Abstract
Background and Purpose
This study aimed to explore several peripheral blood-based markers related to the inflammatory response in a total of 210 patients with acute ischemic stroke (AIS) caused by large artery occlusion in the anterior circulation who received endovascular therapy (EVT) from an observational study of clinical significance of circulating non-coding RNA in acute ischemic stroke (AISRNA).
Methods
We collected baseline characteristics of 210 AIS patients participating in an observational acute stroke cohort: the AISRNA study. The following inflammatory factors were measured in these participants: interleukin-2 [IL-2], IL-4, IL-6, IL-10, tumor necrosis factor-α [TNF-α], and interferon-γ [IFN-γ]. The National Institute of Health Stroke Scale score increase of ≥4 within 24 hours after EVT defined as early neurological deterioration (END).
Results
Compared with patients without END, patients with END had a higher incidence of atrial fibrillation (P=0.012), and also had higher levels of IL-6 and IL-10 (P<0.01). Furthermore, we found that the area under the curves (AUCs) of IL-6 and IL-10 for predicting END were 0.768 (0.697–0.829), and 0.647 (0.570–0.719), respectively. Adjusting for age, sex, and atrial fibrillation, the odds ratios (ORs; 95% confidence interval) for incident END for IL-6 and IL-10 were 1.98 (1.05–6.69) and 1.18 (1.04–1.33), respectively. Additionally, we found significant changes over time in the expression levels of IL-4, IL-6, and IL-10 in patients with END compared with patients without END (P<0.05).
Conclusion
IL-6 and IL-10 levels at admission may be potential markers of END after EVT, and the time course of IL-4, IL-6, and IL-10 is correlated with stroke progression. Further larger studies are needed to confirm the current findings.
Trial Registration
ClinicalTrials.gov NCT04175691. Registered November 21, 2019, https://www.clinicaltrials.gov/ct2/show/NCT04175691
Keywords: acute ischemic stroke, early neurological deterioration, endovascular therapy, inflammatory factors, IL-6
Introduction
Stroke is commonly considered to be a major cause of disability and mortality in adults. Although the early advent of endovascular therapy (EVT) has improved the clinical outcomes of acute ischemic stroke (AIS), over 50% of patients still suffer from disabilities and deficits, which may be the result of neurological and medical complications.1 The precise prediction of clinical outcomes during the acute phase of ischemic stroke after EVT remains challenging. While our previous studies have found that demographics and several clinical characteristics are associated with prognosis after acute ischemic stroke,2–4 the accuracy of prediction remains limited, especially during the acute phase of IS.5 Therefore, the development of precise scores to predict the prognosis in the acute stage may benefit from the identification of individual biomarkers.
A correlation between stroke and the acute inflammatory response can be found in various stages of acute infection. Accumulating evidence has indicated that stroke induces a rapid immunodepression through the autonomic nervous system.6,7 Immune factors, including cytokines, are indicative of stroke-associated infection and are associated with clinical outcome after AIS,8,9 which may be attributed to dynamic changes in the secretion of inflammatory cytokines in the central and peripheral immune responses affecting the progression of AIS.10 These inflammatory cytokines are mostly produced by activated peripheral immune cells and resident microglial cells.11 Mounting evidence showed that Th2 type cytokines including interleukin-10 (IL-10) and IL-4 are involved in the repair of brain injury and the inhibition of stroke-associated inflammation.12,13 Unexpectedly, IL-6, well known for a Th1 type cytokine presenting the pro-inflammatory effect, also represents neurotrophic and regenerative capabilities after cerebral infarction.14 Therefore, these inflammatory cytokines may be associated with stroke pathogenesis and outcome. Previous studies have revealed the functions of inflammatory cytokines in an acute phase of ischemic stroke.9,15 However, the link between inflammatory cytokines and stroke progression remains unclear in AIS patients receiving EVT.
