (BIS) uses a range of frequencies (4 to 1000 kHz) to estimate Z and PhA, from which Xc is calculated, then used in non-linear mathematical models to estimate intra- and extra-cellular resistance values. These values are included in a mixture theory model to estimate fluid volumes [42]. Current application of BIS in sport includes prediction of TBW for inclusion in multi-component models to estimate body composition of adults [43]. An initial use of BIS is assessment of fluid changes associated with glycogen loading and depletion, and exercise-induced muscle damage [44–46].
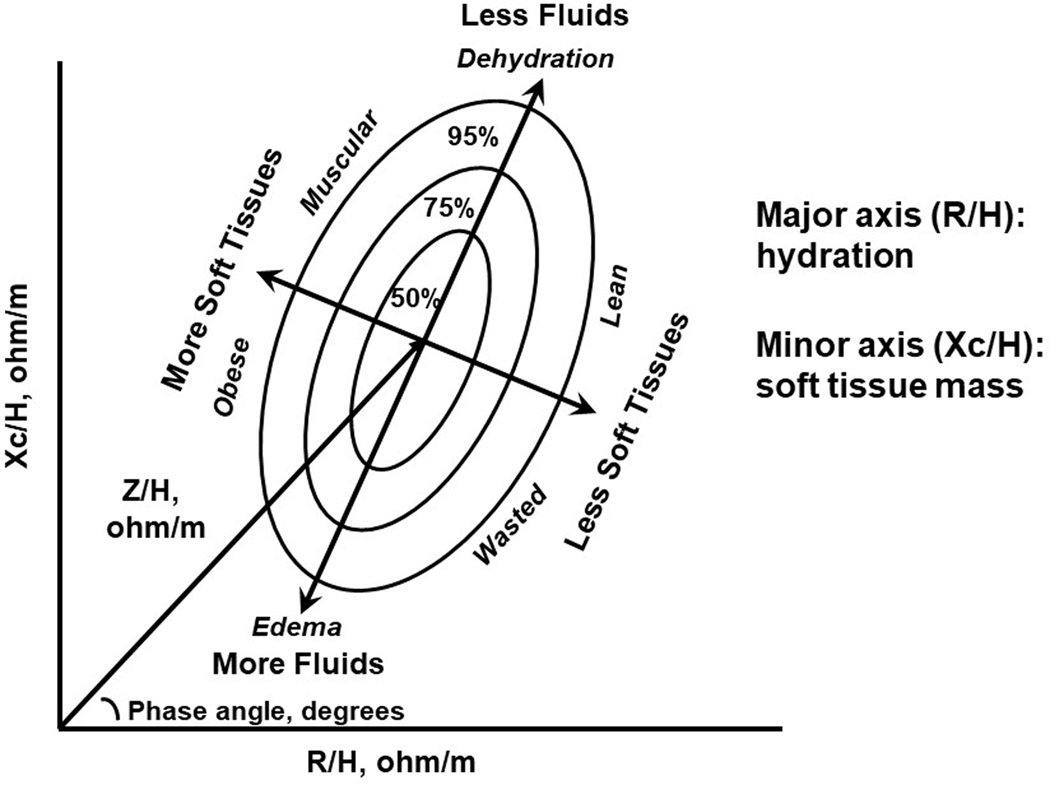
A promising use of BIA in sport is performing bioelectrical impedance vector analysis (BIVA). BIVA illustrates the relationships among 50 kHz, phase-sensitive R and Xc measurements, normalized for height and plotted on a RXc graph, and shows an impedance (Z) vector (Figure 1). Length of the vector (Z/H) is inversely related to TBW, and its position on the RXc graph that is depicted as the PhA, which indicates fluid distribution (ECF/ICF) [47]. Individual or group vectors are compared to the distribution of vectors from healthy, gender-matched individuals (50, 75 and 95% distribution) to enable classification of hydration (hypo-, normal and over-hydration) and cell mass (muscular, obese or lean, depleted). Although developed to identify fluid overload and malnutrition in clinical patients [47,48], BIVA can provide novel information for use in sport.
Figure 1.
Illustration of an RXc plot of healthy adult male Caucasians for bioelectrical impedance vector analysis to classify and track status of soft tissue and fluids. R/H = resistance/height, Xc/H = reactance/height, Z/H = impedance/height. Adapted from Lukaski and Piccoli [47].
Sport phenotype
Metabolic and physical demands of different sports shift the vectors of athletes to the upper left quadrant compared to age- and gender- matched non-athletes [49]. Endurance, compared to power, athletes exhibit a longer mean vector, indicative of less TBW, and smaller PhA suggestive of lower ECF/ICF, hence less LSTM [50–57]. Mean vector position can discriminate performance levels within a sport. Elite athletes have greater PhA values (decreased ECF/ICF) with greater Xc/H (more muscle) and lesser R/H (greater TBW) than inexperienced and less successful athletes [52–54]. Thus, elite athletes tend to have higher LSTM, ICF, and TBW levels. Therefore, BIVA provides a “target” zone of body composition for elite performance in a sport.
Observational studies reveal the vectors of elite athletes were outside of the 50% reference ellipse or the normal range of hydration of the general population [50–60]. Thus, sport-specific RXc plots and tolerance ranges to classify hydration are needed for athletes.
Muscle quality, strength and power
PhA is an indirect measure of ECF/ICF and related to measures of muscular strength [61–63]. Resistance training significantly increased (5 to 8%) total-body PhA independent of gender and smaller increases in FFM (2%) [64–67]. Conversely, detraining significantly decreased PhA (−8%) and FFM (−2%) [65]. The training-induced increase in PhA was attributed to a significant increase in Xc and a measured gain in ICW with a smaller decrease in R and increased TBW. Training-induced improvements in strength, jump height and LSTM among elite athletes are significantly related to increased ICF [68] and generally independent of LSTM [69,70]. The increase in PhA can be explained by an increase in Xc associated with increased ICF and hence muscle cell volume. Thus, BIA measurements are more sensitive in detection of training-induced improvements in muscle size and function than LSTM estimates.
BIVA and acute and chronic hydration changes
BIVA is sensitive to monitor small changes (<500 mL) in fluid volumes [47] and can be a practical monitor of fluid changes in sport. For example, a bout of prolonged exercise in the heat has been shown to decrease BM (2%), significantly increase plasma osmolarity, R/H and Xc/H, and lengthen the impedance vector (4%) [71]. The change in Xc/H was significantly related to the change in plasma osmolarity, an indicator of hypohydration, indicating a loss of ECF. An intense swim training session elicited similar changes in BIA measurements and reduction in BM [72]. The impedance vector lengthened significantly with 5 kg loss in BM after completion of a marathon, then shortened significantly after 48 hours of recovery with a 4 kg gain in BM [73]. These BIVA findings are consistent with fluid loss reported during the long-distance race and replacement of lost fluids during the recovery phase, with corresponding significant increases in R/H and Xc/H after the race, subsequently followed by significant decreases 48 hours after the race. Interestingly, significant predictors of race time were pre-race R/H and Xc/H, changes in R/H and Xc/H post-race, and 48-hour recovery values. Thus, runners with better fluid status (e.g., lower R/H and Xc/H) had faster marathon times.
BIVA also identifies altered fluid distribution predictive of injury risk [74]. Marathon runners at risk of acute kidney injury (AKI) had significantly elevated markers of muscle damage and inflammation with significantly shortened vectors and reduced Xc/H indicating expansion of ECF 48-hours after the race. Runners with Xc/H ≤30.5 ohm predicted AKI stage 1 with 85.5% sensitivity and 91.7% specificity. These findings highlight the potential value of BIVA to detect fluid imbalances associated with inflammation concurrent with muscle damage after prolonged endurance exercise.
Additionally, BIVA allows for tracking serial training-dependent changes in fluid distribution. Vector length shortened significantly during pre-season training then Xc/H and PhA increased significantly at mid-season among male professional soccer players [75]. Investigators speculated that the significant decreases in vector length indicated an increase in TBW and an increase in Xc associated with increased ICW due to glycogen accumulation in muscle. Impedance vectors also shortened significantly among cyclists throughout a 21-d road race along with self-reports of increased fatigue and decreased estimates of power output during heavy mountain climbs and an end-of-race time trial [76]. Researchers surmised that inflammation and muscle edema were causes of fluid accumulation and hence vector shortening. Similar findings were reported in another 21-d road race in which whole-body PhA consecutively decreased with greater decreases in leg PhA [77], consistent with increased ECF/ICF in the legs but with no change in BM. Follow-up studies using tracer dilution methods are needed to confirm the apparent fluid changes and to validate these interpretations.
