Abstract
Epithelial stem cells reside within multiple regions of the lung where they renew various region-specific cells. In addition, there are multiple routes of regeneration after injury through built-in heterogeneity within stem cell populations and through a capacity for cellular plasticity among differentiated cells. These processes are important facets of respiratory tissue resiliency and organism survival. However, this regenerative capacity is not limitless, and repetitive or chronic injuries, environmental stresses, or underlying factors of disease may ultimately lead to or contribute to tissue remodeling and end-stage lung disease. This chapter will review stem cell heterogeneity among pulmonary epithelia in the lower respiratory system, discuss recent findings that may challenge long-held scientific paradigms, and identify several clinically relevant research opportunities for regenerative medicine.
Keywords: Stem cell, Basal stem cell, Club cell, Myoepithelial cell, Submucosal gland, Obliterative bronchiolitis, Chronic rejection, Ionocyte, Goblet cell, Alveolar type II cell
Introduction
The pulmonary epithelium contains multiple distinct functional units with multiple cell types. In the pseudostratified columnar epithelium abundant cell types include: secretory club-like cells, multiciliated cells, and basal cells. Less frequent cell types include: neuroendocrine cells, goblet cells, brush/tuft cells, and the recently discovered ionocytes [1, 2]. Along the cartilaginous tracheobronchial airways, epithelial submucosal glands (SMGs) reside within the airway mesenchyme. SMGs contain secretory mucous and serous cells as well as contractile myoepithelial cells, and glandular ducts contain multiciliated cells as well as basal-like duct cells. The cuboidal epithelium of terminal bronchioles contains secretory club cells, fewer multiciliated cells than those in larger airways, and infrequent airway basal cells. Terminal airways transition into alveoli, which are lined by squamous alveolar type 1 cells and cuboidal alveolar type 2 cells. It is evident that lung epithelial cell types and their fractional distribution in the population change as we move from proximal to distal airways. However, it is also important to acknowledge that stem cells that give rise to these tissues are distinct as well. In this chapter, we discuss the stem cell types within each of these regions and focus on heterogeneity within each population.
Researchers are exploring pulmonary stem cell biology using both human and animal models. Transgenic mouse models allow researchers to study stem cells within their native microenvironment using lineage-labeling approaches. These approaches rely on cell-specific promoter activity to drive expression of Cre recombinase, which provides a spatial selectivity for labeling only specific cell types. Temporal control is accomplished with the use of inducible recombinases such as CreERT2, which is activated with tamoxifen administration. Lineage-tracing experiments have a labeling phase and a fate mapping phase. In the labeling phase, Cre-mediated recombination is usually used to activate the expression of a fluorescent protein. In the fate mapping phase, labeled cells divide and differentiate, and their daughter cells can be followed to establish lineage relationship hierarchies. Lineage labeling experiments can be performed within a normal physiological context to study steady-state turnover or following injury to study regeneration. However, initial labeling specificity is key to interpreting fate mapping results. Cre-drivers used to lineage-label specific cell types must demonstrate specific expression throughout the labeling stage of the experiment. A tamoxifen washout period is often necessary prior to injury to avoid nonspecific labeling of cells that potentially activate the promoter driving Cre as a result of injury. In addition, lineage-labeling cells during developmental windows, when precursor cell identities may still be relatively flexible, could contribute to complexity in interpreting fate mapping results. However, these experiments often provide compelling evidence for the origin of different cell types within a tissue. Overall, despite the constraints of Cre specificity and potential “leakiness” of some Cre-driving promoters, this strategy of stem cell lineage labeling remains to be an accepted standard approach in the field.
In vivo lineage-labeling studies have led to important discoveries of how region-specific stem cell niches maintain quiescence or promote proliferation. For example, a subtype of Axin2-CreERT2 lineage-labeled alveolar type 2 (AT2) cells is regulated by a single fibroblast cell niche that can secrete different Wnt ligands to promote either a quiescent or a proliferative state [3]. Moreover, fluorescent reporters can enable isolation of lineage-traced cells by fluorescence-activated cell sorting (FACS) for characterization in vitro. For example, in vitro colony formation efficiency and differentiation assays can be useful for experiments designed to test the stemness of various cell types within a highly controlled in vitro context (e.g., in the presence of specified growth factors). Within the mammalian pulmonary system, lineage-labeling approaches have been primarily limited to mouse models, and this may be a limitation for studying biology that is not conserved between mice and humans. However, alternative animal models (like the ferret) are becoming increasingly available for lineage-labeling [4]. Tracking lineage-labeled stem cells under native conditions in vivo or in a controlled environment in vitro can help answer the questions regarding proliferative and differentiation capacity as well as self-renewal capability of the stem cells of interest.
Lineage relationships can also be inferred using high-dimensional single-cell datasets. For example, single-cell mRNA sequencing has been used to study human cell lineage hierarchies [2, 5–9]. Powerful new bioinformatic approaches have even led to the novel discovery of a rare airway cell type—the ionocyte—which amounts to less than 1% of cells in the tracheobronchial surface airway epithelium (SAE), yet accounts for more than half of its CFTR mRNA expression [1, 2]. This discovery has critical implications for cystic fibrosis research considering that mutations in CFTR primarily define the pathology. In addition, single-cell approaches can even delineate complexity within what was previously considered a homogeneous cell population. For example, Montoro et al. [1] discovered that in mice there may be distinct subsets of tuft and goblet cells. However, discoveries using single-cell approaches may be confounded by the fact that cells must be enzymatically isolated from tissue samples and analyzed away from their physiological compartment. Thus, experimental in vivo validation is necessary. To address this challenge, sequencing approaches can also be coupled with in vivo lineage analysis to add temporal and spatial dimension into sequencing experiments. Both lineage labeling and sequencing experiments have accelerated the field of stem cell research, and in the modern age of “Big Data,” combining these technologies has expanded our knowledge even further.
Basal Cells
Basal cells serve as multipotent stem cells in the SAE of conducting airways (Fig. 6.1). They reside along the airway basal lamina where they contact multiple luminal cell types and play an important role in homeostasis due to their ability to self-renew and differentiate into various luminal cell lineages [10, 15, 16]. Basal cells can also regenerate the airway epithelium following injury [17–19]. However, basal cell regeneration may be insufficient in some pathologic states. For example, chronic inflammation may eventually overcome basal cell regeneration capacity leading to basal stem cell depletion [20–22]. Thus, we must expand our knowledge of basal cell biology to better understand lung diseases and to refine novel basal cell-targeted regenerative therapies.
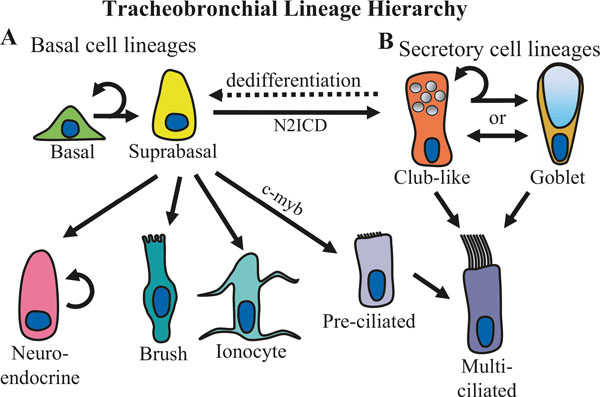
Fig. 6.1.