In the present study, we enrolled a total of 210 patients who received EVT from the Clinical Significance of Circulating Non-coding RNA in Acute Ischemic Stroke (AISRNA) study to investigate a range of inflammatory factors (interleukin-2 [IL-2], IL-4, IL-6, IL-10, tumor necrosis factor-α [TNF-α], and interferon-γ [IFN-γ]) and early neurological deterioration (END) after stroke caused by large artery occlusion in the anterior circulation. Additionally, the influence of END after EVT on time course of inflammation-related biomarkers was further explored.
Materials and Methods
Study Population
All subjects provided informed consent. In the present study, we enrolled consecutive patients with AIS patients caused by large artery occlusion in the anterior circulation who received EVT. Data from a total of 210 AIS patients who received EVT were prospectively collected from an observational study of the AISRNA study (www.clinicaltrials.gov, NCT04175691) to investigate the association of inflammatory factors at admission and their dynamic changes with END for 7 days. The clinical characteristics of the patients are summarized in Table 1. All the patients were from Nanjing First Hospital, Nanjing Medical University which is a stroke center affiliated with China Stroke Association. Eligible patients were enrolled in the present study if they met the following inclusion criteria: (1) AIS patients who received EVT and (2) patients with anterior circulation occlusion. Patients were excluded from the study if they met the following exclusion criteria: (1) patients with intracranial hemorrhage; (2) patients with infection on admission; (3) patients treated with antibiotics or immunodepression medical therapy within the past 4 weeks; (4) patients under 18 years of age; (5) patients with a history of a malignant tumor; and (6) patients who died within 7 days. Clinical outcomes (modified Rankin Scale [mRS]) were further investigated after 3 months. Eligible participants were followed up after 3 months via telephone and contact in an outpatient clinic. Our study protocol was approved by the Nanjing Medical University Ethics Committee and complied with the Declaration of Helsinki.
Table 1.
Baseline Characteristics of the Eligible Patients Stratified by END
| Variable | Total | Non-END | END | p-value |
|---|---|---|---|---|
| n (%) | n=210 | n=187 | n=23 | |
| Demographics | ||||
| Sex (female) | 79 (37.6) | 72 (38.5) | 7 (30.4) | 0.451 |
| Age (years) | 67.98±12.1 | 67.34±12.7 | 70.61±9.6 | 0.528 |
| BMI | 24.71±3.3 | 24.28±3.6 | 25.60±3.8 | 0.132 |
| Medical history | ||||
| Hypertension | 142 (67.6) | 129 (69.0) | 13 (56.5) | 0.228 |
| DM | 45 (21.4) | 39 (20.9) | 6 (26.1) | 0.564 |
| CAD | 29 (13.8) | 26 (13.9) | 3 (13.0) | 0.910 |
| AF | 41 (19.5) | 32 (17.1) | 9 (39.1) | 0.012 |
| IS or TIA | 28 (13.3) | 22 (11.8) | 6 (26.1) | 0.057 |
| Stroke etiologya | ||||
| LAA | 72 (34.3) | 63 (33.7) | 9 (39.1) | 0.407 |
| Cardioembolism | 98 (46.7) | 86 (46.0) | 12 (52.2) | |
| Others | 40 (191.0) | 38 (20.3) | 2 (8.7) | |
| NIHSS on admission | 14 (5–22) | 13 (11–21.5) | 17 (6.5–29) | 0.074 |
| NIHSS after 24h | 11 (4–20) | 10 (3.5–19) | 30 (18–34) | <0.001 |
| 90-day mRS | 2 (0–4) | 1 (0–3) | 4 (2–5) | <0.001 |
| IVT | 88 (41.9) | 78 (41.7) | 10 (43.5) | 0.871 |
| Interval time, min, median (IQR) | ||||
| Onset to groin puncture | 106 (81.5–144) | 104 (82–136) | 111 (73–160.5) | 0.540 |
