Abstract
Background
Drug supply disruptions have increased during the COVID-19 pandemic, especially for medicines used in the ICU. Despite reported shortages in wealthy countries, global analyses of ICU drug purchasing during COVID-19 are limited.
Research Question
Has COVID-19 impacted global drug purchases of first-, second-, and third-choice agents used in intensive care?
Study Design and Methods
We conducted a cross-sectional time series study in a global pharmacy sales dataset comprising approximately 60% of the world’s population. We analyzed pandemic-related changes in units purchased per 1,000 population for 69 ICU agents. Interventional autoregressive integrated moving average models tested for significant changes when the pandemic was declared (March 2020) and during its first stage from April through August 2020, globally and by development status.
Results
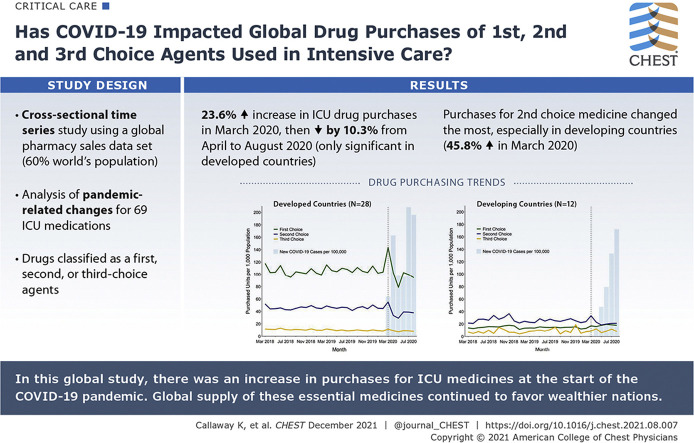
Relative to 2019, ICU drug purchases increased by 23.6% (95% CI, 7.9%-37.9%) in March 2020 (P < .001) and then decreased by 10.3% (95% CI, –16.9% to –3.5%) from April through August (P = .006). Purchases for second-choice medicines changed the most, especially in developing countries (eg, 29.3% increase in March 2020). Despite similar relative changes (P = .88), absolute purchasing rates in developing nations remained low. The observed decrease from April through August 2020 was significant only in developed countries (–13.1%; 95% CI, –17.4% to –4.4%; P < .001). Country-level variation seemed unrelated to expected demand and health care infrastructure.
Interpretation
Purchases for intensive care medicines increased globally in the month of the COVID-19 pandemic declaration, but before peak infection rates. These changes were most pronounced for second-choice agents, suggesting that inexpensive, generic medicines may be purchased more easily in anticipation of pandemic-related ICU surges. Nevertheless, disparities in access persisted. Trends seemed unrelated to expected demand, and decreased purchasing from April through August 2020 may suggest overbuying. National and international policies are needed to ensure equitable drug purchasing during future pandemics.
Key Words: COVID-19, critical care, drugs, global health, ICU
Abbreviations: ARIMA, autoregressive integrated moving average; MIDAS, Multinational Integrated Data Analysis; NMBA, neuromuscular blocking agent; OECD, Organization for Economic Cooperation and Development
Graphical Abstract
The COVID-19 pandemic has disrupted the global drug supply chain substantially.1 , 2 Surges in patients requiring intensive care combined with lockdowns, shipping disruptions, and export bans have strained drug production and distribution.1 , 3 Frequent updates to clinical guidelines and highly publicized touting of potential COVID-19 treatments have made demand difficult to predict.4 , 5
The ICU is vulnerable to drug supply disruptions.6 Many ICU drugs are low-cost generics with few alternatives.2 , 6 Before the pandemic, shortages of injectable drugs were increasing in wealthy nations.7 , 8 , 9 Although data in developing countries are sparse, addressing suboptimal access to medicines in these regions is a high international policy priority.10 , 11
Previous work has described COVID-19-related changes in ICU pharmacy management or has identified themes in which to consider supply chain issues during pandemics.1 , 2 , 6 , 12 However, such discussions have been theoretical and have overrepresented developed countries. Despite concern that COVID-19 could increase disparities in ICU drug supply, we are unaware of any studies that examine actual purchases of these drugs globally. The current analysis used a global monthly pharmacy sales dataset to describe ICU drug purchasing trends in the first stage of the COVID-19 pandemic. We hypothesized that increased purchasing when the pandemic was declared would result in shortages and a shift to alternatives. We also expected purchasing responses to differ between developed and developing countries. Our results have important implications for policies to build equitable ICU drug supply chains for future global emergencies.
Methods
We conducted a cross-sectional time-series study in IQVIA, Inc's Multinational Integrated Data Analysis (MIDAS) database.13 This dataset captures monthly pharmacy purchases for 77 jurisdictions from August 2014 through August 2020. The current study’s sample comprised hospital purchasing data from 41 countries (approximately 59.8% of the world’s population). We excluded countries with only retail data (n = 23) and countries without data on ICU medicines (n = 13) (e-Table 1). Purchases for private and public facilities were aggregated on the national level. One unit represented one vial of medication.13 The dataset did not contain information on individual facilities, care areas, or patients.
We restricted to 69 ICU medicines within six categories: (1) analgesics, including opioids; (2) sedatives for moderate-to-deep sedation; (3) anticoagulants, excluding heparins used for flushing; (4) injectable corticosteroids; (5) inotropes and vasopressors; and (6) neuromuscular blocking agents (NMBAs) (Table 1 ).6 , 14, 15, 16, 17, 18, 19 To proxy medicines ordered for inpatient use, we restricted to parenteral ordinary and long-acting formulations using New Form Codes F and G. For barbiturates and benzodiazepines, we also included rectal systemic forms (New Form Code H).
Table 1.
