Abstract
Background A nasal access guide (NAG) for endoscopic endonasal approaches (EEAs) to the skull-base has been developed and approved for clinical use but its utility has not been formally investigated.
Objective The study aims to assess the effect of a NAG on endoscopic visualization during cadaveric dissection and to perform a workflow analysis with process-based performance measures in the operating room and their effect on clinical outcomes.
Methods Skull-base course participants were observed during hands-on cadaveric dissection with and without NAG. Instances of endoscope withdrawal for lens cleaning and inadequate visualization due to lens soiling were tabulated. Participants completed a Likert-scale survey examining the NAG utility and provided an overall grading. Surgical workflow and process-based performance on patients undergoing EEA to the skull-base was analyzed. Passage of powered and dissecting instruments, removal of endoscopes for cleaning, and dislodgment or migration of the device were reviewed. Postoperative assessments included mucosal trauma and synechiae formation.
Results Instances of endoscope soiling and manual cleaning were significantly reduced by 40% and 61% with the NAG during cadaveric dissection. The overall grading of the device was 2.75/3. Surgical workflow was observed in 35 patients. Average number of passes of endoscopes, instruments, and powered tools during a 10-minute observation period were 3,17, and 5 during the surgical approach, and 3, 18, and 1 during tumor dissection. Dislodgement of the device occurred in 25.7% and migration of the device in 2.8% of cases. Postoperative synechiae, exposed cartilage or septal perforation was not observed in follow up.
Conclusion NAG can significantly reduce inadequate visualization during EEA to the skull-base and has the potential to reduce instances of nasal trauma. Participants assessed its overall utility as being “excellent.”
Keywords: Endoscopic Endonasal Approach, lens cleaning, endoscopic visualization, skull base surgery
Introduction
With the advent of endoscopic endonasal approaches (EEAs) to the skull base, devices have emerged to assist with maintaining adequate endoscopic visualization. Soiling of the endoscope lens may disrupt the flow of the operation 1 and may result in technical errors or misjudgment, potentially causing patient injury. 2 3 In addition, disturbances may lengthen the procedure time and may increase treatment costs, essentially decreasing surgical efficiency. 4 5
The most notable innovation serving this purpose has been the endoscope lens cleaning sheath. 6 In our experience, while this device aids in visualization, it has some shortcomings and it is not a complete solution for EEA skull base cases. Examples of such systems are InstaClear (Olympus, California, United States) and Endo-Scrub (Medtronic, Dublin, Ireland) lens cleaning sheaths. 7 8 The hollow sheath adds an irrigation port to the distal end of the endoscope that allows for in situ cleansing. The washing liquid, generally saline or distilled water, flows through the irrigation channel along the endoscope lens. The current iteration of this device (Endo-Scrub) adds 0.7 mm to the diameter of the endoscope when placed which may increase the likelihood of collateral damage to surrounding structures and restricts the working space for other instruments. In our opinion, another relative disadvantage of the lens cleaning sheath compared with manual irrigation is that it does not offer the added function of clearing debris and blood in the surgical field, especially while drilling.
A nasal access guide (NAG) has been recently developed by SPIWay, LLC (California, United States); the device was approved by the Food and Drug Administration (FDA) for clinical use since February 2018 and has been used in our institution since March 2018 ( Fig. 1 ). The NAG is an anatomically shaped, stretchable, watertight stent that is placed in the bilateral nares. It seats at the nasal vestibule with a conical funnel to protect the external nares and provides a working corridor to the posterior nasal cavity. It was designed to minimize iatrogenic trauma to surrounding mucosa and structures, reduce instances of inadequate visualization caused by obscuration of the endoscope lens, lessen the run-in of blood into the surgical field, and allow for more facile passage of instruments. These features make it particularly well-suited for EEA cases involving the skull base. We believe that this NAG provides visualization maintenance with added functionality. In addition, the guide can be used with lens cleaning sheaths if so desired. This device has not yet been formally investigated. In this study, we examine its utility in the setting of two cadaveric dissection EEA courses and performed a subsequent analysis of workflow and process-based performance measures in the operating room and their effect on clinical outcomes.
Fig. 1.