| Onset to reperfusion | 206.5 (131–272) | 195 (136–218) | 227 (128.5–302.5) | 0.585 |
| EVT to first blood samplingb | 815 (564–1045) | 772 (564–1000) | 847.5 (550.5–1087) | 0.425 |
| mTICI (2b-3) | 184 (87.6) | 165 (88.2) | 19 (82.6) | 0.439 |
| Passes of retriever (<3) | 142 (67.6) | 128 (68.4) | 14 (60.9) | 0.464 |
| Inflammatory factors on admission | ||||
| IL-2 (pg/mL) | 2.52±1.2 | 2.55±1.1 | 2.64±1.4 | 0.526 |
| IL-4 (pg/mL) | 3.12±1.7 | 2.89±1.7 | 3.39±1.6 | 0.681 |
| IL-6 (pg/mL) | 13.54 (6.81–30.60) | 10.08 (6.62–25.68) | 28.04 (15.72–107.06) | 0.002 |
| IL-10 (pg/mL) | 5.16 (4.02–7.26) | 5.05 (4.11–7.50) | 6.02 (3.96–26.45) | <0.001 |
| TNF-α (pg/mL) | 2.18 (1.24–3.11) | 2.12 (1.31–2.85) | 2.30 (1.20–3.96) | 0.206 |
| INF-γ (pg/mL) | 2.42 (1.61–3.24) | 2.35 (1.75–3.18) | 2.23 (0.92–3.30) | 0.227 |
| Laboratory characteristics | ||||
| hs-CRP (μg/mL) | 4.93 (4.32–10.76) | 4.86 (4.20–9.08) | 5.12 (4.43–11.99) | 0.197 |
| HbA1c | 5.82 (5.46–6.50) | 5.81 (5.44–5.82) | 6.01 (5.51–6.66) | 0.442 |
| Fasting glucose (mmol/L) | 6.83 (5.51–8.59) | 6.70 (5.46–8.52) | 6.95 (6.04–8.68) | 0.567 |
| Creatinine (μmol/L) | 67.5 (56.4–90.8) | 66.9 (56.0–90.5) | 69.3 (56.8–95.4) | 0.386 |
| LDL-C (mmol/L) | 2.82±1.1 | 2.80±1.0 | 2.35±0.8 | 0.259 |
Notes: ΔNIHSS 0–24h was defined as the delta NIHSS scale (delta NIHSS 0–24h) = NIHSS on admission – NIHSS after 24 hours. Continuous variables of abnormal distribution are expressed as medians (interquartile range), and normally distributed variables as mean ± standard deviation (SD). Categorical variables are expressed as frequency and percentage. aAccording to the modified TOAST classification. bDefined as time between endovascular treatment and first blood sampling to be measured levels of inflammatory factors.
Abbreviations: END, early neurological deterioration; EVT, endovascular treatment; mRS, modified Rankin scale; IL, interleukin; TNF, tumor necrosis factor; INF, interferon; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; AF, atrial fibrillation; IS, ischemic stroke; TIA, transient ischemic attack; IQR, interquartile range; mTICI, modified Thrombolysis in Cerebral Infarction Score; NIHSS, National Institute of Health Stroke Scale; LAA, large artery atherosclerosis; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IVT, intravenous thrombolysis.
Clinical Data
The data collected included demographics, medical history, stroke severity at admission and 24 h later, stroke etiology, laboratory parameters, modified Thrombolysis in Cerebral Infarction (mTICI) Score, and time of onset to door, groin puncture, and reperfusion. Several data definitions were used in the present study: the National Institute of Health Stroke Scale (NIHSS) was used to assess stroke severity; the delta NIHSS scale (delta NIHSS 0–24h) = NIHSS on admission – NIHSS after 24 h; early neurological deterioration (END) was defined as ΔNIHSS ≥4 points;16 and stroke etiology was determined according to the Trial of Org 10172 in acute stroke treatment (TOAST) criteria.17
Inflammation-Related Biomarkers
Blood samples were collected within 24 h after EVT (day 1) and repeated on days 2, 3 and 7. Plasma samples were centrifuged (2000rpm, 4°C, 20 min) and stored at −80°C. The plasma concentrations of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ were determined with the Navios flow cytometer (Beckman Coulter, California, USA). The upper limit of detection for these factors was 2500 pg/mL.
