ABSTRACT
Background
Environmental enteric dysfunction (EED) is associated with chronic gut inflammation affecting nutrient absorption and development of children, primarily in low- and middle-income countries. Several studies have shown that rice bran (RB) supplementation provides nutrients and modulates gut inflammation, which may reduce risk for undernutrition.
Objective
The aim was to evaluate the effect of daily RB dietary supplementation for 6 mo on serum biomarkers in weaning infants and associated changes in serum and stool metabolites.
Methods
A 6-mo randomized-controlled dietary intervention was conducted in a cohort of weaning 6-mo-old infants in León, Nicaragua. Anthropometric indices were obtained at 6, 8, and 12 mo. Serum and stool ionomics and metabolomics were completed at the end of the 6-mo intervention using inductively coupled plasma MS and ultra-high performance LC–tandem MS. The ɑ1-acid glycoprotein, C-reactive protein, and glucagon-like peptide 2 (GLP-2) serum EED biomarkers were measured by ELISA.
Results
Twenty-four infants in the control group and 23 in the RB group successfully completed the 6-mo dietary intervention with 90% dietary compliance. RB participants had higher concentrations of GLP-2 as compared with control participants at 12 mo [median (IQR): 743.53 (380.54) pg/mL vs. 592.50 (223.59) pg/mL; P = 0.04]. Metabolite profiles showed significant fold differences of 39 serum metabolites and 44 stool metabolites from infants consuming RB compared with control, and with significant metabolic pathway enrichment scores of 4.7 for the tryptophan metabolic pathway, 5.7 for polyamine metabolism, and 5.7 for the fatty acid/acylcholine metabolic pathway in the RB group. No differences were detected in serum and stool trace elements or heavy metals following daily RB intake for 6 mo.
Conclusions
RB consumption influences a suite of metabolites associated with growth promotion and development, while also supporting nutrient absorption as measured by changes in serum GLP-2 in Nicaraguan infants. This clinical trial was registered at https://clinicaltrials.gov as NCT02615886.
Keywords: undernutrition, rice bran, environmental enteric dysfunction biomarkers, metabolome, trace elements, glucagon-like peptide 2, heavy metals
Rice bran modulates glucagon-like peptide 2, serum, and stool metabolites in weaning Nicaraguan infants.
Introduction
The high prevalence of undernutrition in low-and middle-income countries (LMICs) has negative consequences on the growth and development of children during the first 5 y of life (1–3), and stunting and wasting conditions are associated with an increased risk of death (2, 4, 5). Risk factors for undernutrition include, but are not limited to, low birth weight, inadequate breastfeeding, improper complementary feeding, and recurrent infections (4, 6). Diarrheal diseases are also one of the primary causes of undernutrition in children under 5 (1, 4, 6).
Environmental enteric dysfunction (EED) is an acquired condition of the small intestine that most affects children in LMICs (7–9). Although there is little evidence for a specific cause for EED, observational studies suggest that chronic exposure to enteric pathogens from the environment early in life is a major contributor (10–12). EED in children reflects altered gastrointestinal function such as mucosal inflammation, intestinal malabsorption, and increased intestinal permeability, which lead to protein loss (8, 9). It has been challenging to conclusively identify the causes and implications of EED when there is no accepted case definition, and there are no validated, noninvasive diagnostic tests or sets of diagnostic criteria for EED (13). The current gold standard to diagnose EED is upper gastrointestinal endoscopy with biopsy, and this method is quite limited for LMICs due to costs and concerns about safety, which limit its utility for routine diagnosis (14, 15).
In the last few years, a variety of promising EED markers have been studied as indicative of structural, functional, and metabolic changes (16, 17). ɑ1-Acid glycoprotein (AGP) and C-reactive protein (CRP) are markers of systemic inflammation and glucagon-like peptide 2 (GLP-2) is found in the intestinal damage/repair domain and is related to the nutrient absorption function by the intestine. No single biomarker for EED has been universally accepted.
Dietary interventions that provide isolated nutrients (e.g., vitamin A, zinc, iron) or improve the nutrient density of complementary feeds have not been successful in substantially improving growth outcomes in children with EED (18–20). The negative impacts of EED on children involve failure in a number of gut mucosal immune mechanisms that leads to impaired growth and development (21–23). This is a significant issue for Nicaraguans, whereby the prevalence of chronic malnutrition, which includes stunting in children under the age of 5, is 17.3% (24, 25).
Rice bran (RB) is a novel food ingredient with important macro- and micronutrients that has been shown to promote innate resistance against enteric viral and bacterial pathogens that cause diarrhea (26–28). It is a globally accessible food ingredient with a distinct stoichiometry of phytochemicals and prebiotics that induce nonspecific gut mucosal immune responses (26, 27, 29, 30). A recent study showed macronutrients and secondary metabolites present in RB to be beneficial to human health due to their antioxidant, anti-inflammatory, antimicrobial, and cancer-prevention properties (31). Several mechanisms of gut mucosal immune induction have been identified to support how increased dietary RB intake reduces host susceptibility to enteric infections (32). We previously published changes in growth outcomes and differences in fecal EED markers and microbiota as a result of an RB intervention in Nicaraguan weaning infants. Importantly, RB supplementation resulted in a higher length-for-age z-score (LAZ) and lower fecal ɑ1-anti-trypsin as compared with control-group children at 12 mo of age (33). This current study evaluated changes to serum EED biomarkers at 12 mo, targeted serum and stool trace elements, micronutrients and heavy metals, and nontargeted assessment of serum and stool metabolites in weaning infants from León, Nicaragua, where there remains a high burden of diarrheal disease and malnutrition (34).
Methods
Study design
A 6-mo randomized-controlled dietary intervention was conducted in weaning infants residing in León, Nicaragua.
This was an unblinded study in which both the research team, as well as the parents and/or guardians were told if they were part of the intervention group or control. The randomization assignments to intervention and control groups were completed by the study coordinator and ensured balance between groups for sex and health post locations. All infants were recruited from public health rosters provided by the local Health Ministry from the Perla Maria and Sutiava health sectors.
Recruitment of infants began at 4 mo of age, and with eligibility confirmed for enrollment at 6 mo of age. Infants could not have had a diarrheal episode between 4 and 6 mo of age or have taken antibiotics in the prior month to avoid changes to the gut microbiome composition that could confound the effect of RB. Additionally, all eligible participants had no history of malnutrition and received all 3 doses of the rotavirus vaccine per regular administration through the Immunization Program in Nicaragua (35). Initiation of the intervention occurred at 6 mo of age in accordance with the Nicaraguan Ministry of Health (MINSA) recommendation for exclusive breastfeeding during the first 6 mo of life.
The Institutional Review Boards at Colorado State University, Universidad Nacional Autónoma de Nicaragua–León, the University of North Carolina at Chapel Hill, and Virginia Polytechnic Institute and State University approved this study (protocol nos. 14–5233H, Acta no. 129, 14–2501, and 00000657, respectively). Written informed consent was obtained from the infant's parent or responsible guardian prior to any data collection and they allowed access to the complete infant health record. Infants at 6 mo of age who met the eligibility criteria were randomly assigned within the 2 health sectors (Perla Maria Norori and Sutiava) by sex to either the RB dietary intervention or control group, which was not provided with RB. The RB and control groups shared local feeding practices apart from the provided RB. The intervention occurred between March and October 2015 (NCT02615886). The study team used standard reporting forms to monitor which types of food the child consumed during the study period and this information was registered via the questionnaire twice a month. RB food products are not normally consumed in the Nicaraguan population and families did not have any knowledge about RB as a food ingredient for humans before our study. Brown rice was the only other similar food product known to this population, yet brown rice is rarely consumed due to lack of accessibility and high prices as well as limited availability in local grocery stores.
RB packaging for consumption
The USDA–Agricultural Research Service Dale Bumpers National Rice Research Center provided RB that was polished from the US variety Calrose. RB is prone to fat oxidation that can cause the bran to go rancid; thus, heat stabilization was performed by heating the bran at 100ºC for 5 min to inactivate the lipase/lipoxygenase enzymes that cause rancidity (36). The heat-stabilized RB was then sifted to remove any additional debris (rice husk, rice grain). Packaging of the RB was completed by Western Innovations, Inc. (Denver, CO), where 22 kg of RB was weighed into 1-g increments, separated into water-proof sachets, and heat-sealed to ensure the RB would not be contaminated.
Fourteen sachets (1 g/sachet) were filled into a 4” × 3” × 2” box that was labeled for study participants and included nutrient information. These boxes were stored in a cool 8°C, dark, dry place until they were provided to study participants.
Study intervention
As reported previously (33), the study team (doctor, nurse, and study coordinator) provided a 2-wk supply of heat-stabilized RB at each routine home visit and instructed the participant's parent or guardian to add the daily amount of RB to the participant's food. At 6 mo of age, participants in the RB group consumed 1 g RB/d (1 sachet). Between the ages of 7 and 9 mo, participants consumed 2 g RB/d (2 sachets). At 10 mo of age, participants consumed 3 g RB/d (3 sachets). The amount increased to 4 g RB/d (4 sachets) and 5 g RB/d (5 sachets) at 11 and 12 mo of age, respectively. The RB was added to appropriate weaning foods, such as rice cereal, yogurt, fruit and natural juices, vegetables, and soups. At the beginning of the intervention (6 mo of age), infants’ parents or guardians were instructed and monitored daily for 1 wk by study personnel so that guardians knew how to administer and record the amount of RB consumed. Compliance with the RB intervention was calculated from the records that had the amount of RB consumed each day circled in increments of none (0%), half (50%), or all (100%). The study team also collected any unused boxes or sachets during these visits to calculate the amount of RB consumed by children.
Participants in the control group did not receive any RB during the 6-mo study duration and there were no reports of brown rice consumption during that time frame. The study doctor, nurse, and study coordinator visited each participant together every 2 wk during the 6-mo intervention to assess information for any event related to RB consumption that occurred in the preceding 2 wk. Parents or a responsible guardian would contact the study coordinator or nurse at any time by phone call in the case that the infant presented diarrhea or had any possible adverse event. This helped with the prompt collection of the diarrheal sample. The parents or responsible guardian in both study groups could also reach the study team for questions or concerns at any time. Additional household visits occurred when participants were 6, 8, and 12 mo old for anthropometric measures (weight and length) and stool sample collection; a blood sample was obtained from each participant at 12 mo of age. Infant participants were measured for length and weight via a portable stadiometer and weighing balance for children. Length was collected to the nearest centimeter and weight to the nearest 0.1 kg. Anthropometric measures were calculated for LAZ, weight-for-age z-score (WAZ), and weight-for-length z-score (WLZ) scores following the WHO child growth standards using the WHO Anthro software (version 3.2.2) (37). Outcomes related to anthropometric measures were reported previously.
