Abstract
Although vaccination against Coronavirus disease-2019 (COVID-19) is still occurring, several adverse effects temporally related to these vaccines are already being reported, even if through isolated case reports. In the present study, we describe the lesions seen on magnetic resonance imaging (MRI) of three patients who developed neurological symptoms after receiving the ChAdOX1 nCoV-19 vaccine (Oxford/AstraZeneca). The first patient presented with an ischemic stroke in the posterior limb of the left internal capsule, two days after vaccination. The second patient presented with a left facial nerve palsy, seven days after vaccination. The third patient presented with myelitis, eight days after receiving the vaccine. All patients presented the symptoms after the first dose of the vaccine and did not have a history of previous COVID-19. The real incidence of these types of complications is not known yet, but it is important to consider the possibility of COVID-19 vaccine complications, in patients with a recent history of vaccination and recent development of neurological symptoms, even though this association is only casual. Longitudinal studies are necessary to further analyze the incidence of the adverse effects of each vaccine against SARS-CoV-2.
Keywords: COVID-19, Vaccines, Stroke, Facial palsy, Myelitis
1. Introduction
Since the beginning of the current Coronavirus disease-2019 (COVID-19) pandemic, a great interest in vaccines has emerged to prevent the disease. In December 2020, several National Health Agencies approved some vaccines for population use.1 Since the clinical trials of these vaccines, multiple side effects have been reported, ranging from mild symptoms, such as injection site pain, myalgia, fatigue, and fever, to more deleterious ones, including anaphylactic shock.2 In addition, concerns about potential neurological complications of COVID-19 vaccination also arose.3
However, as population-level vaccination is still taking place, it is still difficult to determine whether cases of bleeding, thrombosis, encephalitis and/or myelitis reported after vaccination are just coincidental or really related to vaccination.
This article aims to report 3 cases of neurological diseases that began few days after vaccination against COVID-19.
2. Cases description
2.1. Case 1
A 64-year-old man, with systemic arterial hypertension, presented with right superior and inferior limbs paresia, two days after he received the first dose of the recombinant ChAdOX1 nCoV-19 vaccine. The patient did not have a history of COVID-19, and did not present other cardiovascular risk factors. He was not obese, did not have dyslipidemia or diabetes mellitus, and did not smoke. Also, he did not have a history of previous cardiovascular events. Brain magnetic resonance imaging (MRI), performed on eighth post-vaccination day, demonstrated an acute ischemic stroke in the left nucleo-capsular region, affecting the posterior limb of the internal capsule (Fig. 1 ). The hemogram, including platelets count, was normal. Carotid arteries color-Doppler ultrasonography and echocardiography were also normal. Serum lipids and homocysteine were normal, and the screening for thrombophilia was negative. The patient was hospitalized for a week, under supportive treatment, mainly with aspirin and blood pressure control. The patient had a partial improvement of the symptoms, and still has paresia in the right limbs.
Fig. 1.