Implementation of BIA measurements and BIVA: cautions and limitations
BIA measurements and BIVA are suitable for use in the laboratory or the field. However, water and electrolyte levels affect BIA values, so it is imperative to standardize conditions and measurement protocols to ensure valid estimates of body composition and assessments of acute changes in fluid distribution and to monitor recovery [78]. Specifically, to obtain control or baseline values, athletes should avoid exercise (>24 h) before testing to eliminate the influence of muscle damage or injury, elevated body and skin temperatures, diaphoresis, and sweat and skin electrolyte accumulation. Technical requirements include the use of appropriate electrodes and limb position, a non-conductive measurement surface, and a phase-sensitive impedance device. Care is required to attain normal body and skin temperature and clean electrode sites by removal of sweat and electrolyte build up on the skin with alcohol wipes as sites of electrode placement. A brief, cool water whole-body shower is a means to achieve these ideal conditions. Alternatively, reclining supine in a cool environment and cleaning the skin enables valid measurements in field conditions.
Preliminary data suggest that muscle glycogen loading and depletion also may affect BIA measurements [44,45]. However, these initial observations require confirmation, with additional data on the variability of BIA measurements associated with graded levels of voluntary food and fluid intake with details on timing of food and beverage intake to ensure proper hydration and muscle glycogen content.
Certain assumptions limit the application of BIA in sport. Translation of BIA measurements to fluid volumes and LSTM relies on the two-component model that assumes a constant hydration of the fat-free body (73.2%), although the normal range of hydration is 68 to 75% [79]. Adipose tissue contains ~20% water, thus differences in adipose tissue can contribute to errors in prediction of LSTM [36]. The physical basis for BIA is Ohm’s Law, which states the volume of a conductor depends on the length and cross-sectional area of a cylindrical conductor with constant geometry and composition (specific resistivity). The human body, however, consists of five cylinders (arms, legs and trunk) with different lengths and cross-sectional areas. Thus, BIA measurements have random error due to inter-individual differences in body size, geometry, and composition, resulting in non-uniform conductivity [37]. Although standardizing Z, R, and Xc with height is an initial remedy, the addition of regional anthropometric measurements to BIA measurements might accommodate inter-individual differences in specific-resistivity and improve the sensitivity of BIA and BIVA in sport.
Interpretation of BIA and BIVA findings in sport: consensus
The majority of studies using BIVA in sport report BIA measurements and postulate fluid changes. Reference tracer dilution estimates of fluid volumes are needed to validate the interpretations. Recent reports confirm some significant associations among BIA measurements and reference determinations of fluid volumes in female and male athletes over a competitive season [59]. Vector lengths shortened (p<0.01) and were correlated (p<0.01) with increases in TBW and ICW (r = −0.718 and r = −0.630, respectively) and decreases (p<0.05) in ECW/ICW (r=0.344) after adjusting for age, height, and sex. PhA changes were associated (p<0.01) with TBW and ICW (r=0.458 and r=0.564, respectively) and negatively (p<0.01) related to ECW/ICW (r=−0.436). PhA increased (p<0.01) in the male but not the female athletes in whom TBW decreased. Although these findings are reassuring, future research should use tracer dilution methods and verify interpretations of BIA and BIVA in studies examining fluid alterations in response to different types of training.
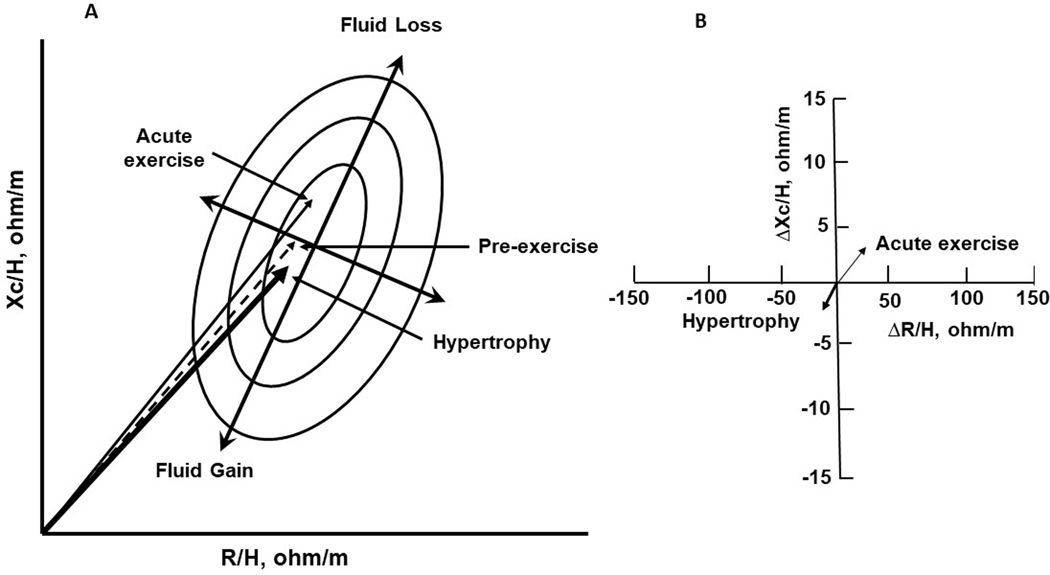
Figure 2 illustrates general patterns of impedance vectors and BIA measurements in response to acute exercise and resistance training. Compared to the pre-exercise vector, acute exercise lengthens the impedance vector and increases R/H, Xc/H, and PhA, suggesting a decrease in ECF and TBW with increased cell density. In contrast, muscle hypertrophy, which is a chronic response, shortens the impedance vector and decreases R/H, Xc/H, and PhA, indicating an increase in ICF and TBW and gain in muscle cell volume. Some key points should be emphasized. Exercise-induced changes in BIA measurements are dynamic, coordinated, and occur concurrently. Importantly, interpretation of PhA must consider hydration status to achieve an understanding of fluid status (ICF compared to ECF). Also, the proportional changes in ECF and ICF are greater than the changes in TBW or BM. Thus, measurements of Xc and R represent key indicators of fluid responses in sport.
Figure 2.
General description of changes in bioelectrical vector positions (A) and bioelectrical impedance measurements (B) in response to acute bout of exercise and muscle hypertrophy after resistance training.
Muscle asymmetry and risk of injury
Lean mass and muscle function
Whereas total and regional body composition describes the physique of an athlete, the relationship between LSTM and muscle-specific and explosive (time- restricted force production during a ballistic task) strength is a significant performance attribute. Combined LSTM measurement and purposeful strength assessments provide information pertaining to athlete’s relative muscle functionality (strength produced per unit lean mass). Many investigations, however, have primarily reported LSTM and strength measurements separately in healthy and previously injured athletes, with fewer studies [80–83,90] evaluating the relationship between these measures. Investigation of the LSTM-strength relationship may be informative to: (a) assess relative muscle functionality in response to training and rehabilitation programs; (b) identify large asymmetries that may limit performance and increase injury risk; and (c) evaluate readiness to RTS following rehabilitation [11,81,83,90].
Open kinetic chain assessments are generally employed to assess muscle strength, most frequently using isokinetic dynamometry, particularly within the rehabilitation setting [84]. Isokinetic testing allows for determination of maximal strength (normalized peak torque, Nm/kg) of specific muscle groups (e.g., quadriceps, hamstrings) at a constant velocity in a given plane of motion (e.g., knee extension/flexion). Thus, this method provides an assessment of contralateral and ipsilateral dynamic strength ratios, and thus identification of asymmetry of specific muscle groups. However, isokinetic testing lacks translation to sport performance characteristic of closed kinetic chain tests, such as vertical jumping (i.e., countermovement [CMJ] and squat [SJ] jumps), which incorporate movements across a full range of motion. Recent widespread availability of, and advancements in, force platform technology provide trainers with immediate information of force and power production and rate of force development for each leg [11]. Although less common, athletes’ muscle function, measured using the preceding methods, has also been examined in combination with LSTM.
It is well known that skeletal muscle CSA and MV, evaluated using CT, MRI, and ultrasound, are strong determinants of muscle strength [85,86]. To examine skeletal muscle in relation to strength/force, researchers have largely evaluated the relationship between either: (a) muscle CSA or MV and isokinetic/isometric strength; or (b) standard frontal view DXA-measured LSTM and force/power produced during vertical jumping or anaerobic testing. Studies evaluating elite soccer players [80,82] reported non-significant correlations between ratios of quadriceps/hamstring CSA and isokinetic knee extensor/flexor strength (r= - 0.33 to 0.28). Other studies found significant, albeit moderate, correlations between quadriceps and hamstring CSA (examined separately) and isokinetic extensor (r=0.54 to 0.60) and flexor (r=0.54 to 0.64) peak torque, respectively [86]. More recently, investigators have used DXA to quantify total-body and lower extremity LSTM, reporting a positive association between LSTM and jump height (r=0.73) and power (r=0.72 to 0.83) during the CMJ and SJ in healthy individuals [81,87,88]. Chiarlitti et al. [18] also noted significant relationships between DXA-measured lower-body LSTM and maximal power during the Wingate anaerobic test and long jump (r=0.54 and 51, respectively) among elite hockey players. Yet, as mentioned, while CT, MRI, and ultrasound can provide muscle cross-sectional area and volume measurements, the use of DXA is becoming more common and may be more advantageous for evaluating skeletal muscle mass due to its widespread availability, quick and non-invasive assessments, and its ability to provide total and regional body composition assessments.