Statistical Analysis
Categorical variables are expressed as frequency and percentage. Continuous variables of an abnormal distribution are expressed as medians (interquartile range [IQR]), and normally distributed variables are expressed as the mean ± standard deviation (SD). The t-test, one-way ANOVA and Mann–Whitney U-tests were used to analyze continuous variables if necessary. Bivariate correlation between delta NIHSS score, 90-day mRS and inflammatory factors was analyzed by the Spearman correlation. Variables from significant factors of the univariable logistic regression analysis were considered in the multivariate logistic regression model to obtain independent factors. Discriminatory capacities of inflammatory factors were assessed by receiver operating characteristic (ROC) curve analyses and area under the curve (AUC). The significance threshold was set at less than 0.05.
Results
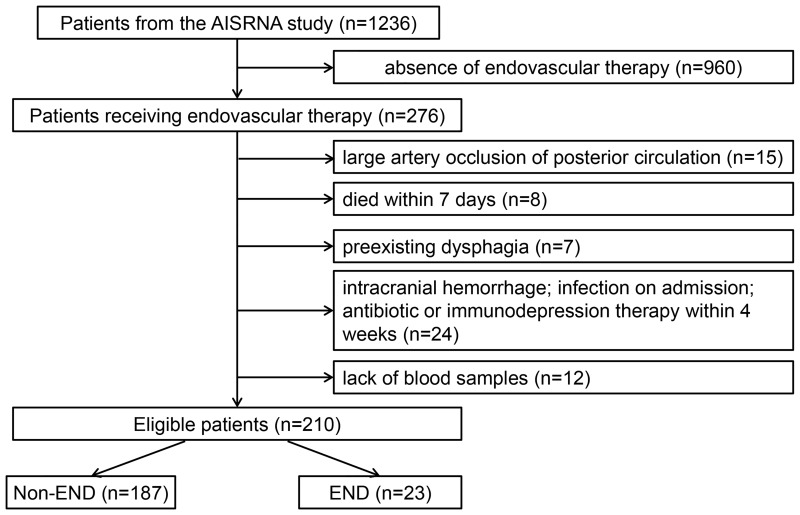
Of the 1236 patients screened from the AISRNA study between November 2019 and February 2021, a total of 210 participants (79 female; mean age, 67.98±12.1 years) met the inclusion criteria (Figure 1). A total of 1026 patients were excluded according to the following criteria: lack of EVT (n=960), posterior circulation occlusion (n=15), died within 7 days (n=8), preexisting dysphagia (n=7), intracranial hemorrhage (n=8), infection on admission (n=10), antibiotic or immunodepression therapy within the past 4 weeks (n=6), and no blood samples (n=12). The baseline characteristics of the eligible patients are summarized in Table 1. Of the 210 patients included, 88 (41.9%) received intravenous thrombolysis.
Figure 1.
Flowchart of the study patients to illustrate study screening, recruitment, and follow-up.
Abbreviation: END, early neurological deterioration.