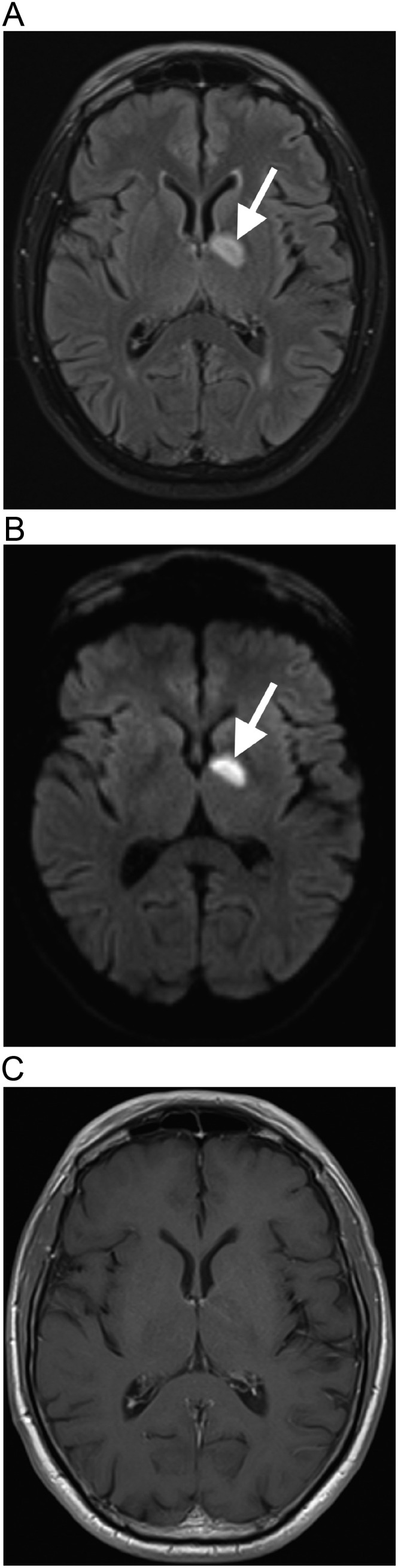
Ischemic stroke after COVID-19 vaccination. Brain MRI demonstrates an acute ischemic stroke in the left basal ganglia region, with hyperintense signal on FLAIR (arrow in a), associated with restricted diffusion, seen on diffusion-weighted imaging (arrow in b), with reduced signal on apparent diffusion coefficient, without gadolinium-enhancement (c), affecting the posterior limb of the internal capsule, in the territory of the left anterior choroidal artery, which is a small-caliber artery not usually seen on MR-angiography. The magnetic resonance angiography is normal (not shown).
2.2. Case 2
A previously healthy 42-year-old man, with no history of COVID-19, presented with pain in the left ear, associated with left facial muscles weakness, accompanied by paresis of the left forehead's muscles, as well as left lagophthalmos and labial hypomobility, compatible with left peripheral facial nerve palsy, seven days after he received the first dose of the recombinant ChAdOX1 nCoV-19 vaccine. There was no history of trauma or other identifiable triggers. Brain MRI, performed 9 days after the vaccination, showed gadolinium enhancement in the canalicular and labyrinthine portions of the left facial nerve and in the left geniculate ganglion (Fig. 2 ). The brain parenchyma was normal. The hemogram was normal. The cerebrospinal fluid (CSF) cell count, as well as protein and glucose levels were normal. The CSF was negative for toxoplasmosis, influenza, herpes simplex virus, varicella zoster virus, cytomegalovirus, and syphilis. The patient presented complete recovery after 7 days of treatment with oral prednisone, 60 mg/day.
