Abstract
The Hippo pathway is a dynamic cellular signalling nexus that regulates differentiation and controls cell proliferation and death. If the Hippo pathway is not precisely regulated, the functionality of the upstream kinase module is impaired, which increases nuclear localisation and activity of the central effectors, the transcriptional co-regulators YAP and TAZ. Pathological YAP and TAZ hyperactivity consequently cause cancer, fibrosis and developmental defects. The Hippo pathway controls an array of fundamental cellular processes, including adhesion, migration, mitosis, polarity and secretion of a range of biologically active components. Recent studies highlight that spatio-temporal regulation of Hippo pathway components are central to precisely controlling its context-dependent dynamic activity. Several levels of feedback are integrated into the Hippo pathway, which is further synergized with interactors outside of the pathway that directly regulate specific Hippo pathway components. Likewise, Hippo core kinases also ‘moonlight’ by phosphorylating multiple substrates beyond the Hippo pathway and thereby integrates further flexibility and robustness in the cellular decision-making process. This topic is still in its infancy but promises to reveal new fundamental insights into the cellular regulation of this therapeutically important pathway. We here highlight recent advances emphasising feedback dynamics and multilevel regulation of the Hippo pathway with a focus on mitosis and cell migration, as well as discuss potential productive future research avenues that might reveal novel insights into the overall dynamics of the pathway.
Keywords: cell migration, feedback, hippo pathway, mitosis, STRIPAK, YAP/TAZ
Introduction
The Hippo pathway was initially identified through genetic loss of function mosaic screens in the fruit fly, Drosophila melanogaster [1–6]. The evolutionary conservation from flies to humans is extensive, although key differences, including gene duplications, exist [7]. The signalling pathway consists of a regulatory serine/threonine kinase cascade that when activated ultimately phosphorylate the co-transcriptional regulators YAP and TAZ [7]. YAP was originally identified independently as an interactor of Yes kinase [8], acidic oligomeric 14-3-3 proteins [9] and as a cofactor of the TEAD transcription factors [10]. TAZ was also isolated as a 14-3-3 protein family-binding protein [11]. Since these original findings, a substantial interest in YAP and TAZ and the biology that they drive has evolved. Seminal studies identified MST1/2, STK25 and the MAP4K family of kinases as activators of the large tumour suppressor kinase1/2 (LATS1/2) [12–16] (Figure 1A). LATS1/2 belong to NDR family of serine/threonine kinases and directly phosphorylate YAP and TAZ on multiple sites. This consequently cause YAP and TAZ to predominantly localise to the cytoplasm and thereby inhibits YAP/TAZ. As YAP and TAZ do not contain DNA-binding domains, their transcriptional activity is dependent on interaction with cognate nuclear transcription factors, predominantly the transcriptional enhanced associate domain (TEAD) family [10,19–21]. Consequently, the dephosphorylation (and thereby inactivation) of upstream components of the Hippo pathway kinase cascade is critical for YAP/TAZ transcriptional activity. The supramolecular PP2A-STRIPAK (Striatin-interacting phosphatase and kinase) complex is central for this regulation [22–25]. This noncanonical phosphatase complex contains both kinase and phosphatase subunits for enhanced regulation, which allows dynamic cellular regulation upon diverse cellular stimuli and functions as an adaptable signalling centre [22]. STRIPAK complexes PP2A to multiple Hippo components, such as the scaffolding protein NF2 as well as LATS1/2 activating kinases [14,24,26–29]. PP2A-STRIPAK consequently activates YAP and TAZ through dephosphorylation and suppression of the upstream Hippo pathway kinase module [14,24,26–30]. The Hippo pathway is a central cellular signalling nexus and regulated by polarity, cell density, machanotransduction and diffusible chemicals [7,31]. STRIPAK-YAP/TAZ signalling is controlled by a range of stimuli, including activation of GPCR signalling by ligands, such as the exemplar Hippo pathway regulator lysophosphatidic acid (LPA) [18,30]. The bioactive lipid LPA activates the STRIPAK complex, which turns off the Hippo pathway kinase cascade [18,30,32,33]. Deactivating STRIPAK inactivates YAP/TAZ activity and reduces tumorigenic potential and elevated expression of STRN3 and STRN4, which function as recruiting STRIPAK factors for LATS activating kinases, are a prominent feature in some cancers [25,26,34–36]. However, PP2A activity is widely decreased in a range of cancers and PP2A activators show therapeutic promise [37,38]. Importantly, additional levels of nuclear YAP/TAZ regulation exist. This additional regulation takes place both via nuclear Hippo pathway independent phosphorylation of YAP/TAZ, such as upon energy stress via AMPK mediated phosphorylation of YAP [39,40], and apparent phosphorylation independent mechanisms [41–43]. The critical role of Hippo pathway signalling in decision-making processes in almost all types of fundamental cell biology has spurred a great interest into this pathway. A precise, dynamic and robust regulation of the pathway is necessary, as otherwise developmental defects [44,45], fibrosis [46,47], impaired regeneration and cancer [30,48–51] occur. This precise regulation is obtained through multiple spatio-temporal level feedback, including transcriptional induction of upstream negative regulators, such as LATS2, AMOTL2, CAV1 and NF2 [17,20,52–59]. This multilevel response consequently reinforces and feeds robustness into the overall regulation of the pathway. Here, we highlight instances of fundamental cellular processes, using mitosis and cell migration as exemplars, that involve multilevel Hippo pathway-mediated integration. This dynamic complexity and synergistic feedback likely function to tightly regulate and safeguard central cellular processes.
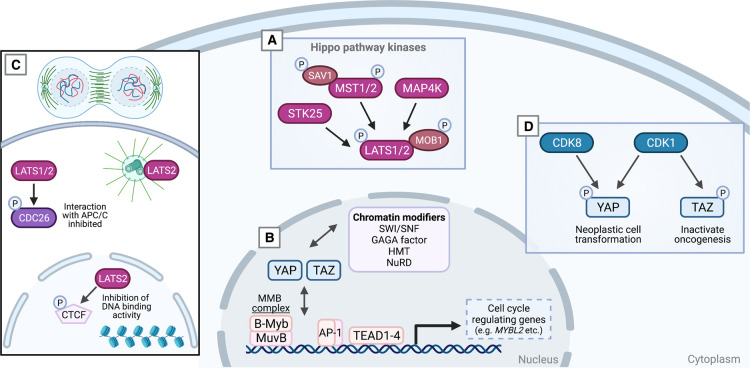
Figure 1. Regulation of mitosis by the Hippo pathway and YAP/TAZ.
(A) Upstream Hippo pathway serine/threonine kinases control activation of LATS1/2 kinases, which in turn directly phosphorylate YAP/TAZ on multiple serine residues and thereby controls YAP/TAZ transcriptional activity though regulating their subcellular localisation. (B) In the nucleus, YAP/TAZ form transcription complexes with various transcription factors to induce expression of cell cycle regulating genes. The cell cycle is further modulated by YAP/TAZ through its interaction with chromatin-modifying proteins, such as nucleosome remodelling and deacetylase (NuRD) complex and histone methyltransferase (HMT) complex, which promote remodelling of chromatin configuration to control gene transcription programmes. (C) LATS1/2 kinases modulate cell cycle exit during late anaphase or telophase through phosphorylation of CDC26 and CTCF. (D) Cyclin-dependent kinases provide an additional level of regulation of mitosis through direct phosphorylation of YAP and TAZ.
Mitosis
Control of cell number is essential to animal development, regenerative processes and in tissue homeostasis. Consequently, dysregulation of cell number may result in tumour formation, developmental defects or organ failure. The Hippo pathway regulates cell number by modulating cell proliferation, cell death and cell differentiation. These functions are shared and evolutionarily conserved from Drosophila to mammals [60]. Chromatin topological organisation is instrumental in gene transcription and overall chromosome compartmentalisation has emerged as critical to higher-order genome organisation [61,62]. During cell division, nuclear chromatin undergoes marked changes with respect to shape and degree of compaction [63–65]. Once segregated by the spindle, chromatin decondenses to re-establish its interphase structure competent for DNA replication and transcription. The precise mitotic chromatin condensation and decondensation is therefore a highly regulated process for mitotically active cells [63]. The Hippo pathway and YAP and TAZ contribute to the regulation of mitosis through interaction with transcription factors, chromatin modifiers and by inducing expression of mitotic genes.