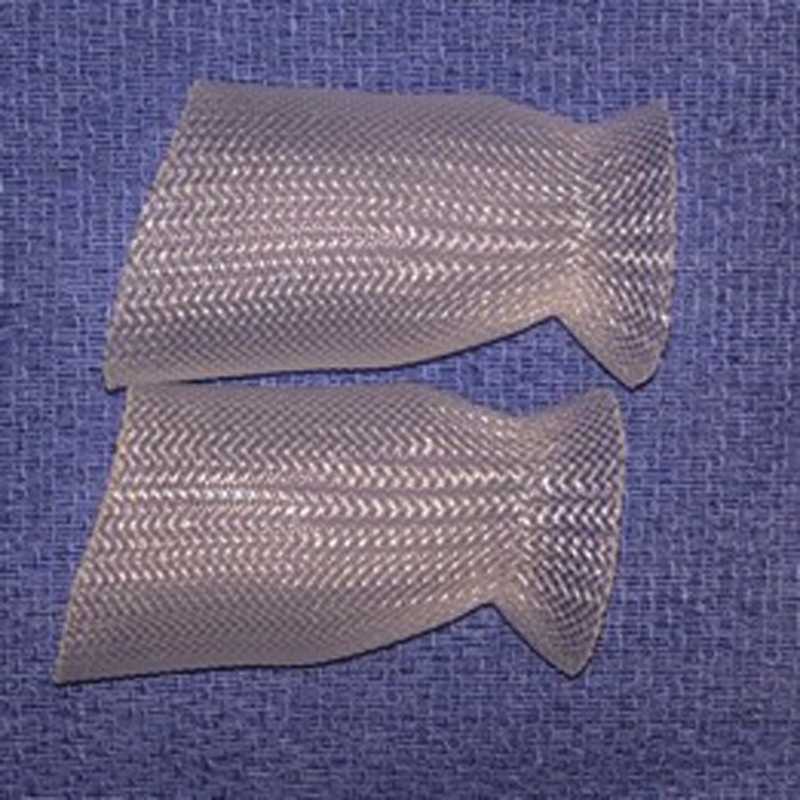
Nasal access guide. The waist of the nasal access guide seats at the nasal vestibule and the widened, flared end is situated between the nasal septum and turbinates. The material is flexible and stretches to accommodate movement of instruments without perceptible friction.
Methods
Neurosurgeons and otolaryngologists of various experience levels (residents, fellows, and attending physicians) attended two cadaveric dissection courses on EEA to the skull base in August and December of 2018. Dissection stations consisting of a two-surgeon team and one cadaver head were observed for 5 minutes with the NAG in place and 5 minutes without it during the same proctored dissection (transclival or transsellar dissections). Observation did not commence until completion of bilateral sphenoidotomies and posterior septectomy. For both conditions (with and without the NAG), independent reviewers tabulated the number of instances the endoscope was withdrawn for lens cleaning and when there was inadequate visualization on the monitor due to soiling of the endoscope lens.
Toward the end of the course, after all participants had trialed the NAG, participants were asked to complete a Likert-scale survey examining nine dimensions of the NAG's utility and assign an overall grading of the device's utility ( Table 1 ). Each dimension was rated as: “excellent” (3 points), “acceptable” (2 points), or “suboptimal” (1 point), for a maximum of 27 points. The overall grading was assessed with the same scale for a maximum of 3 points. The survey also elicited specialty (neurosurgery or otolaryngology) and experience level (resident, fellow, or attending physician) of the participant.
Table 1. Post-use survey of nasal access guide completed by course participants.