Lineage relationships of the surface airway epithelium in the trachea and bronchi. (a) Basal cells self-renew and give rise to all of the cell types present in the surface airway epithelium. Basal cells differentiate into luminal lineages through an intermediate suprabasal cell state [10]. Basal cells can give rise to self-renewing neuroendocrine cells in addition to brush (or tuft) cells and ionocytes [1, 2]. In addition, a subset of basal cells that display intracellular Notch2 activation (N2ICD) gives rise to secretory cells, whereas basal cells that express low levels of c-myb are able to lineage commit toward multiciliated cells [11]. Recent, single-cell RNA sequencing experiments have suggested that basal cells give rise to multiciliated cells through a differentiation of an intermediate population of pre-ciliated cells [12]. If basal cells are ablated, club-like cells can renew basal cells through a process of dedifferentiation (dashed line) [13]. (b) Secretory cells in large airways consist of club-like cells and goblet cells. Club-like cells are similar to bronchiolar club cells in that they express proteins commonly used to identify bronchiolar club cells, such as SCGB1A1 (a.k.a. club cell secretory protein); however, unlike bronchiolar club cells, large airway club-like cells also express mucins such as MUC5B and MUC5AC [14]. Secretory goblet cells have a distinct goblet or cup-like morphology but express many of the same phenotypic markers that club-like cells express; thus, it is unclear if club-like cells and goblet cells are distinct secretory cell fates or if a single population of secretory cells fluctuates between displaying a club-like or goblet cell morphology depending on environmental factors. Multiciliated cell renewal is primarily accomplished by club-like progenitors at steady state [10] and following injury [1, 12]. In addition, it has recently been argued that goblet cells may act as a differentiation intermediate between club-like secretory cells and multiciliated cells [12]
At steady state, airway basal cells are most commonly identified by TP63 (p63) and KRT5 expression. Expression of many keratins may help distinguish luminal epithelial cells from basal cells as well as subtypes of basal cells from one another (Table 6.1). For example, Watson et al. [10] discovered that basal cells that express KRT8 are fated to differentiate into luminal cell types. Recent single-cell RNA sequencing studies have begun to better highlight the expression of various keratins in order to characterize the heterogeneity of different airway cell types including basal cells (Table 6.1) [2, 7, 12]. For example, several studies have found that KRT4 and KRT13 are differentially expressed in a transitional suprabasal cell population as basal cells differentiate into secretory cells [1, 2, 12]. However, in mice, Krt4/Krt13 may demarcate a heterogeneous population of squamous-like cells found within discrete nonciliated regions of the tracheal epithelium (see the discussion on “Hillocks”) [1]. In addition, KRT14 expression is enriched in mitotically active basal cells [23–25]. In the steady state mouse trachea, Krt14 marks a relatively infrequent subset of unipotent and self-renewing basal cells (<20%), but after injury, regenerative Krt14+ basal cells proliferate and become multipotent for all of the major cell lineages of the surface epithelium [26, 27]. KRT14 interacts with KRT5 to establish a structural network of intermediate filaments needed for proliferation [28]. Taken together, composition of keratins can be assessed to distinguish different subpopulations of basal cells, including actively proliferating cells.
Table 6.1.
Keratin expression in airway epithelial cells
A short-term surge in proliferation of basal cells may come at a long-term cost. Evidence supporting this notion in the airway comes from studies of infrequently dividing progenitors. Multiple colony formation efficiency studies have found that infrequently dividing basal cells are highly clonogenic in vitro [27, 29]. Heterogeneity within the stem cell pool to maintain both frequently dividing and infrequently dividing progenitors provides a mechanism of population-level regulation for balancing the capacity to regenerate cells damaged by injury while also safeguarding long-term proliferative potential (Fig. 6.2).
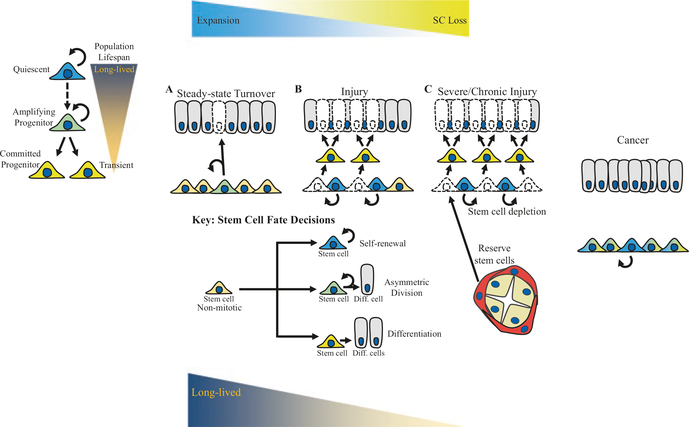
Fig. 6.2.
Proliferative stress requirements of stem cells at steady state and following moderate and severe injury. (a) At steady state, the majority of mitotic stem cells undergo asymmetric cell division (green cells), which is capable of compensating for cell loss during a low rate of turnover without depleting the stem cell population. (b) Following injury, basal cells may undergo symmetric differentiation (yellow cells) in order to more rapidly compensate for the loss of many cells during a higher rate of turnover. Stem cells that undergo symmetric differentiation are removed from the stem cell pool, but other stem cells may compensate for this loss through symmetric self-renewal (blue cells). (c) If the proliferative stress is sustained long enough or the injury is severe enough, the capacity of the stem cell population to self-renew is insufficient to compensate for the loss of stem cells through differentiation. In this case, reserve stem cells from neighboring regenerative stem cell pools may attempt to compensate for stem cell loss. However, in the case of chronic injury, stem cells are ultimately depleted leading to fibrosis and disease
In disease, however, chronic inflammation may lead to proliferative exhaustion of airway basal cells by overstimulating the expansion of KRT14+ basal cells, leading to failed long-term epithelial regeneration and fibrosis. For instance, in the context of chronic rejection following lung transplantation, p63 + KRT5+ basal cells are depleted while KRT14+ basal cells expand in both human patients and in a ferret transplantation model. This shifting basal cell phenotype accompanies a decline in clonogenicity and correlates closely with histologic severity of rejection and progression of obliterative bronchiolitis (OB) [22]. Ghosh et al. [21] discovered a similar decline in p63 + KRT5+ basal cells in chronic obstructive pulmonary disease (COPD) and found that an overall decline in basal cells may identify a subset of prediagnostic, non-COPD patients at heightened risk of developing COPD (pending validation by prospective analysis). Meanwhile, others saw that cigarette smoke may induce expansion of KRT14+ basal cells in COPD-affected patients [30, 31]. Interestingly, in idiopathic pulmonary fibrosis (IPF) it appears that airway basal cells migrate from conducting airways to alveolar regions, where they acquire an aberrant differentiation program and may contribute to fibrosis [6, 32]. Factors that influence the flux states of basal cell heterogeneity within the bounds of normal physiology, during relatively mild perturbations of short-term injury, and during pathoprogressive states of disease remain an important research frontier (Fig. 6.2). Establishment of disease progression markers can become a clinically useful early diagnosis tool of pathologies associated with injury-dependent stem cell depletion, such as OB and COPD.