Blood was collected via venipuncture in a yellow-top tube (HumaTube Serum Gel-C/A 73030; Human Diagnostics Worldwide, Germany) at the 12-mo visit only. Blood was centrifuged at 4 ºC and 750 × g for 10 min to separate for serum and stored in a −80 ºC freezer until analysis. Stool was collected from a study-provided disposable diaper. Stool was placed on ice following collection and frozen at −80 ºC until analysis. All biospecimen samples were stored at −80 ºC at National Autonomous University of Nicaragua–Leon (UNAN-Leon) and then shipped to Colorado State University on dry ice, where they were relocated into a −80 ºC freezer prior to analysis.
A study questionnaire was completed by the participant's caregiver (e.g., mother, father, or grandparent) at baseline to collect the mother's educational level, drinking water source, household flooring type, and animals present in the household. Additional surveys were administered twice monthly to assess for duration of breastfeeding, types and timing of introductions to complementary foods, and any antibiotic use. The breastfeeding questions included whether or not the child was receiving breast milk and/or had been receiving any formula (38). The complementary feeding history included a list of 11 common Nicaraguan foods that are introduced to infants during weaning. Infants’ parents or guardians recorded how often the infant consumed each of the 11 foods. The questionnaire also recorded if a participant had received treatment with antibiotics since the last visit, the reason for taking the antibiotic, the name of the antibiotic, as well as the length of time the participant had been taking the antibiotic. Analysis of breastfeeding and formula feeding patterns, complementary feeding practices, as well as associations with nutritional status for both groups at 6 mo old (i.e., baseline) were reported previously (38).
Serum analysis for EED markers
Serum collected at 12 mo of age was analyzed for systemic inflammation markers AGP and CRP, and the intestinal damage/repair domain GLP-2 (16, 39). Serum was diluted according to the commercial kit's instructions for ELISA determination of EED biomarker concentrations. Concentrations of AGP were determined at a 10,000-fold final dilution (R&D Systems). Samples were diluted to 3000-fold for determination of CRP concentrations (ThermoFisher Scientific). GLP-2 concentrations were determined directly without any dilution step (EMD Millipore Corporation). Final concentrations were determined from averages of replicate assays and duplicate optical density (OD) readings and interpolated using Graphpad Prism 6.0 (GraphPad Prism Software) according to standards measured on each 96-well plate.
To generate the standard curve for each set of samples assayed, an average duplicate OD reading was obtained for each standard, control, and sample that was used to calculate the concentration. Samples below the limit of detection (LOD) were considered nondetected and replaced with zero. No arbitrary values were assigned below the LOD as this may introduce an artificial lack of variance. We did not encounter any samples with concentrations above the upper LOD during the EED marker analyses.
Serum trace elements and heavy metals
Serum was analyzed for elemental concentrations via inductively coupled plasma MS (ICP-MS) at the Proteomics and Metabolomics Facility at Colorado State University. For sample preparation, 150 μL serum was added to a 13- × 100-mm culture tube and mixed with 643 μL of 70% nitric acid (BDH Aristar® Plus) followed by 30 μL internal standard solution (10 ppm each of Sc, Ga, Y, In, and Bi). Samples were left overnight to digest at room temperature and were then heated in a sand bath for 2 h at 120 ºC. After samples cooled, 100 μL hydrogen peroxide (30% Ultrex® II Ultrapure reagent; J.T. Baker) was added to each sample and was heated again in a sand bath for 1 h at 120 ºC and then allowed to cool to room temperature. Solution was transferred to a 15-mL centrifuge tube and diluted to 15 mL using pure water. Samples had an internal standard concentration of 20 ppb in 3% nitric acid.
Elemental concentrations were measured using an Elan DRC (dynamic reaction cell) II mass spectrometer (PerkinElmer) connected to a SeasprayTM MEINHARD nebulizer and a quartz cyclonic spray chamber. Samples were introduced using an ASX-520 autosampler (CETAC Technologies). Lithium (Li), beryllium (Be), boron (B), sodium (Na), phosphorus (P), sulfur (S), magnesium (Mg), potassium (K), calcium (Ca), tungsten (W), iron (Fe), and lead (Pb) were measured in standard mode. Cadmium (Cd), selenium (Se), and arsenic (As) were measured in DRC mode using oxygen as the reactive gas.
Before analysis, the nebulizer gas flow and lens voltage were optimized for maximum indium signal intensity (75,597 counts/s)—0.86 and 8.25, respectively. A daily performance check was also run, which ensured that the instrument was operating properly and obtained a CeO+:Ce+ of 0.026 and a Ba++:Ba of 0.013. A calibration curve was obtained by analyzing 7 dilutions of a multi-element stock solution made from a mixture of single-element stock standards (Inorganic Ventures). To correct for instrument drift, a quality-control solution (pooled serum sample prepared by mixing 1 mL of each digested individual sample) was run every 10th sample.
Data were processed whereby each element was subjected to internal standard corrections and subsequently drift corrected (40). Corrections were chosen based on minimizing the CV for the quality-control samples. After drift correction, samples were corrected for the dilution factor. LODs and limits of quantification were calculated 3 times or 10 times the SD of the blank divided by the slope of the calibration curve, respectively (41). Final concentrations are given in parts per billion (μg/L). Measured calculations below the limits of quantification were assigned to the limits of quantification value for that element.
Stool trace elements and heavy metals
Stool samples were sent to Metabolon, Inc. (Durham, NC, USA), for ionomics. Stool was homogenized in 1% nitric acid with zirconium beads at a rate of 20 mg/1 mL. One hundred microliters of stool homogenate was transferred to a 48-well plate and digested with nitric acid, hydrochloric acid, and hydrogen peroxide. The digest was dried and reconstituted in 1 mL 0.5% nitric acid. The final unit for the concentration was μg/kg (dry stool). Metal ions were measured using ICP-MS. The samples were diluted in deionized-H2O and introduced into the ICP-MS instrument via an ESI Prep-Fast autosampler including dilution with aqueous nitric acid. The Thermo ICAP-RQ instrument was operated in positive ionization and used a multipoint external calibration curve that preceded the sample into the instrument.
Serum and stool metabolomics
Serum and stool were sent to Metabolon, Inc., for nontargeted metabolite profiling via ultra-high performance LC–tandem MS (UPLC-MS). All samples were accessioned into the Metabolon Library Information Management Systems (LIMS) and stored at −80 ºC until metabolome analysis. They were prepared using the automated MicroLab Star® system (Hamilton Company, Switzerland). Eight to 10 recovery standards were added prior to the first step in the extraction process for quality-control purposes.
Extraction was performed using 80% ice-cold methanol under vigorous shaking for 2 min (Glen Mills GenoGrinder 2000) followed by centrifugation to remove protein and dissociate small molecules bound to protein or trapped in the precipitated protein matrix. The resulting extract was divided into 5 fractions: 2 for analysis by 2 separate reverse-phase UPLC-MS methods with positive ion mode electrospray ionization, 1 for analysis by reverse-phase UPLC-MS methods with negative ion mode electrospray ionization, 1 for hydrophilic interaction LC UPLC-MS with negative ion mode electrospray ionization, and 1 sample for backup. All samples were placed briefly on a concentration evaporator (TurboVap® Zymark) to remove organic solvent.
UPLC-MS methods utilized a Waters ACQUITY UP-LC and a ThermoScientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution.
Raw data were extracted, peak-identified, and processed for quality control using Metabolon's hardware and software (systems built on a web-service platform utilizing Microsoft's .NET technologies, which run on high-performance application servers and fiber-channel storage arrays in clusters to provide active failover and load-balancing), rescaled to set the median equal to 1.
Metabolic pathway visualizations
To visualize networks of metabolic pathways from serum metabolites, the relative abundance of each metabolite was evaluated in a pathway analysis software and metabolite classification system (MetabolyncTM plug-in for Cytoscape, version 2.8.3) (42). Pathway enrichment scores (PESs) were calculated using the equation 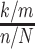 , where “k” represents the number of significant metabolites in a pathway (P ≤ 0.05), “m” the total number of identified metabolites in that pathway, “n” the total number of significant metabolites in the dataset, and “N” the total number of identified metabolites in the complete dataset (43, 44). Metabolic pathways with a PES < or >1 indicated that the pathway contained ≥1 metabolites with a statistically significant fold-difference (between RB group and control at 12 mo) compared with all other pathways within the matrix (45). Each metabolite is symbolized as a circle node, whose size corresponds to the z-score using the relative abundance mean value. This node is extending from a central submetabolic pathway node and the hexagon node represents the super metabolic pathway.
, where “k” represents the number of significant metabolites in a pathway (P ≤ 0.05), “m” the total number of identified metabolites in that pathway, “n” the total number of significant metabolites in the dataset, and “N” the total number of identified metabolites in the complete dataset (43, 44). Metabolic pathways with a PES < or >1 indicated that the pathway contained ≥1 metabolites with a statistically significant fold-difference (between RB group and control at 12 mo) compared with all other pathways within the matrix (45). Each metabolite is symbolized as a circle node, whose size corresponds to the z-score using the relative abundance mean value. This node is extending from a central submetabolic pathway node and the hexagon node represents the super metabolic pathway.
Statistical analysis
Statistical analyses for anthropometric measures (length and weight), serum EED biomarkers, and serum/stool elemental concentrations were completed using SAS version 9.4 (SAS Institute). Normality was evaluated by visual inspection. For anthropometric variables, 2-sample t tests were used to compare means for the 2 treatment groups (RB and control) separately at birth and 6 mo (prior to start of treatment).
For EED biomarkers and serum/stool trace element and heavy metals concentrations, the nonparametric Wilcoxon rank-sum test was used to test for differences between the treatment groups.
Serum and stool metabolite data normalization by each compound was corrected in run-day blocks by registering the medians to equal 1 (1.00) and normalizing each data point proportionately. Following log transformation and imputation of missing values, if any, with the minimum observed value for each compound, a Welch's 2-sample t test was used on serum metabolites to identify biochemicals that differed significantly between experimental groups at 12 mo of age. For stool metabolites, a 2-factor ANOVA with repeated measures identified biochemicals exhibiting significant interaction and main effects for experimental parameters of cohort, time point, and treatment. A P value of <0.05 was used for statistical significance and estimated false discovery rate (q-value) was calculated for nontargeted analysis of metabolites to take into account the multiple comparisons that are typical of metabolomic-based studies. Metabolomics statistical analyses were performed in ArrayStudio using median scaled-log transformed data.