Drugs Commonly Used in the ICU by Class and Response Category
| Drug Classa | First Choice | Second Choice | Third Choice |
|---|---|---|---|
| Analgesics | Fentanyl/hydromorphone Morphine/remifentanil |
IV NSAIDsb IV acetaminophen/paracetamol Sufentanil/alfentanil Other IV analgesicsc |
… |
| Sedatives for moderate-to-deep sedation | Dexmedetomidine Midazolam Propofol |
Lorazepam Ketamine |
Phenobarbitald Pentobarbitald |
| Anticoagulantse | Heparin Low-molecular-weight heparinsf |
Bivalirudin | |
| Corticosteroids | Dexamethasone | Methylprednisolone Hydrocortisone |
Prednisolone |
| Inotropes and vasopressors | Dobutamine Milrinone/enoximone Norepinephrine |
Dopexamine Levosimendan Dopamine Epinephrine Vasopressin/lypressin Phenylephrine Midodrine Terlipressin |
Ephedrinef Isoprenaline |
| Neuromuscular blocking agents | Cisatracurium besilate Rocuronium bromide |
Pancuronium Vecuronium bromide |
Succinylcholineg |
NSAID = nonsteroidal antiinflammatory drug.
Except for benzodiazepines and barbiturates, only parenteral ordinary (New Form Code F) and parenteral long-acting (New Form Code G) formulations were included.
NSAID category comprised aceclofenac, acetylsalicylic acid, clonixin, dexketoprofen trometamol, diclofenac, etodolac, etofenamate, etoricoxib, flurbiprofen axetil, ibuprofen, indomethacin, ketoprofen, ketorolac, lornoxicam, meloxicam, naproxen, nimesulide, parecoxib, piroxicam, and tenoxicam.
Other analgesic category comprised aminophenazone, amobarbital, cyanocobalamin, lidocaine, metamizole sodium, orphenadrine, salicylic acid, secobarbital, and tramadol.
Included rectal systemic formulations (New Form Code H).
Excluded heparins used for flushing.
Low-molecule-weight heparins included dalteparin, enoxaparin, and fondaparinux.
Ephedrine and succinylcholine may be used for intermittent injections only.
We classified each drug as a first-, second-, or third-choice agent using published guidelines.14, 15, 16, 17 This categorization represented a best case scenario in which institutional purchasing aligned with recent evidence.14 , 20 , 21 First-choice therapies were evidence-based treatments used during nonemergencies. Second-choice therapies were sufficient alternatives to first-choice drugs. Third-choice therapies should be reserved for occasions when no other medications are available. These agents may compromise standard of care.
This study was approved by the University of Pittsburgh Institutional Review Board (study identifier, 20060221). Data were downloaded from IQVIA MIDAS by the first and senior authors and stored on a secure Box, Inc server. All authors had full access.
Outcomes
Our primary outcome was changes in per-population purchases of ICU medicines in 2020 relative to 2019. We first examined monthly purchasing trends from August 2014 through August 2020. Rates were standardized per 1,000 population using mid-year estimates from the United Nations 2019 Urbanization Prospectus.22 We also reported monthly, new COVID-19 case counts per 100,000 population.23
Main Analysis
Our main hypothesis was that global ICU drug purchases increased at the beginning of the pandemic, potentially leading to shortages and a shift to therapeutic alternatives. Therefore, we examined changes in ICU drug purchasing rates in March 2020, when the World Health Organization declared COVID-19 a pandemic. Our effect estimate was the change in purchases per 1,000 population relative to March 2019. We computed bootstrap-based CIs using 10,000 samples.24 Although SARS-CoV-2 transmission was widespread only in a few countries in March 2020, previous reports suggest potential stockpiling or proactive purchasing as countries prepared for ICU surges and drug shortages.25
We used a pulse interventional autoregressive integrated moving average (ARIMA) model to test for scenarios in which purchasing increased suddenly in March 2020 and then returned to prepandemic levels. This method is preferred in drug use research because purchasing trends often are autocorrelated and violate linear regression assumptions.26, 27, 28 We performed a seasonal difference by lagging each series by 12 months (d = 12). To maximize stability, prepandemic trends were estimated using all 6 years of available data (August 2014-February 2020). Moving average (q), autoregressive (p), and seasonal terms were added as appropriate based on the lowest Akaike information criterion and residual autocorrelation function, partial autocorrelation function, and white noise probability plots. We tested the stationarity assumption using augmented Dickey Fuller tests (α = 0.1).28 We did not report estimated coefficients from the ARIMA models (eg, p or q coefficients), because these are not interpretable directly in relationship to changes in purchasing rates.
We also examined changes in ICU drug purchases during the first COVID-19 surge from April through August 2020. The effect estimate compared the aggregated purchasing rate within these 5 months with the same months in 2019. We tested the significance of this change with a ramp ARIMA model because we expected that purchases would drop suddenly in April 2020 (eg, when a drug went into shortage) and then would remain at this lower level.