YAP and TAZ modulate mitosis through the formation of a transcriptional complex with TEAD and AP-1, which promote proliferation and regulate expression of a range of cell cycle genes, including genes driving G1/S phase transition, DNA replication and quality control [20,66–70]. Moreover, YAP induces the expression of the transcription factor MYM proto-oncogene like 2 (B-MYB) (encoded by MYBL2) [71], a subunit of the multi-protein Myb-MuvB (MMB) complex. The MuvB core (composed of LIN9, LIN37, LIN52, LIN54 and RBBP4) upon entry into the cell cycle dissociates from p130 in the mitotic repressive DREAM complex [72] and binds to B-Myb during S phase to activate transcription of genes expressed late in the cell cycle. Consequently, overexpression of B-Myb shifts this equilibrium in favour of the mitosis promoting MMB complex and disrupts the DREAM complex [72,73]. Hyperactivation of B-Myb, therefore, cause elevated rate of mitosis and hyperproliferation in a range of cancers [74]. In addition to transcriptionally inducing MYBL2 (encoding B-Myb), YAP/TAZ also complex with B-Myb, which facilitate Myb-MuvB (MMB) chromatin binding and activation of the complex [71,75–77] (Figure 1B). Additional Hippo pathway-mediated regulation of the MMB complex takes place via LATS2. LATS2 phosphorylates and thereby activates the dual-specificity serine/threonine and tyrosine kinase DYRK1A, which in turn phosphorylates the LIN52 subunit of MuvB and consequently promotes the assembly of the DREAM complex [78,79]. Of note, global phosphoproteomic studies in glioblastoma cells highlight that DYRK1A may regulate the Hippo pathway, including via phosphorylation of NF2 and MAP4K4 [80]. Similarly, in flies the DYRK-family kinase Minibrain (Mnb) promotes Yorkie (Yki), the fly ortholog of YAP and TAZ-dependent tissue growth [81]. In addition to mediating their activity via transcription factors, YAP/TAZ also interact and function with multiple chromatin-modifying proteins, including SWI/SNF, GAGA factor, Mediator complex, Histone methyltransferase complex and the NuRD complex [82]. Consequently, depending on the cellular context, YAP/TAZ dynamically interact with a wide array of transcriptional regulators to control cell proliferation. However, the exact mechanism of how this process is fine-tuned remains to be elucidated.
The Hippo pathway additionally regulates mitosis via the zinc finger transcription factor CCCTC-binding factor (CTCF). CTCF is partially retained on mitotic chromosomes and immediately resumes full binding in ana/telophase [83–86]. CTCF and CTCF motifs function as cis-elements and therefore provide critical roles in nucleosome positioning. Consequently, this governs the inheritance of nucleosome positioning at regulatory regions throughout the cell cycle, especially those associated with fast gene reactivation following replication and mitosis [84]. LATS2 likely translocate between the nucleus and the cytoplasm, and a visible fraction localises to the centrosome [87–89]. LATS kinases phosphorylate CTCF in the zinc finger (ZF) linkers and disable its DNA-binding activity [90] (Figure 1C). Chromatin structural transitions during mitosis are tightly controlled as perturbances in this dynamic process can lead to genome dysfunction and culminate in loss of cellular fitness [63,91]. While YAP/TAZ interact with transcription factors to regulate mitotic entry, LATS1/2 exert negative feedback at the cytoplasmic level through direct inhibition of YAP/TAZ, as well as at the nuclear level through disassociation of CTCF from chromatin domains containing YAP target genes [90].
The core Hippo pathway kinases LATS1 and LATS2 further bind to and regulate additional master regulators of mitotic exit. LATS1 binds CDC25B [92] and LATS1/2 phosphorylate CDC26 (known as APC12) [93] (Figure 1C). Phosphorylation of CDC26 inhibits the interaction between CDC26 and anaphase-promoting complex 6 (APC6) and thereby modify the tetratricopeptide repeat subcomplex APC/C, which promotes mitotic exit through reduction in cyclin-dependent kinase (CDK) activity [93,94]. CDKs additionally regulate mitosis via YAP [95] and TAZ [96] as YAP and TAZ are directly phosphorylated on multiple residues by CDK1. CDK1-mediated TAZ phosphorylation inactivates TAZ oncogenic activity [96], whereas CDK1-mediated YAP phosphorylation induces neoplastic cell transformation [95] (Figure 1D). Interestingly, it appears that this mitotic phosphorylation of YAP and TAZ cause functionally opposite cellular responses, and this, therefore, appears to be an example where YAP and TAZ divergence exist. Additionally, CDK8 directly phosphorylate YAP and promote its activation and support tumour growth in colon cancer cells [97] (Figure 1D). The precise details and intricate network of how CDKs mediate YAP/TAZ phosphorylation during mitosis is currently not fully understood [95–97]. Importantly, Hippo pathway activity is critical for cytokinesis, as cytokinesis failure triggers small G protein-mediated activation of the Hippo pathway tumour suppressor kinase LATS2 [98]. LATS1/2-deficient cells or overexpression of YAP/TAZ cause spindle and centrosome defects, which result in failures in correct chromosome segregation and highlights the role of LATS1/2 in coordinating accurate cytokinesis [87,95,96,99,100]. In addition, CDK1 phosphorylates and activates LATS upon microtubule disruption and subsequent mitotic stress induced by chemicals. This genotoxicity causes LATS to stabilise replication forks by controlling CDK2-mediated phosphorylation of BRCA2 [101,102]. CDK1 activity maintains adhesion during interphase [103], which might provide an additional level of CDK1-mediated YAP/TAZ regulation during mitosis [104]. Furthermore, through an apparent transcription-independent mechanism, phosphorylation of YAP by CDK1 is required for tight control of cytokinesis [105]. YAP co-localises to the central spindle and midbody ring with proteins required for cell contraction as well as the polarity scaffold protein PATJ [105]. YAP is therefore required for accurate cytokinesis and cell division to occur. It is worth noting that YAP is not essential for mitosis as multiple proliferative cell model systems have been genetically engineered to be without YAP, as well as some in vivo systems such as the zebrafish develop to adulthood and are fertile. In brief, the cell cycle affects YAP and TAZ through both LATS1/2, CDK1 and CDK8-mediated phosphorylation and coordination of these kinases are central to implement upstream signals for precise timely progression through the cell cycle. This dynamic multilevel regulation of YAP/TAZ and the LATS kinases likely ensures fidelity and robustness and thereby safeguard the timing of proper cell division [89,91,98,101,106–108].
Cell migration
Directional cellular migration is essential during embryo development and is necessary for tissue homeostasis, such as epithelial turnover and in regenerative processes [109,110]. Since original studies carried out more than 110 years ago in both developing and injured embryonic chick embryos [111], the need to discover underlying molecular and mesoscale level mechanisms has been evident. Since then, discoveries obtained from live-cell imaging, in vivo model systems, biochemistry and ‘omics’ approaches have provided remarkable insights into both single and collective cellular migration to obtain multicellular mesoscale migration [109,110,112,113]. Migration and navigation through diverse three-dimensional environments require orchestrated and temporal change in cellular shape and directional movement of distinct cell populations [109,110,113]. Cell migration takes place both as individual cells, but also as collective migration driven by cell–cell contacts and directed by both chemical and biophysical cues [109,110,112,113]. Collective cell migration of epithelial cells requires coordination of actin cytoskeleton dynamics and YAP/TAZ activity, which is driven by YAP-mediated feedback interactions that involve down-regulation of E-cadherin and activation of Rac1 [114–116]. Interestingly, E-Cadherin is a prominent upstream cell–cell junction regulator of YAP/TAZ in mammalian epithelial cells [115,116].