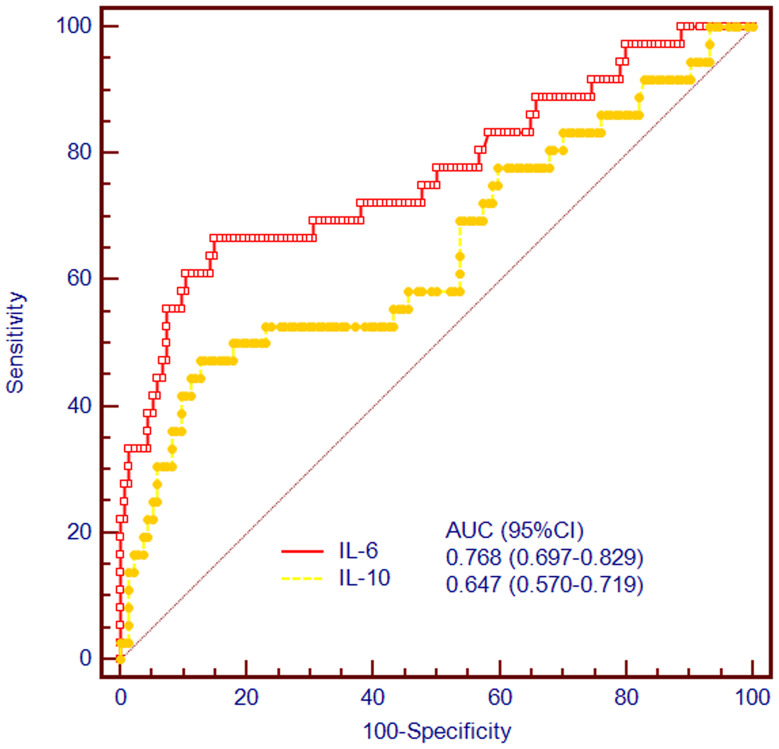
A total of 23 (11.0%) patients suffered END after EVT. The association between inflammatory factors at admission and incidence of END is shown in Table 1. The NIHSS on admission was not significantly different between the groups (P=0.074). However, compared with non-END, NIHSS after 24 h and 90-day mRS were significantly higher in patients with END (P<0.001). We found that END was associated with increased IL-6 and IL-10 levels having a higher proportion of atrial fibrillation (P=0.012). As the delta NIHSS was considered END, we performed a correlation analysis to investigate the correlation between inflammatory factors and delta NIHSS 0–24 h. The results showed that the delta NIHSS was correlated with the expression of IL-6 (r=0.260, P=0.016) and IL-10 (r=−0.238, P=0.028) (Figure S1B and S1C), but a correlation was not observed with the expression of IL-2, IL-4, INF-γ, and TNF-α (P>0.05, Figure S1A, and S1D–F). Furthermore, we also performed a correlation analysis to explore the association between inflammatory factors and 90-day mRS. The findings suggested that serum concentration of IL-6 was significantly associated with 90-day mRS after EVT (r=0.171, P=0.025, Figure S2B), but not IL-2, IL-4, IL-10, INF-γ, and TNF-α (P>0.05, Figure S2A, and S2C–F). Additionally, we performed an ROC curve analysis to explore the predictive powers of these factors. We observed that the AUCs of IL-6 and IL-10 for predicting END were 0.768 (0.697–0.829), and 0.647 (0.570–0.719), respectively (Figure 2), revealing that IL-6 outperformed IL-10 in predicting END (P<0.05).
Figure 2.
Discriminatory capacities of IL-6 and IL-10 for END after EVT. The area under the curves of IL-6 and IL-10 for predicting END were 0.791 (0.689–0.871), and 0.564 (0.452–0.671), respectively (P<0.001).
Abbreviations: END, early neurological deterioration; EVT, endovascular therapy.
Given that association of inflammatory factors with END, we conducted a binary logistic regression to analyze the impact of inflammatory factors on END. The results of the univariate analyses showed that the IL-6 and IL-10 levels were prognostic factors for END, and the findings remained stable after adjustment (Table 2).
Table 2.