Results
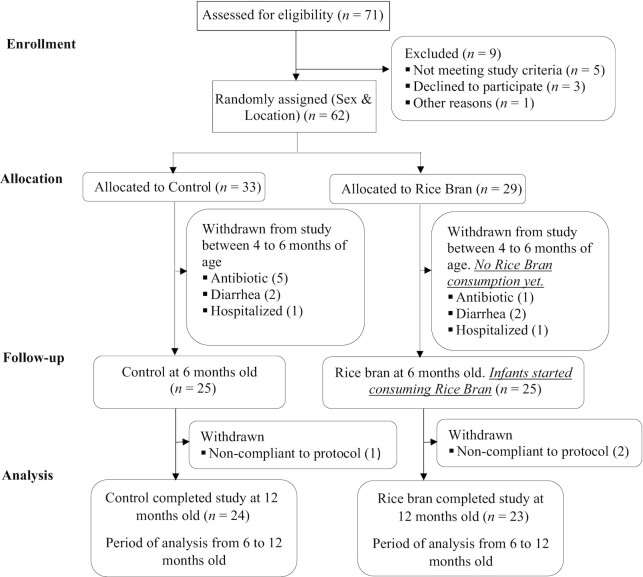
A total of 62 healthy, 4-mo-old infants were recruited and randomly assigned to 1 of 2 intervention groups, but they did not receive RB until 6 mo of age. Participants were followed when they were 4 to 6 mo old to ensure they continued to meet inclusion criteria before starting the intervention. A total of 12 infants were withdrawn during this time period due to antibiotic use (n = 6), diarrhea episode (n = 4), and hospitalization (n = 2). Twenty-four infants in the control group and 23 infants in the RB group successfully completed the 6-mo dietary intervention. A total of 3 participants were withdrawn after the intervention started due to noncompliance (i.e., not providing study samples or not regularly consuming RB). One of the withdrawn RB participants experienced vomiting after consuming the RB and was reported as an unanticipated problem to our institutional review boards. The CONSORT (Consolidated Standards of Reporting Trials) flow of participants is shown in Figure 1.
FIGURE 1.
Study recruitment and participation based on CONSORT statement guidelines. CONSORT, Consolidated Standards of Reporting Trials.
Study participant characteristics
Baseline characteristics for all participants are shown in Table 1. No significant differences were observed between sex and geographic location of health post (P = 0.67). For breastfeeding status, 96% of infants in the control group and 83% in the RB group were consuming breast milk at 6 mo of age. Table 1 illustrates a difference in the birth weight and length, whereby the control group infants were slightly heavier and longer than the RB group at birth, with significance for weight (P = 0.05). At 6 mo of age, when the dietary intervention started, no significant difference between weight and length was observed (P = 0.58 and P = 0.88, respectively). Dietary compliance was averaged for consuming RB and during the 6-mo intervention (90%). No adverse events were reported during this period.
TABLE 1.
Baseline participant characteristics of Nicaraguan infants1
| Variable | Rice bran (n = 23) | Control (n = 24) | P |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 12 (52.0) | 14 (58.0) | 0.67 |
| Female | 11 (48.0) | 10 (42.0) | |
| Water source, n (%) | |||
| Indoor municipal | 23 (100) | 24 (100) | |
| Untreated ground water | 0 (0) | 0 (0) | |
| Delivery type, n (%) | |||
| Vaginal | 16 (69.6) | 11 (45.8) | 0.09 |
| Cesarean | 7 (30.4) | 13 (54.2) | |
| Sanitation system, n (%) | |||
| None | 1 (4.3) | 0 (0) | 0.11 |
| Community latrine | 0 (0) | 0 (0) | |
| Latrine | 9 (39.1) | 4 (16.7) | |
| Indoor toilet | 13 (56.5) | 20 (83.3) | |
| Mother's education, n (%) | |||
| None | 0 (0) | 1 (4.2) | 0.59 |
| Some primary | 7 (30.4) | 3 (12.5) | |
| Completed primary | 2 (8.7) | 3 (12.5) | |
| Some secondary | 5 (21.7) | 8 (33.3) | |
| Completed secondary | 5 (21.7) | 4 (16.7) | |
| University | 4 (17.4) | 5 (20.8) | |
| Breastfeeding status, n (%) | |||
| 6 mo | 19 (82.6) | 23 (95.8) | 0.14 |
| Household animals,2 n (%) | |||
| Poultry | 9 (37.5) | 3 (12.5) | 0.36 |
| Livestock | 2 (8.7) | 2 (8.3) | |
| Domesticated pets | 16 (69.6) | 17 (70.8) | |
| None | 5 (21.7) | 7 (29.2) | |
| Anthropometry3 | |||
| Weight at birth, kg | 2.94 ± 0.38 | 3.17 ± 0.39 | 0.05 |
| Weight at 6 mo, kg | 7.93 ± 0.89 | 8.09 ±1.10 | 0.58 |
| Length at birth, cm | 49.55 ± 3.03 | 50.67 ± 1.93 | 0.15 |
| Length at 6 mo, cm | 66.26 ± 2.90 | 66.38 ± 2.10 | 0.87 |
P values for sex, delivery type, breastfeeding status, and household animals were calculated by chi-square test. Anthropometric P values were calculated by 2-sample t test.
More than 1 category per house.
Values are means ± SDs.
Diarrheal episodes among infants from 6 to 12 mo of age
Overall, 9 episodes of diarrhea were reported among the infants between months 6 and 8. In the RB group there were 5 episodes (21.7%) and there were 4 episodes (16.7%) in the control group. Diarrhea episodes were associated with a maximum of 6 stools per 24-h period, on average. Vomiting was present among 55.5% of episodes and fever among 44.4%. Of all diarrheal episodes reported, 100.0% received oral rehydration solution. Enteric pathogens were detected among 66.7% of the stool samples from infants who experienced a diarrheal episode. The most commonly detected organisms in the diarrheal samples were rotavirus (33.3%; 2 out of 5 episodes in RB and 1 out of 4 episodes in controls), enteropathogenic Escherichia coli (22.2%; 0 out of 5 RB participants and 2 out of 4 controls) and adenovirus (11.1%; 1 out of 5 RB participants and 0 out of 4 controls).
Biomarkers of EED
Table 2 illustrates serum mean concentrations for each EED biomarker. For serum biomarkers, the RB participants had significantly increased GLP-2 concentrations at 12 mo compared with controls (P = 0.03); CRP and AGP did not show significant differences between the RB and the control group. Stool EED biomarkers were reported previously (33).
TABLE 2.
EED biomarkers in serum at 12 mo of age1
| EED biomarker (serum, 12 mo) | Control group (n = 24) | Rice bran group (n = 23) | P 2 |
|---|---|---|---|
| C-reactive protein, mg/L | 2.32 (3.52) | 2.06 (2.06) | 0.96 |
| ɑ1-Acid glycoprotein, mg/mL | 0.73 (0.84) | 1.16 (0.96) | 0.08 |
| Glucagon-like peptide 2, pg/mL | 593 (224) | 744 (381) | 0.04 |
Values are medians (IQR). EED, environmental enteric dysfunction.
Nonparametric Wilcoxon rank-sum test was used to test for differences between the treatment groups.
Serum and stool concentrations of trace elements and heavy metals at 12 mo of age
Tables 3 and 4 illustrate serum and stool concentrations of trace elements, micronutrients, and heavy metals at 12 mo of age compared between control and RB groups. No significant differences were detected for serum analytes between study groups. Trends for higher micronutrients such as calcium, iron, manganese, potassium, sodium, and sulfur in serum of infants consuming RB were noted, whereas heavy metals such as arsenic, barium, and lead were trending lower.
TABLE 3.
Concentrations of trace elements and heavy metals in serum at 12 mo of age
| Control (n = 24) | Rice bran (n = 23) | ||||
|---|---|---|---|---|---|
| Trace element | Median | IQR | Median | IQR | P 1 |
| Micronutrient, ppb | |||||
| Calcium (Ca) | 84,200.00 | 18,300.00 | 89,100.00 | 20,000.00 | 0.30 |
| Cobalt (Co) | 1.54 | 0.51 | 1.50 | 0.90 | 0.86 |
| Copper (Cu) | 1190.00 | 445.00 | 1180.00 | 287.00 | 0.63 |
| Iron (Fe) | 1390.00 | 1190.00 | 1530.00 | 1050.00 | 0.74 |
| Lithium (Li) | 18.20 | 5.38 | 18.80 | 6.76 | 0.80 |
| Magnesium (Mg) | 18,800.00 | 4710.00 | 19,000.00 | 4780.00 | 0.83 |
| Manganese (Mn) | 10.60 | 15.10 | 13.00 | 14.10 | 0.38 |
| Molybdenum (Mo) | 4.18 | 0.87 | 4.04 | 1.37 | 0.44 |
| Phosphorus (P) | 100,000.00 | 26,300.00 | 107,000.00 | 17,700.00 | 0.39 |
| Potassium (K) | 166,000.00 | 38,500.00 | 172,000.00 | 23,600.00 | 0.93 |
| Selenium (Se) | 71.10 | 19.30 | 71.00 | 34.00 | 0.32 |
| Sodium (Na) | 2,620,000.00 | 254,000.00 | 2,720,000.00 | 286,000.00 | 0.32 |
| Strontium (Sr) | 38.60 | 10.70 | 43.90 | 13.20 | 0.31 |
| Sulfur (S) | 907,000.00 | 201,000.00 | 971,000.00 | 185,000.00 | 0.34 |
| Zinc (Zn) | 846.00 | 201.00 | 845.00 | 285.00 | 0.76 |
| Heavy metal, ppb | |||||
| Arsenic (As) | 15.10 | 2.41 | 14.70 | 1.76 | 0.42 |
| Barium (Ba) | 64.50 | 8.11 | 61.10 | 15.00 | 0.46 |
| Cadmium (Cd) | 3.82 | 1.57 | 3.88 | 3.05 | 0.89 |
| Lead (Pb) | 5.95 | 1.41 | 5.76 | 1.19 | 0.53 |
| Nickel (Ni) | 5.16 | 1.93 | 5.18 | 1.00 | 0.64 |
| Other, ppb | |||||
| Aluminum (Al) | 11,300.00 | 1220.00 | 11,000.00 | 2750.00 | 0.48 |
| Tungsten (W) | 0.54 | 0.00 | 0.54 | 0.24 | 0.55 |
| Vanadium (V) | 82.70 | 35.70 | 75.00 | 47.10 | 0.61 |
Nonparametric Wilcoxon rank-sum test was used.
TABLE 4.