The actin cytoskeleton is central to cell migration [117]. Dynamic polymerisation of actin monomers (G-actin) allow the generation of polar and branched actin fibres (F-actin) [117]. This process is spatiotemporally controlled by nucleation and elongation factors [117]. Consequently, actin polymerisation, retrograde actin network flow, treadmilling and contractility greatly mediated by the actin motor protein MyosinII play central roles in cell migration [110,117]. Cell migration integrates both context and cell type-specific chemical, cell–cell and cell–extracellular matrix (ECM) stimuli [109,110,113]. As a result, aberrant cell migration causes developmental defects, impaired regenerative processes and metastasis [109,110,113]. The Hippo pathway via the transcriptional mediators YAP/TAZ-TEAD function as a cellular rheostat for cellular migration, as it incorporates, mediates and dictates the necessary feedback to provide the cellular dynamics for directional migration [18,21,55,114,118–125]. Molecularly this feedback take place at several levels, which include sensing and integrating events at the plasma membrane and via the cytoskeleton [12]. YAP/TAZ are at the core of cue integration required for actin dynamics and cell migration while dysregulation of these dynamics allow cancer cells to change shape, invade surrounding tissues and metastasis [21,122–124,126]. YAP/TAZ are activated by F-actin-mediated mechanical tension and this YAP/TAZ tension sensing is inhibited by actin polymerisation inhibitor latrunculin B. Inhibition of F-actin formation causes LATS1/2 to complex with scaffolding proteins, become activated and subsequently inactivate YAP/TAZ [104,127,128]. This indicates that F-actin inhibits, and G-actin potentially facilitates this complex formation. RhoA regulates the level and dynamics of F-actin depolymerisation, such as increasing cytoskeleton rigidity and promoting a metastatic phenotype. YAP directly regulates actin dynamics through transcriptional up-regulations of Rho GTPase-activating proteins (GAPs) ARHGAP29 and ARHGAP18 [124,129,130], which suppresses RhoA activity and thereby destabilises F-actin. In contrast, YAP/TAZ can also induce Rho guanine exchange factor (RhoGEF) ARHGEF17 that activates RhoA activity [131] (Figure 2A). This combined escalates G- and F-actin turnover and promotes migration [124]. Thus, actin cytoskeleton dynamics regulate YAP/TAZ activity but YAP/TAZ also provide feedback into this modulation through the expression of actin regulating factors.
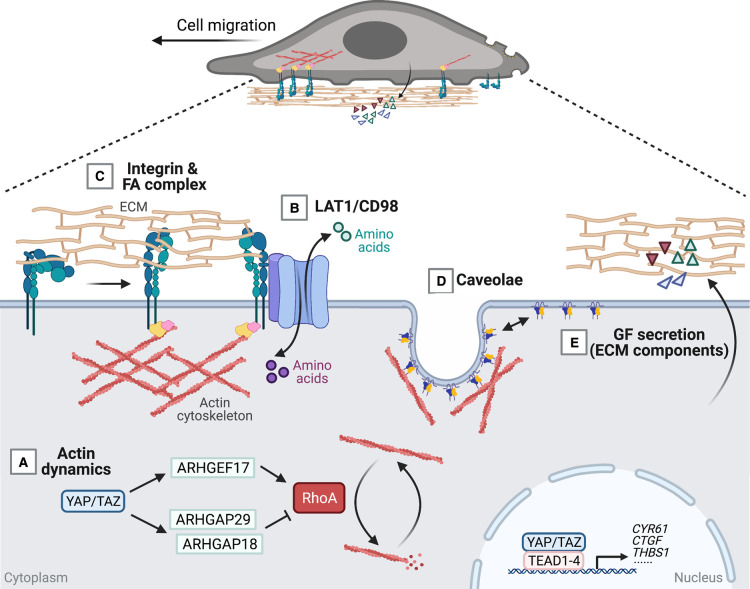
Figure 2. YAP/TAZ activity is central to coordinating cell migration.
Cell migration requires the coordination of various plasma membrane components that link extracellular matrix to the cell cytoskeleton. (A) YAP/TAZ influence the dynamics of the actin cytoskeleton through the expression of Rho GTPase-activating proteins (ARHGAP18, ARHGAP29) and Rho guanine exchange factor (ARHGEF17), which in turn modulates RhoA activity. (B) LAT1/CD98 is a disulfide-linked heterodimer composed of SLC3A2 and SLC7A5 that promotes cell migration and survival by coupling nutrient availability and integrin activity. LAT1/CD98 exports glutamine in exchange of importing amino acids, such as leucine, isoleucine and arginine. SLC3A2 of the LAT1/CD98 heterodimer binds integrins and amplifies integrin-mediated signalling. (C) At the leading edge of the cell, activation of integrin receptors through binding of extracellular matrix (ECM) components promotes focal adhesion complex assembly and the contractile forces generated by the actin cytoskeleton enables movement of the cell. (D) Plasma membrane subdomain caveolae expression is regulated by YAP/TAZ and plays a mechanoprotective role by flattening in response to mechanical forces, such as cellular stretching and osmotic swelling. In migrating cells, caveolae frequently localise to the rear due to low membrane tension. (E) YAP/TAZ-TEAD-mediated transcriptional activity promotes the production of matricellular proteins, such as CYR61 and CTGF. These signalling factors promote cell adhesion, migration and proliferation.
Plasma membrane receptors and subdomains are critical in transducing changes in the extracellular environment to cellular effects. YAP/TAZ-TEAD induce ECM receptors including the expression of the heterodimeric CD98/LAT1 [132] (encoded by SLC3A2 and SLC7A5), a dual function amino-acid transporter and integrin coreceptor, which thereby link mechanotransduction directly to cellular metabolism [132–136] (Figure 2B). Moreover, focal adhesions (FAs) together with integrins, which is integral to linking the ECM to the cytoskeleton, serve as components of the FAs spatio-temporal dynamics [119,137]. YAP/TAZ-TEAD through the generation of (FA) docking proteins and regulators (de)sensitise the dynamic transmission of forces between the cytoskeleton and ECM and thereby mediates the changes in cellular tension necessary for migration (Figure 2C). In addition to plasma membrane receptors, YAP/TAZ-TEAD are also essential for the expression of the machanotransductive and endocytic competent plasma membrane domains termed caveolae [56,57]. Caveolae [138,139] locate and form in migrating cells to the rear due to low membrane tension where they activate RhoA and control contractibility [140] (Figure 2D). Cells genetically modified to have no caveolae consequently have directional cellular migration defects [140,141].
The Hippo pathway also interacts with additional cytoskeletal regulators and is modulated by multiple levels of mechanical stimuli, including shear stress responses, cell–cell interactions, and the stiffness of the microenvironment [31,48]. YAP/TAZ-TEAD regulate the expression and secretion of a range of ECM components and additional growth factors, such as AREG, CYR61, CTGF and THBS1 [20,142–144] (Figure 2). This allows the formation of chemoattractant gradient that promotes cell migration while relaying extracellular mechanical signals to transcriptional regulation (Figure 2E). In addition to secretory extracellular factors, the mechanical extracellular environment regulates Hippo pathway activity. Upon low stiffness Ras-related GTPase (RAP2) binds to and activates ARHGAP29, which results in MAP4K and subsequent LATS1/2 activation. Consequently, YAP/TAZ are inhibited and RAP2 serves as an intracellular mechanosensor to relay stress from the ECM [143]. This combined integration with the actin cytoskeleton likely fine-tune a spatiotemporally co-ordinated actomyosin system necessary for directional cell migration.