Logistic Regression Analyses for Inflammatory Factors Associated with END
| Variable | Modela | OR (95% Confidence Interval) | p-value |
|---|---|---|---|
| IL-2 | 1 | 1.14 (0.62–2.14) | 0.512 |
| 2 | … | … | |
| 3 | … | … | |
| IL-4 | 1 | 1.03 (1.00–1.04) | 0.052 |
| 2 | … | … | |
| 3 | … | … | |
| IL-6 | 1 | 1.68 (1.03–2.64) | 0.012 |
| 2 | 2.04 (1.10–6.62) | 0.016 | |
| 3 | 1.98 (1.05–6.69) | 0.035 | |
| IL-10 | 1 | 1.10 (1.05–1.30) | 0.023 |
| 2 | 1.15 (1.05–1.32) | 0.018 | |
| 3 | 1.18 (1.04–1.33) | 0.022 | |
| TNF | 1 | 1.33 (0.74–1.89) | 0.658 |
| 2 | … | … | |
| 3 | … | … | |
| INF | 1 | 0.94 (0.56–1.87) | 0.789 |
| 2 | … | … | |
| 3 | … | … |
Note: a Model 1: unadjusted; model 2: adjusted for age and sex; model 3 adjusted for model 2 with atrial fibrillation.
Abbreviations: END, early neurological deterioration; IL, interleukin; TNF, tumor necrosis factor; INF, interferon; OR, odds ratio.
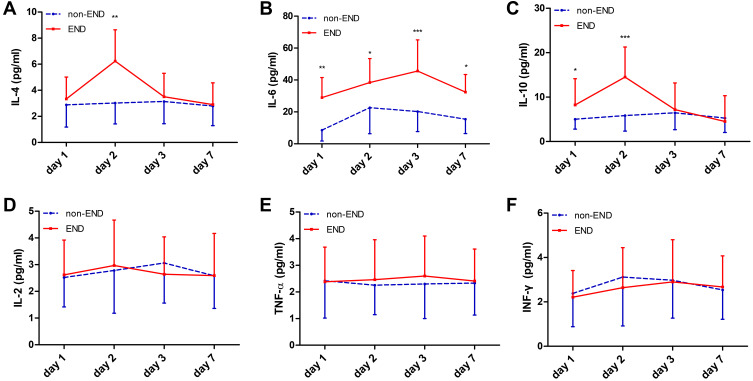
To further study the clinical utility of inflammatory factors after EVT, we analyzed the time courses of the inflammatory factors within 7 days for patients with and without END (Figure 3). A total of 97 blood samples were collected for day 1, day 2, day 3 and 7 among these patients. We found significant changes over time in the expression levels of IL-4, IL-6, and IL-10 in patients with END compared without END (Figure 3A–C, P<0.05), and the IL-6 levels were obviously increased from days 1 to 7 after EVT (Figure 3B, P<0.05). Furthermore, we observed IL-4 and IL-10 peak levels at day 2, and then rapidly decreased at day 3 (Figure 3A and C). However, No significant change over time was found in the expression levels of IL-2 (Figure 3D), TNF-α (Figure 3E), and INF-γ (Figure 3F) between patients with END and patients without END (P>0.05).
Figure 3.
Time course of inflammatory factors after stroke onset (days 1 to 7). Significant changes over time were observed in the expression levels of IL-4, IL-6, and IL-10 in patients with END compared with patients without END (P<0.05). IL-4 levels peaked at day 2 and then rapidly decreased at day 3 (A). IL-6 levels were obviously increased from days 1 to 7 after EVT (B). IL-10 (C) levels also peaked at day 2 and then rapidly decreased at day 3. No significant change over time was found in the expression levels of IL-2 (D), TNF-α (E), and INF-γ (F) between patients with END and patients without END (P>0.05). *P<0.05, **P<0.01, ***P<0.001.
Abbreviation: END, early neurological deterioration.
Discussion
This study of EVT participants from the AISRNA study showed a strong association of IL-6 and IL-10 levels at admission with risk of END. Our study suggests that patients with increased IL-6 and IL-10 levels had a higher risk of developing post-EVT END. By contrast, there was no correlation of baseline levels of IL-2, Il-4, INF-γ, and TNF-α with END. The predictive power of IL-6 for END was superior to that of other inflammatory factors. Additionally, the IL-4, IL-6, and IL-10 levels presented a marked rising trend in patients with END.