Subgroup Analyses
Our secondary hypothesis was that drug purchasing responses differed by development status. We repeated the ARIMA analyses within developed (n = 28) and developing (n = 12) countries, based on the United Nations’ 2019 World Economic Situation Prospectus.29 This classification accounts for several factors that impact population health, including national income, life expectancy, and educational attainment. MIDAS captured most pharmacy sales in both groups (98% on average). To test whether observed changes differed between developed and developing countries, we took the natural logarithm of each series and then ran the pulse and ramp ARIMA models on a third series defined as: logged developed rate minus logged developing rate. China was analyzed separately with intervention dates starting in February 2020, corresponding to the World Health Organization’s global health emergency declaration.30
Results
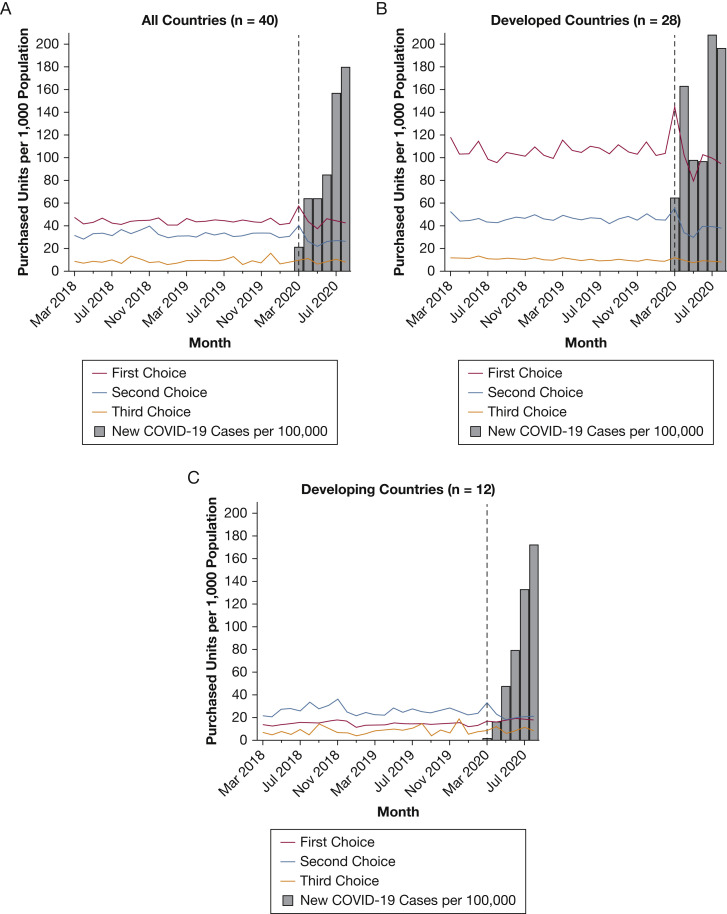
Monthly purchasing rates by drug class and development status are shown in Figure 1 (March 2018-August 2020) and e-Figure 1 (August 2014-August 2020). Before March 2020, global purchases of ICU medicines remained stable, with a mean purchasing rate of 82.2 units per 1,000 population from August 2014 through February 2020. The types of drugs purchased differed substantially by development status. In the 2 years before the pandemic, average monthly purchasing rates of first-choice agents were 105.6 vs 14.5 per 1,000 in developed vs developing countries. In contrast, second-choice medicines dominated in the developing group. Third-choice medications showed low purchasing rates in both groups (Fig 1). Developed countries also had more hospital beds per capita and spent more on health care (e-Table 1).
Figure 1.
A-C, Graphs showing drug purchasing trends, March 2018 through August 2020, by response category and development status: all countries (A), developed countries (B), and developing countries (C). Purchasing rates from authors’ analysis of Multinational Integrated Data Analysis monthly hospital-based purchasing data, March 2018 through August 2020. COVID-19 data from Johns Hopkins University (compiled by Our World in Data). Black dashed line indicates March 2020. Refer to e-Figure 1 for monthly trends across the entire study period (August 2014-August 2020). Development status is drawn from the United Nations’ 2020 World Economic Situation Prospectus. Economies in transition were included in the developing group.
Changes in March 2020
e-Tables 2 and 3 present the pulse ARIMA models. Each outcome met the stationarity assumption. e-Figure 2 presents residual diagnostics for the main drug categories. Relative to 2019, global ICU medication purchases per 1,000 population increased by 23.6% (95% CI, 7.9%-37.9%) in March 2020 (Table 2 ). This result was statistically significant in the pulse ARIMA models (P < .001) (Table 3 ). We did not observe a statistically significant difference between developed and developing countries (P = .88). Developed countries showed a relative increase in ICU drug purchases of 19.6% (95% CI, 10.2%-27.9%). Developing countries also trended toward increasing ICU drug supply, with an increase of 31.7% (95% CI, –8.1%-52.6%) (Table 2). The number of new reported COVID-19 cases per 100,000 population did not reach peak levels until July and August 2020 (Fig 1).
Table 2.
Unadjusted Relative Changes in Units Purchased per 1,000 Population, 2020 vs 2019
| Drug Class | Pandemic Declaration (March 2020) |
First Stage of the Pandemic (April-August 2020) |
||||
|---|---|---|---|---|---|---|
| Purchases per 1,000 Population |
% Change (95% CI)a | Purchases per 1,000 Population |
% Change (95% CI)b | |||
| March 2019 | March 2020 | April-August 2019 | April-August 2020 | |||
| All countries (N = 40) | 86.7 | 107.1 | 23.6 (7.9-37.9) | 431.0 | 386.0 | –10.3 (–16.9 to –3.5) |
| First choice | 46.3 | 57.5 | 24.3 (13.0-31.9) | 220.0 | 214.0 | –2.8 (–13.4 to 16.5) |
| Second choice | 31.1 | 40.2 | 29.3 (2.6-56.2) | 159.0 | 127.0 | –20.2 (–27.9 to –15.2) |
| Third choice | 9.3 | 9.4 | 1.1 (–19.9 to 13.3) | 51.2 | 45.0 | –12.2 (–18.4 to –5.3) |
| Analgesic | 32.7 | 42.6 | 30.1 (4.8-53.2) | 163.0 | 135.0 | –17.2 (–23.9 to –12.2) |
