Abstract
An international, multicenter study compared trimethoprim-sulfamethoxazole MICs for 743 Streptococcus pneumoniae isolates (107 to 244 isolates per country) by E test, using Mueller-Hinton agar supplemented with 5% defibrinated horse blood or 5% defibrinated sheep blood, with MICs determined by the National Committee for Clinical Laboratory Standards broth microdilution reference method. Agreement within 1 log2 dilution and minor error rates were 69.3 and 15.5%, respectively, on sheep blood-supplemented agar and 76.9 and 13.6%, respectively, with horse blood as the supplement. Significant interlaboratory variability was observed. E test may not be a reliable method for determining the resistance of pneumococci to trimethoprim-sulfamethoxazole.
Increasing resistance of Streptococcus pneumoniae to trimethoprim-sulfamethoxazole (T-S) in North America is well documented, and resistance rates of 18 to 26% have recently been reported (1, 2, 4, 7, 13, 16). Resistance to this drug may be even more common in South America, where it is frequently used (8, 15).
An epidemiological surveillance study of invasive S. pneumoniae isolates recovered from children in six Latin American countries (the SIREVA project) was initiated in 1993 (3, 11). This study resulted in a large bank of invasive pneumococcal isolates collected from 1993 to 1996. These isolates were used to collaboratively evaluate E test for the determination of pneumococcal T-S MICs. The objectives of this study were to determine the accuracy of E test compared to broth microdilution (BMD) for testing T-S MICs and to explore the effect on performance of supplementing Mueller-Hinton agar with horse blood, as recommended by the manufacturer of the E test (technical guide 5C; AB Biodisk, Solna, Sweden), rather than with sheep blood.
The evaluation was coordinated by the Canadian National Centre for Streptococcus (NCS) and conducted in each of six participating laboratories located in Argentina, Brazil, Chile, Colombia, Mexico, and Uruguay. The pneumococcal isolates were recovered from blood, cerebrospinal fluid, or pleural fluid of Latin American children ≤5 years old (3, 11).
Common lot numbers of Mueller-Hinton II agar (MHA) (BBL-Becton Dickinson), cation-adjusted Mueller-Hinton II broth (BBL-Becton Dickinson), and Columbia agar base (Unipath Oxoid), used for the preparation of blood agar plates, were supplied to each laboratory. Trimethoprim (Glaxo Wellcome) and sulfamethoxazole (Roche) reference powders, of known potency and from a single lot, were gifts of the respective manufacturers. Two different lots of E-test strips containing T-S in a gradient of 0.002 to 32.0 μg/ml (trimethoprim concentration) were available for the evaluation. Each laboratory tested only one E-test lot. Fresh defibrinated sheep and horse blood were obtained from suppliers within each country. MHA was supplemented with either 5% defibrinated sheep blood (SB-MHA) or 5% defibrinated horse blood (HB-MHA).
The E test was performed according to the manufacturer’s instructions. A single organism suspension was used to inoculate the two agar media and to prepare a 1:100 dilution for the BMD method. The E-test reading was rounded up to the next highest doubling dilution MIC for comparison with BMD. A table for this process was provided to ensure interlaboratory consistency.
The BMD reference method was performed according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines for testing of S. pneumoniae using cation-adjusted Mueller-Hinton broth supplemented with locally prepared 3% lysed horse blood (14). S. pneumoniae ATCC 49619 was included with each test batch for both methods.
E-test results were compared to those determined by the BMD reference method. Data were analyzed for interpretive errors; false susceptibility by E test was classified as a very major error, false resistance by E test was classified as a major error, and an error involving an intermediate category by one method and either a susceptible or resistant result by the other was classified as a minor error (5). The data were also compared with the BMD reference method for MIC agreement within 1 log2 dilution. Acceptable performance was defined as ≤3% very major errors and a combination of major and minor errors of ≤7% (5).