Outlook
The Hippo pathway contains several levels of regulatory and feedback mechanisms. Prominent examples of feedback mechanisms regulating YAP/TAZ activity takes place through transcriptional up-regulation of LATS2, AMOTL2 and NF2 [55,58,59,145], modifiers of the actin cytoskeleton and plasma membrane components, such as caveolae [56,57]. It is evident that multiple levels of both positive and negative cellular feedback regulation are centred on the Hippo pathway in most, if not all, central cellular processes. Here, we have focussed on two of these processes, mitosis (Figure 1) and cellular migration (Figure 2). Many more examples are described elsewhere, such as integration of processes and organelles at the plasma membrane [12], cell size [132], mechanotransduction [31], fibrosis [46,47], cell polarity [146–148], metabolism [40,132,134–136,149], integration with auto- [150] and mito-phagy [151], in great part through interaction with numerous other cellular pathways [7]. These multilevel feedback loops are likely in place to dampen noise and prevent signal fluctuations, and to further integrate inputs from the cellular microenvironment [31,152–154]. An interesting initial perplexing observation is that many types of solid cancers are addicted to hyperactive YAP/TAZ [52,155]. However, it is well established that the pathway has an exceptionally low general somatic point mutation load [156], which means that the course to oncogenesis is currently not fully established. Corruption of the integrated and multilevel cellular feedback and regulation is therefore a likely cause as it offsets cellular and tissue homeostasis, which can result in developmental defects, fibrosis or cancer [30,44–51]. These dynamic molecular couplings serve in non-pathological conditions as flexible integrators of context-dependent stimuli, and likely provide robustness and thereby safeguard fundamental cellular processes from failing. YAP/TAZ, therefore, regulate both the expression of receptors and prominent signalling molecules, including chemical and matrix components [7,12]. YAP/TAZ play a central role by changing both the microenvironment chemical gradients and the intracellular response to already established physiochemical gradients within the microenvironment. New insights will undoubtedly be gained by implementing genetically encoded tagged versions of the Hippo pathway components expressed via genome-editing strategies to allow for the expression of these versions at endogenous levels. Further implementation of the use of distinct spectral sensitivity, for instance using light LOV domains [157] or drug-induced dimerisation, such as the knock sideways techniques [158], will be powerful. However, the initial focus must be on fully validating these systems to ensure that they function as the endogenous protein. This validation is facilitated at least for some of the components where highly specific antibodies are readily available for immunofluorescence imaging [159,160]. Comparative analysis that the cellular stability of the genome-tagged proteins, the steady-state expression levels and localisation dynamics closely follow that of the endogenous untagged version is critical. The realisation that widely used GFP derivatives (27 kDa) are relatively large proteins compared with YAP (70 kDa) and TAZ (55 kDa) is imperative. Some of these fluorescent proteins have additional dimerisation properties, which might combine with potential additional properties such as their ability to form biological condensates [161–163] as well as the ability to pass through the size filtering nuclear pore complexes [164]. Consequently, tagging Hippo pathway components might functionally impair YAP/TAZ and thus highlights that detailed design and characterisation must be carried out. The use of intra- or nano-bodies activatable optogenetic tools combining the specificity and orthogonality of intrabodies with the spatio-temporal precision of optogenetics might overcome some of these limitations [165–168]. Taken advantage of the full spectrum of these exciting techniques will be especially powerful to delineate the dynamics of the pathway, while also allowing to distinguish the intrinsic challenge within the Hippo pathway to determine between transcription dependent and independent mechanisms. We forsee that by using advanced experimental approaches, additional multilevel cellular regulation and integration centred on the Hippo pathway will become apparent in the years to come [55,56,58,59,145]. Realisations of these molecular couplings will provide fundamental insights into cellular processes while also providing new foundational therapeutic opportunities to target the challenging Hippo pathway.
Perspectives
Multilevel dynamic feedback is critical to safe-proofing fundamental cellular processes — the Hippo pathway is at the centre of this cellular decision-making process.
It remains challenging to distinguish Hippo pathway transcription independent from dependent functions, as YAP/TAZ regulates more than 1000 genes [69,126,169,170].
The use of newly developed technologies allows for establishing ‘moonlighting’ functions. For instance, what other substrates do STRIPAK and the range of Hippo pathway kinases have besides regulation of the core Hippo pathway? Spatio-temporal cooperation between components within the Hippo pathway and additional cellular components adds further complexity to the Hippo pathway. It is likely that in the years to come, additional layers of these feedback loops and synergistic multilevel regulation will be revealed and these discoveries are likely to be of therapeutic importance. It might therefore be useful to think about the pathway as a network instead of a linear pathway.
Acknowledgements
Work ongoing in the Gram Hansen lab is supported by a University of Edinburgh Chancellor's Fellowship as well as by Worldwide Cancer Research and LifeArc-CSO. Additional funding has been obtained from the Bone Cancer Research Trust (BCRT), Sarcoma U.K. (SUK202.2016), the Wellcome Trust-University of Edinburgh Institutional Strategic Support Fund (ISSF3) and the Jonathan Haw Fund/ Kinross Trust. J.P. is funded by a MRC Precision Medicine DTP Studentship. Figures were made using BioRender.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
C.G.H and J.P. wrote the manuscript. J.P. prepared the figures.
Open Access Statement
Open access for this article was enabled by the participation of University of Edinburgh in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Abbreviation
- CDK
cyclin-dependent kinase
- CTCF
CCCTC-binding factor
- ECM
extracellular matrix
- FAs
focal adhesions
- GAPs
GTPase-activating proteins
- LPA
lysophosphatidic acid
- MMB
multiprotein Myb-MuvB
References
- 1.Gokhale, R. and Pfleger, C.M. (2019) The power of drosophila genetics: the discovery of the Hippo pathway. Methods Mol. Biol. 1893, 3–26 10.1007/978-1-4939-8910-2_1 [DOI] [PubMed] [Google Scholar]
- 2.Bryant, P.J., Watson, K.L., Justice, R.W. and Woods, D.F. (1993) Tumor suppressor genes encoding proteins required for cell interactions and signal transduction in Drosophila. Dev. Suppl. 119, 239–249 PMID: [PubMed] [Google Scholar]
- 3.Justice, R.W., Zilian, O., Woods, D.F., Noll, M. and Bryant, P.J. (1995) The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 10.1101/gad.9.5.534 [DOI] [PubMed] [Google Scholar]
- 4.Xu, T., Wang, W., Zhang, S., Stewart, R.A. and Yu, W. (1995) Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 10.1242/dev.121.4.1053 [DOI] [PubMed] [Google Scholar]
- 5.Tapon, N., Harvey, K.F., Bell, D.W., Wahrer, D.C., Schiripo, T.A., Haber, D.et al. (2002) Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 10.1016/S0092-8674(02)00824-3 [DOI] [PubMed] [Google Scholar]
- 6.Kango-Singh, M., Nolo, R., Tao, C., Verstreken, P., Hiesinger, P.R., Bellen, H.J.et al. (2002) Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 10.1242/dev.00168 [DOI] [PubMed] [Google Scholar]
- 7.Hansen, C.G., Moroishi, T. and Guan, K.L. (2015) YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513 10.1016/j.tcb.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudol, M. (1994) Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene 9, 2145–2152 PMID: [PubMed] [Google Scholar]
- 9.Basu, S., Totty, N.F., Irwin, M.S., Sudol, M. and Downward, J. (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 10.1016/S1097-2765(02)00776-1 [DOI] [PubMed] [Google Scholar]