Fig. 2.
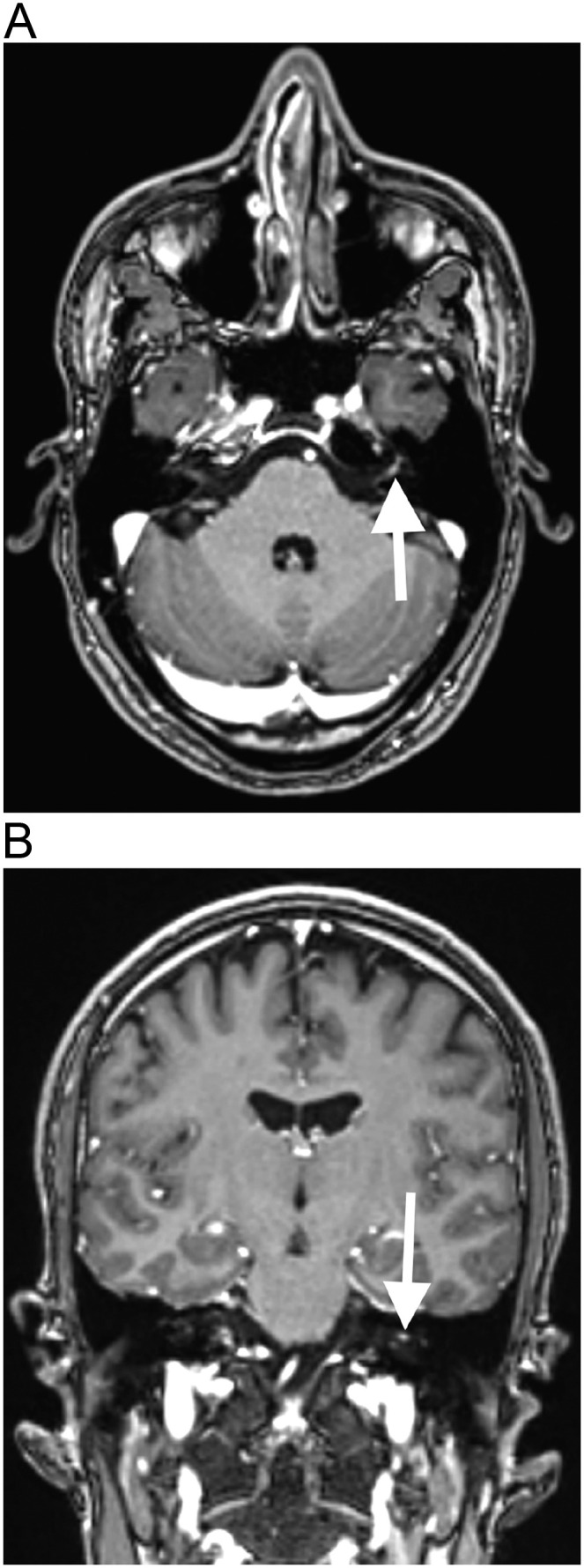
Left facial nerve palsy after COVID-19 vaccination. Brain MRI shows gadolinium enhancement in the canalicular and labyrinthine portions of the left facial nerve, on T1–3D MPRAGE in the axial (arrow in a) and coronal (arrow in b) planes.
2.3. Case 3
A previously healthy 65-year-old man, also with no history of COVID-19, presented with tetraparesia, 8 days after receiving the first dose of the recombinant ChAdOX1 nCoV-19 vaccine. Spine MRI, performed on fifteenth post-vaccination day, demonstrated a lesion with hyperintense signal on T2-weighted imaging and short tau inversion recovery (STIR), with no gadolinium-enhancement, extending from the level of C4 to C6, suggestive of myelitis (Fig. 3 ). Although the patient also had cervical degenerative discopathy, the spinal cord lesion extended beyond the level of the degenerative disc disease, was lateralized to the left portion of the spinal cord, and, in the axial plane, there was no significant compression of the spinal cord. Also, the patient had no symptoms secondary to spinal cord compression prior to this event. Brain MRI was normal. The CSF analysis revealed an increased protein level (70 mg/dL), with normal cell count, and was negative for toxoplasmosis, influenza, herpes simplex virus, varicella zoster virus, cytomegalovirus, and syphilis. Oligoclonal bands (OCBs) were absent, anti-aquaporin 4 and anti-myelin oligodendrocyte antibodies were negative, in the CSF. The patient was hospitalized and treated with 1 g/day of methylprednisolone, for 5 days, with almost complete improvement of the symptoms, with subsequent steroid oral tapering.
Fig. 3.