Researchers are actively investigating the therapeutic potential of engrafting basal cells as a therapy to treat lung diseases. Isolation and cultural expansion of patient-derived basal cells has enormous potential. For example, basal cell therapy has the potential to correct cystic fibrosis (CF) lung disease, which is caused by mutation(s) in the CF transmembrane conductance regulator (CFTR). CF patient basal cells can be harvested and the CFTR mutations in these cells corrected in vitro before the cells are delivered back into the afflicted patients. Recent cell transplantation studies done in mice have shown promising results using this approach and have provided benchmarks for researchers to use toward developing these therapies for humans. For example, based on data extrapolated from mouse studies, research has estimated that a therapeutic dose of 60 million cells is needed for effective treatment [33]. Obtaining such a large number of cells without detriment to cell viability is not trivial, and until recently, it was not clear how well CF cells would grow. However, Hayes Jr. et al. recently demonstrated several landmark findings that promote the feasibility of basal cell therapy for the treatment of CF. For example, both non-CF basal cells as well as CF basal cells can be amplified enough to achieve the estimated therapeutic dose (over 60 million cells), and these culturally expanded basal cells retain the capacity to differentiate into secretory and ciliated cell types [34]. Still, many critical questions remain. For example, barriers to cell engraftment must be identified and overcome. In mice, for example, researchers found that it was critically important to injure the airway epithelium before transplanted cells could efficiently engraft [33]. However, Hayes Jr. et al. [34] propose an interesting suggestion to administer basal cells to lung transplant recipients to prevent primary graft dysfunction, which causes profound epithelial sloughing in 25–29% of lung allografts within 3–14 days following transplantation [35]. However, reaching the estimated therapeutic dose of 60 million cells within 3–14 days is currently pushing beyond technological capabilities. It is feasible, however, for clinicians to store culturally expanded cells obtained from patients by brush biopsy well prior to the lung transplantation procedure; thus, it may be necessary to interrogate how cryo-storage affects basal cell viability and performance. In aggregate, the therapeutic potential of basal cell therapy to treat human disease is poised for clinical usage within the coming years. However, basic questions regarding human airway basal cell biology still linger.
Many aspects of basal cell biology have been clarified using mouse models. However, there are species-specific differences in the biology and distribution of basal cells between mice and larger mammals, including humans. The importance of some of these differences remains unclear. For example, in the mouse lung, the distribution of Krt5 + p63+ basal cells is largely limited to the trachea and bronchi, whereas in human lungs, these cells are distributed throughout conducting airways down to the terminal bronchioles [17, 18]. It might be due to the size of the lung: the bigger it is, the more basal stem cells the organism would need to maintain it. It is still unclear if and how basal cells differ across airway levels in terms of various properties such as proliferative rate or differentiation capacity. However, Okuda et al. [14] demonstrated that large airway epithelial (LAE) cells and small airway epithelial (SAE) cells held an architecture and protein expression phenotype that were indicative of their site of origin upon establishing well-differentiated air–liquid interface (ALI) cultures. This suggests that basal cells retain a site-specific imprint on their multipotency in vitro. Therefore, animal models that are similar to humans in their basal cell distribution throughout the respiratory tree may better recapitulate critically important mechanisms that control stem cell dynamics than rodent models would. For example, ferret airway basal cell distribution resembles human airways, and even the distal airways in the ferret have basal cells similar to that in human lungs [22].
Submucosal Gland Progenitors
Submucosal glands (SMGs) are specialized epithelial invaginations of the superficial epithelium located throughout human cartilaginous airways. SMGs secrete fluids containing hundreds of proteins involved in maintaining sterility of the airway including multiple gel-forming mucins, antimicrobials, surfactants, immune regulators, and many other enzymes [36]. Structurally, submucosal glands are made of compound tubuloacinar epithelia containing four domains: ciliated ducts, collecting ducts, mucous tubules, and serous acini [37]. Ciliated ducts are contiguous with the SAE and contain similar cell types. For example, both multiciliated cells and secretory cells are present in ciliated ducts, and duct cells are similar to SAE basal cells. Although several studies have suggested that glandular duct cells are distinct from basal cells that reside in the superficial epithelium [38–40], duct cell-specific lineage analysis is needed to further support the hypothesis that ductal cells are truly distinct from basal cells. Collecting ducts contain a simple columnar epithelium that is poorly defined and may vary in different airways and between different species. Collecting ducts also tend to be larger in proximal airways than in distal airways and are absent in mice [36]. Branching tubules of mucous cells terminate with bulbous acini of serous cells that comprise the most distal secretory components of the glands [41]. In addition, contractile myoepithelial cells line the mesenchymal surfaces of glands except for the ducts [42, 43], and the glandular epithelium is maintained by myoepithelial stem/progenitor cells (Fig. 6.3).
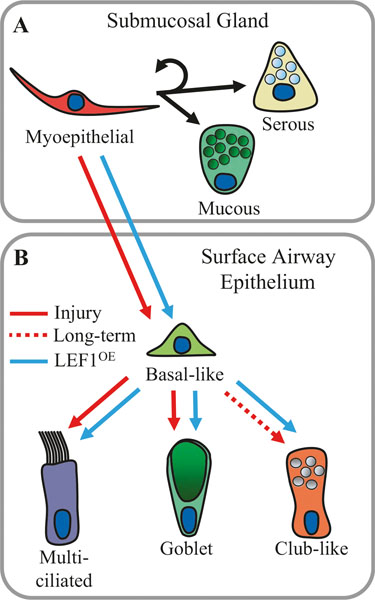
Fig. 6.3.
Submucosal gland myoepithelial cell lineages at steady state and following injury. The tracheobronchial airways possess epithelial submucosal glands that secrete mucous and serous fluids that help regulate mucociliary clearance on the airway surface. (a) Myoepithelial cells within the glands are self-renewing stem cells. At steady state, myoepithelial cells can differentiate into glandular serous and mucous cells [44–46]. (b) Following injury to the airway surface epithelium by naphthalene or SO2 (red arrows), myoepithelial cells activate a Lef1 transcriptional program that promotes their migration to the airway surface where they are capable of establishing long-lived basal cell progenitors [45, 46]. In addition, ectopic overexpression of LEF1 (blue arrows) is sufficient to initiate this process without injury. Initially, following injury, myoepithelial-derived basal-like cells are less likely to renew into SCGB1A1+ secretory club-like cells and have a lineage bias toward multiciliated and secretory goblet cells (mucus-positive cells). However, with increasing time after injury and distance away from the submucosal glands, myoepithelial-derived basal-like cells become increasingly able to differentiate into SCGB1A1+ secretory club-like cells (dashed red line). In addition, ectopic overexpression of LEF1 accelerates this process [45]
Several studies have shown that SMGs are a niche for slowly cycling stem cells that can long-term retain pulsed nucleotide analogs and/or tetracycline-inducible H2B-GFP [19, 27, 29, 47, 48]. Compared to nearby SAE basal cells, glandular progenitors exhibit a greater proliferative capacity in colony formation efficiency assays grown at the air–liquid interface and in denuded tracheal xenografts [27]. Furthermore, in vitro clonal analysis has revealed that glandular progenitors are able to differentiate into both SAE and SMG cell types [27, 39], suggesting that glandular stem cells may contribute to the renewal of both the surface epithelium and SMGs. Moreover, lineage-labeling of myoepithelial cells in mice has revealed that myoepithelial cells are multipotent stem cells that can self-renew and give rise to glandular mucous cells, serous cells, and duct cells during development and at homeostasis [44] (Fig. 6.3). Recently, it was shown that following severe injury to the SAE of both mice and pigs, myoepithelial cells act as reserve stem cells capable of repopulating surface basal cells and other SAE cell types by extension [45, 46]. Myoepithelial cells can repopulate surface basal cells with lasting regenerative capacity. However, at least in mice, myoepithelial-derived basal cells are less likely to generate surface secretory cells that express Scgb1a1 or Scgb3a2 than basal cells that normally reside in the surface epithelium. On the other hand, myoepithelial-derived basal cells were likely to give rise to multiciliated cells and Muc5B secretory cells [45]. Interestingly, although MUC5B is expressed in both glandular mucous cells and SAE secretory cell types, SCGB1A1 is not expressed in SMGs [14]. This may reflect a propensity to retain a gland-like lineage bias and may help provide mechanistic insight into epithelial remodeling that occurs with recurrent epithelial injury. However, it is just as likely that glandular stem cells may play a more significant role in human airways since SMGs are much more abundant in human airways than in mouse ones. The evidence outlined above makes a compelling case that myoepithelial cells (MEC) can act as reserve multipotent stem cells that can contribute to repair of surface airway epithelium (SAE); however, the regenerative contribution of MECs in the SAE might be skewed toward multiciliated cells and Muc5B+ secretory cells.