| 3 | 2 | 1 | |
|---|---|---|---|
| Attribute | “Excellent” | “Acceptable” | “Suboptimal” |
| Ease of deployment | Access guide easily folded, deployed, and unfurled. | Access guide folded, deployed, and unfurled with moderate effort. | Cannot fold, deploy, or unfurl access guide. |
| Scope and instrument movement/friction | Negligible friction between access guide and surgical tools (i.e., endoscope and instruments) during maneuvers. | Moderate friction between access guide and surgical tools (i.e., endoscope and instruments) during maneuvers. | Friction inhibits use of surgical tools (i.e., endoscope and instruments) with access guide. |
| Flex/Stretch ability | Access guide requires negligible additional force to stretch with nares, does not hinder tool maneuvers. | Access guide requires moderate force to stretch with nares slightly hindering tool maneuvers. | Access guide does not stretch with nares, completely hindering desired tool maneuvers. |
| Length of nasal access guide | Length of access guide was the right length to bypass structures and provide a working corridor without obstructing view. | Length of access guide was okay. The length to bypass structures and provide a working corridor without obstructing view could be improved. | Length of access guide must be improved. It did not allow me to effectively bypass structures and provide a working corridor without obstructing view. |
| Migration resistance | Access guide does not migrate during all maneuvers with surgical tools. | Access guide migrates moderately during high-load maneuvers with surgical tools. | Access guide migrates significantly during all maneuvers with surgical tools. |
| Effect on manual endoscope lens cleaning | Access guide significantly reduced need to withdraw scope for manual cleaning. | Access guide somewhat helped to reduce need to withdraw scope for manual cleaning. | Access guide made no difference in or increased need to withdraw scope for manual cleaning. |
| Effect on lens-camera/monitor visualization | Access guide significantly reduced frequency of lens smudging obscuring adequate visualization. | Access guide somewhat reduced frequency of lens smudging obscuring adequate visualization. | Access guide made no difference in or increased frequency of lens smudging obscuring adequate visualization. |
| Protection of collateral structures | Access guide significantly helped to reduce inadvertent trauma to surrounding mucosa and structures. | Access guide somewhat helped to reduce inadvertent trauma to surrounding mucosa and structures. | Access guide made no difference in or increased inadvertent trauma to surrounding mucosa and structures. |
| Post use condition | No damage or deformation to the access guide. | Moderate damage or deformation to the access guide. | Any material tears or complete detachment from access guide. |
| Overall performance | Access guide was easy to use and provided significant utility, improved performance during maneuvers. | Access guide was somewhat easy to use and provided some utility, somewhat improved performance during maneuvers. | Access guide was difficult to use and/ or did not provide any utility, more trouble to use than not to use. |
Note: Participants were instructed to circle or mark the box indicative of their assessment. Cumulative scores were tabulated, for a possible point total of 27 for the survey and 3 for the overall assessment.
To examine the impact of anatomical variations on utility of the NAG during dissection, predissection computed tomography scans of the cadaver heads were reviewed for the following: presence of Onodi cell, concha bullosa, caudal septum deviation, narrow nasal vestibule, and sellar pneumatization. Judgments regarding “narrow nasal vestibule” and “septal deviation” were subjective. However, these determinations were blinded to any results of the study. These findings were correlated with survey and observation findings.
Surgical workflow and process-based performance on 35 patients undergoing transsellar/suprasellar EEA to the skull base was analyzed (IRB approval-STUDY19070177). Independent observation at the time of surgery for 10 minutes during surgical approach and 10 minutes during tumor resection with the NAG in place was performed ( Fig. 2 ). For both observation times (during surgical approach and resection), the independent reviewer tabulated the number of passes of endoscope, instances the endoscope was withdrawn for lens cleaning, passes of dissecting instruments and suctions, and passes of powered instrumentation. Additionally, the reviewer recorded instances when the NAG was damaged or dislodged. The use of packing depended on the presence of an intraoperative cerebrospinal fluid leak and the type of reconstruction. Silastic Doyle septal splints were placed in all patients. Packing and splints were removed at a maximum of 7 days. Minimal debridement was performed at that time. Patients were followed 2 weeks postoperatively and evaluated for any mucosal trauma or intranasal synechia formation. Any scarring that was observed at 2 weeks would be due to mucosal trauma that occurred during surgery and was not prevented by the packing or splints.
Fig. 2.