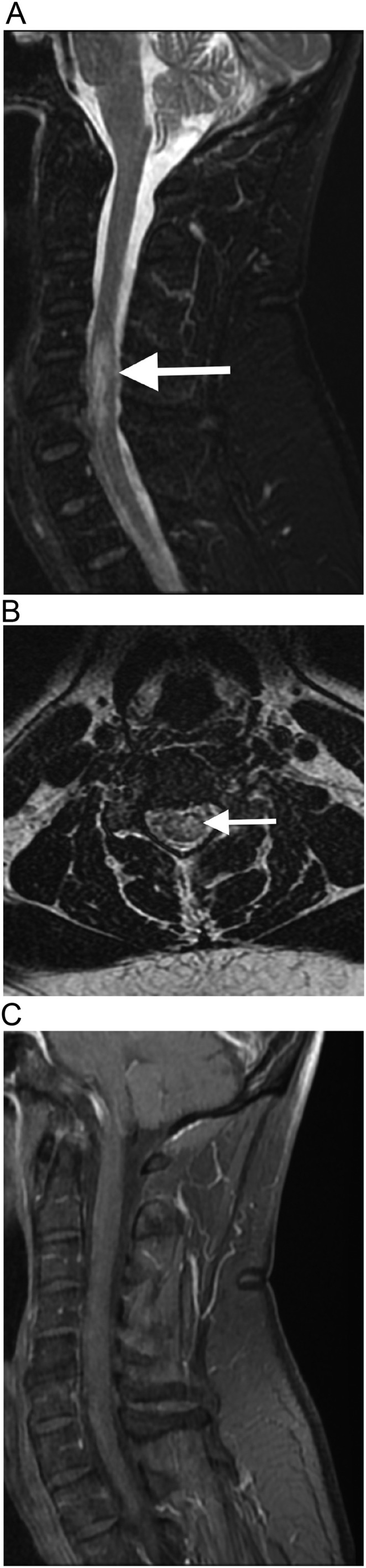
Myelitis after COVID-19 vaccination. Cervical spine MRI shows a hyperintense lesion on STIR, in the sagittal plane (arrow in a), and on T2-weighted imaging, in the axial plane (arrow in b), extending from C4 to C6 level, lateralized to the left portion of the spinal cord. The lesion does not present gadolinium-enhancement (c). Although the patient also have cervical degenerative discopathy, the lesion extends beyond the level of the degenerative disc disease, and, in the axial plane, there is no significant compression of the spinal cord.
3. Discussion
We reported three patients with neurological symptoms associated with brain or spine MRI alterations, temporally-related with a ChAdOX1 nCoV-19 vaccine (Oxford/AstraZeneca) dose. One patient had an ischemic stroke, two days after vaccination; the second patient had a facial nerve palsy, seven days after vaccination; and the third one had a cervical myelitis.
Several adverse effects of the vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), have been reported. The majority of these adverse effects correspond to mild and limited symptoms, such as fever, chills, fatigue, headache, joint and muscle pain, malaise, and nausea.4 However, since phase III trials there are concerns about more serious adverse effects.5
Thrombotic complications have been reported in association with BNT162b2 (Pfizer/BioNtech), mRNA-1273 (Moderna), and ChAdOx1 nCov-19 (Oxford/AstraZeneca) vaccines, with a total rate of 0.21 case of venous or arterial thrombotic events per 1 million person vaccinated-days. Some of these patients had a heparin-induced thrombocytopenia-like syndrome, suggesting an immunological event as a potential origin of thrombosis,6 in which antibodies were found to cause massive platelet activation.7 The ChAdOx1 nCoV-19 vaccine has a replication-deficient chimpanzee adenovirus (ChAdOx1), containing the full-length spike glycoprotein of SARS-CoV-2 (nCoV-19) gene. Then, this hypothesis relates to the reaction between the cationic platelet factor 4 (PF-4), and the anionic free DNA, found in the recombinant adenovirus vaccine.7 However, the frequency and the thrombosis-related deaths, remain extremely low if viewed against the global impact of COVID-19 itself. Walter et al.8 reported a case of ischemic stroke in a patient with vaccine-induced thrombosis, without thrombocytopenia, associated with elevated serum IgG antibodies against PF4-polyanion complexes. Although, we did not asses the presence of antibodies against PF4–polyanion complexes in the patient with a cerebrovascular event presented in this study, he may have presented a form of vaccine-induced thrombosis, without thrombocytopenia, as in the case reported by Walter et al.,8 as he developed neurological symptoms temporally related to ChAdOx1 nCov-19 vaccine.