| Sedative | 18.2 | 23.4 | 28 (6.1-38.7) | 91.8 | 89.7 | –2.3 (–10.4 to 5.2) |
| Anticoagulant | 17.3 | 18.2 | 5.3 (–2.5 to 24.1) | 86.5 | 78.6 | –9.1 (–22.1 to 13.5) |
| Corticosteroid | 11.3 | 12.7 | 12.5 (1.3-25.6) | 55.4 | 45.9 | –17.1 (–26.7 to –10.7) |
| Inotropes and vasopressors | 5.1 | 6.7 | 33.6 (18.8-47.4) | 24.2 | 25.0 | 3.6 (–7.6 to 15.3) |
| NMBA | 2.1 | 3.5 | 68.7 (29.7-93.2) | 9.7 | 11.8 | 21.8 (0.8-61.0) |
| Developed countries (n = 28) | 175.7 | 210.1 | 19.6 (10.2-27.9) | 804.0 | 699.0 | –13.1 (–17.4 to –4.4) |
| First choice | 115.4 | 143.7 | 24.5 (13-32.8) | 532.0 | 479.0 | –10.0 (–16.0 to 5.1) |
| Second choice | 48.8 | 55.2 | 13.2 (2.6-27.2) | 226.0 | 178.0 | –21.1 (–27.1 to –16.8) |
| Third choice | 11.6 | 11.2 | –2.8 (–4.8 to 9.7) | 47.0 | 41.9 | –10.8 (–18.9 to –8.7) |
| Analgesic | 56.5 | 67.5 | 19.4 (5.9-28.6) | 263.0 | 214.0 | –18.7 (–23.0 to –13.6) |
| Sedative | 36.4 | 47 | 29.2 (16.5-39.8) | 155.0 | 144.0 | –7.3 (–9.8 to 1.6) |
| Anticoagulant | 47.0 | 49.5 | 5.3 (–2.6 to 29.3) | 228.0. | 198.0 | –13.2 (–25.9 to 12.9) |
| Corticosteroid | 20.7 | 22.5 | 8.7 (–0.5 to 25.2) | 90.1 | 72.5 | –19.5 (–31.0 to –14.3) |
| Inotropes and vasopressors | 9.9 | 14.1 | 42.9 (20.9-59.1) | 44.8 | 44.3 | –1.0 (–8.0 to 4.1) |
| NMBA | 5.3 | 9.6 | 80.8 (34.6-105.8) | 23.6 | 26.4 | 11.7 (1.4-21.9) |
| Developing countries (n = 12) | 44.5 | 58.6 | 31.7 (–8.1 to 52.6) | 254.0 | 239.0 | –5.8 (–24.0 to 0.3) |
| First choice | 13.5 | 16.9 | 25.0 (–2.2 to 40.2) | 72.4 | 89.4 | 23.4 (–7.9 to 40.2) |
| Second choice | 22.7 | 33.2 | 45.8 (–8.5 to 77.7) | 128.0 | 103.0 | –19.4 (–36.8 to –11.0) |
| Third choice | 8.3 | 8.6 | 3.7 (–33.6 to 16.7) | 53.3 | 46.5 | –12.7 (–27.5 to 1.8) |
| Analgesic | 21.5 | 30.9 | 43.8 (–8.5 to 74.4) | 116.0 | 98.2 | –145.4 (–33.0 to –8.5) |
| Sedative | 9.7 | 12.2 | 26.7 (–24.6 to 44.6) | 61.7 | 64.2 | 4.0 (–20.7 to 10.0) |
| Anticoagulant | 3.3 | 3.5 | 7.6 (–20.8 to 19.4) | 19.5 | 22.5 | 15.5 (0.7-31.4) |
| Corticosteroid | 6.8 | 8.1 | 18.5 (–5.8 to 35.4) | 38.9 | 33.4 | –14.3 (–33.0 to –1.1) |
| Inotropes and vasopressors | 2.8 | 3.3 | 18.5 (3.2-25.5) | 14.4 | 15.9 | 10.7 (–13.4 to 31.2) |
| NMBA | 0.59 | 0.71 | 20.4 (5.6-33.9) | 3.1 | 5.0 | 58.8 (–17.8 to 195.0) |
Authors’ analysis of Multinational Integrated Data Analysis monthly hospital-based purchasing data, August 2014 through August 2020. NMBA = neuromuscular blocking agent.
Relative estimates defined as (monthly rate in March 2020 minus monthly rate in March 2019) divided by (monthly rate in March 2019). CIs are derived from 10,000 bootstrap samples.
Relative estimates defined as (summed rate from April through August 2020 minus summed rate from April through August 2019) divided by (summed rate from April through August 2019). CIs are derived from 10,000 bootstrap samples.
Table 3.
ARIMA Tests of Changes, 2020 vs Previous Years
| Drug Class |
P Value, March 2020 vs Previous Yearsa |
P Value, April-August 2020 vs Previous Yearsb |
||||||
|---|---|---|---|---|---|---|---|---|
| All Countries (N = 40) | Developed Countries (n = 28) | Developing Countries (n = 12) | Difference, Developed vs Developing Countriesc | All Countries (N = 40) | Developed Countries (n = 28) | Developing Countries (n = 12) | Difference, Developed vs Developing Countriesd | |
| All ICU drugs | < .001 | < .001 | .05 | .88 | .006 | < .001 | .94 | .05 |
| First choice | < .001 | < .001 | .014 | .031 | < .001 | < .001 | < .001 | < .001 |
| Second choice | < .001 | < .001 | < .001 | .11 | < .001 | < .001 | .009 | .17 |
| Third choice | .93 | .25 | .84 | .58 | .87 | .11 | .98 | .79 |
| Analgesic | < .001 | < .001 | .005 | .27 | < .001 | < .001 | .014 | .28 |
| Sedative | .25 | < .001 | .96 | .93 | .97 | < .001 | .65 | .37 |
| Anticoagulant | .18 | .05 | .42 | .31 | < .001 | < .001 | .034 | < .001 |
| Corticosteroid | < .001 | .006 | < .001 | .83 | < .001 | < .001 | .09 | .017 |
| Inotropes and vasopressors | < .001 | < .001 | .39 | .007 | .08 | .36 | .014 | .01 |
| NMBA | < .001 | < .001 | .96 | .021 | < .001 | .09 | < .001 | < .001 |
Authors’ analysis of Multinational Integrated Data Analysis monthly hospital-based purchasing data, August 2014 through August 2020. Boldface values indicate P < .05. NMBA = neuromuscular blocking agent.