A recent methodological advancement and alternative to the standard frontal DXA scan method is the lateral view scan method that recently demonstrated accuracy and reliability in quantifying upper-leg compartmental (anterior/posterior) LSTM using the GE Lunar iDXA [89]. Moderate to strong correlations were reported between collegiate athletes’ anterior and posterior upper-leg compartmental LSTM and isokinetic extensor (r=0.56 to 0.72) and flexor (r=0.53 to 0.84) peak torque, respectively, with stronger correlations reported at 60°/s, and SJ-measured peak force, rate of force development, and jump height (r=0.58 to 0.95) [90]. Thus, the combination of muscle-specific and explosive strength assessments and the lateral DXA scan measurements may offer unique advantages compared to standard frontal view DXA scan evaluations. Specifically, this scan method may allow for identifying location-specific ipsilateral upper-leg (quadriceps, hamstring) LSTM asymmetries that contribute to an understanding of the role asymmetries may play in negatively impacting function and sport performance and increasing injury risk. Importantly, this lateral scan method demonstrated accuracy on the GE Lunar iDXA but not on the Hologic Horizon [91]. Divergent findings are likely due to differences in scanner geometry (i.e., fan beam vs. narrow fan beam); scanner software and capabilities in creating manual regions of interest; and the manufacturers’ differing proprietary algorithms to quantify composition. Longitudinal investigations are warranted to ascertain the utility of this lateral scan method to examine changes in upper-leg compartmental LSTM in relation to muscle function in healthy and previously injured athletes. Additionally, investigation is warranted to evaluate the feasibility of using the lateral scan method to examine upper extremity compartmental composition. These assessments may provide insight into structural etiological factors, such as regional asymmetries, that may contribute to decreased performance and increased injury risk.
Effects of LSTM asymmetries and function on performance
Technical descriptions of asymmetries differ across studies. Contralateral limb asymmetries can be characterized as weaker vs. stronger [92], dominant vs. non-dominant [84], right vs. left [81], and injured vs. non-injured [83,93]. Ipsilateral asymmetries are designated as between antagonist muscle groups of the same limb (e.g., quadriceps vs. hamstrings) [84,94]. Some degree of asymmetry is considered normal and results from limb dominance (i.e., laterality) and sport-specific training (e.g., kicking vs. non-kicking leg strength, throwing vs. non-throwing arm LSTM) [19,95,96]. Strength, force, and power asymmetries >10% have been associated with a negative impact on performance measures (e.g., decreased vertical jump height and change of direction speed) [81,96–98], although equivocal findings have been noted [99]. However, a threshold of LSTM asymmetry that negatively impacts performance is still largely unknown.
There are limited data examining the relationship between contralateral LSTM asymmetries and function on performance. In non-injured collegiate athletes, Bell et al. [81] evaluated asymmetries with the limb symmetry index (LSI) calculated as LSI=[(right–left)/0.5(right+left)x100%] and found that contralateral lower extremity LSTM asymmetry accounted for 20% and 25% of the variance in CMJ-measured force and power asymmetries, respectively. Additionally, power asymmetries >10%, likely partially due to LSTM asymmetry, resulted in a decreased jump height of 9 cm (effect size = d >0.8). In Australian footballers, similarly calculated asymmetries between “kicking” and “support” limbs identified a 3% deficit in DXA-measured leg LSTM asymmetry that was associated with an 8% decrement in strength asymmetries and reduced kicking accuracy; greater symmetry was related to better kicking performance [95]. These data suggest that LSTM asymmetries may partially contribute to strength and power asymmetries and can, in turn, negatively impact performance-related outcomes. It is noteworthy, however, that Bell et al. [81] reported 95% of their athlete sample to demonstrate a large range of force (−11.8% to 16.8%) and power (−9.98% to 11.45%) asymmetries. Thus, additional research is needed to establish sensitive and specific thresholds at which asymmetries in strength, force, and LSTM negatively affect performance.
Asymmetries and injury risk
Measurement of regional LSTM, strength, and force asymmetries as predictors of injury risk and incidence is critical. Reducing the risk of primary injury (i.e., first) and secondary injury (i.e., re-injury of ipsilateral limb or injury of contralateral limb) and determining the optimal symmetry thresholds and timing for RTS clearance are major challenges in sports medicine [100,101]. The lower extremity is the most commonly reported injury site in athletes [102,103]. Anterior cruciate ligament (ACL) injury and localized muscle injuries, such as a hamstring strain injury (HSI), are among the most common injuries, particularly in sports requiring sprinting, jumping, and cutting (e.g., track, soccer, American football, basketball). Various modifiable and non-modifiable risk factors have been suggested to increase athletes’ primary and secondary ACL and HSI injury risk, including muscle strength asymmetries, dysfunctional neuromuscular activation, degree of joint laxity and flexibility, age, and gender, among others [104–106]. Specific to ACL injury, hamstring flexibility and decreased hamstring vs. quadriceps strength of the same leg (“quadriceps dominance”) are primary injury risk factors [107–109], while secondary injury risk factors include prior ACL injury, persistent quadriceps strength asymmetry and muscle atrophy (e.g., CSA deficits), and neuromuscular dysfunction [109–113].
Return to sport (RTS)
Strength and force are common indicators to evaluate progress during rehabilitation and to clear an athlete for RTS. However, despite RTS guidelines recommending sport-specific neuromuscular screening and quadriceps strength and jump asymmetry <10–15% [112–115], athletes have exhibited ≥15% asymmetries in the preceding measures at the time of RTS and >10–15% asymmetries up to two years following ACL reconstruction [83,116–119]. In fact, quadriceps strength asymmetry has been reported as a major limitation up to two years following ACL reconstruction [117] and is associated with an increased secondary injury risk [110]. In vertical jump evaluations, Paterno et al. [119] reported athletes to exhibit ~15% force asymmetries during the landing phase of a drop vertical jump, with greater force placed on the non-injured leg, while the previously injured leg produced lower force during the jump’s takeoff phase. Although these researchers did not examine LSTM asymmetries or report how force asymmetries may predict secondary injury risk, their findings suggest dependence on the non-injured leg as a compensatory mechanism, possibly increasing athletes’ secondary injury risk [120,121]. Considering athletes’ secondary ACL injury rate is up to 30% within two years following RTS, sole examination of strength and force asymmetries may be insufficient for determining athletes’ readiness to RTS [122,123]. Thus, an assessment approach that evaluates LSTM in combination with function, ultimately to examine relative muscle functionality, may be critical to guide RTS decisions.
While many strength/force investigations have been reported in previously injured athletes, fewer investigations have noted the relationship between asymmetries in LSTM and strength following primary ACL injury, with many of these investigations having been conducted in physically active non-athletes. In this sample population ~14 months post-ACL injury, Konishi et al. [112] reported lower isokinetic extensor peak torque produced per unit quadriceps MV in the previously injured vs. non-injured leg (0.086±0.019 Nm/cm3 vs. 0.100±0.016 Nm/cm3; p<0.05), and in both legs vs. controls (p<0.01), similar to others’ observations [111,113]. Thomas et al. [113] also reported that quadriceps CSA deficits six months following ACL reconstruction explained ~31% of the variance in quadriceps strength deficits in the previously injured leg (p=0.011). Thus, quadriceps mass and strength deficits post-reconstruction may increase secondary injury risk, but this hypothesis has not yet been proven.
Investigators more recently have used DXA to examine the relationship between asymmetries in LSTM and force produced during vertical jumping in athletes with previous ACL injury. Jordan et al. [83] examined asymmetry differences by calculating an asymmetry index for contralateral DXA-measured upper-leg LSTM and impulse (area under the force vs. time curve) during the CMJ and SJ in elite alpine skiers two years following ACL reconstruction versus healthy skier controls. Observations revealed moderate relationships between the asymmetry index of upper leg LSTM and CMJ and SJ impulse (r=0.57 to 0.66; P<0.01). Moreover, significant impulse asymmetry index differences between ACL and control groups were noted during the CMJ’s concentric phase (6.5% vs. 0.5%, respectively) and the second half of the SJ’s concentric phase (8.8% vs. −1.0%, respectively). More recently, Raymond-Pope et al. [124] reported female adolescent athletes one-year following ACL reconstruction and RTS had significant asymmetries in anterior and posterior upper leg LSTM, measured using the lateral DXA scan method, in addition to asymmetries of ~20% and 21% in quadriceps isokinetic peak torque and squat jump peak vertical ground reaction force, respectively. Relative muscle functionality ratios also revealed muscle function deficits in the previously injured leg vs. the non-injured leg in relation to LSTM during isokinetic and squat jump testing indicating persistent muscle dysfunction. Further investigation is warranted following ACL and muscle injuries to examine the relationship between DXA-derived LSTM and muscle function in different sample populations, how relative muscle functionality changes throughout rehabilitation and following RTS, and how asymmetries in LSTM and function may predict injury risk.