Glands in mice are confined to the proximal trachea extending from the cricoid cartilage to no further than the first few cartilage rings of the proximal trachea [19], although age, gender, and mouse strain may contribute to variations in gland size and abundance [49–51]. On the other hand, ferrets, like humans, have SMGs throughout their cartilaginous airways [52]. Recently, it has been shown that SMGs are destroyed in transplanted lungs in both human and ferret allografts as they develop a form of chronic lung allograft dysfunction (CLAD) known as obliterative bronchiolitis (OB). Depletion of the SMG stem cell niche occurs, as allografts progressively lose clonogenic surface basal cells in both distal and proximal airways [22]. The existing paradigm regarding pathoprogression of OB has suggested that disease pathology is limited to distal airways. For example, some researchers have suggested that bronchiolar club cells are selectively affected in OB [53], and indeed, there is evidence of bronchiolar club cell loss in OB lungs [22, 53, 54]. However, recent findings in human and ferret allografts have suggested that the depletion of stem cells occurs more globally in both large and small airway basal cell populations as well as in reserve stem cell niches of SMGs [22]. This may indicate that epithelial remodeling occurs as regenerative stem cell populations are depleted through a process of proliferative exhaustion. Moreover, murine myoepithelial-derived reserve basal cells may not generate SCGB1A1-expressing club cells as readily as do surface-resident basal cells [45], and if this biology is conserved in ferrets and humans, it may suggest a possible mechanism for club cell loss with OB. Depletion of surface epithelial basal cells and reserve stem cells from SMGs might be a root cause of OB. Thus, the ferret is an excellent transplant model to study this pathology because the distribution of airway basal cells and submucosal glands in ferrets is strikingly similar to that of humans, and the progressive loss of these stem cell niches is also similar.
Secretory Cells
Historically, airway secretory cells have been segregated into four distinct cell types based on their morphology and ultrastructure: club cells, goblet cells, serous cells, and pulmonary neuroendocrine cells (PNECs) [55, 56]. However, since these early morphometry studies, PNECs have largely been disassociated from other secretory cell types, and we will briefly discuss their contribution to pulmonary stem cell biology in a later section. In humans, serous cells are mainly present in fetal airways and in adult submucosal glands, although they have been occasionally observed in adult bronchioles by transmission electron microscopy [57]. Their lineage relationships with other cell types have not been explored in recent years. In this section, we will discuss characteristics and controversies surrounding club cells and goblet cells as secretory cell types placing an emphasis on population heterogeneity and their contribution to different epithelial lineages.
Club cells have been identified as a major progenitor cell type of the airway epithelium (Figs. 6.1 and 6.4) [10, 68]. Club cells are commonly identified by their expression of SCGB1A1 (aka club cell secretory protein or CCSP), but classically, they must also be dome-shaped columnar cells lacking periodic acid-Schiff (PAS) staining, and by this definition, Boers et al. found that CCs are largely restricted to terminal and respiratory bronchioles in healthy human airways [69]. By contrast, goblet cells are mucus-producing cells prototypically identified by their “flask-shaped” morphology and prominent PAS-reactive cytoplasmic vacuoles, and by this histologic definition, Boers et al. [69] found that goblet cells are more abundant in proximal airways and are largely absent from terminal and respiratory bronchioles. However, these narrow morphological definitions of secretory club cells and goblet cells do not completely describe the diversity of secretory cell types nor can club cells be specifically identified by SCGB1A1 expression alone.
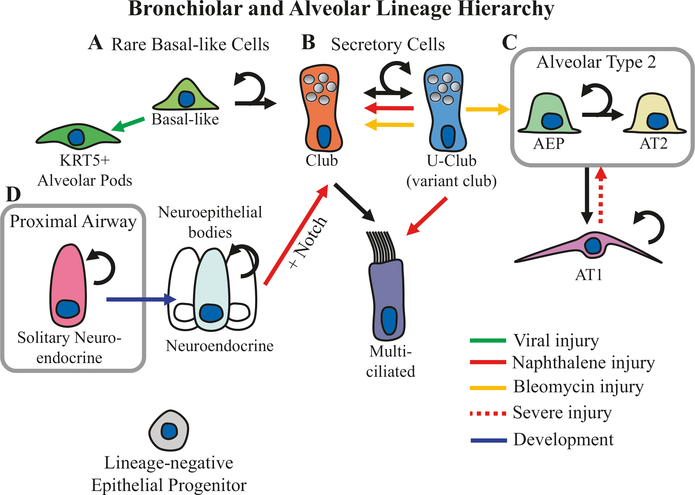
Fig. 6.4.