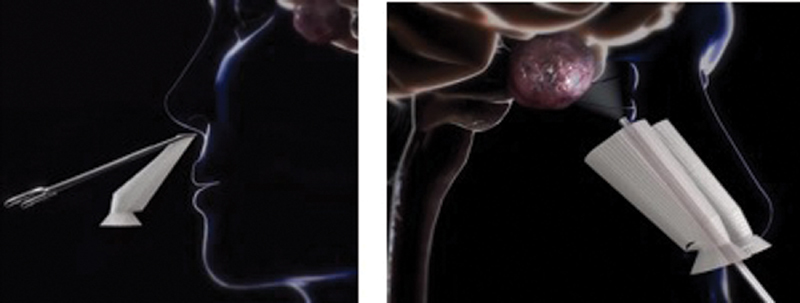
Device insertion ( left ) and nasal access guide deployed ( right ). 18
Mann-Whitney U and Kruskal–Wallis H tests were used to compare survey results between groups. Wilcoxon's Rank-Sum test was used to compare observational findings between use and nonuse of the NAG during dissection and surgery. All analyses were obtained by PSAW Statistics, release version 20.0 (SPSS Inc, Chicago, Illinois, United States), and a two-sided p -value < 0.05 was considered statistically significant. Additionally, to avoid conflicts of interest during the study, author E.W.W. was the principal investigator for the IRB submission and oversaw all aspects of the study.
Results
Cadaveric Dissection Data
A total of 61 out of 70 course participants fully completed the survey and were observed during dissection of fresh frozen cadavers with and without the NAG. A total of 35 dissection stations were observed. Each station had two participants with one serving as the endoscopist and the other as the dissector. 42.6% of participants were otolaryngologists and 57.4% were neurosurgeons ( Table 2 ). Of those participants indicating their experience level ( n = 61), 37.7% were trainees (residents/fellows) and 62.3% were attending physicians.
Table 2. Characteristics of survey takers and survey results.
| Characteristics | Respondents % ( N ) | Overall grading of device ( ± SD) | Survey score of device ( ± SD) | p -Value of survey score comparison |
|---|---|---|---|---|
| Specialty | 0.586 | |||
| Otolaryngology | 42.6 (26) | 2.65 (0.49) | 22.88 (2.89) | |
| Neurosurgery | 57.4 (35) | 2.77 (0.43) | 23.40 (2.53) | |
| Experience level | 0.677 | |||
| Trainee (resident/fellow) | 37.7 (23) | 2.79 (0.43) | 23.36 (2.56) | |
| Attending | 62.3 (38) | 2.68 (0.47) | 23.16 (2.68) |
Abbreviation: SD, standard deviation.
Note: Total possible points of device overall grade is 3 (3, “excellent,” 1, “suboptimal”). Total possible points of device Survey score is 27.
During transsellar or transclival dissections, the mean instances of endoscope lens soiling was significantly reduced by 39.9% while the NAG was in place compared with its absence (3.43 vs. 2.06; p < 0.001) ( Table 3 ). The mean instances of manual endoscope lens cleaning were reduced by 61.0% (1.46 vs. 0.57; p = 0.001). On analysis of the effect of anatomical abnormalities on observational findings, only caudal septum deviation had significant bearing. When this was present, the mean instances of endoscope leans soiling significantly increased (3.64 vs. 1.94; p = 0.007) ( Table 3 ). When the NAG was used in heads with a caudal septum deviation, the mean instances of endoscope lens soiling with the NAG in place were significantly reduced (1.45 vs. 2.82; p = 0.036).
Table 3. Objective assessment of endonasal endoscopic dissection with and without nasal access guide.
| Mean instances of endoscope lens obscuration ( ± SD) | p -Value | Mean instances of manual endoscope lens cleaning ( ± SD) | p -Value | |
|---|---|---|---|---|
| Nasal access guide | ||||
| With device | 2.06 (1.73) | <0.001 | 0.57 (0.74) | 0.001 |
| Without device | 3.43 (2.42) | 1.46 (1.54) | ||
| Anatomical anomalies | ||||
| Onodi cells | 0.434 | 0.748 | ||
| Yes | 3.25 (2.60) | 0.92 (0.90) | ||
| No | 2.62 (2.20) | 1.04 (1.44) | ||
| Concha bullosa | 0.146 | 0.959 | ||
| Yes | 3.35 (2.50) | 0.95 (1.10) | ||
| No | 2.44 (2.12) | 1.05 (1.47) | ||
| Caudal septum deviation | 0.007 | 0.176 | ||
| Yes | 3.64 (2.59) | 1.32 (1.59) | ||
| No | 1.94 (1.61) | 0.74 (1.03) | ||
| Narrow nasal vestibule | 0.230 | 0.741 | ||
| Yes | 3.75 (2.92) | 1.00 (1.07) | ||
| No | ||||
| Sellar configuration | 0.146 | 0.668 | ||
| Conchal | N/A | N/A | ||
| Sellar | 2.64 (2.34) | 1.02 (1.39) | ||
| Presellar | 3.67 (1.51) | 1.00 (0.89) |
The mean survey score was 23.33 (out of 27 possible; SD ± 2.64). Scores were not significantly affected by specialty or experience level. The mean overall grading of the NAG on a scale of 3 (1= “suboptimal,” 3 = ” excellent”) was 2.75.