In summary, studies reporting an association between asymmetries and injury risk have primarily focused on asymmetries in function (strength, force, power) rather than muscle size/LSTM [97,109]. Quantifying regional LSTM and calculating U/L body mass ratios would allow sports practitioners to examine LSTM asymmetries that may hinder sport performance and contribute to functional asymmetries, which may increase athlete’s primary or secondary injury risk [110]. Further, although contralateral asymmetries in DXA-measured LSTM have been more recently noted in the literature in healthy and previously injured athlete populations, with reports of concurrent strength and force assessments, evaluating ipsilateral differences in the upper and lower limbs may be more beneficial in examining muscle-specific changes in response to training and rehabilitation. The ability to quantify athletes’ LSTM in ipsilateral compartments (e.g., quadriceps/hamstrings) using the lateral DXA scan method [89], and to evaluate these measurements in relation to strength/force to determine relative muscle functionality, would allow for developing more effective sport- and position- specific training and rehabilitation programs [97]. Further, the ability to examine longitudinal ipsilateral muscle functionality changes may provide greater insight into factors, such as regional asymmetries, contributing to injury [11]. These evaluations may allow for determining relative muscle functional asymmetry thresholds that negatively affect performance and perhaps predict injury/re-injury risk.
Muscle asymmetry with regional BIA
A promising and practical alternative to DXA for identification of muscle asymmetry is regional BIA. This technology employs multi-electrode systems with source and detector contact electrodes located at the hands and feet or other anatomical locations. Commercial systems introduce either a 50 kHz or multiple-frequency alternating current and may include phase-sensitive measurement devices. Subjects are either in a supine or standing position [125–131]. Regional estimates of FFM and FM of the arms, legs and trunk are generated using proprietary software requiring input of measurements of impedance variables at body segments, BM, height, age, and gender.
Initial validation studies of the various regional BIA devices found significant differences for total-body and regional estimates of LSTM compared to values from standard reference methods [16,125–131]. These reports failed to report regional BIA measurements and specific resistivity largely due to lack of determination of limb geometry. Thus, their findings demonstrate that the proprietary prediction equations for LSTM and muscle mass are questionable. While total-body BIA measurements are significant predictors of gains in muscle function following resistance training [63], an evaluation of regional BIA measurements as indicators of muscle asymmetry merits investigation. Regional BIA can be a novel approach to characterize muscle quality and determine asymmetry of PhA measurements in relation to muscle function and sport-specific performance attributes and injury risk.
Localized muscle injury
Localized muscle injuries are common in sport and account for more than 30% of all injuries. More than 90% of these injuries occur in the lower extremity, affecting the hamstrings (37%), adductors (23%), quadriceps (19%), and gastrocnemius and soleus (13%) muscles [132,133]. Muscle injuries ensue after either excessive tensile force applied to a muscle results in rupture at the myotendinous junction or a sudden compressive force causing contusion of the muscle. MRI and US are commonly used to determine the presence and extent of muscle injury. Recently, localized BIA (L-BIA) was evaluated as a practical addition to evaluate soft tissue injuries.
Localized BIA is an adaptation of the total-body technique. It uses four electrodes placed on the skin near the injury, and it is sufficiently sensitive to detect changes in soft tissue hydration and cell membrane integrity and structure [134]. Nescolarde et al. [135] reported a significant percentage difference in R, Xc and PhA between the injured and non-injured contralateral muscle measured 24-hr after injury. The relative difference increased with MRI-determined severity of the muscle injury (grade I, II and III). The increases in %R difference, indicative of inflammation-induced fluid accumulation, were not proportional to the severity of injury (type1=10.4%, type II=18.4%, type III=14.1%) and consistent with findings that edema is not correlated with MRI-graded injury severity [136,137]. Disruption of muscle structure, characterized by %Xc difference, however, increased significantly with injury severity (type I=17.5%, type II=32.9%, type III=52.9%). These changes exceeded the observed reproducibility of localized BIA measurements of the quadriceps and hamstrings (1.2 to 1.8%) and adductors (2.1 to 3.5%) [135]. Asymmetry (right vs left) of localized Xc of uninjured muscles at usual sites of injury (quadriceps, hamstring and adductor) averaged 13.8% as compared to observed differences in Xc with grade I, II and III injures (17.5, 32.9 and 52.9%, respectively). Thus, disrupted muscle tissue structure was associated with the largest disparity in %Xc between the injured and non-injured paired muscles.
Controversy exists about prognostic indicators for RTS. Edema is one factor and the severity of the muscle injury [138], which depends on the MRI-determined muscle gap or retraction of muscle fibers, is another. Male soccer players exhibited MRI-determined edema in type I (<10% CSA) and II injuries (10 to 50% CSA) [139]. MRI revealed type II injuries with different structural characteristics: a feather-like image without (II-f) or with a muscle gap (II-g). The L-BIA muscle measurements revealed a progressively greater increase in %R difference from type I to type II-f and II-g (10.2, 12.8 and 19.9%, respectively). The largest increase was in %Xc from Type I to II-f and II-g (13.4, 23.5 and 27.5%, respectively) with a comparable trend in PhA (3.2, 11.2 and 20.5%, respectively). Athletes with the type II-g injury had significantly longer RTS (48 d) compared to other athletes with type II-f (14 d) that was greater than type I injuries (10 d). These findings suggest that L-BIA can distinguish severity of muscle tears in type II injuries and reveal the muscle gap that predicts longer RTS. Importantly, L-BIA can provide a practical tool to identify muscle gap that is not always found with MRI [140].
Methodological Errors and Standardization
Assessment of human body composition requires indirect methods that measure a physical characteristic and mathematically transform it into a specific body component (e.g., FM, FFM, LSTM, bone). Awareness and control of the sources of error, therefore, are critical to understanding whether a method can determine a biologically meaningful change. Therefore, body composition studies to benefit athletes require attention to experimental errors and have sensitivity and power to make sound conclusions.
Biological errors are random and comprise deviations from the basic assumptions of a method or model and can impact the validity of an individual’s body composition assessment. Common assumptions include constant hydration of FFM, differences in density of FFM, attenuation of photon energy from x-rays related to soft tissue density and thickness, and other norms. Implementation of standardized preparation protocols prior to testing are advised to limit measurement errors. For example, in the case of DXA, it is recommended that athletes are scanned in an overnight fasted and rested state, do not participate in exercise the day of testing and ideally within ~24 hours of testing, and wear minimal clothing [141].
In contrast, technical errors are non-random and relate to the accuracy and precision of the instrument to perform measurement(s). Accuracy depends on the validity of a measurement. It utilizes an inanimate standard and determines the equivalence of the device measurement to the value of the standard. For example, DXA must be calibrated using an anthropomorphic phantom equivalent for soft tissue and bone. Additionally, BIA measurements can be validated by including an internal or external parallel circuit containing a high-precision resistor and capacitor to assess technical accuracy.
A critical technical factor is the in vivo reproducibility or precision of the measurement. In contrast to accuracy, precision is operator dependent. Precision should be determined for the test administrator(s) in a sample of the individuals under study. Coefficients of variation should also be calculated to determine the inter- and intra- rater reliability of device-specific compositional measurements. Measurement precision is an essential factor in use of a method to assess the body composition of an athlete; it should be considerably less than the expected change [142]. Description of both technical validation and measurement error should be included in any report of body composition estimates in athletes [143,144].
Some specific concerns arise with BIA and DXA. Not all BIA instruments report the same measurements [145]. Low-impedance electrodes are recommended for reliable and accurate assessment of hydration [146]. Similarly, body composition measurements with different DXA instruments are not interchangeable [147]. Proprietary software is another non-random source of error of body composition estimates. Studies of athletes, observational and interventional, should use the same instrument to avoid inter-device variability.
SUMMARY
Differences in total-body composition impact performance in sport. Increased LSTM and muscle can be advantageous in sports requiring strength and power. Much of this evidence comes from traditional methods (e.g., anthropometry, densitometry, ultrasound) that did not consider inter-individual differences in chemical composition of the fat-free body; confirmation using more advanced techniques (e.g., three-component models) tends to support these initial generalized conclusions.
Evolving research seeks to ascertain the role of regional body composition on sport- and position- specific biomechanical attributes, increased risk of injury, graded muscle injury, and objective criteria for return to play for injured athletes. Whereas early research emphasized asymmetry in muscle function (strength, force and power), contemporary efforts seek to determine the interaction of muscle size and mass with function. New findings of discernment of competition level with body composition characteristics derived with innovative methods and tracking of hydration status during training and competition afford new prospects in sport.