Small airway bronchiolar and alveolar lineages. (a) Basal-like progenitors are rare in small airways, yet they play an important role in restoring the structural integrity of alveolar epithelium after catastrophic viral injury (green arrow) by generating KRT5+ alveolar pods [58–60]. Recent evidence also suggests that small airway basal-like cells give rise to bronchiolar club cells [7]. (b) A rare subtype of club cells expressing Uroplakin3a (U-Club cells) is capable of renewing the larger club cell population at steady state. Following naphthalene injury (red arrow), U-club cells can also give rise to multiciliated cells, and following bleomycin injury (yellow arrow), U-Club cells renew alveolar type 2 (AT2) cells [61]. (c) The alveolar epithelium consists of AT2 cells and alveolar type 1 (AT1) cells, and AT2 cells are self-renewing and can lineage commit to AT1 cells [62]. Recently, a population of highly clonogenic stem cells called alveolar epithelial progenitors (AEPs) has been identified within the AT2 cell population [63]. In addition, AT1 cells are able to dedifferentiate following severe injury (dashed red arrow) into AT2 cells [64, 65]. (d) In addition, small airways harbor neuroepithelial bodies, which consist of clusters of self-renewing neuroendocrine cells. Forced induction of notch signaling in the context of injury induces neuroendocrine to club-like cell transdifferentiation [66]. During development (blue arrow), solitary neuroendocrine cells residing in proximal airways migrate to small airway branch points where they organize into neuroepithelial bodies [67]
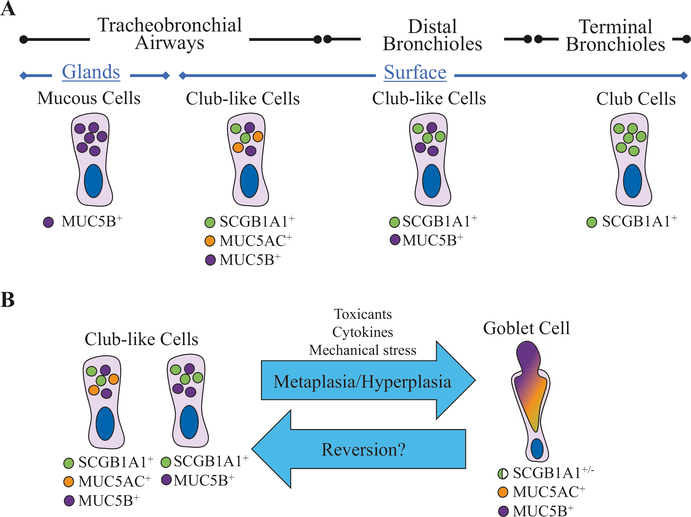
Indeed, club-like secretory cells that do not fit the classical club cell description but do express SCGB1A1 can be found throughout the human conducting airways (Fig. 6.5). For example, Boers et al. [69] described “indeterminate” SCGB1A1-expressing, PAS-positive cells present in human bronchi and to a lesser extent in distal nonterminal and terminal bronchioles. In normal/healthy human airways, SCGB1A1 marks club-like cells of the superficial epithelium from the trachea to the terminal bronchioles, and SCGB1A1 expression is absent only from submucosal glands. Notably, most club-like cells also express the mucins MUC5B and/or MUC5AC at all levels of the respiratory tree except for in terminal bronchioles where mucins are not expressed. In addition, cells with typical goblet cell morphology were rarely observed in healthy human lungs that were selected using rigorous inclusion criteria, which included only nonsmoking donor lungs with minimal exposure to mechanical ventilation [14]. However, various aeroallergens and toxins, viral infections, and even mechanical stresses such as hyperventilation can induce inflammatory mediators that promote goblet cell metaplasia and hyperplasia [70–73]. Considering that the airway surface epithelium is constantly exposed to the external environment, it is unclear to what extent goblet cell abundance and/or morphology can fluctuate within the bounds of normal physiology. For example, this may suggest that the classically defined goblet cell morphology may in fact reflect an elastic response to various forms of harmful environmental stimuli. On the other hand, changes in goblet cell abundance are a hallmark of many chronic respiratory diseases, and goblet cell hyperplasia is a shared feature of asthma [74] and COPD [75], whereas surface goblet cells are hypertrophic in cystic fibrosis (CF) [76]. Taken together, these data may suggest either that goblet cells are a distinct cell fate or that club-like cells may fluctuate in (and perhaps out of) a goblet cell state. Club-like cell plasticity has been observed in dedifferentiating club-like cells that regenerate airway basal cells after basal cell ablation [13]. A better understanding of the lineage relationship between club-like cells and goblet cells may lead to important discoveries regarding disease progression while also advancing basic scientific knowledge regarding the continuum between flexible cell states and committed cell fates. For example, are goblet cells simply stress-induced hyperactive club-like cells or are they a terminally committed cell lineage that is distinct from club-like cells?
Fig. 6.5.
Nuances among pulmonary secretory cells. Recent studies have provided compelling data for anatomical specificity of secretory cell types in the human lung. (a) Based on the expression of MUC5B, MUC5AC, and SCGB1A1 (CCSP), there are at least four distinct secretory cell types. Submucosal glands possess MUC5B-expressing secretory mucous cells. In proximal large airways, secretory club-like cells express MUC5B, MUC5AC, and SCGB1A1. In small bronchial airways, secretory club-like cells express MUC5B and SCGB1A1 but not MUC5AC, and in terminal bronchioles, secretory club-like cells express only SCGB1A1 [14]. (b) Given that club-like cells express many of the phenotypic characteristic of goblet cells, it may be necessary to experimentally challenge the paradigm that goblet cells are indeed a divergent cell type rather than simply being a hypersecretory state of club-like cells. For example, if goblet cell metaplasia/hyperplasia is readily reversible back to a club-like cell phenotype, this may suggest that goblet cells and club-like cells are the same population of secretory cells
Furthermore, it is unclear to what extent bronchiolar club cells differ from club-like cells from other regions of the lung. Bronchial club-like cells and bronchiolar club cells may share a similar origin. KRT5+ basal cells have been identified as progenitors for both club-like cells and club cells based on bioinformatic analyses of single-cell transcriptomes of human bronchial epithelial cells (HBECs) and small airway epithelial cells (biopsied from the 10th- to 12th-generation airways) [2, 7]. Interestingly, basal cells isolated from either large airways or small airways generated well-differentiated ALI cultures that contained secretory cells that phenotypically mirrored the in vivo region-specific cell types [14]. Taken together, this suggests that classically defined club cells in human terminal bronchioles and club-like cells throughout the rest of the tracheobronchial airways share many similar characteristics but may in fact be distinct subtypes of airway secretory cells.
Support for the notion that club cells and club-like cells retain lineage-specific programs has also been demonstrated in mice, and it was hypothesized that this region-specific programming ensures that cellular composition is restored to maintain healthy functionality [77]. How finely are airway progenitors defined by region specificity, and what are the molecular mechanisms responsible for this, remains to be discovered. Perhaps, most importantly, more research is needed to investigate whether this semi-predetermined state of stem cells can be overcome in the interest of developing efficient cell-based therapies.
Airway secretory cell heterogeneity has been reported in many studies of human and other species. For example, ultrastructural studies have reported the presence of club cells within the upper airways of many mammalian species [78–81]. However, some groups have argued that club cells cannot be reliably identified based solely on their ultrastructure, as club cells share no definitive features between different species, and there may be a considerable variability within a single animal subject [82]. This heterogeneity may be critically important for maintaining normal physiology. The larger club cell population contains subsets of cells with variable expression of different detoxifying enzymes such as members of the cytochrome P450 family. Cytochrome P450 enzymes oxidize multiple small-molecule substrates and therefore can produce cytotoxic intermediates, which is the primary reason for injury after naphthalene exposure. Variant club cells that do not express Cyp2f2—a member of the cytochrome P450 family—are resistant to naphthalene and can regenerate the bronchiolar epithelia after injury [83–85]. Recently, a subset of variant club cells that express Upk3a (U-club cells) was reported to contribute to homeostatic renewal of airway epithelial cells and to be able to regenerate alveolar type 1 (AT1) and alveolar type 2 (AT2) cells following bleomycin injury in mice [61]. It is thus evident that heterogeneity of airway secretory cells contributes to the differential ability of these cells to repair various types of injury. Research that helps to delineate the molecular mechanisms that expand progenitor cell potency and differentiation potential may help improve the likelihood that regenerative cell-based therapies can become a mainstream treatment option for acute or chronic airway injuries.
Pulmonary Neuroendocrine Cells (PNECs)
PNECs can exist as solitary cells within proximal airways but in distal airways they are mostly found within clusters called neuroepithelial bodies (NEBs). NEBs may be further specified by their anatomical organization. Nodal NEBs are located at airway branching points, whereas internodal NEBS are located between branching points [67]. Mature NEBs are basally innervated by vagal nerve afferents and serve as an important interface between the central nervous system and the conducting epithelium to regulate breathing [86]. PNECs may have many functions effecting regeneration, including an important role in regulating U-club cells organized around NEBs, and after naphthalene injury, epithelial regeneration by U-club cells and possibly other variant club cells occurs preferentially around nodal NEBs [61, 85, 87]. However, PNECs may also directly participate in regeneration following naphthalene injury coupled with ectopic activation of Notch signaling by transdifferentiating into club cells (Fig. 6.4) [66]. This plasticity may suggest that PNECs have the capacity to serve as potential reserve stem cells for small airways in a similar way that glandular myoepithelial cells do for the superficial epithelia in larger airways. Additionally, small cell lung cancers are thought to arise from PNECs [88–90]. However, it may be difficult to appreciate heterogeneity within the PNEC population given their relative rarity among other cell types. However, one conspicuous question that remains is how solitary PNECs in proximal airways differ from those found within small airway NEBs. However, it has already been discovered that during development, solitary PNECs in proximal airways migrate toward distal airways where they establish static nodal NEBs but not internodal NEBs [67, 91]. This divergent developmental ontogeny between nodal and internodal NEBs may point toward a provocative difference in the physiological function and regenerative capacity of PNECs throughout the conducting airway tree. Overall, PNECs remain relatively understudied in the context of pulmonary stem cell biology, although there is some evidence that PNECs may function as a reserve stem cell population.