- 10.Vassilev, A., Kaneko, K.J., Shu, H., Zhao, Y. and DePamphilis, M.L. (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229–1241 10.1101/gad.888601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai, F., Marignani, P.A., Sarbassova, D., Yagi, R., Hall, R.A., Donowitz, M.et al. (2000) TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19, 6778–6791 10.1093/emboj/19.24.6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausch, V. and Hansen, C.G. (2020) The hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 30, 32–48 10.1016/j.tcb.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 13.Meng, Z., Moroishi, T., Mottier-Pavie, V., Plouffe, S.W., Hansen, C.G., Hong, A.W.et al. (2015) MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 6, 8357 10.1038/ncomms9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim, S., Hermance, N., Mudianto, T., Mustaly, H.M., Mauricio, I.P.M., Vittoria, M.A.et al. (2019) Identification of the kinase STK25 as an upstream activator of LATS signaling. Nat. Commun. 10, 1547 10.1038/s41467-019-09597-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Q., Li, S., Mana-Capelli, S., Roth Flach, R.J., Danai, L.V., Amcheslavsky, A.et al. (2014) The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev. Cell 31, 291–304 10.1016/j.devcel.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng, Y., Wang, W., Liu, B., Deng, H., Uster, E. and Pan, D. (2015) Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev. Cell 34, 642–655 10.1016/j.devcel.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao, B., Li, L., Tumaneng, K., Wang, C.Y. and Guan, K.L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24, 72–85 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu, F.X., Zhao, B., Panupinthu, N., Jewell, J.L., Lian, I., Wang, L.H.et al. (2012) Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 10.1016/j.cell.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, H., Liu, C.Y., Zha, Z.Y., Zhao, B., Yao, J., Zhao, S.et al. (2009) TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J. Biol. Chem. 284, 13355–13362 10.1074/jbc.M900843200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, B., Ye, X., Yu, J., Li, L., Li, W., Li, S.et al. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 10.1101/gad.1664408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamar, J.M., Stern, P., Liu, H., Schindler, J.W., Jiang, Z.G. and Hynes, R.O. (2012) The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl Acad Sci. U.S.A. 109, E2441–E2450 10.1073/pnas.1212021109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong, B.C., Bae, S.J., Ni, L., Zhang, X., Bai, X.C. and Luo, X. (2021) Cryo-EM structure of the Hippo signaling integrator human STRIPAK. Nat. Struct. Mol. Biol. 28, 290–299 10.1038/s41594-021-00564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couzens, A.L., Knight, J.D., Kean, M.J., Teo, G., Weiss, A., Dunham, W.H.et al. (2013) Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 6, rs15 10.1126/scisignal.2004712 [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro, P.S., Josue, F., Wepf, A., Wehr, M.C., Rinner, O., Kelly, G.et al. (2010) Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol. Cell 39, 521–534 10.1016/j.molcel.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 25.Zheng, Y., Liu, B., Wang, L., Lei, H., Pulgar Prieto, K.D. and Pan, D. (2017) Homeostatic control of Hpo/MST kinase activity through autophosphorylation-dependent recruitment of the STRIPAK PP2A phosphatase complex. Cell. Rep. 21, 3612–3623 10.1016/j.celrep.2017.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang, Y., Fang, G., Guo, F., Zhang, H., Chen, X., An, L.et al. (2020) Selective inhibition of STRN3-containing PP2A phosphatase restores Hippo tumor-suppressor activity in gastric cancer. Cancer Cell 38, 115–128.e9 10.1016/j.ccell.2020.05.019 [DOI] [PubMed] [Google Scholar]
- 27.Chen, R., Xie, R., Meng, Z., Ma, S. and Guan, K.L. (2019) STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat. Cell Biol. 21, 1565–1577 10.1038/s41556-019-0426-y [DOI] [PubMed] [Google Scholar]
- 28.Sarmasti Emami, S., Zhang, D. and Yang, X. (2020) Interaction of the Hippo pathway and phosphatases in tumorigenesis. Cancers (Basel) 12, 2438 10.3390/cancers12092438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae, S.J., Ni, L. and Luo, X. (2020) STK25 suppresses Hippo signaling by regulating SAV1-STRIPAK antagonism. eLife 9, e54863 10.7554/eLife.54863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo, J. and Yu, F.X. (2019) GPCR-Hippo signaling in cancer. Cells 8, 426 10.3390/cells8050426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanconato, F., Cordenonsi, M. and Piccolo, S. (2019) YAP and TAZ: a signalling hub of the tumour microenvironment. Nat. Rev. Cancer 19, 454–464 10.1038/s41568-019-0168-y [DOI] [PubMed] [Google Scholar]
- 32.Ho, L.T.Y., Skiba, N., Ullmer, C. and Rao, P.V. (2018) Lysophosphatidic acid induces ECM production via activation of the mechanosensitive YAP/TAZ transcriptional pathway in trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 59, 1969–1984 10.1167/iovs.17-23702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuda, D., Kobayashi, D., Akahoshi, N., Ohto-Nakanishi, T., Yoshioka, K., Takuwa, Y.et al. (2019) Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J. Clin. Invest. 129, 4332–4349 10.1172/JCI121955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang, Y., Chen, M., Zhou, L., Ma, J., Li, Y., Zhang, H.et al. (2019) Architecture, substructures, and dynamic assembly of STRIPAK complexes in Hippo signaling. Cell Discov. 5, 3 10.1038/s41421-018-0077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neal, S.J., Zhou, Q. and Pignoni, F. (2020) STRIPAK-PP2A regulates Hippo-Yorkie signaling to suppress retinal fate in the Drosophila eye disc peripodial epithelium. J. Cell Sci. 133, jcs237834 10.1242/jcs.237834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo, G., Han, H., Vargas, R.E., Yang, B., Li, X. and Wang, W. (2020) MAP4K interactome reveals STRN4 as a Key STRIPAK complex component in Hippo pathway regulation. Cell Rep. 32, 107860 10.1016/j.celrep.2020.107860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard, D., Huang, W., Izadmehr, S., O'Connor, C.M., Wiredja, D.D., Wang, Z.et al. (2020) Selective PP2A enhancement through biased heterotrimer stabilization. Cell 181, 688–701.e16 10.1016/j.cell.2020.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita, K., He, S., Nowak, R.P., Wang, J., Zimmerman, M.W., Fu, C.et al. (2020) Allosteric activators of protein phosphatase 2A display broad antitumor activity mediated by dephosphorylation of MYBL2. Cell 181, 702–715.e20 10.1016/j.cell.2020.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Wang, W., Xiao, Z.D., Li, X., Aziz, K.E., Gan, B., Johnson, R.L.et al. (2015) AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 10.1038/ncb3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo, J.S., Meng, Z., Kim, Y.C., Park, H.W., Hansen, C.G., Kim, S.et al. (2015) Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 17, 500–510 10.1038/ncb3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiao, S., Wang, H., Shi, Z., Dong, A., Zhang, W., Song, X.et al. (2014) A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 10.1016/j.ccr.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 42.Elosegui-Artola, A., Andreu, I., Beedle, A.E.M., Lezamiz, A., Uroz, M., Kosmalska, A.J.et al. (2017) Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 43.Lamar, J.M., Xiao, Y., Norton, E., Jiang, Z.G., Gerhard, G.M., Kooner, S.et al. (2019) SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J. Biol. Chem. 294, 2302–2317 10.1074/jbc.RA118.004364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis, J.R. and Tapon, N. (2019) Hippo signalling during development. Development 146, dev167106 10.1242/dev.167106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng, Y. and Pan, D. (2019) The Hippo signaling pathway in development and disease. Dev. Cell 50, 264–282 10.1016/j.devcel.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, C.L., Choi, S.H. and Mo, J.S. (2019) Role of the Hippo pathway in fibrosis and cancer. Cells 8, 468 10.3390/cells8050468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rognoni, E. and Walko, G. (2019) The roles of YAP/TAZ and the Hippo pathway in healthy and diseased skin. Cells 8, 411 10.3390/cells8050411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moroishi, T., Hansen, C.G. and Guan, K.L. (2015) The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 15, 73–79 10.1038/nrc3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulkarni, A., Chang, M.T., Vissers, J.H.A., Dey, A. and Harvey, K.F. (2020) The Hippo pathway as a driver of select human cancers. Trends Cancer 6, 781–796 10.1016/j.trecan.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 50.Thompson, B.J. (2020) YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays: news and reviews in molecular. Cell. Dev. Biol. 42, e1900162 10.1002/bies.201900162 [DOI] [PubMed] [Google Scholar]