All methods of human body composition assessment are indirect and, thus, have limited accuracy. Optimization of body composition measurement must minimize technical or measurement errors, standardize test procedures and establish stable physiological status to enable high reliability of assessments. Attention to these test conditions will enhance reproducibility of findings are their application to individual athletes.
RESEARCH OPPORTUNITIES
Assessments of FM, FFM, and LSTM provide practical evidence of a role for body composition in sport. Availability and advancement of body composition methods allow valid and reliable estimation of total-body and regional LSTM and muscle mass, and measurements of passive bioelectrical characteristics that permit critical evaluation of their specific roles in performance, function, injury risk, and recovery from injury. Utilizing these body composition methods to assess total and regional LSTM in conjunction with muscle-specific and explosive strength measurements, in addition to sport performance outcomes, provides future research opportunities in multiple areas.
Total body LSTM can be used to predict performance in power sports, with differences in regional distribution associated with improved performance. Additionally, regional LSTM and ratios of these measurements (e.g., U/L LSTM) can provide insight to modify training programs to enhance athletes’ biomechanical function and optimize sport- and position- specific performance. Notably, only a handful of studies have evaluated U/L LSTM ratios, and therefore further investigations are needed to quantify these ratios across sports and positions within each sport to optimize athletes’ sport- and position- specific performance. Additionally, future studies should seek to identify relationships between total and regional body composition measurements and sport- and position- specific characteristics linked to improved performance (i.e., kicking, shooting, sprinting, etc.). Future investigations should employ longitudinal total and regional LSTM assessments in athletes using large sample sizes across sports and identify relationships among LSTM changes with variations in strength and sport performance outcomes. This assessment would enable the development of prediction equations, which may allow for optimizing athletes’ sport performance.
Investigation of the effects of regional LSTM asymmetry on training, performance, and injury/re-injury risk is a research priority. Thus, it is reasonable to determine the thresholds of contralateral and ipsilateral LSTM asymmetry that negatively affect training and performance; and identify sensitive and specific thresholds at which asymmetries in strength, force, and LSTM adversely impact training and performance and contribute to injury/re-injury risk. Although asymmetries in muscle-specific and explosive strength and LSTM may contribute to injury risk, investigations are needed to determine interactions of ipsilateral and contralateral asymmetries, strength, and sport-specific functional measures on injury/re-injury risk.
Novel bioelectrical measurements of body regions as indicators of muscle quality merit investigation. The combination of low-impedance surface electrodes with advanced modeling of conductor volumes can discriminate physiological characteristics of the upper-and lower-limbs and trunk. These measurements provide qualitative assessments of regional tissue hydration, and muscle damage that can guide physical training and identify imbalances between contralateral limbs as related to severity of injury. There is a conspicuous lack of data indicating the validity of BIA to detect changes in ECF/ICF with muscle glycogen loading and depletion as well as the sensitivity to identify changes in ECF including plasma volume in response to physical activity. Future studies could provide ranges of acceptable regional bioimpedance values related to performance goals and thresholds indicative of cellular perturbation and, hence, increased risk of impaired performance or injury. They also should ascertain the validity of regional bioelectrical measurements as practical indices of muscle asymmetry.
These areas of investigation offer new applications of body composition measurements to support the health and performance of athletes. Additionally, these newer, more advanced, and non-invasive methods for evaluating skeletal muscle mass allow athletes, trainers, sports medicine professionals, and researchers to track changes in athletes’ skeletal muscle mass over time, such as over the course of a season or career. This information, combined with evaluation of sport- and position- specific performance and strength/force measurements, can be used to examine the efficacy of a new training, rehabilitation, or diet regimen to enhance athletes’ health and sport performance, identify and minimize injury/re-injury risk, and predict a safe return to play.
Footnotes
Conflict of Interest
The authors report no conflicts of interest. They also guarantee, beyond the absence of any conflict of interest, that the manuscript was created consistent with the ethical standards of the International Journal of Sports Medicine [148].
References
- 1.PubMed.gov (Searched December 28, 2020).
- 2.Wilmore JH. Body composition in sport and exercise: directions for future research. Med Sci Sports Exerc 1983; 15: 21–31. [PubMed] [Google Scholar]
- 3.Meyer NL, Sundgot-Borgen J, Lohman TG, et al. Body composition for health and performance: a survey of body composition assessment practice carried out by the Ad Hoc Research Working Group on Body Composition, Health and Performance under the auspices of the IOC Medical Commission. Br J Sports Med 2013; 47: 1044–1053. [DOI] [PubMed] [Google Scholar]
- 4.Ackland TR, Lohman TG, Sundgot-Borgen J, et al. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sports Med 2012; 42: 227–249. [DOI] [PubMed] [Google Scholar]
- 5.Lukaski HC. Body composition: perspectives. In: Lukaski HC, Ed. Body Composition: Health and Performance in Exercise and Sport. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2017: 3–12. [Google Scholar]
- 6.Silva AM. Structural and functional body components in athletic health and performance phenotypes. Eur J Clin Nutr 2019; 73: 215–224. [DOI] [PubMed] [Google Scholar]
- 7.Bosch TA, Burruss TP, Weir NL, et al. Abdominal body composition differences in NFL football players. J Strength Cond Res 2014; 28: 3313–3319. [DOI] [PubMed] [Google Scholar]
- 8.Boileau RA, Horswill CA. Body composition in sports: Measurement and applications for weight gain and loss. In: Exercise and Sport Science. Garrett WE Jr and Kirkendall DT, Eds. Philadelphia, PA: Lippincott Williams & Wilkins, 2000: 319–338. [Google Scholar]
- 9.O’Connor H, Olds T, Maughan RJ. Physique and performance for track and field events. J Sports Sci 2007; 25(Suppl 1): S49–S60. [DOI] [PubMed] [Google Scholar]
- 10.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr 2014; 38: 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dengel DR, Raymond CJ, Bosch TA. Assessment of muscle mass. In Lukaski HC, Ed. Body composition: Health and performance in exercise and sport. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2017: 27–47. [Google Scholar]
- 12.Burkhart TA, Arthurs KL, Andrews DM (2009). Manual segmentation of DXA scan images results in reliable upper and lower extremity soft and rigid tissue mass estimates. J Biomech 2009; 27: 197–206. [DOI] [PubMed] [Google Scholar]
- 13.Fosbøl MØ, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Funct Imag 2015; 35: 81–97. [DOI] [PubMed] [Google Scholar]
- 14.Moon JR, Kendall KL. Endurance athletes. In: Lukaski HC, ed. Body Composition: Health and Performance in Exercise and Sport. Boca Raton: CRC Press; 2017: 171–210. [Google Scholar]
- 15.Bosch TA, Carbuhn AF, Stanforth PR, et al. Body composition and bone mineral density of Division 1 Collegiate football players: A Consortium of College Athlete Research Study. J Strength Cond Res 2019; 33: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dengel DR, Bosch TA, Burruss TP, et al. Body composition and bone mineral density of National Football League players. J Strength Cond Res 2014; 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Raymond CJ, Dengel DR, Bosch TA. Total and segmental body composition examination in collegiate football players using multifrequency bioelectrical impedance analysis and dual x-ray absorptiometry J Strength Cond Res 2018; 32: 772–782. [DOI] [PubMed] [Google Scholar]
- 18.Chiarlitti NA, Delisle-Houde P, Reid RE, et al. Importance of body composition in the national hockey league combine physiological assessments. J Strength Cond Res 2018; 32: 3135–3142. [DOI] [PubMed] [Google Scholar]
- 19.Czeck MA, Raymond-Pope CJ, Bosch TA, et al. Total and regional body composition of NCAA Division I collegiate baseball athletes. Int J Sports Med 2019; 40: 447–452. [DOI] [PubMed] [Google Scholar]
- 20.Czeck MA, Raymond-Pope CJ, Stanforth PR, et al. Total and regional body composition of NCAA Division I collegiate female softball athletes. Int J Sports Med 2019; 40: 645–649. [DOI] [PubMed] [Google Scholar]