The NCS supplied a pilot sample of 15 to 20 isolates to each of the six laboratories. Each laboratory determined the BMD MICs for its respective set of isolates, and these were compared with MIC data provided by the NCS. All laboratories obtained 100% correlation within 1 log2 dilution of the NCS results prior to beginning the evaluation.
Five of six laboratories (from countries designated A, B, C, D, and E) evaluated the performance of E test on SB-MHA and HB-MHA for a total of 743 pneumococcal isolates. The number of isolates examined in each laboratory varied from 107 to 244 (Table 1). Of the 743 isolates, 265 were susceptible (MIC, ≤0.5/9.5 μg/ml), 185 were intermediate (1/19 to 2/38 μg/ml), and 293 were resistant (≥4/76 μg/ml) to T-S by BMD (14). The laboratory in country F was unable to obtain fresh horse blood during the study period and therefore evaluated E test only on SB-MHA. These data were excluded from the cumulative totals.
TABLE 1.
Interlaboratory variation in minor errors and agreement within 1 log2 dilution of E test performed on SB-MHA and HB-MHA compared with BMD
| Country | No. of isolates tested | No. (%) of isolates showing:
|
|||
|---|---|---|---|---|---|
| Minor interpretative errors
|
Agreement within 1 log2 dilution
|
||||
| SB-MHA | HB-MHA | SB-MHA | HB-MHA | ||
| A | 122 | 5 (4.1) | 3 (2.4) | 95 (77.9) | 115 (94.3) |
| B | 244 | 34 (13.9) | 29 (11.9) | 138 (56.6) | 159 (65.2) |
| C | 107 | 46 (43.0) | 40 (37.4) | 53 (49.5) | 52 (48.6) |
| D | 150 | 15 (10.0) | 14 (9.3) | 119 (79.3) | 128 (85.3) |
| E | 120 | 15 (12.5) | 15 (12.5) | 110 (91.7) | 117 (97.5) |
| Total | 743 | 115 (15.5) | 101 (13.6) | 515 (69.3) | 571 (76.9) |
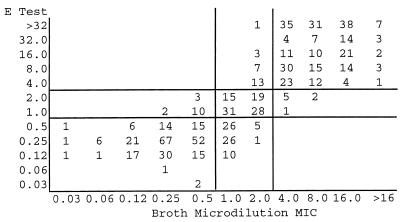
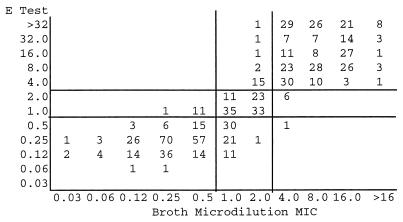
Scattergrams comparing E test MICs with SB-MHA (Fig. 1) or HB-MHA (Fig. 2) to BMD MICs, according to NCCLS interpretive criteria for T-S (14), demonstrate similar performances for the two media (correlation coefficients of 0.56 and 0.52, respectively). However, wide interlaboratory variances in agreement and minor error rates were observed (Table 1). One very major error on HB-MHA was reported; no major errors were detected.
FIG. 1.
Scattergram comparing BMD with E-test MIC determinations performed on SB-MHA for 743 S. pneumoniae isolates. Horizontal and vertical lines represent susceptible, intermediate, and resistant breakpoints. MICs, in micrograms per milliliter, are for trimethoprim only. Correlation coefficient = 0.56.
FIG. 2.
Scattergram comparing BMD with E-test MIC determinations performed on HB-MHA for 743 S. pneumoniae isolates. Horizontal and vertical lines represent susceptible, intermediate, and resistant breakpoints. MICs, in micrograms per milliliter, are for trimethoprim only. Correlation coefficient = 0.52.
The laboratory in country F tested 202 pneumococci on SB-MHA only. It reported MIC agreement within 1 log2 dilution for 151 (74.8%) isolates, 17 (8.4%) minor errors, and no very major or major errors.