- 51.Salem, O. and Hansen, C.G. (2019) The Hippo pathway in prostate cancer. Cells 8, 370 10.3390/cells8040370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Y., Xu, X., Maglic, D., Dill, M.T., Mojumdar, K., Ng, P.K.et al. (2018) Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 25, 1304–1317.e5 10.1016/j.celrep.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao, B., Li, L., Lu, Q., Wang, L.H., Liu, C.Y., Lei, Q.et al. (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25, 51–63 10.1101/gad.2000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirate, Y., Hirahara, S., Inoue, K., Suzuki, A., Alarcon, V.B., Akimoto, K.et al. (2013) Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 23, 1181–1194 10.1016/j.cub.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moroishi, T., Park, H.W., Qin, B., Chen, Q., Meng, Z., Plouffe, S.W.et al. (2015) A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 29, 1271–1284 10.1101/gad.262816.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rausch, V., Bostrom, J.R., Park, J., Bravo, I.R., Feng, Y., Hay, D.C.et al. (2019) The Hippo pathway regulates caveolae expression and mediates flow response via caveolae. Curr. Biol. 29, 242–255.e6 10.1016/j.cub.2018.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strippoli, R., Sandoval, P., Moreno-Vicente, R., Rossi, L., Battistelli, C., Terri, M.et al. (2020) Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 11, 647 10.1038/s41419-020-02822-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He, C., Lv, X., Huang, C., Hua, G., Ma, B., Chen, X.et al. (2019) YAP1-LATS2 feedback loop dictates senescent or malignant cell fate to maintain tissue homeostasis. EMBO Rep. 20, e44948 10.15252/embr.201744948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen, Q., Zhang, N., Xie, R., Wang, W., Cai, J., Choi, K.S.et al. (2015) Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 29, 1285–1297 10.1101/gad.264234.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imajo, M., Ebisuya, M. and Nishida, E. (2015) Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 17, 7–19 10.1038/ncb3084 [DOI] [PubMed] [Google Scholar]
- 61.Szabo, Q., Bantignies, F. and Cavalli, G. (2019) Principles of genome folding into topologically associating domains. Sci. Adv. 5, eaaw1668 10.1126/sciadv.aaw1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ou, H.D., Phan, S., Deerinck, T.J., Thor, A., Ellisman, M.H. and O'Shea, C.C. (2017) ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357, eaag0025 10.1126/science.357.6346.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonin, W. and Neumann, H. (2016) Chromosome condensation and decondensation during mitosis. Curr. Opin. Cell Biol. 40, 15–22 10.1016/j.ceb.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 64.Gibcus, J.H., Samejima, K., Goloborodko, A., Samejima, I., Naumova, N., Nuebler, J.et al. (2018) A pathway for mitotic chromosome formation. Science 359, eaao6135 10.1126/science.aao6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao, S.S., Huntley, M.H., Durand, N.C., Stamenova, E.K., Bochkov, I.D., Robinson, J.T.et al. (2014) A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang, J., Wu, S., Barrera, J., Matthews, K. and Pan, D. (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 67.Lavado, A., Park, J.Y., Pare, J., Finkelstein, D., Pan, H., Xu, B.et al. (2018) The Hippo pathway prevents YAP/TAZ-driven hypertranscription and controls neural progenitor number. Dev. Cell 47, 576–591.e8 10.1016/j.devcel.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koo, J.H., Plouffe, S.W., Meng, Z., Lee, D.H., Yang, D., Lim, D.S.et al. (2020) Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 34, 72–86 10.1101/gad.331546.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanconato, F., Forcato, M., Battilana, G., Azzolin, L., Quaranta, E., Bodega, B.et al. (2015) Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Agostino, S., Sorrentino, G., Ingallina, E., Valenti, F., Ferraiuolo, M., Bicciato, S.et al. (2016) YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 17, 188–201 10.15252/embr.201540488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pattschull, G., Walz, S., Grundl, M., Schwab, M., Ruhl, E., Baluapuri, A.et al. (2019) The Myb-MuvB complex is required for YAP-dependent transcription of mitotic genes. Cell Rep. 27, 3533–3546.e7 10.1016/j.celrep.2019.05.071 [DOI] [PubMed] [Google Scholar]
- 72.Sadasivam, S. and DeCaprio, J.A. (2013) The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat. Rev. Cancer 13, 585–595 10.1038/nrc3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iness, A.N., Felthousen, J., Ananthapadmanabhan, V., Sesay, F., Saini, S., Guiley, K.Z.et al. (2019) The cell cycle regulatory DREAM complex is disrupted by high expression of oncogenic B-Myb. Oncogene 38, 1080–1092 10.1038/s41388-018-0490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musa, J., Aynaud, M.M., Mirabeau, O., Delattre, O. and Grunewald, T.G. (2017) MYBL2 (B-Myb): a central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis. 8, e2895 10.1038/cddis.2017.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadasivam, S., Duan, S. and DeCaprio, J.A. (2012) The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev. 26, 474–489 10.1101/gad.181933.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grundl, M., Walz, S., Hauf, L., Schwab, M., Werner, K.M., Spahr, S.et al. (2020) Interaction of YAP with the Myb-MuvB (MMB) complex defines a transcriptional program to promote the proliferation of cardiomyocytes. PLoS Genet. 16, e1008818 10.1371/journal.pgen.1008818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabochette, P., Vega-Lopez, G., Bitard, J., Parain, K., Chemouny, R., Masson, C.et al. (2015) YAP controls retinal stem cell DNA replication timing and genomic stability. eLife 4, e08488 10.7554/eLife.08488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tschop, K., Conery, A.R., Litovchick, L., Decaprio, J.A., Settleman, J., Harlow, E.et al. (2011) A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. 25, 814–830 10.1101/gad.2000211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Litovchick, L., Florens, L.A., Swanson, S.K., Washburn, M.P. and DeCaprio, J.A. (2011) DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev. 25, 801–813 10.1101/gad.2034211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Recasens, A., Humphrey, S.J., Ellis, M., Hoque, M., Abbassi, R.H., Chen, B.et al. (2021) Global phosphoproteomics reveals DYRK1A regulates CDK1 activity in glioblastoma cells. Cell Death Discov. 7, 81 10.1038/s41420-021-00456-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Degoutin, J.L., Milton, C.C., Yu, E., Tipping, M., Bosveld, F., Yang, L.et al. (2013) Riquiqui and minibrain are regulators of the Hippo pathway downstream of Dachsous. Nat. Cell Biol. 15, 1176–1185 10.1038/ncb2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hillmer, R.E. and Link, B.A. (2019) The roles of Hippo signaling transducers Yap and Taz in chromatin remodeling. Cells 8, 502 10.3390/cells8050502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burke, L.J., Zhang, R., Bartkuhn, M., Tiwari, V.K., Tavoosidana, G., Kurukuti, S.et al. (2005) CTCF binding and higher order chromatin structure of the H19 locus are maintained in mitotic chromatin. EMBO J. 24, 3291–3300 10.1038/sj.emboj.7600793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Owens, N., Papadopoulou, T., Festuccia, N., Tachtsidi, A., Gonzalez, I., Dubois, A.et al. (2019) CTCF confers local nucleosome resiliency after DNA replication and during mitosis. eLife 8, e47898 10.7554/eLife.47898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oomen, M.E., Hansen, A.S., Liu, Y., Darzacq, X. and Dekker, J. (2019) CTCF sites display cell cycle-dependent dynamics in factor binding and nucleosome positioning. Genome Res. 29, 236–249 10.1101/gr.241547.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang, H., Emerson, D.J., Gilgenast, T.G., Titus, K.R., Lan, Y., Huang, P.et al. (2019) Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature 576, 158–162 10.1038/s41586-019-1778-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yabuta, N., Okada, N., Ito, A., Hosomi, T., Nishihara, S., Sasayama, Y.et al. (2007) Lats2 is an essential mitotic regulator required for the coordination of cell division. J. Biol. Chem. 282, 19259–19271 10.1074/jbc.M608562200 [DOI] [PubMed] [Google Scholar]
- 88.Nishiyama, Y., Hirota, T., Morisaki, T., Hara, T., Marumoto, T., Iida, S.et al. (1999) A human homolog of Drosophila warts tumor suppressor, h-warts, localized to mitotic apparatus and specifically phosphorylated during mitosis. FEBS Lett. 459, 159–165 10.1016/S0014-5793(99)01224-7 [DOI] [PubMed] [Google Scholar]