Surgical Workflow and Process-Based Performance Data
Thirty-five study subjects were included with 19 female and 16 male patients. Mean age was 53 years at the time of surgery. Diagnoses included 24 patients (67.6%) with pituitary adenoma, four patients (11.5%) with craniopharyngioma, three patients (8.7%) with meningioma, three patients (8.7%) with Rathke's cleft cyst, and one patient (3.5%) with chordoma. Thirty-two patients underwent primary surgery and three patients had revision surgery. Average duration of surgery was 4.1 hours. Seventeen (48.5%) patients had septal deviation and nine (25.7%) required concurrent septoplasty. Rate of nasoseptal flap reconstruction was 71.4%; 72% of these patients also underwent a reverse septal flap for coverage of the septal donor site. The NAG was deployed before sphenoidotomy in 24 patients (70.3%) and after sphenoidotomy in 11 cases (29.7%). Powered instrumentation (drill) was used in all cases. Both surgeons were right-handed, standing on the right side of the patient.
During a 10-minute observation period, the average number of passes of endoscopes, dissecting instruments, and powered tools was three, 17, and five during the surgical approach, and three, 18, and one during tumor dissection, respectively. Most passes occurred in the patient's left (nonvisualized) nostril (87%). Average number of irrigations to clean the endoscope was nine during surgical approach and three during tumor resection.
Minor damage of the NAG on the side of drilling was occasionally observed but did not interfere with use in ten patients (28.5%); dislodgement of the device occurred in nine cases (25.7%) and migration of the device (extranasal portion pushed into the nasal passage) occurred in one case, (2.8%). Intraoperative mucosal trauma (septum abrasion) was reported in four cases prior to deployment of the device (11.4%). Instruments were easily passed through the guide; sharp right-angle instruments were a cause for dislodgement upon withdrawal of the instrument on the blind (left) side. The NAG effectively prevented any additional trauma to nasal mucosa after deployment. Postoperative synechiae, exposed cartilage, or septal perforation was not observed at 2-week follow-up in any patient. There were no significant differences with pathology-based subgroup analysis of studied variables.
Discussion
Endonasal endoscopic surgery of the skull base has become widely accepted as the preferred technique for a variety of skull base pathology. The primary advantage of endoscopic endonasal surgery is enhanced visualization of the surgical field. 9 Poor visualization, due to soiling of the endoscope lens or increased blood in the surgical field from mucosal trauma interferes with precise surgery and increases the risk of surgical error. Removal of the scope for manual cleaning of the lens disrupts the workflow and is inefficient. 10 Efforts to maintain continuous endoscopic visualization have been primarily directed toward cleansing of the lens using irrigation systems. 1 4 6 11 12
In this study, we introduce the NAG, which is an anatomically shaped, stretchable, watertight stent that is placed in the bilateral nares. It provides a conical funnel to protect the external nares and a working corridor to the posterior nasal cavity. The device provides a protected corridor to the surgical field and has been designed to minimize iatrogenic collateral damage, facilitate improved visualization, allow for easier passage of instruments, and lessen the run-in of fluids into the view of the surgical field with resultant overall improvement in surgical efficiency. The NAG is easy to deploy and accommodates a wide range of nasal apertures. The material is flexible and stretches to accommodate movement of instruments without perceptible friction.
The use of NAGs in cadaveric and live patient settings in this study demonstrates that its use significantly reduces instances of inadequate visualization due to soiling of the endoscope during EEA of the skull base, especially in the presence of septal deviation. During the cadaveric skull base course, participants who trialed the device in cadavers rated different facets of its utility favorably and most often, assessed its overall utility as being “excellent.”