Alveolar Progenitors
Lung alveolar epithelium is composed of squamous alveolar type 1 (AT1) cells and cuboidal alveolar type 2 (AT2) cells. AT2 cells are the predominant stem cell for alveoli, as they can both self-renew and generate AT1 cells (Fig. 6.4) [62, 92, 93]. However, AT1 cells may also have a limited capacity to proliferate after injury [64, 65]. AT2 cells are typically identified by their expression of SFTPC, ABCA3, and/or LAMP3, while AT1 cells can be marked with PDPN, AGER, AQP5, and HOPX [94]. Both AT1 and AT2 cells are capable of at least some regenerative proliferation, but the bulk of this function is executed by AT2 cuboidal alveolar cells.
Recently, a subset of AT2 cells (SFTPC+/HOPX−) that express AXIN2 at steady state has been identified as a highly regenerative and clonogenic alveolar stem cell population. These airway epithelial progenitors (AEP) are divergent from other AT2 cells, possessing a distinct chromatin structure and transcriptomic profile, and AEPs can be isolated from human lungs by their expression of the surface marker TM4SF1 [63]. Unlike SOX2-derived TP63+ KRT5+ airway basal-like progenitors that primarily restore epithelial barrier function by forming “KRT5+ alveolar pods” following influenza injury [58], AEPs restore the alveolar epithelium by regenerating both AT1 and AT2 cells (Fig. 6.4) [63]. At steady state single fibroblasts directly neighboring AEPs maintain their “stemness” with paracrine Wnt signals that act to prevent AEPs from transdifferentiating into AT1 cells. However, after injury, AEPs exit their stromal niche to engage in regeneration [3]. Noncell autonomous signals that steer lineage commitment of AEPs into AT1 or AT2 cells may be elucidated in forthcoming studies. Further understanding of processes that regulate this biology will bring an efficient cell-based therapy to repair alveolar damage within reach. Likewise, given that AEPs have only recently been discovered, it is still unknown how AEPs are affected by disease or contribute to disease such as cancer. Interestingly, in a previous murine adenocarcinoma study that induced KRAS mutations in AT2 cells, only a rare subset of AT2 cells initiated tumors [92]. Whether or not these tumor-initiating AT2 cells are the newly appreciated AEPs remains to be seen. Taken together, this emerging body of research on AEPs suggests that this specific subset of AXIN2-positive AT2 cells might be the driver of “stemness” in this population.
In addition to AT2 cells, AT1 cells may also have a limited capacity to proliferate under the right conditions. Lineage-labeled Hopx+ AT1 cells were able to proliferate and generate AT2 cells following partial pneumonectomy and also established colonies in 3D culture [64]. Additionally, HOPX expression was dynamically downregulated both in a mouse model of pulmonary fibrosis induced by bleomycin injury and in human IPF lungs. Therefore, HOPX expression may promote progenitor cell quiescence at homeostasis and work to restrain excessive proliferation following injury [65].
Hillocks
Morphologically, hillocks were recently described in the steady-state mouse trachea as distinct structures with a stratified epithelial architecture lacking multiciliated cells and demarcated by Krt4 and Krt13 expression [1, 2]. Hillocks contain specific basal (Trp63 + Krt13+) and club (Scgb1a1 + Krt13+) cell types, which have a transcription profile that is distinct from those of other tracheal cells [1]. As to their function within the mouse trachea, Montoro et al. [1] offered evidence that hillock cells have heightened turnover and increased expression of genes involved in squamous barrier function and immunomodulation. Published in a separate manuscript at the same time, Plasschaert et al. corroborated the existence of a distinct population of Krt4+/Krt13+ cells in mouse tracheal epithelia and found that KRT4/KRT13 expression defined a major axis of heterogeneity within the basal cell population of culturally expanded and differentiated human bronchial epithelial cells. However, Plasschaert et al. [2] interpreted this population of KRT4+/KRT13+ cells to be an intermediate population between basal stem cells and differentiated luminal secretory cells. To ascertain if hillocks are metaplastic zones in flux or represent a distinct niche, further work is needed to better understand their origin and purpose. In addition, the question of how hillocks relate to human airway epithelial physiology at steady state cannot yet be clearly answered because this structure has not been described outside of the mouse trachea.
Conclusions
In this chapter, we discussed how pulmonary stem cell types differ depending on their anatomical compartment of origin, how heterogeneity within each population may preferentially give rise to various differentiated cell types, and how the type of injury and its severity may affect the regenerative process. Different compartments within the lung have their own stem cell niches. The superficial epithelium of conducting airways is maintained by basal stem cells and to some extent is also maintained by club-like cells in proximal airways but in terminal bronchioles, it is maintained by club cells. Submucosal glands are maintained by myoepithelial cells that can also act as reserve stem cells for the superficial airway epithelium in the context of severe injury. Hillocks of KRT4+/KRT13+ cells are a novel structure found in the mouse trachea and may potentially represent yet another distinct stem cell niche maintained by hillock-specific progenitors. A subset of PNECs organized within nodal NEBs at airway bifurcations may also contribute to epithelial renewal as a reserve stem cell niche for distal airways. Finally, the alveolar epithelium is maintained by a population of cuboidal AT2 cells that contains a subpopulation of quiescent AEP stem cells.
Stem cells renew differentiated cells that are normally present within their respective compartments at steady state, and they may also expand more rapidly following an injury to compensate for increased cell death. Additionally, in case of severe or chronic injury reserve stem cells, such as myoepithelial cells in submucosal glands, they can migrate outside of their niche and aid in the repair of other tissues such as the surface airway epithelium. However, the reparative contribution of reserve stem cells from alternative compartments may skew the epithelial population composition toward that of the compartment where the reserve stem cell originated. This is likely due to epigenetic programming that is partially retained within reserve stem cells. However, with time reserve myoepithelial stem cells become increasingly similar to native surface basal cells as they migrate away from their glandular origin, and this process can be accelerated with overexpression of LEF1 in myoepithelial cells. From a therapeutic standpoint, determination and possible modification of these epigenetic marks might be an important step toward development of stem cell supplementation therapies.
Another important aspect of stem cell biology to consider is their long-term ability to self-renew. Impaired self-renewal of stem cells is a hallmark of OB and COPD; therefore, research on what drives this stem cell niche depletion/exhaustion is highly clinically relevant considering that most lung transplantation patients develop OB within 5 years of the surgery [95]. If regeneration is accomplished not by the region-specific stem cell but rather by a reserve stem cell population from an alternative compartment, then the function of the newly repaired epithelia may by impaired. Epithelium that is repaired by a reserve stem cell may have a slightly different composition that is skewed toward the compartment where reserve stem cells originated. For example, regeneration of small airway epithelium by reserve stem cells that migrated from large airways may lead to an overabundance of mucous-secreting cells in the small airways, which could lead to obstruction of airflow or impaired mucociliary clearance. Overall heterogeneity of lung stem cells should be an important consideration in designing cell- and drug-based therapies. As the research in this field advances, we expect to see an increasing number of clinical applications of stem cells and pharmaceuticals that target stem cells.