- 21.Raymond-Pope CJ, Solfest AL, Carbuhn A, et al. Total and regional body composition of NCAA Division I collegiate basketball athletes. Int J Sports Med 2020; 41: 242–247. [DOI] [PubMed] [Google Scholar]
- 22.Dengel DR, Keller KA, Stanforth PR, et al. Body composition and bone mineral density of Division 1 collegiate track and field athletes, a consortium of college athlete research (C-CAR) study. J Clin Densitom 2020; 23: 303–313. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch KR, Smith-Ryan AE, Trexler ET, et al. Body composition and muscle characteristics of Division I track and field athletes. J Strength Cond Res 2016; 30: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dengel OH, Raymond-Pope CJ, Bosch TA, et al. Body composition and visceral adipose tissue in female collegiate equestrian athletes. Int J Sports Med 2019; 40: 404–408. [DOI] [PubMed] [Google Scholar]
- 25.Harty PS, Zabriskie HA, Stecker RA, et al. Position-specific body composition values in female collegiate rugby union athletes. J Strength Cond Res 2019. doi: 10.1519/jsc.0000000000003314 [DOI] [PubMed] [Google Scholar]
- 26.Sanfilippo J, Krueger D, Heiderscheit B, et al. Dual-energy x-ray absorptiometry body composition in NCAA Division I athletes: exploration of mass distribution. Sports Health 2019; 11: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roelofs E, Bockin A, Bosch T, et al. Body composition of National Collegiate Athletic Association (NCAA) Division I female soccer athletes through competitive seasons. Int J Sports Med 2020. doi: 10.1055/a-1177-0716. [DOI] [PubMed] [Google Scholar]
- 28.Carbuhn AF, Fernandez TE, Bragg AF, et al. Sport and training influence bone and body composition in women collegiate athletes. J Strength Cond Res. 2010; 24: 1710–1717. [DOI] [PubMed] [Google Scholar]
- 29.Delisle-Houde P, Chiarlitti NA, Reid RE, et al. Relationship between physiologic tests, body composition changes, and on-ice playing time in Canadian collegiate hockey players. J Strength Cond Res 2018; 32: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 30.Milanese C, Cavedon V, Corradini G, et al. Seasonal DXA-measured body composition changes in professional male soccer players. J Sports Sci 2015; 33: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 31.Peart A, Wadsworth D, Washington J, et al. Body composition assessment in female National Collegiate Athletic Association Division I softball athletes as a function of playing position across a multiyear time frame. J Strength Cond Res. 2019; 33: 3049–3055. [DOI] [PubMed] [Google Scholar]
- 32.Roelofs EJ, Smith-Ryan AE, Trexler ET, et al. Seasonal effects on body composition, muscle characteristics, and performance of collegiate swimmers and Divers. J Athl Train 2017; 52: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvestre R, Kraemer WJ, West C, et al. Body composition and physical performance during a National Collegiate Athletic Association Division I men’s soccer season. J Strength Cond Res 2006; 20: 962. [DOI] [PubMed] [Google Scholar]
- 34.Stanforth PR, Crim BN, Stanforth D, et al. Body composition changes among female NCAA Division 1 athletes across the competitive season and over a multiyear time frame. J Strength Cond Res 2014; 28: 300–307. [DOI] [PubMed] [Google Scholar]
- 35.Trexler ET, Smith-Ryan AE, Mann JB, et al. Longitudinal body composition changes in NCAA Division I college football players. J Strength Cond Res. 2017; 31: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prokop NW, Reid RE, Andersen RE. Seasonal changes in whole body and regional body composition profiles of elite collegiate ice-hockey players. J 5trength Cond Res 2016; 30: 684–692. [DOI] [PubMed] [Google Scholar]
- 37.Moon JR. Body composition in athletes and sports nutrition: an examination of the bioimpedance analysis technique. Eur J Clin Nutr. 2013; 67 Suppl 1: S54–S59. [DOI] [PubMed] [Google Scholar]
- 38.Lukaski HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur J Clin Nutr 2013; 67 Suppl 1: S2–S9. [DOI] [PubMed] [Google Scholar]
- 39.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr 2004; 23: 1430–1453. [DOI] [PubMed] [Google Scholar]
- 40.Sun SS, Chumlea WC, Heymsfield SB, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiological surveys. Am J Clin Nutr 2003; 77: 331–340. [DOI] [PubMed] [Google Scholar]
- 41.Maughan RJ, Shirreffs SB. Hydrometry, hydration status and performance. In: Lukaski HC, Ed. Body Composition: Health and Performance in Exercise and Sport. Orlando, FL: CRC Press, Taylor & Francis Group; 2017: 49–68. [Google Scholar]
- 42.Matthie JR. Bioimpedance measurements of human body composition: Critical analysis and outlook. Expert Rev Med Devices 2008; 5: 239–261. [DOI] [PubMed] [Google Scholar]
- 43.Nickerson BS, Tinsley GM, Esco MR. Validity of field and laboratory three-compartment models in healthy adults. Med Sci Sports Exerc 2019; 51:1032–1039. [DOI] [PubMed] [Google Scholar]
- 44.Shiose K, Yamada Y, Motonaga K, et al. Segmental extracellular and intracellular water distribution and muscle glycogen after 72-h carbohydrate loading using spectroscopic techniques. J Appl Physiol 2016; 121: 205–211. [DOI] [PubMed] [Google Scholar]
- 45.Shiose K, Yamada Y, Motonaga K, et al. Muscle glycogen depletion does not alter segmental extracellular and intracellular water distribution measured using bioimpedance spectroscopy. J Appl Physiol 2018; 124: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 46.Shiose K, Tanabe Y, Ohnishi T, et al. Effect of regional muscle damage and inflammation following eccentric exercise on electrical resistance and the body composition assessment using bioimpedance spectroscopy. J Physiol Sci 2019; 69: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukaski HC, Piccoli A. Bioelectrical impedance vector analysis for assessment of hydration in physiological states and clinical conditions. In: Preedy VR, Ed. Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. London: Springer; 2012: 287–305. [Google Scholar]
- 48.Lukaski HC, Vega Diaz N, Talluri A, et al. Classification of hydration in clinical conditions: indirect and direct approaches using bioimpedance. Nutrients 2019; 11: 809. doi: 10.3390/nu11040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castizo-Olier J, Irurtia A, Jemni M, et al. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS One, 2018; 13:e0197957. doi: 10.1371/journal.pone.0197957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koury JC, Trugo N MF, Torres AG. Phase angle and bioelectrical impedance vectors in adolescent and adult male athletes. Int J Sports Physiol Perform 2014; 9: 798–804. [DOI] [PubMed] [Google Scholar]
- 51.Piccoli A, Pastori G, Codognotto M, et al. Equivalence of information from single frequency v. bioimpedance spectroscopy in bodybuilders. Br J Nutr 2007; 97:182–192. [DOI] [PubMed] [Google Scholar]
- 52.Micheli ML, Pagani L, Marella M, et al. Bioimpedance and impedance vector patterns as predictors of league level in male soccer players. Int J Sports Physiol Perform 2014; 9: 532–539. [DOI] [PubMed] [Google Scholar]
- 53.Carrasco-Marginet M, Castizo-Olier J, Rodríguez-Zamora L, et al. Bioelectrical impedance vector analysis (BIVA) for measuring the hydration status in young elite synchronized swimmers. PLoS One 2017; 12:e0178819. doi: 10.1371/journal.pone.0178819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giorgi A, Vicini M, Pollastri L, et al. Bioimpedance patterns and bioelectrical impedance vector analysis (BIVA) of road cyclists. J Sports Sci 2018; 36: 2608–2613. [DOI] [PubMed] [Google Scholar]
- 55.Campa F, Toselli S. Bioimpedance vector analysis of elite, sub-elite, and low-level male volleyball players. Int J Sports Physiol Perform 2018; 13: 1250–1253. [DOI] [PubMed] [Google Scholar]
- 56.Castizo-Olier J, Carrasco-Marginet M, Roy A, et al. Bioelectrical impedance vector analysis (BIVA) and body mass changes in an ultra-endurance triathlon event. J Sports Sci Med 2018; 17: 571–579. [PMC free article] [PubMed] [Google Scholar]
- 57.Mascherini G, Castizo-Olier J, Irurtia A, et al. Differences between the sexes in athletes’ body composition and lower limb bioimpedance values. Muscles Ligaments Tendons J 2018; 7: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campa F, Matias C, Gatterer H, et al. Classic bioelectrical impedance vector reference values for assessing body composition in male and female athletes. Int J Environ Res Public Health 2019; 16:5066. doi: 10.3390/ijerph16245066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campa F, Matias CN, Marini E, et al. Identifying athlete body fluid changes during a competitive season with bioelectrical impedance vector analysis. Int J Sports Physiol Perform 2019; 11:1–7. [DOI] [PubMed] [Google Scholar]
- 60.Marini E, Campa F, Buffa R, et al. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin Nutr 2020; 39: 447–454. [DOI] [PubMed] [Google Scholar]