The effect of experience level (attending physicians vs. trainees) on the performance of the NAG was not objectively assessed. Unfortunately, there were no adequate data to examine this statistically. However, this analysis would have been complicated by the fact that some dissection stations had both surgeons from the same specialty. It should be noted that while experience level could have potentially been a confounding variable in the measured effect of the nasal sleeve, differences in objective nasal sleeve performance measures were ascertained by comparing participants to themselves with or without use of the nasal sleeve. In this way, differences can nearly be completely attributed to use of the nasal sleeve. Additionally, a binarial approach was used in both the cadaveric dissections and live patient surgeries. However, even with a uninostril approach, instruments fed through the ipsilateral nostril relative to the endoscope still have the potential to cause inadvertent damage to mucosal structures unless the endoscope is used to guide the instrument through the nasal passage. Even on the side of the endoscope, instruments are typically passed blindly into the nasal cavity as the endoscope usually remains stationary near the surgical site. We have observed that the risk of mucosal trauma is greater in trainees who lack the “muscle memory” of repetitive actions and misjudge the surgical trajectory. Cadaveric models of surgical instrument motion confirm differences in novice and expert surgeons as shown in the study by Harbison et al where more experienced surgeons are more aware of the surrounding anatomy and operate with lower overall instrument travel indicative of greater efficiency. 13
Moreover, use of NAG in a clinical setting demonstrated the potential for prevention of collateral damage from blind passage of instruments during EEA. In particular, during a short observation period of 10 minutes, surgical instruments and powered tools were passed on average 17 and 18 times during the surgical approach and tumor dissection, respectively. For a surgery that may last from 2 to 4 hours, this translates into hundreds of passes, especially in the blinded nasal cavity (left side for right-handed surgeons). Intraoperatively, unnecessary mucosal trauma increases bleeding into the surgical field which obscures visualization of critical structures. Postoperatively, unnecessary mucosal trauma prolongs healing and may result in additional crusting, synechiae, and septal perforation. Nasal mucosalization following surgery takes on average 2 to 4 weeks. 14 15 In this study, the follow-up period was purposely limited to 2 weeks because this is the time when early synechia formation between turbinates and septal tissues can be detected and treated; longer observations may confirm mature synechia. 16
In our experience, the NAG greatly facilitates rapid passage of instruments through the nasal cavity, increasing the efficiency of surgery. It protects the nasal aperture from burn injuries from powered instrumentation such as drills and ultrasonic aspirators. For malignant tumors with seeding potential such as chordomas, we speculate that it may decrease the risk of tumor seeding in the nasal cavity from tumor spillage due to our observation that tumor fragments are sometimes dropped within the NAG as instruments are removed. 17
There were no related complications or unsatisfactory features associated with the use of the device. Potential limitations of this study include limited experience with the device in the cadaveric model, short observation periods, and lack of comparative analyses prior to and after introduction of the NAG devices. Further comparative multi-institutional studies are necessary to clearly demonstrate the clinical benefits of the NAG for visualization and prevention of postoperative complications.
Conclusion
NAG can significantly reduce instances of inadequate visualization during EEA to the skull base and have demonstrated the ability to reduce instances of nasal trauma. Participants who trialed the device rated different facets of its utility favorably and most often assessed its overall utility as being “excellent.”
Conflict of Interest P.A.G. and C.H.S. are shareholders of SPIWay, LLC, makers of the nasal access guide. The other authors report no conflict of interest.
These authors contributed equally to this work.