Contributor Information
Thomas J. Lynch, Department of Anatomy and Cell Biology, Division of Cardiothoracic Surgery, Carver College of Medicine, University of Iowa, Iowa City, IA, USA Department of Surgery, Carver College of Medicine, University of Iowa, Iowa City, IA, USA.
Vitaly Ievlev, Department of Anatomy and Cell Biology, Division of Cardiothoracic Surgery, Carver College of Medicine, University of Iowa, Iowa City, IA, USA.
Kalpaj R. Parekh, Department of Anatomy and Cell Biology, Division of Cardiothoracic Surgery, Carver College of Medicine, University of Iowa, Iowa City, IA, USA Department of Surgery, Carver College of Medicine, University of Iowa, Iowa City, IA, USA.
References
- 1.Montoro DT et al. (2018) A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560:319–324. 10.1038/s41586-018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plasschaert LW et al. (2018) A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560:377–381. 10.1038/s41586-018-0394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ (2018) Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359:1118–1123. 10.1126/science.aam6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu M et al. (2019) Highly efficient transgenesis in ferrets using CRISPR/Cas9-mediated homology-independent insertion at the ROSA26 locus. Sci Rep 9(1):1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treutlein B et al. (2014) Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509:371–375. 10.1038/nature13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y et al. (2016) Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1:e90558. 10.1172/jci.insight.90558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo WL et al. (2018) Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med 198(11):1375–1388. 10.1164/rccm.201710-2107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie T et al. (2018) Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep 22:3625–3640. 10.1016/j.celrep.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AJ et al. (2018) Basal stem cell fate specification is mediated by SMAD signaling in the developing human lung. bioRxiv. 2018:461103. 10.1101/461103 [DOI] [Google Scholar]
- 10.Watson JK et al. (2015) Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep 12:90–101. 10.1016/j.celrep.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardo-Saganta A et al. (2015) Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 16:184–197. 10.1016/j.stem.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz Garcia S et al. (2018) Single-cell RNA sequencing reveals novel cell differentiation dynamics during human airway epithelium regeneration. Cold Spring Harbor Laboratory: bioRxiv. 10.1101/451807 [DOI] [Google Scholar]
- 13.Tata PR et al. (2013) Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503:218–223. 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda K et al. (2018) Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med 199(6):715–727. 10.1164/rccm.201804-0734OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira VH et al. (2013) Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. elife 2:e00966. 10.7554/eLife.00966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rock JR et al. (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106:12771–12775. 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock JR, Randell SH, Hogan BL (2010) Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3:545–556. 10.1242/dmm.006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL (2013) Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol 2:131–148. 10.1002/wdev.58 [DOI] [PubMed] [Google Scholar]
- 19.Lynch TJ, Engelhardt JF (2014) Progenitor cells in proximal airway epithelial development and regeneration. J Cell Biochem 115:1637–1645. 10.1002/jcb.24834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh M et al. (2013) Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol 49:1127–1134. 10.1165/rcmb.2013-0049OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh M et al. (2018) Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am J Respir Crit Care Med 197:885–896. 10.1164/rccm.201704-0667OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swatek AM et al. (2018) Depletion of airway submucosal glands and TP63(+)KRT5(+) basal cells in obliterative bronchiolitis. Am J Respir Crit Care Med 197:1045–1057. 10.1164/rccm.201707-1368OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM (2011) Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22:4068–4078. 10.1091/mbc.E10-08-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papafotiou G et al. (2016) KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7:11914. 10.1038/ncomms11914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole BB et al. (2010) Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol 177:362–376. 10.2353/ajpath.2010.090870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh M et al. (2011) Context-dependent differentiation of multipotential keratin 14-e xpressing tracheal basal cells. Am J Respir Cell Mol Biol 45:403–410. 10.1165/rcmb.2010-0283OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch TJ et al. (2016) Wnt signaling regulates airway epithelial stem cells in adult murine submucosal glands. Stem Cells 34:2758–2771. 10.1002/stem.2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S (2009) An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One 4:e5623. 10.1371/journal.pone.0005623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh M et al. (2013) Regulation of trachebronchial tissue-specific stem cell pool size. Stem Cells 31:2767–2778. 10.1002/stem.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schamberger AC, Staab-Weijnitz CA, Mise-Racek N, Eickelberg O (2015) Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci Rep 5:8163. 10.1038/srep08163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herfs M et al. (2012) Proinflammatory cytokines induce bronchial hyperplasia and squamous metaplasia in smokers: implications for chronic obstructive pulmonary disease therapy. Am J Respir Cell Mol Biol 47:67–79. 10.1165/rcmb.2011-0353OC [DOI] [PubMed] [Google Scholar]
- 32.Smirnova NF et al. (2016) Detection and quantification of epithelial progenitor cell populations in human healthy and IPF lungs. Respir Res 17:83. 10.1186/s12931-016-0404-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh M, Ahmad S, White CW, Reynolds SD (2017) Transplantation of airway epithelial stem/progenitor cells: a future for cell-based therapy. Am J Respir Cell Mol Biol 56:1–10. 10.1165/rcmb.2016-0181MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes D Jr et al. (2018) Cell therapy for cystic fibrosis lung disease: regenerative basal cell amplification. Stem Cells Transl Med 8(3):225–235. 10.1002/sctm.18-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitson BA et al. (2007) Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 26:1004–1011. 10.1016/j.healun.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 36.Widdicombe JH, Wine JJ (2015) Airway gland structure and function. Physiol Rev 95:1241–1319. 10.1152/physrev.00039.2014 [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Driskell RR, Engelhardt JF (2004) Airway glandular development and stem cells. Curr Top Dev Biol 64:33–56. 10.1016/S0070-2153(04)64003-8 [DOI] [PubMed] [Google Scholar]
- 38.Hegab AE et al. (2012) Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med 1:719–724. 10.5966/sctm.2012-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegab AE et al. (2011) Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells 29:1283–1293. 10.1002/stem.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegab AE et al. (2014) Aldehyde dehydrogenase activity enriches for proximal airway basal stem cells and promotes their proliferation. Stem Cells Dev 23:664–675. 10.1089/scd.2013.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khansaheb M et al. (2011) Properties of substance P-stimulated mucus secretion from porcine tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol 300:L370–L379. 10.1152/ajplung.00372.2010 [DOI] [PubMed] [Google Scholar]
- 42.Meyrick B, Reid L (1970) Ultrastructure of cells in the human bronchial submucosal glands. J Anat 107:281–299 [PMC free article] [PubMed] [Google Scholar]
- 43.Meyrick B, Sturgess JM, Reid L (1969) A reconstruction of the duct system and secretory tubules of the human bronchial submucosal gland. Thorax 24:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson PJ, Lynch TJ, Engelhardt JF (2017) Multipotent myoepithelial progenitor cells are born early during airway submucosal gland development. Am J Respir Cell Mol Biol 56:716–726. 10.1165/rcmb.2016-0304OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch TJ et al. (2018) Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22:653–667.e655. 10.1016/j.stem.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tata A et al. (2018) Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22:668–683.e666. 10.1016/j.stem.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH (2001) Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24:662–670. 10.1165/ajrcmb.24.6.4217 [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Driskell RR, Engelhardt JF (2006) Stem cells in the lung. Methods Enzymol 419:285–321. 10.1016/S0076-6879(06)19012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Innes BA, Dorin JR (2001) Submucosal gland distribution in the mouse has a genetic determination localized on chromosome 9. Mamm Genome 12:124–128 [DOI] [PubMed] [Google Scholar]