Concentrations of trace elements and heavy metals in stool at 12 mo of age
| Control (n = 23) | Rice bran (n = 23) | ||||
|---|---|---|---|---|---|
| Trace element | Median | IQR | Median | IQR | P 1 |
| Micronutrient, ppb | |||||
| Calcium (Ca) | 25,700,000.00 | 43,200,000.00 | 33,500,000.00 | 35,600,000.00 | 0.54 |
| Cadmium (Cd) | 95.00 | 79.00 | 122.00 | 112.00 | 0.52 |
| Cobalt (Co) | 285.00 | 235.00 | 321.00 | 218.00 | 1.00 |
| Copper (Cu) | 30,900.00 | 23,700.00 | 29,100.00 | 31,500.00 | 0.74 |
| Chromium (Cr) | 961.00 | 820.00 | 1890.00 | 1530.00 | 0.03 |
| Iron (Fe) | 274,000.00 | 259,000.00 | 220,000.00 | 311,000.00 | 0.74 |
| Magnesium (Mg) | 5,300,000.00 | 2,520,000.00 | 6,590,000.00 | 3,580,000.00 | 0.06 |
| Manganese (Mn) | 46,000.00 | 38,000.00 | 70,600.00 | 63,200.00 | 0.04 |
| Molybdenum (Mo) | 781.00 | 955.00 | 704.00 | 771.00 | 0.76 |
| Potassium (K) | 10,900,000.00 | 4,730,000.00 | 11,000,000.00 | 5,130,000.00 | 0.71 |
| Selenium (Se) | 487.00 | 478.00 | 552.00 | 504.00 | 0.96 |
| Sodium (Na) | 2,330,000.00 | 3,840,000.00 | 1,670,000.00 | 1,720,000.00 | 0.17 |
| Strontium (Sr) | 45,100.00 | 26,800.00 | 48,300.00 | 34,200.00 | 0.48 |
| Zinc (Zn) | 352,000.00 | 440,000.00 | 346,000.00 | 298,000.00 | 0.74 |
| Heavy metal, ppb | |||||
| Arsenic (As) | 123.00 | 74.40 | 148.00 | 98.60 | 0.59 |
| Barium (Ba) | 26,700.00 | 10,200.00 | 31,900.00 | 19,900.00 | 0.28 |
| Lead (Pb) | 332.00 | 322.00 | 451.00 | 747.00 | 0.29 |
| Nickel (Ni) | 1800.00 | 1540.00 | 2380.00 | 1790.00 | 0.12 |
| Other, ppb | |||||
| Aluminum (Al) | 415,000.00 | 535,000.00 | 418,000.00 | 644,000.00 | 0.94 |
| Antimony (Sb) | 48.30 | 20.60 | 54.10 | 29.90 | 0.46 |
| Silver (Ag) | 36.30 | 50.90 | 36.50 | 84.20 | 0.96 |
| Thallium (Tl) | 20.10 | 16.90 | 18.20 | 19.40 | 0.99 |
| Vanadium (V) | 4940.00 | 4320.00 | 4300.00 | 4320.00 | 0.54 |
Nonparametric Wilcoxon rank-sum test was used.
The stool profile showed significant differences for only manganese and chromium (Table 4), with increased excretion in the RB group. The RB group stool samples trended towards greater excretion of aluminum, barium, lead, nickel, strontium, and vanadium when compared with the control group.
RB consumption influences serum metabolome in Nicaraguan infants
Serum metabolite analysis of infants at 12 mo of age resulted in the detection of 1081 biochemicals, of which 772 compounds were of known structural identity and 309 compounds of unknown structural identity. Tables 5 and 6 show 39 metabolites with significant fold-differences between children consuming RB compared with controls. Significant fold-differences occurred for 15 amino acids, 3 peptides, 13 lipids, 4 nucleotides, and 4 plant/food components in infants consuming RB compared with controls. Amino acid metabolites of significant nutritional importance and that increased with RB intake were lysine (1.91-fold, N-acetyllysine), tryptophan (1.18-fold, tryptophan; 1.81-fold, serotonin), proline (1.31-fold, prolylhydroxyproline), and methionine (1.27-fold) metabolic pathways. Within the broad chemical class of lipids, we observed differences among phosphatidylcholine (1.30-fold, 1-palmitoy1-2-palmitoleoyl-GPC; 1.14-fold, 1-palmitoy1-2-oleoyl-GPC), sphingolipid (0.64-fold, N-behenoyl-sphingadienine), and secondary bile acids (0.40-fold, glycodeoxycholate sulfate). Significantly increased metabolites from fatty acid metabolism associated with RB intake included di-homo-linolenoyl-choline (1.64-fold) and oleoylcholine (1.50-fold). Many of those significant serum metabolites between RB and control group listed in Tables 5 and 6 were reported to be components of the RB food metabolome (31).
TABLE 5.
Serum amino acid and peptide metabolites significantly modulated by RB supplementation compared with control at 12 mo of age1
| Chemical class | Metabolic pathway | Metabolite | HMDB2 | Fold-difference3 | P | q Value |
|---|---|---|---|---|---|---|
| Amino acids | Glycine, serine, and threonine metabolism | Sarcosine | HMDB00271 | 1.46↑ | 0.000 | 0.30 |
| Lysine metabolism | N2-acetyllysine** | HMDB00446 | 1.91↑ | 0.011 | 0.78 | |
| 5-(galactosylhydroxy)-L-lysine | — | 1.17↑ | 0.015 | 0.78 | ||
| Tryptophan metabolism | Tryptophan** | HMDB00929 | 1.18↑ | 0.028 | 0.78 | |
| N-acetylkynurenine (2) | — | 2.80↑ | 0.000 | 0.30 | ||
| Serotonin** | HMDB00259 | 1.81↑ | 0.021 | 0.78 | ||
| Indolepropionate | HMDB02302 | 0.69↓ | 0.034 | 0.78 | ||
| 5-bromotryptophan | — | 1.20↑ | 0.039 | 0.78 | ||
| Leucine, isoleucine, and valine metabolism | 3-hydroxyisobutyrate | HMDB00336 | 1.52↑ | 0.008 | 0.74 | |
| Methionine, cysteine, SAM, and taurine metabolism | Methionine** | HMDB00696 | 1.27↑ | 0.008 | 0.74 | |
| Urea cycle; arginine and proline metabolism | N-acetylarginine** | HMDB04620 | 1.41↑ | 0.002 | 0.30 | |
| N-acetylcitrulline | HMDB00856 | 2.10↑ | 0.001 | 0.30 | ||
| Prolylhydroxyproline | HMDB06695 | 1.31↑ | 0.001 | 0.30 | ||
| Polyamine metabolism | N1, N12-diacetylspermine | HMDB02172 | 0.66↓ | 0.036 | 0.78 | |
| 4-acetamidobutanoate** | HMDB03681 | 1.23↑ | 0.046 | 0.78 | ||
| Peptides | γ-Glutamyl amino acid | γ-Glutamylmethionine** | HMDB29155 | 1.44↑ | 0.032 | 0.78 |
| γ-Glutamylthreonine** | HMDB29159 | 1.47↑ | 0.012 | 0.78 | ||
| Polypeptide | HWESASXX* | — | 2.14↑ | 0.046 | 0.78 |
The table displays metabolites with statistically significant differences between RB and control groups. *Indicates compounds with level 2 annotation that have not been officially confirmed based on a standard but have relative confidence for identity. **Indicates dual identification from RB food metabolome. Upward arrows indicate increased fold-difference in RB compared to control. Downward arrows indicate decreased fold-difference in RB compared to control. RB, rice bran; SAM, S-adenoysl methionine.
HMDB refers to the Human Metabolome Database. Access numbers are provided for each metabolite identified in the database.
Fold-difference between study groups was calculated by dividing the scaled relative abundance in children randomly assigned to RB vs. control.
TABLE 6.
Serum lipid, nucleotide, and xenobiotic metabolites significantly modulated by RB supplementation compared with control at 12 mo of age1
| Chemical class | Metabolic pathway | Metabolite | HMDB2 | Fold-difference3 | P 4 | q Value5 |
|---|---|---|---|---|---|---|
| Lipids | Medium-chain fatty acid | Heptanoate (7:0)** | HMDB00666 | 0.73↓ | 0.033 | 0.78 |
| Fatty acid, dicarboxylate | Glutarate (pentanedioate) | HMDB00661 | 2.00↑ | 0.031 | 0.78 | |
| Undecanedioate** | HMDB00888 | 0.77↓ | 0.002 | 0.30 | ||
| Fatty acid metabolism (acyl choline) | Oleoylcholine | — | 1.50↑ | 0.017 | 0.78 | |
| Dihomo-linolenoyl-choline | — | 1.64↑ | 0.010 | 0.78 | ||
| Endocannabinoid | N-oleoylserine | — | 1.21↑ | 0.039 | 0.78 | |
| Phosphatidylcholine (PC) | 1-palmitoyl-2-palmitoleoyl-GPC | HMDB07969 | 1.30↑ | 0.046 | 0.78 | |
| 1-palmitoyl-2-oleoyl-GPC | HMDB07972 | 1.14↑ | 0.022 | 0.78 | ||
| Diacylglycerol | Linoleoyl-linolenoyl-glycerol [2] | HMDB07250 | 0.53↓ | 0.008 | 0.74 | |
| Sphingolipid metabolism | N-behenoyl-sphingadienine | — | 0.64↓ | 0.029 | 0.78 | |
| Androgenic steroids | Epiandrosterone sulfate | — | 0.55↓ | 0.027 | 0.78 | |
| Androstenediol (3α, 17α) monosulfate (3) | — | 0.59↓ | 0.037 | 0.78 | ||
| Secondary bile acid metabolism | Glycodeoxycholate sulfate | — | 0.40↓ | 0.018 | 0.78 | |
| Nucleotides | Purine metabolism, adenine containing | Adenine** | HMDB00034 | 1.56↑ | 0.017 | 0.78 |
| N1-methyladenosine | HMDB03331 | 1.21↑ | 0.020 | 0.78 | ||
| Pyrimidine metabolism, uracil containing | Pseudouridine** | HMDB00767 | 1.18↑ | 0.024 | 0.78 | |
| 5-methyluridine (ribothymidine)** | HMDB00884 | 1.18↑ | 0.028 | 0.78 | ||
| Xenobiotics | Xanthine metabolism | Caffeine | HMDB01847 | 1.85↑ | 0.032 | 0.78 |
| 1-methylurate | HMDB03099 | 1.69↑ | 0.023 | 0.78 | ||
| Umbelliferone sulfate | — | 0.00↓ | 0.015 | 0.78 | ||
| Eugenol sulfate | — | 0.21↓ | 0.017 | 0.78 |
The table displays metabolites with statistically significant differences between RB and control groups.
**Indicates dual identification from RB food metabolome. Upward arrows indicate increased fold-difference in RB compared to control. Downward arrows indicate decreased fold-difference in RB compared to control. RB, rice bran.
HMDB refers to the Human Metabolome Database. Access numbers are provided for each metabolite identified in the database.
Fold-difference between study groups was calculated by dividing the scaled relative abundance in children randomly assigned to RB vs. control.
Serum P values calculated by Welch's 2-sample t test.
q-Value threshold accounts for multiple comparisons and false-discovery rate.