Most of the minor errors (68 of 115 on SB-MHA and 63 of 101 on HB-MHA) resulted from a susceptible E-test result for isolates that were intermediate according to MICs determined by BMD. Over 60% of the isolates for which MICs failed to correlate within 1 log2 dilution were resistant to T-S (≥4/76 μg/ml) by BMD. With few exceptions, these isolates were consistently more resistant by E test regardless of the blood supplement.
Resistance to T-S of penicillin-susceptible S. pneumoniae isolates often occurs (1, 13, 15, 16), so susceptibility to the 1-μg oxacillin screen does not predict susceptibility to T-S. Furthermore, detection of resistance to T-S by disk diffusion may not be reliable (6, 10). The BMD method is recommended for MIC testing of pneumococci (14), but this method is technically demanding and expensive. E test offers a reliable alternative for some antibiotics (9, 12, 17, 18), and we have evaluated its performance for testing of pneumococci and T-S.
Two previous evaluations of T-S MICs by E test using SB-MHA reported correlations within 1 log2 dilution for 42% (9) and 88% (18) of their isolates, a range similar to our interlaboratory observations of 49.5 to 91.7%.
The E test failed to meet our acceptability criterion of ≤7% combined major-plus-minor errors (5). Our average minor error rates of 15.5 and 13.6% for SB-MHA and HB-MHA, respectively, suggest improved performance over a previous evaluation that reported 38.2% minor errors (18). Similar to that study, the majority of our minor errors resulted from E-test MICs that were lower than those determined by BMD. This trend has important clinical implications if isolates are erroneously reported as susceptible.
Conversely, all six of our participating laboratories observed elevated E-test MICs compared to BMD MICs for T-S-resistant pneumococci. Since there would be no change in the resistant interpretation for these isolates, this problem is not relevant to patient care, but it does present a technical concern. It may be that higher antibiotic concentrations on the E-test strip are inaccurate, difficult to read, or incompletely diffused into the medium.
One important observation of this study is the significant interlaboratory variability in E-test performance, even when training, materials, and protocols have been standardized. Good correlation between the NCS and all participants for the BMD method with the initial pilot sample supports interlaboratory reliability of the performance of the reference method. The two E-test lot numbers that were used did not correlate with the observed interlaboratory variation.
Others have reported poor interlaboratory reproducibility for T-S disk zone sizes even when the same MHA base was used to test S. pneumoniae ATCC 49619 (6). The faint haze of growth sometimes observed around the T-S disk may affect interpretation (6), and this difficulty may also compromise the interpretation of E-test results. However, trailing endpoints were observed infrequently by our participants; therefore, this factor is an unlikely explanation for the differences in performance.
Each laboratory supplied its own fresh sheep and horse blood, and variability in these products, as suggested by others (18), may have been one reason for the wide range in error rates reported by our participants. One explanation for this may be the variable presence of components that interfere with folate metabolism (9).
Slightly improved E-test performance with the use of HB-MHA was reported by four of five participants. Our data support the manufacturer’s recommendation of using horse blood for pneumococci when testing T-S. However, it is difficult to obtain fresh horse blood in some Latin American countries, and implementing a specific medium for testing a single drug-organism combination may not be practical.
The reasons for our interlaboratory variability require further investigation, but the variability suggests that the decision to use E test for pneumococci and T-S requires on-site evaluation against the BMD reference method with locally obtained supplies.
Acknowledgments
We thank the World Health Organization, the Pan American Health Organization, and the Canadian International Development agency for financial support.
We thank José Luis Di Fabio for guidance and unfailing confidence in the SIREVA network.