References
- 1.Cassera M A, Goers T A, Spaun G O, Swanström L L. Efficacy of using a novel endoscopic lens cleaning device: a prospective randomized controlled trial. Surg Innov. 2011;18(02):150–155. doi: 10.1177/1553350611399297. [DOI] [PubMed] [Google Scholar]
- 2.Wiegmann D A, ElBardissi A W, Dearani J A, Daly R C, Sundt T M., III Disruptions in surgical flow and their relationship to surgical errors: an exploratory investigation. Surgery. 2007;142(05):658–665. doi: 10.1016/j.surg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Sutton E, Youssef Y, Meenaghan N. Gaze disruptions experienced by the laparoscopic operating surgeon. Surg Endosc. 2010;24(06):1240–1244. doi: 10.1007/s00464-009-0753-3. [DOI] [PubMed] [Google Scholar]
- 4.Kreeft D, Arkenbout E A, Henselmans P WJ, van Furth W R, Breedveld P. Review of techniques to achieve optical surface cleanliness and their potential application to surgical endoscopes. Surg Innov. 2017;24(05):509–527. doi: 10.1177/1553350617708959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macia I, Gossot D. Maintaining a clear vision during long-lasting thoracoscopic procedures. Interact Cardiovasc Thorac Surg. 2010;11(05):522–524. doi: 10.1510/icvts.2010.241760. [DOI] [PubMed] [Google Scholar]
- 6.Kubo S, Kikawada T, Hasegawa H, Tominaga S, Yoshimine T. Irrigation-suction straw sheath system for a rigid endoscope during endonasal endoscopic pituitary surgery. Minim Invasive Neurosurg. 2005;48(06):373–375. doi: 10.1055/s-2005-915630. [DOI] [PubMed] [Google Scholar]
- 7.Olympus. Instaclear Lens Cleaner SystemILCS. Accessed April 16, 2020 at:http://olympusmedical.com.sg/products/all-products/other-products/ent-products/instaclear-lens-cleaner-system/index.html
- 8.Medtronic. Inferior Turbinate BladesE-SLCS. Accessed April 16, 2020 at:https://www.medtronic.com/us-en/healthcare-professionals/products/ear-nose-throat/sleep-disordered-breathing/inferior-turbinate-blades/related-products.html
- 9.Alfieri A. Endoscopic endonasal transsphenoidal approach to the sellar region: technical evolution of the methodology and refinement of a dedicated instrumentation. J Neurosurg Sci. 1999;43(02):85–92. [PubMed] [Google Scholar]
- 10.Cappabianca P, Alfieri A, Thermes S, Buonamassa S, de Divitiis E.Instruments for endoscopic endonasal transsphenoidal surgery Neurosurgery 19994502392–395., discussion 395–396 [DOI] [PubMed] [Google Scholar]
- 11.Jho H D, Alfieri A. Endoscopic endonasal pituitary surgery: evolution of surgical technique and equipment in 150 operations. Minim Invasive Neurosurg. 2001;44(01):1–12. doi: 10.1055/s-2001-13590. [DOI] [PubMed] [Google Scholar]
- 12.Tatsuki H, Yokobori T, Katayama C. A novel one-step lens cleaning device using air and water flow for endoscopic surgery. PLoS One. 2018;13(07):e0200749. doi: 10.1371/journal.pone.0200749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbison R A, Berens A M, Li Y, Bly R A, Hannaford B, Moe K S. Region-specific objective signatures of endoscopic surgical instrument motion: a cadaveric exploratory analysis. J Neurol Surg B Skull Base. 2017;78(01):99–104. doi: 10.1055/s-0036-1588061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saafan M E, Ragab S M, Albirmawy O A, Elsherif H S. Powered versus conventional endoscopic sinus surgery instruments in management of sinonasal polyposis. Eur Arch Otorhinolaryngol. 2013;270(01):149–155. doi: 10.1007/s00405-012-1969-8. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein J M, Lebowitz R A, Jacobs J B. Initial report on postoperative healing after endoscopic sinus surgery with the microdebrider. Otolaryngol Head Neck Surg. 1998;118(06):800–803. doi: 10.1016/S0194-5998(98)70272-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zeng Q, Ke X, Yang Y, Hu G, Zhang X. Influence of chitosan-based dressing on prevention of synechia and wound healing after endoscopic sinus surgery: a meta-analysis. Am J Rhinol Allergy. 2017;31(06):401–405. doi: 10.2500/ajra.2017.31.4469. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes Cabral D T, Zenonos G A, Fernandez-Miranda J C, Wang E W, Gardner P A. Iatrogenic seeding of skull base chordoma following endoscopic endonasal surgery. J Neurosurg. 2018;129(04):947–953. doi: 10.3171/2017.6.JNS17111. [DOI] [PubMed] [Google Scholar]
- 18.SPIWay. SPIWay Endonasal Access GuideE-SLCS at:http://spiway.com/the-spiway.html