- 89.McPherson, J.P., Tamblyn, L., Elia, A., Migon, E., Shehabeldin, A., Matysiak-Zablocki, E.et al. (2004) Lats2/Kpm is required for embryonic development, proliferation control and genomic integrity. EMBO J. 23, 3677–3688 10.1038/sj.emboj.7600371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo, H., Yu, Q., Liu, Y., Tang, M., Liang, M., Zhang, D.et al. (2020) LATS kinase-mediated CTCF phosphorylation and selective loss of genomic binding. Sci. Adv. 6, eaaw4651 10.1126/sciadv.aaw4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bolgioni, A.F. and Ganem, N.J. (2016) The interplay between centrosomes and the Hippo tumor suppressor pathway. Chromosome Res. 24, 93–104 10.1007/s10577-015-9502-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukai, S., Yabuta, N., Yoshida, K., Okamoto, A., Miura, D., Furuta, Y.et al. (2015) Lats1 suppresses centrosome overduplication by modulating the stability of Cdc25B. Sci. Rep. 5, 16173 10.1038/srep16173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masuda, K., Chiyoda, T., Sugiyama, N., Segura-Cabrera, A., Kabe, Y., Ueki, A.et al. (2015) LATS1 and LATS2 phosphorylate CDC26 to modulate assembly of the tetratricopeptide repeat subcomplex of APC/C. PLoS One 10, e0118662 10.1371/journal.pone.0118662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sivakumar, S. and Gorbsky, G.J. (2015) Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat. Rev. Mol. Cell Biol. 16, 82–94 10.1038/nrm3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang, S., Zhang, L., Liu, M., Chong, R., Ding, S.J., Chen, Y.et al. (2013) CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res. 73, 6722–6733 10.1158/0008-5472.CAN-13-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang, L., Chen, X., Stauffer, S., Yang, S., Chen, Y. and Dong, J. (2015) CDK1 phosphorylation of TAZ in mitosis inhibits its oncogenic activity. Oncotarget 6, 31399–31412 10.18632/oncotarget.5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou, J., Zeng, Y., Cui, L., Chen, X., Stauffer, S., Wang, Z.et al. (2018) Zyxin promotes colon cancer tumorigenesis in a mitotic phosphorylation-dependent manner and through CDK8-mediated YAP activation. Proc. Natl Acad. Sci. U.S.A. 115, E6760–E67E9 10.1073/pnas.1800621115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganem, N.J., Cornils, H., Chiu, S.Y., O'Rourke, K.P., Arnaud, J., Yimlamai, D.et al. (2014) Cytokinesis failure triggers Hippo tumor suppressor pathway activation. Cell 158, 833–848 10.1016/j.cell.2014.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim, W., Cho, Y.S., Wang, X., Park, O., Ma, X., Kim, H.et al. (2019) Hippo signaling is intrinsically regulated during cell cycle progression by APC/C(Cdh1). Proc. Natl Acad. Sci. U.S.A. 116, 9423–9432 10.1073/pnas.1821370116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iida, S., Hirota, T., Morisaki, T., Marumoto, T., Hara, T., Kuninaka, S.et al. (2004) Tumor suppressor WARTS ensures genomic integrity by regulating both mitotic progression and G1 tetraploidy checkpoint function. Oncogene 23, 5266–5274 10.1038/sj.onc.1207623 [DOI] [PubMed] [Google Scholar]
- 101.Pefani, D.E., Latusek, R., Pires, I., Grawenda, A.M., Yee, K.S., Hamilton, G.et al. (2014) RASSF1A-LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat. Cell Biol. 16, 962–971, 1–8 10.1038/ncb3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuoka, S., Ballif, B.A., Smogorzewska, A., McDonald, III, E.R., Hurov, K.E., Luo, J.et al. (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 10.1126/science.1140321 [DOI] [PubMed] [Google Scholar]
- 103.Jones, M.C., Zha, J. and Humphries, M.J. (2019) Connections between the cell cycle, cell adhesion and the cytoskeleton. Phil. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180227 10.1098/rstb.2018.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao, B., Li, L., Wang, L., Wang, C.Y., Yu, J. and Guan, K.L. (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 10.1101/gad.173435.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bui, D.A., Lee, W., White, A.E., Harper, J.W., Schackmann, R.C., Overholtzer, M.et al. (2016) Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP. Sci. Signal. 9, ra23 10.1126/scisignal.aaa9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang, S., Chen, Q., Liu, Q., Li, Y., Sun, X., Hong, L.et al. (2017) Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell 31, 669–684.e7 10.1016/j.ccell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aylon, Y., Michael, D., Shmueli, A., Yabuta, N., Nojima, H. and Oren, M. (2006) A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 20, 2687–2700 10.1101/gad.1447006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hergovich, A., Kohler, R.S., Schmitz, D., Vichalkovski, A., Cornils, H. and Hemmings, B.A. (2009) The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr. Biol. 19, 1692–1702 10.1016/j.cub.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 109.Kechagia, J.Z., Ivaska, J. and Roca-Cusachs, P. (2019) Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- 110.Yamada, K.M. and Sixt, M. (2019) Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 10.1038/s41580-019-0172-9 [DOI] [PubMed] [Google Scholar]
- 111.Ruth, E.S. (1911) Cicatrization of wounds in vitro. J. Exp. Med. 13, 422–424 10.1084/jem.13.4.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collinet, C. and Lecuit, T. (2021) Programmed and self-organized flow of information during morphogenesis. Nat. Rev. Mol. Cell Biol. 22, 245–265 10.1038/s41580-020-00318-6 [DOI] [PubMed] [Google Scholar]
- 113.Paul, C.D., Mistriotis, P. and Konstantopoulos, K. (2017) Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 10.1038/nrc.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park, J., Kim, D.H., Shah, S.R., Kim, H.N., Kshitiz, Kim, P.et al. (2019) Switch-like enhancement of epithelial-mesenchymal transition by YAP through feedback regulation of WT1 and Rho-family GTPases. Nat. Commun. 10, 2797 10.1038/s41467-019-10729-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim, N.G., Koh, E., Chen, X. and Gumbiner, B.M. (2011) E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl Acad. Sci. U.S.A. 108, 11930–11935 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benham-Pyle, B.W., Pruitt, B.L. and Nelson, W.J. (2015) Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science 348, 1024–1027 10.1126/science.aaa4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Svitkina, T. (2018) The actin cytoskeleton and actin-based motility. Cold Spring Harb. Perspect. Biol. 10, a018267 10.1101/cshperspect.a018267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang, Z., Wu, Y., Wang, H., Zhang, Y., Mei, L., Fang, X.et al. (2014) Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl Acad. Sci. U.S.A. 111, E89–E98 10.1073/pnas.1319190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nardone, G., La Cruz J, O.-D., Vrbsky, J., Martini, C., Pribyl, J., Skladal, P.et al. (2017) YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 8, 15321 10.1038/ncomms15321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mason, D.E., Collins, J.M., Dawahare, J.H., Nguyen, T.D., Lin, Y., Voytik-Harbin, S.L.et al. (2019) YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol. 218, 1369–1389 10.1083/jcb.201806065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen, J., Cao, B., Wang, Y., Ma, C., Zeng, Z., Liu, L.et al. (2018) Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J. Exp. Clin. Cancer Res. 37, 175 10.1186/s13046-018-0850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu, F.X., Luo, J., Mo, J.S., Liu, G., Kim, Y.C., Meng, Z.et al. (2014) Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25, 822–830 10.1016/j.ccr.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chan, S.W., Lim, C.J., Guo, K., Ng, C.P., Lee, I., Hunziker, W.et al. (2008) A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 68, 2592–2598 10.1158/0008-5472.CAN-07-2696 [DOI] [PubMed] [Google Scholar]
- 124.Qiao, Y., Chen, J., Lim, Y.B., Finch-Edmondson, M.L., Seshachalam, V.P., Qin, L.et al. (2017) YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 19, 1495–1502 10.1016/j.celrep.2017.04.075 [DOI] [PubMed] [Google Scholar]
- 125.Kim, J., Kwon, H., Shin, Y.K., Song, G., Lee, T., Kim, Y.et al. (2020) MAML1/2 promote YAP/TAZ nuclear localization and tumorigenesis. Proc. Natl Acad Sci. U.S.A. 117, 13529–13540 10.1073/pnas.1917969117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park, H.W., Kim, Y.C., Yu, B., Moroishi, T., Mo, J.S., Plouffe, S.W.et al. (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162, 780–794 10.1016/j.cell.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin, F., Yu, J., Zheng, Y., Chen, Q., Zhang, N. and Pan, D. (2013) Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154, 1342–1355 10.1016/j.cell.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ibar, C., Kirichenko, E., Keepers, B., Enners, E., Fleisch, K. and Irvine, K.D. (2018) Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J. Cell Sci. 131, jcs214700 10.1242/jcs.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maeda, M., Hasegawa, H., Hyodo, T., Ito, S., Asano, E., Yuang, H.et al. (2011) ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol. Biol. Cell 22, 3840–3852 10.1091/mbc.e11-04-0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Porazinski, S., Wang, H., Asaoka, Y., Behrndt, M., Miyamoto, T., Morita, H.et al. (2015) YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature 521, 217–221 10.1038/nature14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin, C., Yao, E., Zhang, K., Jiang, X., Croll, S., Thompson-Peer, K.et al. (2017) YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife 6, e21130 10.7554/eLife.21130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hansen, C.G., Ng, Y.L., Lam, W.L., Plouffe, S.W. and Guan, K.L. (2015) The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 25, 1299–1313 10.1038/cr.2015.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boulter, E., Estrach, S., Tissot, F.S., Hennrich, M.L., Tosello, L., Cailleteau, L.et al. (2018) Cell metabolism regulates integrin mechanosensing via an SLC3A2-dependent sphingolipid biosynthesis pathway. Nat. Commun. 9, 4862 10.1038/s41467-018-07268-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Najumudeen, A.K., Ceteci, F., Fey, S.K., Hamm, G., Steven, R.T., Hall, H.et al. (2021) The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat. Genet. 53, 16–26 10.1038/s41588-020-00753-3 [DOI] [PubMed] [Google Scholar]