Inflammation is a hallmark of stroke etiology and progression. Poststroke inflammation is considered a requisite pathological process involved in ischemic brain injury.18 A series of detrimental complications occur and the blood-brain barrier (BBB), damaged after the initial brain injury, is crossed by activated peripheral immune cells including monocytes and T cells.19 Furthermore, poststroke inflammatory response is associated with stroke severity at admission as determined by NIHSS.20 Additionally, patients who suffered END were more susceptible to a poor functional outcome after 90 days.21 However, the association between poststroke inflammatory response and END remains uncertain. Therefore, we performed a prospective study from the AISRNA study to further investigate the influence of inflammatory factors on END after stroke.
The biological function of IL-6 in AIS remains controversial.22 IL-6 is considered as multipotent functions involving in brain damage and nerve regeneration.23 Firstly, IL-6 would enhance the brain injury and weaken the proliferation of neural stem cells in the acute phase of ischemic stroke. Secondly, IL-6 could inhibit collagen deposition to protect glial cells and repair brain injury in the subacute phage of cerebral ischemia.24 A previous study has demonstrated that the IL-6 levels are increased in peripheral blood samples during the first 7 days after stroke onset.25 For example, increased IL-6 is associated with the infarct size and stroke severity at admission12,26 as well as risk of incident stroke,27 but another study reported the opposite, that early levels of IL-6, as a neuroprotective factor, are inversely correlated with lesion size and functional outcome.28 Our findings also suggest that increased IL-6 was associated with risk of END after EVT. Thus, this controversial phenomenon needs to be further investigated. However, there is less agreement on the time point of the peak of IL-6 levels. Some studies reported IL-6 peak levels at day 3,29,30 which is similar to our findings. Others describe high levels of IL-6 that ranged from a few hours until one day or a week after stroke.28,31,32
IL-10 is a major anti-inflammatory cytokine that is secreted from monocytes and T cells, suggesting its participation in a plethora of immunomodulatory functions. High expression of IL-10 promotes glial and neuronal cell survival and weakens inflammatory responses in the brain.33 In a permanent middle cerebral artery occlusion model, IL-10 suppresses proinflammatory molecules and reduces infarct volume.34 Several investigators also reported that IL-10 may serve a neuroprotective role and predict clinical outcome after stroke.12,35,36 However, other studies have suggested that IL-10 is a marker of incident stroke.37,38 Our previous studies from experimental rats and human stroke patients observed that IL-10 is positively associated with stroke risk.39,40 In the present study, we also reported a positive correlation between IL-10 and END after EVT, suggesting that increased IL-10 levels at admission were associated with END after EVT. We observed IL-10 peak levels at day 2, and patients with END had decreased IL-10 levels compared with those with non-END at 7 days. This phenomenon may be due to a stress response of IL-10 regarding END, which leads to high levels of IL-10, but its levels were subsequently decreased after day 2. Therefore, IL-10 may be involved in the process of END, and its molecular mechanisms remain to be explored in further studies.
IL-4, an anti-inflammatory cytokine, can drive the differentiation of Th2 cell, which play beneficial roles in inhibiting poststroke inflammation, repairing damaged brain tissues and inducing neurotrophic factors in astrocytes.41,42 A previous study has demonstrated that IL-4 is significantly correlated with stroke severity and functional outcome in AIS patients.12 However, our results showed no association of IL-4 with END after EVT, but IL-4 levels were increased in patients with END at day 2 and then rapidly decreased at day 3. At present, we are also investigating the molecular mechanisms of IL-4 and IL-10 regarding dynamic change in stroke progression.
Several limitations should be acknowledged in the study. First, this study included a small number of patients from a single center. We are seeking for subcenters to complete the AISRNA study. Second, we did not analyze the association between the initial infarct burden and END after EVT. Third, although the incidence of atrial fibrillation in END patients was higher than those in non-END patients, the effect of inflammatory factors on END remained stable after adjustment for atrial fibrillation. Therefore, a randomized controlled study is urgent to confirm this finding. Finally, molecular mechanisms regarding dynamic changes of these inflammatory factors should be further explored in patients undergoing EVT.