- 61.Bourgeois B, Fan B, Johannsen N, et al. Improved strength prediction combining clinically available measures of skeletal muscle mass and quality. J Cachexia Sarcopenia Muscle 2019; 10: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hetherington-Rauth M, Baptista F, Sardinha LB. BIA-assessed cellular hydration and muscle performance in youth, adults, and older adults. Clin Nutr 2019; pii: S0261–5614(19)33167-X. doi: 10.1016/j.clnu.2019.11.040. [DOI] [PubMed] [Google Scholar]
- 63.Sardinha LB. Physiology of exercise and phase angle: another look at BIA. Eur J Clin Nutr 2018; 72: 1323–1327. [DOI] [PubMed] [Google Scholar]
- 64.Fukuda DH, Stout JR, Moon JR, et al. Effects of resistance training on classic and specific bioelectrical impedance vector analysis in elderly women. Exp Gerontol 2016; 74:9–12. [DOI] [PubMed] [Google Scholar]
- 65.Dos Santos L, Cyrino ES, Antunes M, et al. Changes in phase angle and body composition induced by resistance training in older women. Eur J Clin Nutr 2016; 70: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro AS, Avelar A, Dos Santos L, et al. Hypertrophy-type resistance training improves phase angle in young adult men and women. Int J Sports Med 2017; 38: 35–40. [DOI] [PubMed] [Google Scholar]
- 67.Souza MF, Tomeleri CM, Ribeiro AS, et al. Effect of resistance training on phase angle in older women: A randomized controlled trial. Scand J Med Sci Sports 2017; 27: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 68.Silva AM, Matias CN, Santos DA, et al. Increases in intracellular water explain strength and power improvements over a season. Int J Sports Med 2014; 35: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 69.Nabuco HCG, Silva AM, Sardinha LB, et al. Phase angle is moderately associated with short-term maximal intensity efforts in soccer players. Int J Sports Med 2019; 40: 739–743. [DOI] [PubMed] [Google Scholar]
- 70.Tomeleri CM, Ribeiro AS, Cavaglieri CR, et al. Correlations between resistance training-induced changes on phase angle and biochemical markers in older women. Scand J Med Sci Sports 2018; 28: 2173–2182. [DOI] [PubMed] [Google Scholar]
- 71.Gatterer H, Schenk K, Laninschegg L, et al. Bioimpedance identifies body fluid loss after exercise in the heat: a pilot study with body cooling. PLoS One 2014; 9(10):e109729. doi: 10.1371/journal.pone.0109729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrasco-Marginet M, Castizo-Olier J, Rodríguez-Zamora L, et al. Bioelectrical impedance vector analysis (BIVA) for measuring the hydration status in young elite synchronized swimmers. PLoS One 2017; 12:e0178819. doi: 10.1371/journal.pone.0178819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castizo-Olier J, Carrasco-Marginet M, Roy A, et al. Bioelectrical impedance vector analysis (BIVA) and body mass changes in an ultra-endurance triathlon event. J Sports Sci Med 2018; 17: 571–579. [PMC free article] [PubMed] [Google Scholar]
- 74.Nescolarde L, Roca E, Bogónez-Franco P, et al. Relationship between bioimpedance vector displacement and renal function after a marathon in non-elite runners. Front Physiol 2020; 11: 352. doi: 10.3389/fphys.2020.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mascherini G, Gatterer H, Lukaski H, et al. Changes in hydration, body-cell mass and endurance performance of professional soccer players through a competitive season. J Sports Med Phys Fitness 2015; 55: 749–755. [PubMed] [Google Scholar]
- 76.Pollastri L, Lanfranconi F, Tredici G, et al. Body water status and short-term maximal power output during a multistage road bicycle race (Giro d’Italia 2014). Int J Sports Med 2016; 37: 329–333. [DOI] [PubMed] [Google Scholar]
- 77.Marra M, Da Prat B, Montagnese C, et al. Segmental bioimpedance analysis in professional cyclists during a three-week stage race. Physiol Meas 2016; 37: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Correa CH, Eraso JC. Bioelectrical impedance analysis (BIA): a proposal for standardization of the standard method for adults. J Phys Conf Ser, 2012; 407: 012018. doi: 10.1088/1742-6596/407/1/012018. [DOI] [Google Scholar]
- 79.Moore FD, Boyden CM Body cell mass and limits of hydration of the fat-free body: their relation to estimated skeletal weight. Ann NY Acad Sci 1963; 110: 62–71. [DOI] [PubMed] [Google Scholar]
- 80.Akagi R, Tohdoh Y, Takahashi H Strength and size ratios between reciprocal muscle groups in the thigh and lower leg of male collegiate soccer players. Clin Physiol Funct Imaging. 2014; 34: 121–125. [DOI] [PubMed] [Google Scholar]
- 81.Bell DR, Sanfilippo JL, Binkley N, Heiderscheit BC. Lean mass asymmetry influences force and power asymmetry during jumping in collegiate athletes. J Strength Cond Res 2014; 28: 884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denadai BS, Oliveira FBD, Camarda SRDA, et al. Hamstrings-to-quadriceps strength and size ratios of male professional soccer players with muscle imbalance. Clin Physiol Funct Imaging. 2016; 36: 159–164. [DOI] [PubMed] [Google Scholar]
- 83.Jordan MJ, Aagaard P, Herzog W. Lower limb asymmetry in mechanical muscle function: A comparison between ski racers with and without ACL reconstruction. Scand J Med Sci Sports 2015; 25: e301–e309. [DOI] [PubMed] [Google Scholar]
- 84.Newton RU, Gerber A, Nimphius S, et al. Determination of functional strength imbalance of the lower extremities. J Strength Cond Res 2006; 20: 971–977. [DOI] [PubMed] [Google Scholar]
- 85.Fukunaga T, Miyatani M, Tachi M, et al. Muscle volume is a major determinant of joint torque in humans. Acta Physiol 2001; 172: 249–255. [DOI] [PubMed] [Google Scholar]
- 86.Masuda K, Kikuhara N, Takahashi H, et al. The relationship between muscle cross-sectional area and strength in various isokinetic movements among soccer players. J Sports Sci. 2003; 21: 851–858. [DOI] [PubMed] [Google Scholar]
- 87.Jones B, Emmonds S, Hind K, et al. Physical qualities of international female rugby league players by playing position. J Strength Cond Res 2016; 30: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 88.Stephenson ML, Smith DT, Heinbaugh EM, et al. Total and lower extremity lean mass percentage positively correlates with jump performance. J Strength Cond Res 2015; 29: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 89.Raymond CJ, Bosch TA, Busch FK, et al. Accuracy and reliability of assessing lateral compartmental leg composition using DXA. Med Sci Sports Exerc 2017; 49: 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raymond-Pope CJ, Dengel DR, Fitzgerald JS, et al. Association of compartmental leg lean mass measured by dual X-ray absorptiometry with force production. J Strength Cond Res 2020; 34: 1690–1699. [DOI] [PubMed] [Google Scholar]
- 91.Raymond-Pope CJ, Bosch TA, Dengel DR. Assessing agreement of lateral leg muscle and bone composition using dual x-ray absorptiometry. J Clin Densitom 2020; 23: 451–458. doi: 10.1016/j.jocd.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Impellizzeri FM, Rampinini E, Maffiuletti N, et al. A vertical jump force test for assessing bilateral strength asymmetry in athletes. Med Sci Sports Exerc 2007; 39: 2044–2050. [DOI] [PubMed] [Google Scholar]
- 93.Barber SD, Noyes FR, Mangine RE, et al. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop Relat Res 1990; 225 :204–214. [PubMed] [Google Scholar]
- 94.Lockie RG, Shultz AB, Jeffriess MD, et al. The relationship between bilateral differences of knee flexor and extensor isokinetic strength and multi-directional speed. Isokinet Exerc Sci 2012; 20: 211–219. [Google Scholar]
- 95.Hart NH, Nimphius S, Spiteri T, et al. Leg strength and lean mass symmetry influences kicking performance in Australian football. J Sci Med Sport. 2014; 13: 157–165. [PMC free article] [PubMed] [Google Scholar]
- 96.Maloney SJ. The relationship between asymmetry and athletic performance: A critical review. J Strength Cond Res 2019; 33: 2579–2593. [DOI] [PubMed] [Google Scholar]
- 97.Bishop C, Brashill C, Abbott W, et al. Jumping asymmetries are associated with speed, change of direction speed, and jump performance in elite academy soccer players. J Strength Cond Res 2019. doi: 10.1519/JSC.0000000000003058. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman JR, Ratamess NA, Klatt M, et al. Do bilateral power deficits influence direction-specific movement patterns? Res Sports Med 2007; 15: 125–132. [DOI] [PubMed] [Google Scholar]
- 99.Lockie RG, Callaghan SJ, Berry SP, et al. Relationship between unilateral jumping ability and asymmetry on multidirectional speed in team-sport athletes. J Strength Cond Res 2014; 28: 3557–3566. [DOI] [PubMed] [Google Scholar]
- 100.Ekstrand J, Healy JC, Waldén M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2012; 46: 112–117. [DOI] [PubMed] [Google Scholar]
- 101.Opar DA, Williams MD, Shield AJ. Hamstrings strain injuries: Factors that lead to injury and re-injury. Sports Med 2012; 42: 209–226. [DOI] [PubMed] [Google Scholar]
- 102.Knapik JJ, Bauman CL, Jones BH, et al. Preseason strength and flexibility imbalances associated with athletic injuries in female collegiate athletes. Am J Sports Med 1991; 19: 76–81. [DOI] [PubMed] [Google Scholar]