During the trial phase, ChAdOX1 nCoV-19 vaccine had been associated with myelitis twice. One case was considered a possible related event, whereas the other one was found to be secondary to multiple sclerosis.9., 10. Previous authors reported cases of transverse myelitis,9., 11. including longitudinally extensive transverse myelitis,12 related with ChAdOX1 nCoV-19 vaccine with excellent recovery.9 The pathophysiology of myelitis associated with vaccines against COVID-19 remains unknown, but it is hypothesized that SARS-CoV-2 antigens, perhaps also present in the vaccine or its chimpanzee adenovirus adjuvant (in the case of ChAdOx1 nCoV-19 vaccine), may induce immune mechanisms leading to myelitis.13 Although rare, inflammatory complications of the central nervous system have already been associated with other vaccines, such as those against H1N1 influenza, Japanese encephalitis, or yellow fever, with different types of encephalitis and myelitis, including acute disseminated encephalomyelitis.14 One of our patients also presented with myelitis temporally related with COVID-19 vaccination, with a good response to treatment.
Previous cases of facial nerve palsy have been reported after BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and CoronaVac (Sinovac Biotech) vaccines against COVID-19.15., 16. Colella et al.15 described the first peer-reviewed case report of Bell's palsy following Pfizer-BioNTech vaccination, however, they did not report if the patient had neuroimaging alterations. Some studies on adverse effects of COVID-19 vaccines presented different results according with the studied vaccine. While Wan et al.16 reported an overall increased risk of facial palsy after CoronaVac vaccination, a case-control study reported no association between facial palsy and BNT162b2 vaccine.17 Facial palsy may be associated with an autoimmune phenomenon, through molecular mimicry by the vaccine antigen, activation of dormant autoreactive T cells, or to an immune-mediated segmental demyelination similar to Guillain-Barré syndrome (GBS). Other possible pathophysiology mechanism is a reactivation of latent herpes simplex type 1 infection of the geniculate ganglia of facial nerve, secondary to the immunological challenge imposed by the vaccine.16 Although the second patient presented in this article did not receive BNT162b2, mRNA-1273 or CoronaVac vaccines, he received the ChAdOX1 nCoV-19 vaccine (Oxford/AstraZeneca) and developed symptoms of left peripheral facial palsy, 7 days after the first dose of this vaccine, with symptoms improvement after steroids treatment, in a similar way of the previous patients.15 Although it is not yet possible to gauge the likelihood of a cause-effect relationship and the possible role of comorbidities between COVID-19 vaccines and facial palsy, and other neurological adverse events, the timing and mode of onset suggests that these effects may be related to vaccination.
Waheed et al.2 reported a case of GBS during the first week after receiving the first dose of BNT162b2 (Pfizer) vaccine, associated with gadolinium-enhancement of the cauda equina nerve roots. Although our patients had not developed GBS, and they were given a different vaccine, the possibility of GBS should be considered in patients with post-vaccine neurological complications and compatible signs and symptoms.
The majority of significant neurological adverse effects associated with vaccines against COVID-19 are still just anecdotally reported. The real incidence of these types of complications is not known yet. It must be taken into account that the reported associations may be only casual, and it is necessary to assess whether their incidence in the vaccinated population is really above the expected incidence with other vaccines or in the general population, as well as the severity of the adverse effects.9 Also, it is also important to state that all vaccines, including those against COVID-19 and other diseases, may rarely present adverse effects.14 The three cases presented in this study were related to the same vaccine, because this is one of the most used vaccines in our country, and not necessarily because this specific vaccine generates more adverse effects, compared to others.
Until the day this article was written, more than 142 million doses of the vaccines against COVID-19 were already applied in Brazil,18 with an average of more than 1 million doses applied per day, during the month of July 2021.19 Although the number of applied doses from each manufacturer is not disclosed, 75.9 million doses form Astrazeneca/Oxford, 57.7 million from Coronovac/Sinovac, 16.3 million from Pfizer/BioNTech, and 4.7 million from Janssen/Johnson&Johnson vaccines were distributed in Brazil (but not necessarily already applied).20 In addition, ChAdOX1 nCoV-19 vaccine has been used since the beginning of the campaign, in January 2021. BNT162b2 (Pfizer) has only recently been introduced in Brazil, and mRNA-1273 (Moderna) and Gam-COVID-Vac (Sputnik V) vaccines are not currently being used in our country. Then, it is expected to have a greater number of adverse effects related to ChAdOX1 nCoV-19 vaccine in Brazil.