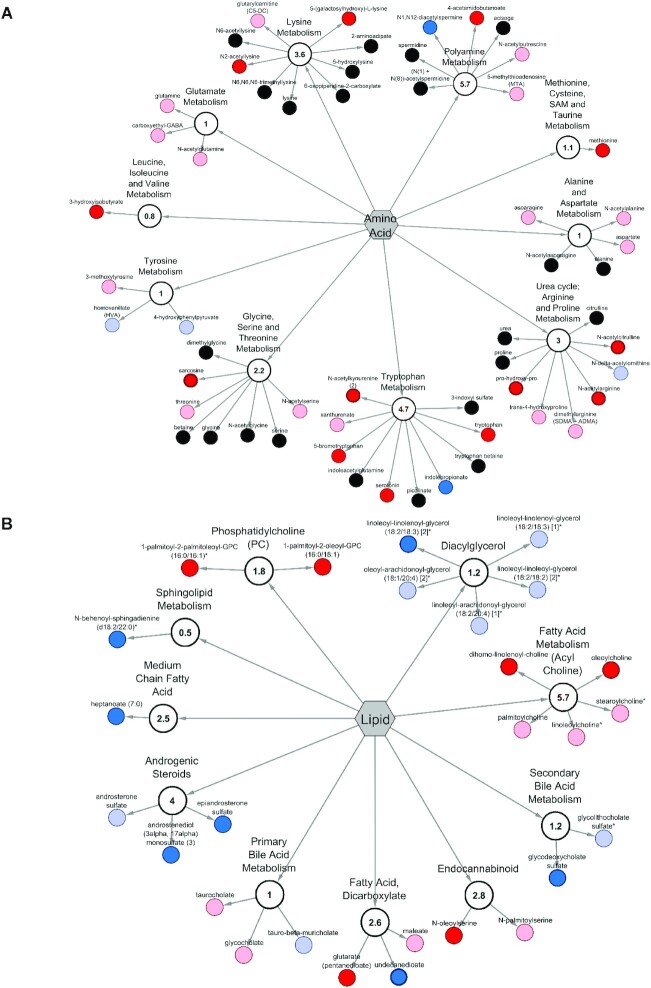
Figure 2 visualizes the spectrum of serum metabolites and metabolic pathways affected by RB consumption using a metabolite pathway network analysis. Figure 2A focuses on amino acids, with a significant pathway enrichment score of 4.7 for the tryptophan metabolic pathway and 5.7 for polyamine metabolism. Lipids (fatty acid metabolism/acylcholine) represent another significant metabolic pathway affected by RB consumption, with a pathway enrichment score of 5.7 (Figure 2B).
FIGURE 2.
Cytoscape network analysis of serum lipid and amino acid metabolites in RB-fed infants at 12 mo compared with the control group at 12 mo. (A) Pathway-specific network visualization for serum lipid metabolites. (B) Pathway-specific network visualization for serum amino acid metabolites. Scores < or >1 represent PESs that indicate that the pathway contains ≥1 metabolites with statistically significant fold-differences between the RB group and control at 12 mo compared with all other pathways within the matrix. Red nodes indicate significant increased difference (P < 0.05) between the RB and the control groups, with a metabolite ratio of ≥1. Light red nodes indicate narrowly missed statistical increase cutoffs for significance (0.05 < P < 0.10) between the RB and control groups. Blue nodes indicate significant decrease differences (P < 0.05) between the RB and the control groups, with a metabolite ratio of <1. Light-blue nodes indicate narrowly missed statistical decrease cutoffs for significance (0.05 < P < 0.10) between the RB and control groups, and black nodes indicate nonsignificant differences between the RB and control groups. Each metabolite is represented as a node extending from a central submetabolic pathway node, also identified as the PES. The central hexagon is the super metabolic pathway. PES, pathway enrichment score; RB, rice bran. SAM, S-adenosyl methionine. *Indicates dual identification from RB food metabolome.
RB consumption influences the stool metabolome in Nicaraguan infants
Daily supplementation with RB for 6 mo in Nicaraguan infants was examined at 12 mo of age for changes in the stool metabolome compared with controls. The stool biochemical profile contained 1449 compounds, of which 1017 biochemicals were of known structural identity and 432 biochemicals had not been structurally identified. Tables 7 and 8 show 44 metabolites with significant differences between the 2 groups. A significant fold-difference in stool metabolites was reported for 4 amino acids, 1 peptide, 1 carbohydrate, 19 lipids, 3 nucleotides, 3 cofactors and vitamins, and 13 xenobiotics from infants consuming RB compared with the control group. Certain stool metabolites increased with RB intake, such as tyrosine (2.71-fold, dopamine 3-O-sulfate), disaccharides (2.06-fold, sucrose), and fatty acid–dicarboxylate metabolic pathways (2.18-fold, 2-hydroxyadipate; 1.80-fold, pimelate). These metabolites were significantly different at 12 mo of age. We also observed significant decreases in stool lysophospholipid metabolism pathway and secondary bile acid metabolism and identified significant increases within the fatty acid metabolism pathway for palmitoylcholine (2.31-fold), oleoylcholine (3.54-fold), linoleoylcholine (1.88-fold), and stearoylcholine (1.48-fold).
TABLE 7.
Stool amino acid, peptide, carbohydrate, and lipid metabolites significantly modulated by RB supplementation compared with control at 12 mo of age1
| Chemical class | Metabolic pathway | Metabolite | HMDB2 | Fold- difference3 | P 4 | q Value5 |
|---|---|---|---|---|---|---|
| Amino acids | Tyrosine metabolism | Dopamine 3-O-sulfate | HMDB06275 | 2.71↑ | 0.045 | 0.99 |
| Vanillic alcohol sulfate | — | 1.93↑ | 0.038 | 0.99 | ||
| Tryptophan metabolism | Skatol | HMDB00466 | 0.65↓ | 0.007 | 0.63 | |
| Methionine, cysteine, SAM, and taurine | Methionine sulfoxide** | HMDB02005 | 0.68↓ | 0.032 | 0.88 | |
| Peptides | Acetylated peptides | 4-hydroxyphenylacetylglycine | — | 2.48↑ | 0.013 | 0.67 |
| Carbohydrates | Disaccharides and oligosaccharides | Sucrose** | HMDB00258 | 2.06↑ | 0.047 | 0.99 |
| Lipids | Fatty acid, dicarboxylate | 2-hydroxyadipate** | HMDB00321 | 2.18↑ | 0.013 | 0.67 |
| 3-methyladipate** | HMDB00555 | 0.47↓ | 0.045 | 0.99 | ||
| Pimelate (heptanedioate)** | HMDB00857 | 1.80↑ | 0.030 | 0.87 | ||
| Fatty acid metabolism (acyl choline) | Palmitoylcholine | — | 2.31↑ | 0.023 | 0.82 | |
| Oleoylcholine | — | 3.54↑ | 0.001 | 0.25 | ||
| Linoleoylcholine* | — | 1.88↑ | 0.040 | 0.99 | ||
| Stearoylcholine* | — | 1.48↑ | 0.013 | 0.67 | ||
| Lysophospholipid | 1-oleoyl-GPC (18:1)** | HMDB02815 | 0.31↓ | 0.024 | 0.82 | |
| 1-linoleoyl-GPC (18:2)** | HMDB10386 | 0.41↓ | 0.047 | 0.99 | ||
| 1-palmitoyl-GPE (16:0)** | HMDB11503 | 0.38↓ | 0.027 | 0.86 | ||
| 1-linoleoyl-GPE (18:2)*,** | HMDB11507 | 0.49↓ | 0.003 | 0.43 | ||
| Glycolipid metabolism | 1-linoleoyl-2-linolenoyl-digalactosylglycerol (18:2/18:3)* | — | 0.52↓ | 0.005 | 0.61 | |
| Diacylglycerol | Linolenoyl-linolenoyl-glycerol (18:3/18:3) [1]* | HMDB07278 | 0.58↓ | 0.001 | 0.25 | |
| Secondary bile acid metabolism | Deoxycholate | HMDB00626 | 0.31↓ | 0.022 | 0.82 | |
| Glycodeoxycholate | HMDB00631 | 0.47↓ | 0.005 | 0.61 | ||
| Taurolithocholate | HMDB00722 | 0.52↓ | 0.000 | 0.006 | ||
| Glycoursodeoxycholate | HMDB00708 | 0.37↓ | 0.031 | 0.87 | ||
| Dehydrolithocholate | HMDB00502 | 0.25↓ | 0.025 | 0.82 | ||
| 6-oxolithocholate | — | 0.18↓ | 0.011 | 0.67 |
Metabolites with statistically significant differences between RB and control groups are shown. *Indicates compounds with level 2 annotation that have not been officially confirmed based on a standard but have relative confidence for identity. **Indicates dual identification from RB food metabolome. Upward arrows indicate increased fold-difference in RB compared to control. Downward arrows indicate decreased fold-difference in RB compared to control. RB, rice bran.
HMDB refers to the Human Metabolome Database. Access numbers are provided for each metabolite identified in the database.
Fold-difference between study groups was calculated by dividing the scaled relative abundance of RB vs. control.
Stool P values calculated by ANOVA.
q-Value threshold accounts for false-discovery rate.
TABLE 8.
Stool nucleotide, cofactor, and vitamin and xenobiotic metabolites significantly modulated by RB supplementation compared with control at 12 mo of age1
| Chemical class | Metabolic pathway | Metabolite | HMDB2 | Fold-difference3 | P 4 | q Value5 |
|---|---|---|---|---|---|---|
| Nucleotides | Purine metabolism, (hypo)xanthine/inosine containing | Xanthosine** | HMDB00299 | 0.35↓ | 0.011 | 0.67 |
| Purine metabolism, adenine containing | N6-dimethylallyladenine | — | 0.44↓ | 0.005 | 0.61 | |
| Pyrimidine metabolism, uracil containing | 5-methyluridine (ribothymidine)** | HMDB00884 | 0.37↓ | 0.029 | 0.87 | |
| Cofactors and vitamins | Nicotinate and nicotinamide metabolism | Nicotinamide** | HMDB01406 | 1.82↑ | 0.004 | 0.57 |
| Hemoglobin and porphyrin metabolism | L-urobilin | HMDB04159 | 0.41↓ | 0.047 | 0.99 | |
| Vitamin B-6 metabolism | Pyridoxine (vitamin B-6)** | HMDB02075 | 4.65↑ | 0.001 | 0.25 | |
| Xenobiotics | Xanthine metabolism | Caffeic acid sulfate | HMDB41708 | 2.18↑ | 0.040 | 0.99 |
| Food component/plant | Retinal | HMDB01358 | 0.71↓ | 0.009 | 0.67 | |
| Coumaroylquinate (4) | — | 1.40↑ | 0.031 | 0.87 | ||
| Ferulylglycine (1) | — | 2.32↑ | 0.041 | 0.99 | ||
| Quinate** | HMDB03072 | 2.92↑ | 0.045 | 0.99 | ||
| 1,2-dilinolenoyl-digalactosylglycerol (18:3/18:3) | — | 0.49↓ | 0.008 | 0.63 | ||
| 1-palmitoyl-2-linolenoyl-digalactosylglycerol (16:0/18:3) | — | 0.40↓ | 0.021 | 0.81 | ||
| Drug | 2-hydroxyacetaminophen sulfate* | — | 3.16↑ | 0.009 | 0.67 | |
| 2-methoxyacetaminophen sulfate* | — | 2.57↑ | 0.048 | 0.99 | ||
| Clotrimazole | HMDB01922 | 0.52↓ | 0.020 | 0.81 | ||
| Loratadine | HMDB05000 | 1.21↑ | 0.011 | 0.67 | ||
| 2-acetamidophenol sulfate | — | 2.62↑ | 0.000 | 0.12 | ||
| Chemical | 4-thiouracil | — | 0.52↓ | 0.021 | 0.81 |
Metabolites with statistically significant differences between RB and control groups are shown. *Indicates compounds with level 2 annotation that have not been officially confirmed based on a standard but have relative confidence for identity. **Indicates dual identification from RB food metabolome. Upward arrows indicate increased fold-difference in RB compared to control. Downward arrows indicate decreased fold-difference in RB compared to control. RB, rice bran.