- 103.Roos KG, Marshall SW, Kerr ZY, et al. Epidemiology of overuse injuries in collegiate and high school athletics in the United States. Am J Sports Med 2015; 43: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 104.Liu H, Garrett WE, Moorman CT, et al. Injury rate, mechanism, and risk factors of hamstring strain injuries in sports: A review of the literature. J Sport Health Sci 2012; 1: 92–101. [Google Scholar]
- 105.Myer GD, Ford KR, Khoury J, et al. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br J Sports Med 2011; 45: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silvers HJ, Mandelbaum BR. Prevention of anterior cruciate ligament injury in the female athlete. Br J Sports Med 2007; 41(suppl 1): i52–i59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. Am J Sports Med. 2005; 33: 492–501. [DOI] [PubMed] [Google Scholar]
- 108.Hewett TE, Myer GD, Ford KR, et al. Mechanisms, prediction, and prevention of ACL injuries: cut risk with three sharpened and validated tools. J Orthop Res 2016; 34: 1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shultz SJ, Schmitz RJ. Current understandings and directions for future research. In: Noyes F, Barber-Westin S Eds. ACL Injuries in the Female Athlete. Berlin: Springer, 2018: 641–666. [Google Scholar]
- 110.Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: The Delaware-Oslo ACL cohort study. Br J Sports Med 2016; 50: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konishi Y, Ikeda K, Nishino A, et al. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament repair. Scand J Med Sci Sports 2007; 17: 656–661. [DOI] [PubMed] [Google Scholar]
- 112.Konishi Y, Oda T, Tsukazaki S, et al. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc 2011; 19: 641–645. [DOI] [PubMed] [Google Scholar]
- 113.Thomas AC, Wojtys EM, Brandon C, et al. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport 2016; 19: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther 2012; 42: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kvist J Rehabilitation following anterior cruciate ligament injury: Current recommendations for sports participation. Sports Med 2004; 34: 269–280. [DOI] [PubMed] [Google Scholar]
- 116.Myer GD, Paterno MV, Ford KR, et al. Rehabilitation after anterior cruciate ligament reconstruction: Criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006; 36(6): 385–402. [DOI] [PubMed] [Google Scholar]
- 117.Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2012; 42: 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grindem H, Eitzen I, Engebretsen L, et al. Nonsurgical or surgical treatment of ACL injuries: knee function, sports participation, and knee reinjury: The Delaware-Oslo ACL Cohort Study. J Bone Joint Surg 2014; 96: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paterno MV, Ford KR, Myer GD, et al. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sports Med 2007; 17: 258–262. [DOI] [PubMed] [Google Scholar]
- 120.Paterno MV, Schmitt LC, Ford KR, et al. Effects of sex on compensatory landing strategies upon return to sport after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2011; 41: 553–559. [DOI] [PubMed] [Google Scholar]
- 121.Hewett TE, Myer GD, Ford KR, et al. Mechanisms, prediction, and prevention of ACL injuries: cut risk with three sharpened and validated tools. J Orthop Res 2016; 34: 1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wiggins AJ, Grandhi RK, Schneider DK, et al. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med 2016; 44: 1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Webster KE, Hewett TE. What is the evidence for and validity of Return-to-Sport testing after anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis. Sports Med 2019; 49: 917–929. [DOI] [PubMed] [Google Scholar]
- 124.Raymond-Pope CJ, Dengel DR, Fitzgerald JS, et al. Correction: anterior cruciate ligament reconstructed female athletes exhibit relative muscle dysfunction after return to sport. Int J Sports Med 2020; doi: 10.1055/a-1273-8269. [DOI] [PubMed] [Google Scholar]
- 125.Buckinx F, Reginster JY, Dardenne N, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015; 16: 60. doi: 10.1186/s12891-015-0510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bosy-Westphal A, Schautz B, Later W, et al. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr 2013; 67 Suppl 1: S14–S21. [DOI] [PubMed] [Google Scholar]
- 127.Bosy-Westphal A, Jensen B, Braun W, et al. Quantification of total-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr 2017; 71: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wingo BC, Barry VG, Ellis AC, et al. Comparison of segmental body composition estimated by bioelectrical impedance analysis and dual-energy X-ray absorptiometry. Clin Nutr ESPEN 2018; 28: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Esco MR, Snarr RL, Leatherwood MD, et al. Comparison of total and segmental body composition using DXA and multifrequency bioimpedance in collegiate female athletes. J Strength Cond Res 2015; 29: 918–925. [DOI] [PubMed] [Google Scholar]
- 130.McLester CN, Nickerson BS, Kliszczewicz BM, et al. Reliability and agreement of various InBody body composition analyzers as compared to dual-energy x-ray absorptiometry in healthy men and women. J Clin Densitom 2018: S1094–6950(18)30221-X. doi: 10.1016/j.jocd.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Schoenfeld BJ, Nickerson BS, Wilborn CD, et al. Comparison of multifrequency bioelectrical impedance analysis vs dual x-ray absorptiometry for assessing body composition changes after participation in a 10-wk resistance training program. J Strength Cond Res 34: 678–688, 2020. [DOI] [PubMed] [Google Scholar]
- 132.Ekstrand J, Hägglund M, Waldén M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med 2011; 45: 553–558. [DOI] [PubMed] [Google Scholar]
- 133.Engebretsen L, Soligard T, Steffen K, et al. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br J Sports Med 2013; 47: 407–414. [DOI] [PubMed] [Google Scholar]
- 134.Lukaski HC, Moore M. Bioelectrical impedance assessment of wound healing. J Diabetes Sci Technol 2012; 6: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nescolarde L, Yanguas J, Lukaski H et al. Effects of muscle injury severity on localized bioimpedance measurements. Physiol Meas 2015; 36: 27–42. [DOI] [PubMed] [Google Scholar]
- 136.Reurink G, Goudswaard GJ, Tol JL, et al. MRI observations at return to play of clinically recovered hamstring injuries. Br J Sports Med 2014; 48: 1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reurink G, Brilman EG, de Vos RJ, et al. Magnetic resonance imaging in acute hamstring injury: can we provide a return to play prognosis? Sports Med 2015; 45: 133–146. [DOI] [PubMed] [Google Scholar]
- 138.Pedret C, Rodas G, Balius R, et al. Return to play after soleus muscle injuries. Orthop J Sports Med 2015; 3:2325967115595802. doi: 10.1177/2325967115595802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nescolarde L, Yanguas J, Terricabras J, et al. Detection of muscle gap by L-BIA in muscle injuries: clinical prognosis. Physiol Meas 2017; 21: L1–L9. [DOI] [PubMed] [Google Scholar]
- 140.Valle X, Alentorn-Geli E, Tol JL, et al. Muscle injuries in sports: a new evidence-informed and expert consensus-based classification with clinical application. Sports Medicine 2017; 47: 1241–1253. [DOI] [PubMed] [Google Scholar]
- 141.Nana A, Slater GJ, Stewart AD, Burke LM. Methodology review: using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int J Sport Nutr Exerc Metab 2015; 25: 198–215. [DOI] [PubMed] [Google Scholar]
- 142.Earthman CP. Body composition tools for assessment of adult malnutrition at the bedside: a tutorial on research considerations and clinical applications. J Parenter Enteral Nutr 2015; 39: 787–822. [DOI] [PubMed] [Google Scholar]
- 143.Hind K, Slater G, Oldroyd B, et al. Interpretation of dual-energy x-ray absorptiometry-derived body composition change in athletes: a review and recommendations for best practice. J Clin Densitom 2018; 21: 429–443. [DOI] [PubMed] [Google Scholar]
- 144.Zemski AJ, Hind K, Keating SE, et al. Same-day vs consecutive-day precision error of dual-energy x-ray absorptiometry for interpreting body composition change in resistance-trained athletes. J Clin Densitom 2019; 22: 104–114. [DOI] [PubMed] [Google Scholar]
- 145.Silva AM, Matias CN, Nunes CL, et al. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur J Clin Nutr 2019; 73: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 146.Nescolarde L, Lukaski H, DeLorenzo A, et al. Different displacement of bioimpedance vector due to Ag/AgCl electrode effects. Eur J Clin Nutr 2016; 70: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 147.Jankowski LG, Warner S, Gaither K, et al. Cross-calibration, least significant change and quality assurance in multiple dual-energy x-ray absorptiometry scanner environments: 2019 ISCD Official Position. J Clin Densitom 2019; 22:472–483. [DOI] [PubMed] [Google Scholar]
- 148.Harriss DJ, Macsween A, Atkinson G. Ethical standards in sport and exercise science research: 2020 update. Int J Sports Med 2019; 40: 813–817. [DOI] [PubMed] [Google Scholar]