Despite only the temporal association between vaccination with adverse effects does not necessarily imply causation, there is much to learn about the effects of vaccination against COVID-19. Population-based studies are needed to evaluate adverse effects of these vaccines and whether, in fact, there is an increase in the number of neurological adverse effects with the use of these vaccines. Nevertheless, one certainty remains: the benefit of getting vaccinated outweighs any possible risk.21
Therefore, it is important to keep in mind the imaging aspect of the central nervous system lesions related to the vaccine against COVID-19, even if it is only a casual association. Risks and benefits exist for all vaccines, but there is no doubt that mass vaccination is the best option for controlling the current pandemic. Longitudinal studies are necessary to further analyze the incidence of the adverse effects of each vaccine against SARS-CoV-2.
Funding
No funding was received for this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author contributions
Diogo Goulart Corrêa: Conceptualization; Investigation; Writing the original draft.
Luis Alcides Quevedo Cañete: Investigation; Writing the original draft.
Gutemberg Augusto Cruz dos Santos: Methodology; Investigation.
Romulo Varella de Oliveira: Formal analysis; Investigation.
Carlos Otávio Brandão: Formal analysis; Reviewing the original draft;
Luiz Celso Hygino da Cruz Jr.: Formal analysis; Reviewing the original draft; Supervision.
Declaration of competing interest
None.
References
- 1.Coronavirus disease (COVID-19): vaccines. 2020. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines Accessed 26 April 2021.
- 2.Waheed S., Bayas A., Hindi F., Rizvi Z., Espinosa P.S. Neurological complications of COVID-19: guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2) doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahase E. Covid-19: Oxford researchers halt vaccine trial while adverse reaction is investigated. BMJ. 2020;370 doi: 10.1136/bmj.m3525. [DOI] [PubMed] [Google Scholar]
- 6.Smadja D.M., Yue Q.Y., Chocron R., Sanchez O., Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58(1) doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review of the potential mechanisms and proposed management. Sci Prog. 2021;104(2) doi: 10.1177/00368504211025927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter U., Fuchs M., Grossmann A. Adenovirus-vectored COVID-19 vaccine-induced immune thrombosis of carotid artery: a case report. Neurology. 2021 doi: 10.1212/WNL.0000000000012576. [Online first] [DOI] [PubMed] [Google Scholar]
- 9.Singh Malhotra H., Gupta P., Prabhu V., Garg R.K., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021 doi: 10.1093/qjmed/hcab069. [Online first] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voysey M., Clemens S., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vegezzi E., Ravaglia S., Buongarzone G., et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J Neuroimmunol. 2021 doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Román G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katal S., Pouraryan A., Gholamrezanezhad A. COVID-19 vaccine is here: practical considerations for clinical imaging applications. Clin Imaging. 2021;76:38–41. doi: 10.1016/j.clinimag.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colella G., Orlandi M., Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021:1–3. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan E.Y.W., Chui C.S.C., Lai F.T.T. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451-5. [Online first] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shemer A., Pras E., Einan-Lifshitz A., Dubinsky-Pertzov B., Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021 doi: 10.1001/jamaoto.2021.1259. [online first] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministério da Saúde COVID-19 Vacinação - Doses aplicadas. 2021. https://qsprod.saude.gov.br/extensions/DEMAS_C19Vacina/DEMAS_C19Vacina.html Accessed August 5.
- 19.Daily COVID-19 vaccine doses administered. 2021. https://ourworldindata.org/grapher/daily-covid-19-vaccination-doses?country=~BRA Accessed August 6.
- 20.Confira os últimos números da vacinação contra a Covid-19. 2021. https://www.gov.br/casacivil/pt-br/assuntos/noticias/2021/julho/confira-os-ultimos-numeros-da-vacinacao-contra-a-covid-19 Accessed August 5.
- 21.Cirillo N., Doan R. The association between COVID-19 vaccination and Bell's palsy. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00467-9. [Online first] [DOI] [PMC free article] [PubMed] [Google Scholar]