- 135.Estrach, S., Lee, S.A., Boulter, E., Pisano, S., Errante, A., Tissot, F.S.et al. (2014) CD98hc (SLC3A2) loss protects against ras-driven tumorigenesis by modulating integrin-mediated mechanotransduction. Cancer Res. 74, 6878–6889 10.1158/0008-5472.CAN-14-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan, R., Zhao, X., Lei, J. and Zhou, Q. (2019) Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature 568, 127–130 10.1038/s41586-019-1011-z [DOI] [PubMed] [Google Scholar]
- 137.Tan, S.J., Chang, A.C., Anderson, S.M., Miller, C.M., Prahl, L.S., Odde, D.J.et al. (2020) Regulation and dynamics of force transmission at individual cell-matrix adhesion bonds. Sci. Adv. 6, eaax0317 10.1126/sciadv.aax0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hansen, C.G. and Nichols, B.J. (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 20, 177–186 10.1016/j.tcb.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 139.Hubert, M., Larsson, E. and Lundmark, R. (2020) Keeping in touch with the membrane; protein- and lipid-mediated confinement of caveolae to the cell surface. Biochem. Soc. Trans. 48, 155–163 10.1042/BST20190386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hetmanski, J.H.R., de Belly, H., Busnelli, I., Waring, T., Nair, R.V., Sokleva, V.et al. (2019) Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 51, 460–475.e10 10.1016/j.devcel.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grande-Garcia, A., Echarri, A., de Rooij, J., Alderson, N.B., Waterman-Storer, C.M., Valdivielso, J.M.et al. (2007) Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 177, 683–694 10.1083/jcb.200701006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamashiro, Y., Thang, B.Q., Ramirez, K., Shin, S.J., Kohata, T., Ohata, S.et al. (2020) Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl Acad. Sci. U.S.A. 117, 9896–9905 10.1073/pnas.1919702117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meng, Z., Qiu, Y., Lin, K.C., Kumar, A., Placone, J.K., Fang, C.et al. (2018) RAP2 mediates mechanoresponses of the Hippo pathway. Nature 560, 655–660 10.1038/s41586-018-0444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang, J., Ji, J.Y., Yu, M., Overholtzer, M., Smolen, G.A., Wang, R.et al. (2009) YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat. Cell Biol. 11, 1444–1450 10.1038/ncb1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dai, X., Liu, H., Shen, S., Guo, X., Yan, H., Ji, X.et al. (2015) YAP activates the Hippo pathway in a negative feedback loop. Cell Res. 25, 1175–1178 10.1038/cr.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan, B., Yatim, S., Peng, S., Gunaratne, J., Hunziker, W. and Ludwig, A. (2020) The mammalian crumbs complex defines a distinct polarity domain apical of epithelial tight junctions. Curr. Biol. 30, 2791–2804.e6 10.1016/j.cub.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 147.Chen, C.L., Gajewski, K.M., Hamaratoglu, F., Bossuyt, W., Sansores-Garcia, L., Tao, C.et al. (2010) The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl Acad. Sci. U.S.A. 107, 15810–15815 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Varelas, X., Samavarchi-Tehrani, P., Narimatsu, M., Weiss, A., Cockburn, K., Larsen, B.G.et al. (2010) The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell 19, 831–844 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 149.Alsamman, S., Christenson, S.A., Yu, A., Ayad, N.M.E., Mooring, M.S., Segal, J.M.et al. (2020) Targeting acid ceramidase inhibits YAP/TAZ signaling to reduce fibrosis in mice. Sci. Transl. Med. 12, eaay8798 10.1126/scitranslmed.aay8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang, D., He, J., Huang, B., Liu, S., Zhu, H. and Xu, T. (2020) Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 11, 880 10.1038/s41419-020-03069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cho, Y.K., Son, Y., Saha, A., Kim, D., Choi, C., Kim, M.et al. (2021) STK3/STK4 signalling in adipocytes regulates mitophagy and energy expenditure. Nat. Metab. 3, 428–441 10.1038/s42255-021-00362-2 [DOI] [PubMed] [Google Scholar]
- 152.Calvo, F., Ege, N., Grande-Garcia, A., Hooper, S., Jenkins, R.P., Chaudhry, S.I.et al. (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M.et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 154.Pankova, D., Jiang, Y., Chatzifrangkeskou, M., Vendrell, I., Buzzelli, J., Ryan, A.et al. (2019) RASSF1A controls tissue stiffness and cancer stem-like cells in lung adenocarcinoma. EMBO J. 38, e100532 10.15252/embj.2018100532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Steinhardt, A.A., Gayyed, M.F., Klein, A.P., Dong, J., Maitra, A., Pan, D.et al. (2008) Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 39, 1582–1589 10.1016/j.humpath.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kandoth, C., McLellan, M.D., Vandin, F., Ye, K., Niu, B., Lu, C.et al. (2013) Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Redchuk, T.A., Kaberniuk, A.A. and Verkhusha, V.V. (2018) Near-infrared light-controlled systems for gene transcription regulation, protein targeting and spectral multiplexing. Nat. Protoc. 13, 1121–1136 10.1038/nprot.2018.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Robinson, M.S., Sahlender, D.A. and Foster, S.D. (2010) Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev. Cell 18, 324–331 10.1016/j.devcel.2009.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kingston, N.M., Tilston-Lunel, A.M., Hicks-Berthet, J. and Varelas, X. (2019) Immunofluorescence microscopy to study endogenous TAZ in mammalian cells. Methods Mol. Biol. 1893, 107–113 10.1007/978-1-4939-8910-2_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rausch, V. and Hansen, C.G. (2019) Immunofluorescence study of endogenous YAP in mammalian cells. Methods Mol. Biol. 1893, 97–106 10.1007/978-1-4939-8910-2_8 [DOI] [PubMed] [Google Scholar]
- 161.Cai, D., Feliciano, D., Dong, P., Flores, E., Gruebele, M., Porat-Shliom, N.et al. (2019) Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 21, 1578–1589 10.1038/s41556-019-0433-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu, Y., Wu, T., Gutman, O., Lu, H., Zhou, Q., Henis, Y.I.et al. (2020) Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 10.1038/s41556-020-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alberti, S., Gladfelter, A. and Mittag, T. (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Timney, B.L., Raveh, B., Mironska, R., Trivedi, J.M., Kim, S.J., Russel, D.et al. (2016) Simple rules for passive diffusion through the nuclear pore complex. J. Cell Biol. 215, 57–76 10.1083/jcb.201601004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dong, J.X., Lee, Y., Kirmiz, M., Palacio, S., Dumitras, C., Moreno, C.M.et al. (2019) A toolbox of nanobodies developed and validated for use as intrabodies and nanoscale immunolabels in mammalian brain neurons. eLife 8, e48750 10.7554/eLife.48750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Redchuk, T.A., Karasev, M.M., Verkhusha, P.V., Donnelly, S.K., Hulsemann, M., Virtanen, J.et al. (2020) Optogenetic regulation of endogenous proteins. Nat. Commun. 11, 605 10.1038/s41467-020-14460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Valon, L., Marin-Llaurado, A., Wyatt, T., Charras, G. and Trepat, X. (2017) Optogenetic control of cellular forces and mechanotransduction. Nat. Commun. 8, 14396 10.1038/ncomms14396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dowbaj, A.M., Jenkins, R.P., Williamson, D., Heddleston, J.M., Ciccarelli, A., Fallesen, T.et al. (2021) An optogenetic method for interrogating YAP1 and TAZ nuclear-cytoplasmic shuttling. J. Cell Sci. 134, jcs.253484 10.1242/jcs.253484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walko, G., Woodhouse, S., Pisco, A.O., Rognoni, E., Liakath-Ali, K., Lichtenberger, B.M.et al. (2017) A genome-wide screen identifies YAP/WBP2 interplay conferring growth advantage on human epidermal stem cells. Nat. Commun. 8, 14744 10.1038/ncomms14744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Debaugnies, M., Sanchez-Danes, A., Rorive, S., Raphael, M., Liagre, M., Parent, M.A.et al. (2018) YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 19, e45809 10.15252/embr.201845809 [DOI] [PMC free article] [PubMed] [Google Scholar]