HMDB refers to the Human Metabolome Database. Access numbers are provided for each metabolite identified in the database.
Fold-difference between study groups was calculated by dividing the scaled relative abundance of RB vs. control.
Stool P values calculated by ANOVA.
q-Value threshold accounts for false-discovery rate.
RB compounds identified in infant serum and stool
A list of serum or fecal metabolites that differed between RB and control groups and that were also reported in the RB food metabolome is shown in Tables 5–8. The RB metabolite profile that was fed to infants had 448 identified compounds, of which 13 were significantly detected in serum of RB infants when compared with controls at 12 mo of age. There were 14 potentially RB-derived compounds in stool that were significantly different from the control group. We observed that 5-methyluridine, a metabolite found in RB, showed a significant fold-change in both stool and serum after intake at 12 mo (increased 1.18-fold in serum and decreased 0.37-fold in stool). Table 5highlights amino acids and peptides. Table 6 shows lipids, nucleotides and xenobiotics found in the RB food that showed significant differences in serum after nutritional intervention. Amino acid compounds that increased with RB intake and that were likely derived from RB intake were tryptophan (1.18-fold, tryptophan; 1.81-fold, serotonin) and methionine (1.27-fold, methionine). We also found significant fold-differences in 1 amino acid, 1 carbohydrate, 7 lipids, 1 nucleotide, 2 cofactors and vitamins, and 1 xenobiotic derived from RB in stool. The amino acid metabolite skatol decreased by 0.77-fold in the tryptophan metabolic pathway and sucrose significantly increased by 2.07-fold in the disaccharides metabolic pathway. Within the class of lipids, decreased fold-change was observed for 1-oleoyl-GPC (18:1; 0.31-fold), 1-linoleoyl-GPC (18:2; 0.41-fold), 1-palmitoyl-GPE (16:0; 0.38-fold), and 1-linoleoyl-GPE (18:2; 0.49-fold). In addition, we also found a significant increase in quinate (2.92-fold) by the food component/plant metabolic pathway and pyridoxine (4.65-fold) by the vitamin B-6 metabolic pathway.
Discussion
This study demonstrated that RB supplementation favorably modulated the GLP-2 serum EED biomarker and impacted a variety of chemical classes of serum and stool metabolites without major changes to targeted trace element concentrations. In our study, serum metabolites from the tryptophan metabolic pathway were significantly increased with RB supplementation, including tryptophan (1.18-fold), N-acetylkynurenine (2.8-fold), serotonin (1.81-fold), and 5-bromotryptophan (1.2-fold). Tryptophan is an essential amino acid and principal component of the human diet with relevance to the enteric neurological system while also playing a substantial role in the functionality of the gut–brain axis (46–48). Several studies have demonstrated direct relations between the concentration of tryptophan and its metabolites with various disorders such as irritable bowel syndrome (46), obesity (49), cardiovascular diseases (50), anorexia nervosa (51, 52), and others (53). Furthermore, serotonin is thought to mediate the vomiting reflex in children with rotavirus and norovirus (54). Kosek et al. (55) reported associations between tryptophan concentration and linear growth in 2 longitudinal birth cohorts, and increased concentrations of tryptophan were associated with LAZ gain in those infants. The association between serum tryptophan concentrations and LAZ in this cohort merits continued attention for RB supplementation to the diet, particularly as infants showed more growth at 8 and 12 mo than infants in the control group (33). N-acetylkynurenine, a derivative of N-acetyl tryptophan, and observed in this RB intervention group, has been associated with regulating inflammation by inhibiting macrophage activation. Recent research has examined the anti-inflammatory properties of N-acetylkynurenine and its role in autoimmune disease treatment and tissue remodeling (56).
Serotonin, which is synthesized from tryptophan and is a metabolite associated with metabolic alteration in infants with EED, also increased 1.81-fold. Whether elevated serum serotonin metabolite reflects increased gut permeability is unclear; nevertheless, tryptophan increases nutrient absorption important for growth and gut function (57, 58). This supports the positive growth outcomes observed in infants consuming RB, where, after only 2 mo of the intervention, there was a statistically significant increase at 8 mo of age for LAZ (P < 0.01). Likewise, at 12 mo of age, infants who received the daily RB supplement were still longer (by length) and showed a significantly higher serum GLP-2 biomarker, indicating improved intestinal health (anthropometric outcomes shown in a previous published article) (33). Notably, we also found an important decrease in the metabolite indolepropionate (0.69-fold) resulting from tryptophan metabolism. Indolepropionate is a well-established antioxidant (59) and biomarker of Clostridium sporogenes (60), although studies have shown that serum concentrations of indolepropionate are positively correlated with dietary fiber intake and negatively correlated with CRP concentrations (61).
Serum metabolite analysis supports the effects of RB consumption with significant increased fold-differences among essential amino acids, such as methionine, a dietary amino acid required for normal growth and development of humans (62). Methionine is the most limiting amino acid in the diet, and it is often found at low concentrations in cereal grains (63). Despite this, methionine increased 1.27-fold after RB intake. In addition, methionine has been associated with linear growth in infants from Malawi (64). The increase in certain essential amino acids in the RB group is important because LAZ was significantly greater in the RB group as compared with controls at 8 mo. WAZ was not significantly different between infants that consumed RB compared with controls. Nevertheless, it is possible that the short period of time in which RB consumption and individual factors, such as weight variability at birth and feeding patterns, could have influenced these results (65, 66).
There is evidence of a direct relation between EED and growth deficits in children (9, 23, 67). Thus, it is necessary to better understand the relation among EED biomarkers with nutritional status after RB intake in children (8, 68). Serum biomarkers CRP and AGP are systemic indicators of inflammation and increases have been associated with yearly decline in infants’ LAZ scores (16). In addition, GLP-2 is an intestinotrophic hormone, secreted by enteroendocrine L cells of the intestinal epithelium. It has been shown to increase epithelial proliferation, inhibit apoptosis, enhance barrier function, and increase digestion, nutrient absorption, and blood flow (69–72). In this study population, the higher serum GLP-2 at 12 mo in the RB group compared with the control group reflects reduced inflammation correlated with RB consumption. Such results are of exceptional significance as GLP-2 is a critical mediator of lipid absorption, and for its ability to reduce inflammation and repair intestinal damage by increasing nutrient absorption (73–75). Reparative and cytoprotective properties of RB could also provide further powerful health benefits to these infants (76).
Interestingly, we noticed decreases in several serum lipid metabolites in the RB group compared with the control group at 12 mo of age. These findings demonstrate that lipid metabolites present in RB may have been metabolized or bioavailable to developing organ systems. To support this hypothesis, we noticed that RB intake increased 3-hydroxyisobutyrate metabolite (1.52-fold), which is a ketone body and can be used for energy production, as carbon sources for lipogenesis by the amino acid metabolic pathway (77), and is involved in energy metabolism to develop brain and lung cells (78). We also observed that glutarate (pentanedioate) lipid metabolite increased significantly (2.00-fold), which is likely due to its production in tryptophan and lysine metabolism as it relates to RB consumption (79). These findings support the growing evidence that RB supplementation not only improves nutrient intake and metabolite profiles important to infant growth and development but also enhances intestinal health and reduces EED.
The stool metabolites such as tyrosine (2.71-fold, dopamine 3-O-sulfate), disaccharides (2.06-fold, sucrose), and fatty acid–dicarboxylate (2.18-fold, 2-hydroxyadipate; 1.80-fold, pimelate) metabolic pathways were also noteworthy for relevance to reduced gut inflammation. Improvements in nutritional status and immune function may relate to increased vitamins (4.65-fold, pyridoxine). Important decreases in stool lipid metabolites were observed that indicated lipid absorption in reference to the RB compositional lipid profile, as well as other metabolites such as amino acids, peptides, nucleotides, and xenobiotics (Tables 7 and 8). We also noticed significant differences in stool lysophospolipid metabolites [1-oleoyl-GPC (18:1), 1-linoleoyl-GPC (18:2), 1-palmitoyl-GPE (16:0), and 1-linoleoyl-GPE (18:2)] and secondary bile acid metabolites (deoxycholate, glycodeoxycholate, taurolithocholate, glycoursodeoxycholate, dehydrolithocholate and 6-oxolithocholate), which have been established as sensitive indicators of gastrointestinal function and microbiome studies (80). Other stool metabolites with significant fold-change that increased with RB intake were sucrose (2.06-fold), 2-hydroxyadipate (2.18-fold), and pimelate (1.80-fold). Sucrose is a disaccharide of 6-O-sinapoyl sucrose metabolism occurring in human gut microbiota and a significant fold-increase is correlated with the growth of beneficial bacteria (Bifidobacterium) and supports gut development and protection in infants (81). Skatole is a critical precursor for serotonin and melatonin by the tryptophan metabolic pathway (82). Skatole biosynthesis may have physiological importance herein and based on reported roles in infant fecal microbiota metabolism and neurodevelopment (83).
Nicotinamide changes have been shown for different foods and showed significant fold-differences related to RB intake and possibly also related to tryptophan metabolism. The modified microbiome metabolism and GLP-2 serum biomarker expression could have been increased via shunted tryptophan metabolism toward anti-inflammatory pathways (84). This is related to the observed increase in quinate (2.92-fold), which has shown evidence in controlling oxidative and inflammatory stress conditions by modulating other metabolic pathways (85). Stool pyridoxine had the highest 4.65-fold difference in the stool metabolome, and merits attention for bioaccessibility of pyridoxine. Pyridoxine availability to the host depends on many factors such as temperature, pH of the gastrointestinal tract, and dietary fiber for bonding to polysaccharide and polypeptides (86). Therefore, we suggest that RB supplementation not only increased pyridoxine and nutrient uptake but also enhanced other metabolic pathways for reducing EED risk and especially in the function of the nervous system (87). Pyridoxine, or vitamin B-6, has a role in cognitive development, immune function, and hemoglobin formation (88). There is also other evidence that vitamin B-6 may have an antioxidant function and concentrations have been correlated with amino acid metabolism, nutrition, growth, and infant development (88).
Finally, we observed that 5-methyluridine, which is an endogenous methylated nucleoside found in human fluids and has also been explored as a therapeutic target for hypomethylating agents (89), was detected with significant differences in serum and stool samples. According to this finding, 5-methyluridine, which is found in RB, increased (1.18-fold) in serum after intake and significant differences were also found in stool. In addition, increases in serum 5-methyluridine were positively associated with height-for-age z-score in young infants from rural Malawi (90), and other studies have suggested the importance of this metabolite in cancer prevention (91). It is pertinent to note that high q values in the serum and stool metabolite outcomes were a concern but metabolites with significant P values were encouraging for dual detection of RB metabolites that revealed changes between intervention and control. Future research is warranted to deepen our understanding of the importance of RB in infant nutrition during these critical stages of growth and development.