- 50.Nettesheim P, Martin DH (1970) Appearance of glandlike structures in the tracheobronchial tree of aging mice. J Natl Cancer Inst 44:687–693 [PubMed] [Google Scholar]
- 51.Wansleeben C, Bowie E, Hotten DF, Yu YR, Hogan BL (2014) Age-related changes in the cellular composition and epithelial organization of the mouse trachea. PLoS One 9:e93496. 10.1371/journal.pone.0093496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajighasemi-Ossareh M et al. (2013) Distribution and size of mucous glands in the ferret tracheobronchial tree. Anat Rec (Hoboken) 296:1768–1774. 10.1002/ar.22783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly FL et al. (2012) Epithelial Clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant 12:3076–3084. 10.1111/j.1600-6143.2012.04201.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilpin SE et al. (2012) Altered progenitor cell and cytokine profiles in bronchiolitis obliterans syndrome. J Heart Lung Transplant 31:222–228. 10.1016/j.healun.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 55.Jeffery PK, Gaillard D, Moret S (1992) Human airway secretory cells during development and in mature airway epithelium. Eur Respir J 5:93–104 [PubMed] [Google Scholar]
- 56.Jeffery PK, Li D (1997) Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J 10:1655–1662 [DOI] [PubMed] [Google Scholar]
- 57.Rogers AV, Dewar A, Corrin B, Jeffery PK (1993) Identification of serous-like cells in the surface epithelium of human bronchioles. Eur Respir J 6:498–504 [PubMed] [Google Scholar]
- 58.Ray S et al. (2016) Rare SOX2(+) airway progenitor cells generate KRT5(+) cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Reports 7:817–825. 10.1016/j.stemcr.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuo W et al. (2015) p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 517:616–620. 10.1038/nature13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan AE et al. (2015) Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517:621–625. 10.1038/nature14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guha A, Deshpande A, Jain A, Sebastiani P, Cardoso WV (2017) Uroplakin 3a(+) cells are a distinctive population of epithelial progenitors that contribute to airway maintenance and post-injury repair. Cell Rep 19:246–254. 10.1016/j.celrep.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 62.Barkauskas CE et al. (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123:3025–3036. 10.1172/JCI68782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zacharias WJ et al. (2018) Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555:251–255. 10.1038/nature25786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain R et al. (2015) Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6:6727. 10.1038/ncomms7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ota C et al. (2018) Dynamic expression of HOPX in alveolar epithelial cells reflects injury and repair during the progression of pulmonary fibrosis. Sci Rep 8:12983. 10.1038/s41598-018-31214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao E et al. (2018) Notch signaling controls transdifferentiation of pulmonary neuroendocrine cells in response to lung injury. Stem Cells 36:377–391. 10.1002/stem.2744 [DOI] [PubMed] [Google Scholar]
- 67.Noguchi M, Sumiyama K, Morimoto M (2015) Directed migration of pulmonary neuroendocrine cells toward airway branches organizes the stereotypic location of neuroepithelial bodies. Cell Rep 13:2679–2686. 10.1016/j.celrep.2015.11.058 [DOI] [PubMed] [Google Scholar]
- 68.Rawlins EL et al. (2009) The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4:525–534. 10.1016/j.stem.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boers JE, Ambergen AW, Thunnissen FB (1999) Number and proliferation of Clara cells in normal human airway epithelium. Am J Respir Crit Care Med 159:1585–1591. 10.1164/ajrccm.159.5.9806044 [DOI] [PubMed] [Google Scholar]
- 70.Chen G et al. (2014) Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189:301–313. 10.1164/rccm.201306-1181OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doherty T, Broide D (2007) Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol 19:676–680. 10.1016/j.coi.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 72.Kuperman DA et al. (2002) Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 8:885–889. 10.1038/nm734 [DOI] [PubMed] [Google Scholar]
- 73.Reid AT et al. (2018) Persistent induction of goblet cell differentiation in the airways: therapeutic approaches. Pharmacol Ther 185:155–169. 10.1016/j.pharmthera.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 74.Ordonez CL et al. (2001) Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 163:517–523. 10.1164/ajrccm.163.2.2004039 [DOI] [PubMed] [Google Scholar]
- 75.Kim V et al. (2015) Chronic bronchitis and current smoking are associated with more goblet cells in moderate to severe COPD and smokers without airflow obstruction. PLoS One 10:e0116108. 10.1371/journal.pone.0116108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voynow JA, Fischer BM, Roberts BC, Proia AD (2005) Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 172:1013–1018. 10.1164/rccm.200410-1398OC [DOI] [PubMed] [Google Scholar]
- 77.Chen H et al. (2012) Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30:1948–1960. 10.1002/stem.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidel V et al. (2013) Distribution and morphology of Clara cells in common marmosets (Callithrix jacchus). J Med Primatol 42:79–88. 10.1111/jmp.12038 [DOI] [PubMed] [Google Scholar]
- 79.Pack RJ, Al-Ugaily LH, Morris G (1981) The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat 132:71–84 [PMC free article] [PubMed] [Google Scholar]
- 80.Plopper CG et al. (1983) Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res 5:281–294 [DOI] [PubMed] [Google Scholar]
- 81.Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG (1980) The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res 208:65–84 [DOI] [PubMed] [Google Scholar]
- 82.Widdicombe JG, Pack RJ (1982) The Clara cell. Eur J Respir Dis 63:202–220 [PubMed] [Google Scholar]
- 83.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG (1995) Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Phys 269:L800–L818. 10.1152/ajplung.1995.269.6.L800 [DOI] [PubMed] [Google Scholar]
- 84.Stripp BR, Maxson K, Mera R, Singh G (1995) Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Phys 269:L791–L799. 10.1152/ajplung.1995.269.6.L791 [DOI] [PubMed] [Google Scholar]
- 85.Reynolds SD, Giangreco A, Power JH, Stripp BR (2000) Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 156:269–278. 10.1016/S0002-9440(10)64727-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD (2015) Vagal sensory neuron subtypes that differentially control breathing. Cell 161:622–633. 10.1016/j.cell.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR (2001) Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 24:671–681. 10.1165/ajrcmb.24.6.4498 [DOI] [PubMed] [Google Scholar]
- 88.Meuwissen R et al. (2003) Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4:181–189 [DOI] [PubMed] [Google Scholar]
- 89.Park KS et al. (2011) Characterization of the cell of origin for small cell lung cancer. Cell Cycle 10:2806–2815. 10.4161/cc.10.16.17012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song H et al. (2012) Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 109:17531–17536. 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuo CS, Krasnow MA (2015) Formation of a neurosensory organ by epithelial cell slithering. Cell 163:394–405. 10.1016/j.cell.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desai TJ, Brownfield DG, Krasnow MA (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507:190–194. 10.1038/nature12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rock JR et al. (2011) Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108:E1475–E1483. 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nikolic MZ, Sun D, Rawlins EL (2018) Human lung development: recent progress and new challenges. Development 145(16):pii: dev163485. 10.1242/dev.163485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aguilar PR, Michelson AP, Isakow W (2016) Obliterative bronchiolitis. Transplantation 100:272–283. 10.1097/TP.0000000000000892 [DOI] [PubMed] [Google Scholar]