P value derived from pulse intervention on original series.
P value derived from ramp intervention on original series.
P values derived from pulse intervention for logged and differenced series.
P values derived from ramp intervention for logged and differenced series.
Globally, purchases for second-choice medicines showed the largest relative increases in March 2020 (29.3%; 95% CI, 2.6%-56.2%). Although the development groups did not differ significantly in the ARIMA analysis (P = .11), the relative increase in developing countries was more than three times higher than that in developed regions (45.8% vs 13.2%, respectively). We observed a statistically significant difference in purchases for first-choice drugs (P = .031). However, the relative changes were similar (24.5% and 25.0%). Purchases for third-choice drugs did not change (Table 2, Table 3).
Changes From April Through August 2020
Despite growing COVID-19 incidence, global ICU medication purchases decreased by 10.3% (95% CI, –16.9% to –3.5%) between April and August 2020 relative to 2019. Like the March 2020 results, the largest change occurred for second-choice drugs, which decreased by 20.2% (95% CI, –27.9% to –15.2%) (Table 2).
e-Table 3 presents the ramp ARIMA models. The observed purchasing decrease from April through August 2020 was statistically significant only among developed countries (P < .001) (Table 3). In contrast, first-choice purchases increased by 23.4% in developing countries (95% CI, –7.9% to 40.2%) (Table 2). Both groups decreased purchases for second-choice drugs. Neither group significantly changed purchases for third-choice drugs (Table 2, Table 3).
Differences by Drug Category
Relative changes by drug category are shown in Tables 2 and 3. e-Tables 4 and 5 show results for subclasses within each category. Among the six analyzed ICU drug categories, analgesics showed the highest purchasing rates (Table 2, e-Fig 1). Like the overall results, global purchases increased when the pandemic was declared (30.1%; 95% CI, 4.8%-53.2%) and then decreased from April through August (–17.2%; 95% CI, –23.9% to -12.2%). These changes did not differ by development status (ARIMA P ≥ .27) (Table 3). However, developed and developing countries bought different types of analgesics. In March 2020, developed countries significantly increased opioid purchases, whereas developing countries purchased more nonsteroidal antiinflammatory drugs. From April through August 2020, developing countries continued to purchase more opioids, sufentanil, and alfentanil, while analgesic purchases decreased in developed nations (e-Tables 4, 5).
Global corticosteroid purchasing rates increased by 12.5% (95% CI, 1.3%-25.6%) in March 2020, followed by a decrease of 17.1% (95% CI, –26.7% to –10.7%) from April through August (Table 2). Second-choice corticosteroids showed the largest changes. The observed decrease from April through August 2020 again was statistically significant only for developed countries (P < .001) (Table 3). This difference seemed to be driven by dexamethasone and prednisolone (e-Tables 4, 5).
NMBAs, inotropes, and vasopressors increased significantly in developed, but not developing countries, in March 2020 (P ≤ .021 for difference). However, this pattern flipped from April through August 2020, when purchases increased in developing, but not in developed, countries (P ≤ .01 for difference) (Table 3). Developing countries also significantly increased purchases for first-choice anticoagulants and first-choice sedatives from April through August (e-Tables 4, 5).
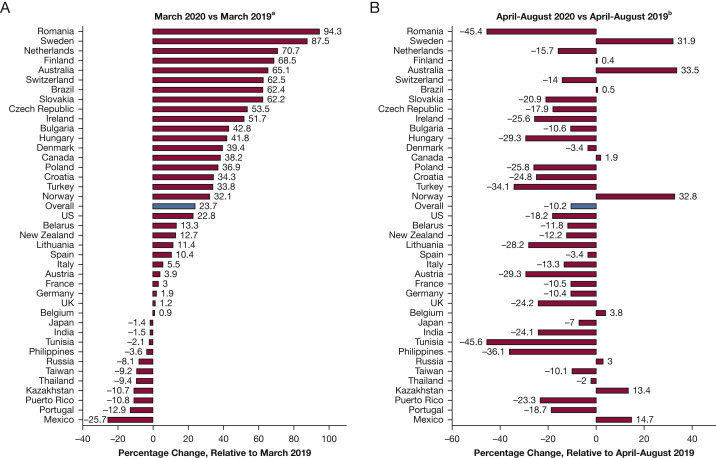
Differences by Country
Figure 2 presents country-specific relative changes in the overall ICU drug purchasing rate in March 2020 and April through August 2020 compared to 2019. Consistent with the overall results, 28 countries increased per-population purchases of ICU medicines in March 2020. We observed increases of ≥ 50% among several small European countries, as well as in Australia and Brazil. Only 11 countries decreased ICU drug purchases in March 2020; except for Japan and Portugal, all of these were in the developing group. Like the overall results, most countries decreased ICU drug purchases from April through August 2020. However, we observed increases of > 10% in five countries during these months: Sweden (31.9%), Australia (33.5%), Norway (32.8%), Kazakhstan (13.4%), and Mexico (14.7%).
Figure 2.
A-B, Forest plot showing relative changes in units purchased per 1,000 population, 2020 vs 2019, by country: March 2020 vs March 2019 (A) and April-August 2020 vs April-August 2019 (B). Authors’ analysis is of Multinational Integrated Data Analysis monthly hospital-based purchasing data, August 2014 through August 2020. aRelative estimates defined as: (monthly rate in March 2020 minus monthly rate in March 2019) divided by (monthly rate in March 2019). bRelative estimates defined as: (summed rate from April through August 2020 minus the summed rate from April through August 2019) divided by (summed rate from April through August 2019).