Study limitations
This study had limitations such as the small cohort size and short study duration of the diet intervention. The sample size was comparable to other studies with metabolome outcomes (33, 92, 93). Furthermore, we only analyzed 3 established serum EED marker. The evaluation of other EED-related biomarkers was previously conducted with stool. Gut epithelial integrity and permeability can be measured in urine using lactulose to mannitol ratios (94), although urine was not collected in our study. Also, no allergies to the rice bran were expected (95). EED and systemic inflammation biomarkers and metabolome analysis were measured and evaluated for differences between groups at the end of the study (12 mo of age). Future phase 2 studies with RB in diverse populations using an intent-to-treat study design are warranted.
Conclusions
The consumption of heat-stabilized RB in weaning infants was associated with positive changes to GLP-2, serum, and stool metabolites. RB supplementation and changes in serum GLP-2 has implications as a candidate biomarker of nutrient absorption with other serum metabolites associated with improved intestinal health. RB supplementation warrants further investigation as a practical intervention strategy to reduce EED prevalence and risk for children from LMICs, particularly where rice is grown as a staple crop.
ACKNOWLEDGEMENTS
The authors thank Claudia Cortez, Silvia Altamirano, Ridder Guido, and Remigio Carvajal for their assistance in data collection. The authors’ responsibilities were as follows—SV and EPR: designed the research and maintained study oversight; SB-D and LY: contributed to the study design; ECB and LEZ: conducted research and sample collection; IZ, SV, JP, and CP: analyzed stool and serum samples; AMW and LEZ: performed data analysis; LEZ, ECB, IR, AMW, and EPR: wrote and edited the manuscript; EPR: had primary responsibility for the final product; and all authors: read and approved the final manuscript.
Notes
Supported by the Bill and Melinda Gates Foundation–Grand Challenges Explorations in Global Health award (OPP1043255) and a US Fulbright Faculty Development scholarship award. LEZ was supported by an international research capacity-building award from the Fogarty International Center, D43TW010923. SB-D is supported by grant K24AI141744 from the National Institute of Allergy and Infectious Diseases. AMW graduate training was supported by the National Science Foundation under Grant No. 1828902 and National Institute of Food and Agriculture from the United States Department of Agriculture (NIFA-USDA) (2016-67001-24538).
Author disclosures: The authors report no conflicts of interest. The funders had no role in study design, implementation, data collection, analysis, and interpretation of the data, decision to publish, or preparation of the manuscript.
Abbreviations used: AGP, ɑ1-acid glycoprotein; CRP, C-reactive protein; EED, environmental enteric dysfunction; GLP-2, glucagon-like peptide 2; ICP-MS, inductively coupled plasma MS; LAZ, length-for-age z-score; LMIC, low-and middle-income country; LOD, limit of detection; OD, optical density; PES, pathway enrichment score; RB, rice bran; UPLC-MS, ultra-high performance LC–tandem MS; WAZ, weight-for-age z-score.
Contributor Information
Luis E Zambrana, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA; Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León (UNAN-León), León, Nicaragua.
Annika M Weber, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA.
Erica C Borresen, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA.
Iman Zarei, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA.
Johann Perez, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León (UNAN-León), León, Nicaragua.
Claudia Perez, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León (UNAN-León), León, Nicaragua.
Iker Rodríguez, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA; Biotic Products Development Center, National Polytechnic Institute, Morelos, Mexico.
Sylvia Becker-Dreps, Departments of Family Medicine and Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Lijuan Yuan, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA.
Samuel Vilchez, Center of Infectious Diseases, Department of Microbiology and Parasitology, Faculty of Medical Sciences, National Autonomous University of Nicaragua, León (UNAN-León), León, Nicaragua.
Elizabeth P Ryan, Email: e.p.ryan@colostate.edu, Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, USA.
References
- 1.WHO. Complementary Feeding. May 2017. Available from: https://www.who.int/health-topics/complementary-feeding#tab=tab_1 (accessed 12 June 2017). [Google Scholar]
- 2.Black ER, Allen LH, Bhutta AZ. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet North Am Ed. 2008:18. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 3.Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet North Am Ed. 2021;397(10282):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhutta ZA, Salam RA. Global nutrition epidemiology and trends. Ann Nutr Metab. 2012;61:9. [DOI] [PubMed] [Google Scholar]
- 5.Myatt M, Khara T, Schoenbuchner S, Pietzsch S, Dolan C, Lelijveld N, Briend A. Children who are both wasted and stunted are also underweight and have a high risk of death: a descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch Public Health. 2018;76:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph SA, Casapía M, Blouin B, Maheu-Giroux M, Rahme E, Gyorkos TW. Risk factors associated with malnutrition in one-year-old children living in the Peruvian Amazon. PLoS Negl Trop Dis. 2014;8(12):e3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusch TG, Rosenberg IH, Denno MD, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JPet al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34(3):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keusch TG, Denno DM, Black ER, Dugga C, Guerrant R, Lavery JV, Nataro JP, Resenber IR, Ryan ET, Tarr PIet al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59(4):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syed S, Ali A, Duggan C. Environmental enteric dysfunction in children: a review. J Pediatr Gastroenterol Nutr. 2016;63(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korpe SP, Petri WA. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18(6):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis. 2018;12(1):e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis-Auguste J, Kelly P. Tropical enteropathies. Curr Gastroenterol Rep. 2017;19(7):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denno DM, Tarr PI, Nataro JP. Environmental enteric dysfunction: a case definition for intervention trials. Am J Trop Med Hyg. 2017;97(6):1643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tickell KD, Atlas HE, Walson JL. Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 2019;17(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahfuz M, Das S, Mazumder RN, Masudur Rahman M, Haque R, Bhuiyan MMR, Akhter H, Sarker MSA, Mondal D, Muaz SSAet al. Bangladesh Environmental Enteric Dysfunction (BEED) study: protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ Open. 2017;7(8):e017768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal NT, Sadiq K, Syed S, Akhund T, Umrani F, Ahmed S, Yakoob MY, Rahman N, Qureshi S, Xin Wet al. Promising biomarkers of environmental enteric dysfunction: a prospective cohort study in Pakistani children. Sci Rep. 2018;8(1):2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer JM, Ghosh S, Ausman LM, Webb P, Bashaasha B, Agaba E, Turyashemererwa FM, Tran HQ, Gewirtz AT, Erhardt Jet al. Markers of environmental enteric dysfunction are associated with poor growth and iron status in rural Ugandan Infants. J Nutr. 2020;150(8):2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caulfield EL, Huffman SL, Piwoz GE. Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food Nutr Bull. 1999;20(2):183. [Google Scholar]
- 19.Dewey KG, Cohen RJ, Brown KH, Rivera LL. Age of introduction of complementary foods and growth of term, low-birth-weight, breast-fed infants: a randomized intervention study in Honduras. Am J Clin Nutr. 1999;69(4):679–86. [DOI] [PubMed] [Google Scholar]
- 20.Smith HE, Ryan KN, Stephenson KB, Westcott C, Thakwalakwa C, Maleta K, Cheng JY, Brenna JT, Shulman RJ, Trehan Iet al. Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12–35 months in a randomized controlled clinical trial. J Nutr. 2014;144(12):2059–65. [DOI] [PubMed] [Google Scholar]
- 21.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet North Am Ed. 1991;338:907. [DOI] [PubMed] [Google Scholar]
- 22.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:7. [DOI] [PubMed] [Google Scholar]
- 23.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RLet al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UNICEF. United Nations Children Fund. Available from: https://www.unicef.org/infobycountry/nicaragua_statistics.html 2018. [Google Scholar]
- 25.UNICEF; World Bank Group . Levels and trends in child malnutrition. New York, NY: UNICEF; 2015. [Google Scholar]
- 26.Lin CC, Chen HH, Chen YK, Chang HC, Lin PY, Pan IH, Chen DY, Chen CM, Lin SY. Rice bran feruloylated oligosaccharides activate dendritic cells via Toll-like receptor 2 and 4 signaling. Molecules. 2014;19(4):5325–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Wen K, Tin C, Li G, Wang H, Kocher J, Pelzer K, Ryan E, Yuan L. Dietary rice bran protects against rotavirus diarrhea and promotes Th1-type immune responses to human rotavirus vaccine in gnotobiotic pigs. Clin Vaccine Immunol. 2014;21(10):1396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei S, Ramesh A, Twitchell E, Wen K, Bui T, Weiss M, Yang X, Kocher J, Li G, Giri-Rachman Eet al. High protective efficacy of probiotics and rice bran against human norovirus infection and diarrhea in gnotobiotic pigs. Front Microbiol. 2016;7:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy KV, Maheswaraiah A, Naidu KA. Rice bran oil and n-3 fatty acid-rich garden cress (Lepidium sativum) seed oil attenuate murine model of ulcerative colitis. Int J Colorectal Dis. 2014;29(2):267–9. [DOI] [PubMed] [Google Scholar]
- 30.Herfel T, Jacobi S, Lin X, van Heugten E, Fellner V, Odle J. Stabilized rice bran improves weaning pig performance via a prebiotic mechanism. J Anim Sci. 2013;91(2):907–13. [DOI] [PubMed] [Google Scholar]
- 31.Zarei I, Brown DG, Nealon NJ, Ryan EP. Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice. 2017;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson AJ, Ollila CA, Kumar A, Borresen EC, Raina K, Agarwal R, Ryan EP. Chemopreventive properties of dietary rice bran: current status and future prospects. Adv Nutr. 2012;3(5):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrana LE, McKeen S, Ibrahim H, Zarei I, Borresen EC, Doumbia L, Boré A, Cissoko A, Douyon S, Koné Ket al. Rice bran supplementation modulates growth, microbiota and metabolome in weaning infants: a clinical trial in Nicaragua and Mali. Sci Rep. 2019;9(1):13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker-Dreps S, Bucardo F, Vilchez S, Zambrana LE, Liu L, Weber DJ, Pena R, Barclay L, Vinje J, Hudgens MGet al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J. 2014;33(11):1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MINSA . Norma tecnica de inmunizaciones y manual de procedimientos de inmunizaciones. In: Inmunizaciones PAd, ed. Managua (Nicaragua): Ministerio de Salud de Nicaragua; 2013. [Google Scholar]