REFERENCES
- 1.Breiman R F, Butler J C, Tenover F C, Elliot J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 2.Butler J C, Hofmann J, Cetron M S, Elliot J A, Facklam R R, Breiman R F The Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s pneumococcal sentinel surveillance system. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 3.Di Fabio J L, Homma A, De Quadros C. Pan American Health Organization epidemiological surveillance network for Streptococcus pneumoniae. Microb Drug Resist. 1997;3:131–133. doi: 10.1089/mdr.1997.3.131. [DOI] [PubMed] [Google Scholar]
- 4.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elder B L, Hansen S A, Kellogg J A, Marsik F J, Zabransky R J. Verification and validation of procedures in the clinical microbiology laboratory. 1997. Coordinating ed., B. W. McCurdy. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 6.Fuchs P C, Barry A L, Brown S D The Antimicrobial Susceptibility Testing QC Group. Interpretive criteria and quality control parameters for testing of susceptibilities of Haemophilus influenzae and Streptococcus pneumoniae to trimethoprim and trimethoprim-sulfamethoxazole. J Clin Microbiol. 1997;35:125–131. doi: 10.1128/jcm.35.1.125-131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann J, Cetron M S, Farley M M, Baughman W S, Facklam R R, Elliot J A, Deaver K A, Breiman R F. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 8.Hortal M The Pneumococcus Study Group. Capsular type distribution and susceptibility to antibiotics of Streptococcus pneumoniae clinical strains isolated from Uruguayan children with systemic infections. Microb Drug Resist. 1997;3:159–163. doi: 10.1089/mdr.1997.3.159. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen J H, Howell A W, Maher L A. Quantitative antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E-test. J Clin Microbiol. 1991;29:109–114. doi: 10.1128/jcm.29.1.109-114.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgensen J H, Swenson J M, Tenover F C, Ferraro M J, Hindler J A, Murray P R. Development of interpretive criteria and quality control limits for broth microdilution and disk diffusion antimicrobial susceptibility testing of Streptococcus pneumoniae. J Clin Microbiol. 1994;32:2448–2459. doi: 10.1128/jcm.32.10.2448-2459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kertesz D A, Di Fabio J L, de Cunto Brandileone M C, Castañeda E, Echániz-Aviles G, Heitman I, Homma A, Hortal M, Lovgren M, Ruvinsky R O, Talbot J A, Weekes J, Spika J S PAHO Pneumococcal Surveillance Study Group. Invasive Streptococcus pneumoniae infection in Latin American children: results of the Pan American Health Organization Surveillance Study. Clin Infect Dis. 1998;26:1355–1361. doi: 10.1086/516350. [DOI] [PubMed] [Google Scholar]
- 12.Kiska D L, Kerr A, Jones M C, Chazotte N N, Eskridge B, Miller S, Jordan M, Sheaffer C, Gilligan P H. Comparison of antimicrobial susceptibility methods for detection of penicillin-resistant Streptococcus pneumoniae. J Clin Microbiol. 1995;33:229–232. doi: 10.1128/jcm.33.1.229-232.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovgren M, Spika J S, Talbot J A. Invasive Streptococcus pneumoniae infections: serotype distribution and antimicrobial resistance in Canada, 1992 to 1995. Can Med Assoc J. 1998;158:327–331. [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Sessegolo J F, Levin A S S, Levy C E, Asensi M, Facklam R R, Teixeira L M. Distribution of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated in Brazil from 1988 to 1992. J Clin Microbiol. 1994;32:906–911. doi: 10.1128/jcm.32.4.906-911.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simor A E, Louie M, Low D E The Canadian Bacterial Surveillance Network. Canadian national survey of prevalence of antimicrobial resistance among clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2190–2193. doi: 10.1128/aac.40.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skulnick M, Small G W, Lo P, Patel M P, Porter C R, Low D E, Matsumura S, Mazzulli T. Evaluation of accuracy and reproducibility of E test for susceptibility testing of Streptococcus pneumoniae to penicillin, cefotaxime, and ceftriaxone. J Clin Microbiol. 1995;33:2334–2337. doi: 10.1128/jcm.33.9.2334-2337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover F C, Baker C N, Swenson J M. Evaluation of commercial methods for determining antimicrobial susceptibility of Streptococcus pneumoniae. J Clin Microbiol. 1996;34:10–14. doi: 10.1128/jcm.34.1.10-14.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]