The ARIMA models for China can be found in e-Figure 3 and e-Table 6. Unlike the overall results, ICU drug purchases in China did not change significantly at the start of its COVID-19 epidemic in February 2020 (P = .079). However, we did observe significant decreases in purchases for anticoagulants, corticosteroids, and NMBAs. From March through August 2020, per-population purchases for all classes except third-choice medications, inotropes, and vasopressors were significantly lower compared with 2019.
Post Hoc Analyses
Our analyses revealed substantial country-level variation in ICU drug purchasing trends. One potential driver could be the timing of national COVID-19 epidemics. We therefore performed two additional post hoc analyses that compared our main March 2020 results with purchasing behaviors at different time points.
First, we analyzed each pandemic month separately to assess changes in purchasing as the pandemic progressed. These results are shown in e-Table 7. Globally, purchases for most drug categories increased the most in March 2020, compared with any other month of the pandemic. The only exception was purchases for third-choice drugs, which showed a delayed increase of 16.4% (95% CI, –36.2% to 47.4%) in April 2020, compared with 1.1% (95% CI, –19.9% to 13.3%) in March. This delayed effect seemed to be driven by sedative and NMBA purchases in developing countries. Starting in June 2020, developing countries also showed delayed increases in purchases for anticoagulants, inotropes, and vasopressors. Increased purchases for first-choice agents were largest in developing settings in June and July (33.5% and 29.8%, respectively, vs 25% in March). ICU drug purchases decreased in developed countries in every month after March, except sedatives, NMBA, inotropes, and vasopressors, which saw small increases in April.
Second, we calculated country-specific purchasing changes in each month that the government required a nonpharmacologic intervention (eg, business closures, stay-at-home orders).31 Results for this analysis are presented in e-Table 8. Most countries increased purchases of ICU drugs more in March 2020 than in any other nonpharmacologic intervention month. The exceptions were Australia and Kazakhstan, which increased drug purchases by > 85% in January and February 2020, respectively.
Finally, country-level variation in purchasing responses to COVID-19 may be explained by health system infrastructure. To test this hypothesis, we ranked countries by relevant health system characteristics, including COVID-19 incidence and response stringency, ICU bed occupancy, hospital capacity, and health care financing.23 , 32 , 33 These results can be found in e-Figures 4, 5, 6, 7, 8, 9, 10, 11, and 12. We did not observe any clear trends between country-specific purchasing and available health system variables. The exception was Sweden, which appeared to increase purchases from April through August in response to increased ICU occupancy rates (e-Fig 5).
Discussion
The results of this global study supported our hypothesis that global ICU drug purchasing increased at the start of the COVID-19 pandemic. Contrary to our second hypothesis, our results suggest that both developed and developing countries trended toward increased purchases. However, wealthier nations continued to show higher absolute purchasing rates, especially for first-choice drugs. Additionally, post hoc analyses revealed little association between purchasing responses and local COVID-19 situations. These results suggest a need for more equitable and organized drug purchasing policies during future pandemics.
The observed global increases in ICU drug purchases in March 2020 suggest that these drugs may be accessible to purchase as countries prepare for ICU surges and potential shortages. Many of these agents are inexpensive generics with a long history of use. Nevertheless, purchasing rates remained higher in developed vs developing countries. We observed proactive purchasing (and potential overbuying) in developed countries compared with delayed increases among developing nations. Because this dataset does not contain information on unfilled orders, surplus capacity, or absolute supply, we could not quantify the optimal level of purchasing in each country. However, our findings suggest that purchasing behaviors in developed countries may impact the immediate availability of ICU medicines worldwide. Aside from pandemics and other natural disasters, shortages of IV generic drugs are increasingly common and result from chronic vulnerabilities in the global drug supply chain (eg, low incentives for less-profitable drugs, regulatory challenges).34 In addition to these established causes, our results suggest a need to understand better how purchasing decisions in one country impact drug supplies elsewhere.
In the current analysis, developing countries were more likely to purchase second-choice vs first-choice drugs. Access to evidence-based critical care may lag in developing settings for several potential reasons. In this dataset, second-choice drugs were almost one-third of the cost of first-choice agents in developing countries (data not shown). New treatments for critical illness may be difficult to implement in resource-limited settings. Outside of pandemics, ICU case mix in developing regions may differ from that in wealthier countries given high burdens of maternal mortality, motor vehicle injury, and acute infectious disease complications.35 These results suggest a need to prioritize ICU agents in global efforts to ensure equitable access to essential drugs. Nevertheless, in 2019, the World Health Organization’s recommended essential medicines list included only 24 of the 69 agents in this study.36
Post hoc analyses suggested that drug purchasing trends during the COVID-19 pandemic were not clearly related to local COVID-19 situations or to country-level health system characteristics, with a few exceptions. For example, Australia increased ICU drug purchases in every pandemic month, as well as in February 2020. This may be related to national efforts to strengthen the National Medical Stockpile.37 Kazakhstan’s purchasing responses also seemed more aligned to national policies such as international travel restrictions and cancellation of public gatherings (e-Table 8). Sweden’s sustained increase in purchasing seemed to be related to increased ICU occupancy (e-Fig 5), potentially because of its failed herd immunity approach to COVID-19.38 Other than these few examples, country-level purchasing responses were concentrated in March 2020, regardless of national COVID-19 situations. These findings highlight the impact of international public health policy announcements in terms of catalyzing national responses. However, they also underscore a need for more robust country-level prediction tools to align drug purchasing better with expected demand. Such tools could help to prevent so-called panic buying, as well as to monitor progress toward more equitable distribution of medicines.6
We were pleased to find that purchases for third-choice drugs did not change. These results suggest that drug shortages did not result in the poorest choices of care, which may increase adverse outcome rates.39 , 40 Conservation efforts should focus on switching to oral or bolus forms of first- and second-choice medicines.14 , 16 These efforts could avoid the use of agents that lack evidence, as well as situations in which no drugs are available.41 , 42
There are several limitations to the current analysis. Although we proxied inpatient medicines by restricting the analysis to IV drugs, we could not explicitly identify ICU-specific purchases in MIDAS. However, this limitation applied throughout the study period, so should not bias our effect estimates. We also categorized drugs into first-, second-, and third-choice agents using North American guidelines, which may not include all approved drugs in each country.6 , 14, 15, 16, 17, 18, 19 It is also notable that this categorization could be subjective depending on practitioner, facility, and patient need.14 , 21 We similarly used the established “developed” and “developing” classification to group countries. Although countries within each group were similar on measured variables (e-Table 1), it is possible that unmeasured heterogeneity exists. Purchasing rates could be underestimated (especially in developing countries), because we excluded retail purchases. We also did not analyze switches to nonparenteral hospital formulations (eg, oral sedatives). Finally, because our data ended in August 2020, we could not evaluate purchasing changes during the second wave of ICU surges.