- 36.Lakkakula NR, Lima M, Walker T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour Technol. 2004;92(2):157–61. [DOI] [PubMed] [Google Scholar]
- 37.WHO . WHO Anthro and Macros. version 3.2.2. Geneva (Switzerland): WHO Global Nutrition Cluster; 2011. Available from: https://www.nutritioncluster.net/node/4825. [Google Scholar]
- 38.Borresen EC, Guajardo M, Zambrana LE, Perez J, Perez C, Stallones L, Becker-Dreps S, Yuan L, Vilchez S, Ryan EP. Association between infant feeding practices and nutritional status in healthy Nicaraguan infants. J Food Nutr Diet. 2016;1(3):6. [Google Scholar]
- 39.Connor EE, Evock-Clover CM, Wall EH, Baldwin RLt, Santin-Duran M, Elsasser TH, Bravo DM. Glucagon-like peptide 2 and its beneficial effects on gut function and health in production animals. Domest Anim Endocrinol. 2016;56(Suppl):S56–65. [DOI] [PubMed] [Google Scholar]
- 40.Haugen J-E, Tomic O, Kvaal K. A calibration method for handling the temporal drift of solid state gas-sensors. Anal Chim Acta. 2000;407(1-2):23–39. [Google Scholar]
- 41.Broccardo CJ, Schauer KL, Kohrt WM, Schwartz RS, Murphy JP, Prenni JE. Multiplexed analysis of steroid hormones in human serum using novel microflow tile technology and LC–MS/MS. J Chromatogr B. 2013;934:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27(3):431–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li KJ, Borresen EC, Jenkins-Puccetti N, Luckasen G, Ryan EP. Navy bean and rice bran intake alters the plasma metabolome of children at risk for cardiovascular disease. Front Nutr. 2017;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown DG, Borresen EC, Brown RJ, Ryan EP. Heat-stabilised rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: a randomised controlled trial. Br J Nutr. 2017;117(9):1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgerald P, Cassidy Eugene M, Clarke G, Scully P, Barry S, Quigley Eamonn MM, Shanahan F, Cryan J, Dinan Timothy G. Tryptophan catabolism in females with irritable bowel syndrome: relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol Motil. 2008;20(12):1291–7. [DOI] [PubMed] [Google Scholar]
- 47.Keszthelyi D, Troost FJ, Jonkers DM, van Donkelaar EL, Dekker J, Buurman WA, Masclee AA. Does acute tryptophan depletion affect peripheral serotonin metabolism in the intestine?. Am J Clin Nutr. 2012;95(3):603–8. [DOI] [PubMed] [Google Scholar]
- 48.Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, Bjorklund G. How important is tryptophan in human health?. Crit Rev Food Sci Nutr. 2019;59(1):72–88.. doi: 10.1080/10408398.2017.1357534. [DOI] [PubMed] [Google Scholar]
- 49.Strasser B, Berger K, Fuchs D. Effects of a caloric restriction weight loss diet on tryptophan metabolism and inflammatory biomarkers in overweight adults. Eur J Nutr. 2015;54(1):101–7. [DOI] [PubMed] [Google Scholar]
- 50.Yu E, Ruiz-Canela M, Guasch-Ferre M, Zheng Y, Toledo E, Clish CB, Salas-Salvado J, Liang L, Wang DD, Corella Det al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevencion con Dieta Mediterranea (PREDIMED) Study. J Nutr. 2017;147(3):314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haleem DJ. Improving therapeutics in anorexia nervosa with tryptophan. Life Sci. 2017;178:87–93. [DOI] [PubMed] [Google Scholar]
- 52.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94(1):121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Degradation of tryptophan in neurodegenerative disorders. Adv Exp Med Biol. 1999;467:133–8. [DOI] [PubMed] [Google Scholar]
- 54.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman Het al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011;7(7):e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosek MN, Mduma E, Kosek PS, Lee GO, Svensen E, Pan WK, Olortegui MP, Bream JH, Patil C, Asayag CRet al. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg. 2016;95(4):928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rael LT, Bar-Or R, Banton KL, Mains CW, Roshon M, Tanner AH, Lieser MJ, Acuna DL, Bar-Or D. The anti-inflammatory effect of LMWF5A and N-acetyl kynurenine on macrophages: involvement of aryl hydrocarbon receptor in mechanism of action. Biochem Biophys Rep. 2018;15:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semba DR, Shardell M, Trehan I, Moaddel R, Maleta MK, Ordiz IM, Kraemer K, Khadeer M, Ferruci L, Manary MJ. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep. 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semba RD, Shardell M, Trehan I, Moaddel R, Maleta KM, Ordiz MI, Kraemer K, Khadeer M, Ferrucci L, Manary MJ. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep. 2016;6:2800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardeland R, Zsizsik BK, Poeggeler B, Fuhrberg B, Holst S, Coto-Montes A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Boston (MA): Springer; 1999. [DOI] [PubMed] [Google Scholar]
- 60.Jallet JJ, Forrest TP, Macdonald IA, Marrie TJ, Holdeman LV. Production of indole-3-propanoic acid and 3-(p-hydroxyphenyl)propanoic acid by Clostridium sporogenes: a convenient thin-layer chromatography detection system. Can J Microbiol. 1980;26(4):448–53. [DOI] [PubMed] [Google Scholar]
- 61.Tuomainen M, Lindström J, Lehtonen M, Auriola S, Pihlajamäki1 J, Peltonen M, Tuomilehto J, Uusitupa M, de Mello VD, Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes. 2018;8(35):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McBreairty LE, Bertolo RF. The dynamics of methionine supply and demand during early development. Appl Physiol Nutr Metab. 2016;41(6):581–7. [DOI] [PubMed] [Google Scholar]
- 63.Ball OR, Courtney-Martin G, Pencharz BP. The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J Nutr. 2006;136:1682S–93S. [DOI] [PubMed] [Google Scholar]
- 64.Ordiz MI, Semba DR, Moaddel R, Rolle-Kampczyk U, von Bergen M, Herberth G, Khadeer M, Röder S, Manary JM. Serum amino acid concentrations in infants from Malawi are associated with linear growth. Curr Dev Nutr. 2019;3:nzz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO . Global database on child growth and malnutrition. Geneva (Switzerland): WHO; 1997. [Google Scholar]
- 66.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66(9):487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guerrant LR, Leite AM, Pinkerton R, Medeiros P, Cavalcante P, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kaga JCet al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in Northeast Brazil. journalpone. 2016;11(9):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosek M, Guerrant RL, Kang G, Bhutta Z, Yori PP, Gratz J, Gottlieb M, Lang D, Lee G, Haque Ret al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis. 2014;59(Suppl 4):S239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci. 1996;93(15):7911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, Holzenberger M, Gonska T, Brubaker PL. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155(2):370–9. [DOI] [PubMed] [Google Scholar]
- 71.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell Det al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130(1):150–64. [DOI] [PubMed] [Google Scholar]
- 72.Brubaker PL. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Comprehens Physiol. 2018;8(3):1185–210. [DOI] [PubMed] [Google Scholar]
- 73.Terry NA, Ngaba LV, Wilkins BJ, Pi D, Gheewala N, Kaestner KH. Lipid malabsorption from altered hormonal signaling changes early gut microbial responses. Am J Physiol Gastrointest Liver Physiol. 2018;315:G580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iqbal NT, Sadiq K, Syed S, Akhund T, Umrani F, Ahmed S, Yakoob MY, Rahman N, Qureshi S, Xin Wet al. Promising biomarkers of environmental enteric dysfunction: a prospective cohort study in Pakistani children. Sci Rep. 2018;8:2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigalet DL, Martin G, Meddings J, Hartman B, Holst JJ. GLP-2 levels in infants with intestinal dysfunction. Pediatr Res. 2004;56(3):371–6. [DOI] [PubMed] [Google Scholar]
- 76.Drucker DJ. Glucagon-like peptide 2. J Clin Endocrinol Metab. 2001;86(4):1759–64. [DOI] [PubMed] [Google Scholar]
- 77.Edmond J, Auestad N, Robbins RA, Bergstrom JD. Ketone body metabolism in the neonate: development and the effect of diet. Fed Proc. 1985;44(7):2359–64. [PubMed] [Google Scholar]
- 78.Yeh YY, Sheehan PM. Preferential utilization of ketone bodies in the brain and lung of newborn rats. Fed Proc. 1985;44(7):2352–8. [PubMed] [Google Scholar]
- 79.Hong Y-G, Moon Y-M, Hong J-W, No S-Y, Choi T-R, Jung H-R, Yang S-Y, Bhatia SK, Ahn J-O, Park K-Met al. Acid using Escherichia coli whole cell bio-catalyst overexpressing GabTD from Bacillus subtilis. Enzyme Microb Technol. 2018;118:57–65. [DOI] [PubMed] [Google Scholar]
- 80.Gregory KE, Bird SS, Gross VS, Marur VR, Lazarev AV, Walker WA, Kristal BS. Method development for fecal lipidomics profiling. Anal Chem. 2013;85:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JEet al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome. 2017;5(75):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whitehead TR, Price NP, Drake HL, Cotta MA. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl Environ Microbiol. 2008;74(6):1950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friedman M. Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res. 2018;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yaman M, Mızrak ÖF. Determination and evaluation of in vitro bioaccessibility of the pyridoxal, pyridoxine, and pyridoxamine forms of vitamin B6 in cereal-based baby foods. Food Chem. 2019;298:125042. [DOI] [PubMed] [Google Scholar]
- 87.Bowling FG. Pyridoxine supply in human development. Semin Cell Dev Biol. 2011;22(6):611–18. [DOI] [PubMed] [Google Scholar]
- 88.Brown MJ, Ameer MA, Beier K. Vitamin B6 deficiency. Treasure Island (FL): StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 89.Liu Z, Liu S, Xie Z, Blum W, Perrotti D, Paschka P, Klisovic R, Byrd J, Chan KK, Marcucci G. Characterization of in vitro and in vivo hypomethylating effects of decitabine in acute myeloid leukemia by a rapid, specific and sensitive LC-MS/MS method. Nucleic Acids Res. 2007;35(5):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semba RD, Trehan I, Li X, Sale N Jr, Moaddel R, Ordiz MI, Maleta KM, Kraemer K, Manary MJ. Low serum v-3 and v-6 polyunsaturated fatty acids and other metabolites are associated with poor linear growth in young children from rural Malawi. Am J Clin Nutr. 2017;106:1490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang TH, Hu ML. Intracellular levels of S-adenosylhomocysteine but not homocysteine are highly correlated to the expression of nm23-H1 and the level of 5-methyldeoxycytidine in human hepatoma cells with different invasion activities. Nutr Cancer. 2006;55(2):224–31. [DOI] [PubMed] [Google Scholar]
- 92.Zarei I, Oppel RC, Borresen EC, Brown RJ, Ryan EP. Modulation of plasma and urine metabolome in colorectal cancer survivors consuming rice bran. Integr Food Nutr Metab. 2019;6(3):e00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baxter BA, Oppel RC, Ryan EP. Navy beans impact the stool metabolome and metabolic pathways for colon health in cancer survivors. Nutrients. 2018;11(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59(Suppl 4):S213–19. [DOI] [PubMed] [Google Scholar]
- 95.Satoh R, Tsuge I, Tokuda R, Teshima R. Analysis of the distribution of rice allergens in brown rice grains and of the allergenicity of products containing rice bran. Food Chem. 2019;276:761–7. [DOI] [PubMed] [Google Scholar]