Interpretation
In this global study, we observed an increase in purchases for ICU medicines at the start of the COVID-19 pandemic. Although this increase occurred in both developed and developing countries, global supply of these essential medicines continued to favor wealthier nations. Although purchasing of cheap, generic drugs may not change as drastically during public health emergencies compared with other medicines, disparities still exist within the global drug supply chain. National and transnational organizations should focus on building more stable, equitable distribution strategies to prepare for the next pandemic shock.
Take-home Points.
Study Question: How did purchases of first-, second-, and third-choice ICU agents change in developed and developing countries at the start of the COVID-19 pandemic?
Results: Both developed and developing countries increased purchases for ICU drugs when the pandemic was declared, but before peak infection rates. Developed countries continued to show higher purchasing rates and bought more first-choice drugs. Country-level purchasing responses seemed unrelated to local COVID-19 situations.
Interpretation: Inexpensive, generic ICU medicines may be accessible globally at the start of public health emergencies. However, developed countries continue to have better access and may have overbought preferred medicines early in the COVID-19 pandemic.
Acknowledgments
Author contributions: K. J. S. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K. C. K. and K. J. S. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M. T., S. L. K.-G., and I. J. B. contributed substantially to the study design, interpretation, and the writing of the manuscript. S. D. R. contributed substantially to the study design and statistical analyses.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The data for this project was provided through a special COVID-19 research opportunity from the IQVIA, Inc Institute Human Data Science Research Collaborative.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The data for this project was provided through a special COVID-19 research opportunity from the IQVIA Institute Human Data Science Research Collaborative. All authors report receiving funding unrelated to this project from the National Institutes of Health. Dr Tadrous reports funds from Canadian Institutes of Health Research and the Ontario Ministry of Health. Dr Barbash reports funding from Agency for Healthcare Research and Quality (AHRQ). Dr Suda reports funds from AHRQ, the National Institutes of Health, and Veterans Health Administration.
Supplementary Data
References
- 1.Socal M.P., Sharfstein J.M., Greene J.A. The pandemic and the supply chain: gaps in pharmaceutical production and distribution. Am J Public Health. 2021;111(4):635–639. doi: 10.2105/AJPH.2020.306138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socal M.P., Sharfstein J.M., Greene J.A. Critical drugs for critical care: protecting the US pharmaceutical supply in a time of crisis. Am J Public Health. 2020;110(9):1346–1347. doi: 10.2105/AJPH.2020.305803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badreldin HA, Atallah B. Global drug shortages due to COVID-19: impact on patient care and mitigation strategies. Res Soc Adm Pharm. 2021;17(1):1946-1949. [DOI] [PMC free article] [PubMed]
- 4.Samuels E., Kelly M. Analysis: how false hope spread about hydroxychloroquine to treat covid-19—and the consequences that followed. Washington Post. Accessed March 8, 2021. https://www.washingtonpost.com/politics/2020/04/13/how-false-hope-spread-about-hydroxychloroquine-its-consequences/
- 5.A timeline of COVID-19 developments in 2020. American Journal of Managed Care. Accessed March 8, 2021. https://www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020
- 6.Burry L.D., Barletta J.F., Williamson D., et al. It takes a village. Contending with Drug Shortages During Disasters. Chest. 2020;158(6):2414–2424. doi: 10.1016/j.chest.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox E. Drug shortages statistics. American Society of Health-System Pharmacists. https://www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-Statistics
- 8.European Medicines Agency Shortages catalogue. Published September 17, 2018. European Medicines Agency website. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/availability-medicines/shortages-catalogue
- 9.Pauwels K., Simoens S., Casteels M., Huys I. Insights into European drug shortages: a survey of hospital pharmacists. PLoS One. 2015;10(3):e0119322. doi: 10.1371/journal.pone.0119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettus K., Cleary J.F., Lima L de, et al. Availability of internationally controlled essential medicines in the COVID-19 pandemic. J Pain Symptom Manage. 2020;60(2):e48–e51. doi: 10.1016/j.jpainsymman.2020.04.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong M., Moles R.J., Chaar B., Chen T.F. Essential medicines in a high income country: essential to whom? PLoS One. 2015;10(12):e0143654. doi: 10.1371/journal.pone.0143654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson N.C., Quinn N.J., Khalique S., et al. Clinical pharmacists: an invaluable part of the coronavirus disease 2019 frontline response. Crit Care Explor. 2020;2(10):e0243. doi: 10.1097/CCE.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y. 2020 ACTS annual report statistical quality applied to IQVIA’s information offerings. Accessed June 15, 2021. IQVIA website. https://www.iqvia.com/library/publications/acts-2020
- 14.Ammar M.A., Sacha G.L., Welch S.C., et al. Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med. 2021;36(2):157–174. doi: 10.1177/0885066620951426. [DOI] [PubMed] [Google Scholar]
- 15.Aziz S., Arabi Y.M., Alhazzani W., et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46(7):1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siow W.T., Tang S.H., Agrawal R.V., et al. Essential ICU drug shortages for COVID-19: what can frontline clinicians do? Crit Care. 2020;24:260. doi: 10.1186/s13054-020-02971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benge C.D., Burka A.T. Heparin drug shortage conservation strategies. Fed Pract. 2019;36(10):449–454. [PMC free article] [PubMed] [Google Scholar]
- 18.Kanji S., Burry L., Williamson D., et al. Therapeutic alternatives and strategies for drug conservation in the intensive care unit during times of drug shortage: a report of the Ontario COVID-19 ICU Drug Task Force. Can J Anesth Can Anesth. 2020;67(10):1405–1416. doi: 10.1007/s12630-020-01713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanques G., Constantin J.-M., Devlin J.W., et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46(12):2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hick J.L., Einav S., Hanfling D., et al. Surge capacity principles. Chest. 2014;146(4):e1S–e16. doi: 10.1378/chest.14-0733. [DOI] [PubMed] [Google Scholar]
- 21.Einav S., Hick J.L., Hanfling D., et al. Surge capacity logistics: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 suppl):e17S–e43. doi: 10.1378/chest.14-0734. [DOI] [PubMed] [Google Scholar]
- 22.United Nations Population Division World urbanization prospects. United Nations website. Accessed November 17, 2020. https://population.un.org/wup/DataQuery/
- 23.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. Coronavirus pandemic (COVID-19). Our World Data. Published online March 5, 2020. Accessed March 13, 2021. Our World in Data website. https://ourworldindata.org/coronavirus
- 24.SAS Help Center PROC SURVEYSELECT statement. SAS website. Accessed April 18, 2021. https://documentation.sas.com/doc/en/pgmsascdc/9.4_3.4/statug/statug_surveyselect_syntax01.htm
- 25.Hernandez I., Tadrous M., Magnani J.W., Guo J., Suda K.J. Impact of the COVID-19 pandemic on global anticoagulant sales: a cross-sectional analysis across 39 countries. Am J Cardiovasc Drugs. 2021;21(5):581–583. doi: 10.1007/s40256-021-00475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helfenstein U. The use of transfer function models, intervention analysis and related time series methods in epidemiology. Int J Epidemiol. 1991;20(3):808–815. doi: 10.1093/ije/20.3.808. [DOI] [PubMed] [Google Scholar]
- 27.Jandoc R., Burden A.M., Mamdani M., Lévesque L.E., Cadarette S.M. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol. 2015;68(8):950–956. doi: 10.1016/j.jclinepi.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Schaffer A.L., Dobbins T.A., Pearson S.-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol. 2021;21(1):58. doi: 10.1186/s12874-021-01235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhenmin L., Kituyi M., Songwe V., Algayerova O., Barcena A., Alisiahbana A.S., Dashti R. 2020. https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/publication/WESP2020_FullReport_web.pdf
- 30.World Health Organization Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). World Health Organization website. Accessed April 21, 2021. https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov
- 31.Hale T., Angrist N., Goldszmidt R., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021;5(4):529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie H., Mathieu E., Rodés-Guirao L., et al. Coronavirus pandemic (COVID-19). Accessed April 21, 2021. https://ourworldindata.org/coronavirus
- 33.European Centre for Disease Prevention and Control Data on hospital and ICU admission rates and current occupancy for COVID-19. Published April 15, 2021. European Centre for Disease Prevention and Control website. Accessed April 21, 2021. https://www.ecdc.europa.eu/en/publications-data/download-data-hospital-and-icu-admission-rates-and-current-occupancy-covid-19
- 34.Center for Drug Evaluation and Research, United States Food and Drug Administration Drug shortages: root causes and potential solutions. Published online March 11, 2020. Food and Drug Administration website. Accessed June 14, 2021. https://www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions
- 35.Diaz JV, Riviello ED, Papali A, Adhikari NKJ, Ferreira JC. Global critical care: moving forward in resource-limited settings. Ann Glob Health. 85(1). https://doi.org/10.5334/aogh.2413 [DOI] [PMC free article] [PubMed]
- 36.World Health Organization. 2017 WHO model list of essential medicines, 20th list (March 2017, amended August 2017). World Health Organization. https://apps.who.int/iris/handle/10665/273826
- 37.Australian Government Department of Health National medical stockpile. Published March 19, 2020. Australian Government Department of Health website. Accessed July 13, 2021. https://www.health.gov.au/initiatives-and-programs/national-medical-stockpile
- 38.Claeson M., Hanson S. COVID-19 and the Swedish enigma. Lancet. 2021;397(10271):259–261. doi: 10.1016/S0140-6736(20)32750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall R., Bryson G.L., Flowerdew G., et al. Drug shortages in Canadian anesthesia: a national survey. Can J Anaesth J Can Anesth. 2013;60(6):539–551. doi: 10.1007/s12630-013-9920-z. [DOI] [PubMed] [Google Scholar]
- 40.Baumer A.M., Clark A.M., Witmer D.R., Geize S.B., Vermeulen L.C., Deffenbaugh J.H. National survey of the impact of drug shortages in acute care hospitals. Am J Health Syst Pharm. 2004;61(19):2015–2022. doi: 10.1093/ajhp/61.19.2015. [DOI] [PubMed] [Google Scholar]
- 41.Reuters Running low on sedatives, Brazil hospitals tie down patients before intubation. Published April 16, 2021. The Wire Science website. Accessed June 16, 2021. https://science.thewire.in/health/running-low-on-sedatives-brazil-hospitals-tie-down-patients-before-intubation/
- 42.Bergamo M. Supply of medicines used to intubate Covid-19 patient will last 20 days. Published March 19, 2021. Folha de São Paolo Science and Health website. Accessed June 16, 2021. https://www1.folha.uol.com.br/internacional/en/scienceandhealth/2021/03/supply-of-medicines-used-to-intubate-covid-19-patient-will-last-20-days.shtml
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.