In summary, this study illustrates the correlation of inflammatory factors on END and their time course after EVT among AIS patients caused by large artery occlusion in the anterior circulation. We found that the serum concentration of IL-6 and IL-10 at admission may be a potential marker of END after EVT, and the time course of these factors is correlated with END. Additional, further larger studies are needed to confirm the current findings.
Funding Statement
This work was supported by the National Natural Science Foundation of China [No. 81901215 (to Qi-Wen Deng) and No. 81802093 (to Hui-Ling Sun)], the Medjaden Academy & Research Foundation for Young Scientists [No. MJR20201129 (to Qi-Wen Deng)], the Science and Technology Development Plan of Nanjing Foundation [No. 201727004 (to Hong-Chao Shi) and the Nanjing Medical Science and Technique Development Foundation Project [No. YKK18097 (to Feng Zhou)].
Abbreviations
END, early neurological deterioration; IL, interleukin; TNF, tumor necrosis factor; INF, interferon; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; AF, atrial fibrillation; IS, ischemic stroke; TIA, transient ischemic attack; IQR, interquartile range; mTICI, modified Thrombolysis in Cerebral Infarction Score; NIHSS, National Institute of Health Stroke Scale; LAA, large artery atherosclerosis; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Data Sharing Statement
All data supporting our results are available from the corresponding authors upon reasonable.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389(10069):641–654. doi: 10.1016/S0140-6736(16)30962-X [DOI] [PubMed] [Google Scholar]
- 2.Deng QW, Liu YK, Zhang YQ, et al. Low triglyceride to high-density lipoprotein cholesterol ratio predicts hemorrhagic transformation in large atherosclerotic infarction of acute ischemic stroke. Aging. 2019;11(5):1589–1601. doi: 10.18632/aging.101859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng QW, Li S, Wang H, et al. The short-term prognostic value of the triglyceride-to-high-density lipoprotein cholesterol ratio in acute ischemic stroke. Aging Dis. 2018;9(3):498–506. doi: 10.14336/AD.2017.0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng QW, Wang H, Sun CZ, et al. Triglyceride to high-density lipoprotein cholesterol ratio predicts worse outcomes after acute ischaemic stroke. Eur J Neurol. 2017;24(2):283–291. doi: 10.1111/ene.13198 [DOI] [PubMed] [Google Scholar]
- 5.Byblow WD, Stinear CM. It is difficult to make predictions, especially about the future. Stroke. 2017;48(12):3187–3188. doi: 10.1161/STROKEAHA.117.019071 [DOI] [PubMed] [Google Scholar]
- 6.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38(3):1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d [DOI] [PubMed] [Google Scholar]
- 7.Deng QW, Yang H, Yan FL, et al. Blocking sympathetic nervous system reverses partially stroke-induced immunosuppression but does not aggravate functional outcome after experimental stroke in rats. Neurochem Res. 2016;41(8):1877–1886. doi: 10.1007/s11064-016-1899-8 [DOI] [PubMed] [Google Scholar]
- 8.Schuppner R, Dirks M, Grosse GM, et al. ADAMTS-13 activity predicts outcome in acute ischaemic stroke patients undergoing endovascular treatment. Thromb Haemost. 2018;118(4):758–767. [DOI] [PubMed] [Google Scholar]
- 9.Bustamante A, Sobrino T, Giralt D, et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. J Neuroimmunol. 2014;274(1–2):215–224. doi: 10.1016/j.jneuroim.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 10.Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24(5):708–723. doi: 10.1016/j.bbi.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 11.Reaux-le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lin S, Chen X, et al. The prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis. 2019;10(3):544–556. doi: 10.14336/AD.2018.0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Lu G, Wang D, et al. MicroRNA-4443 regulates monocyte activation by targeting tumor necrosis factor receptor associated factor 4 in stroke-induced immunosuppression. Eur J Neurol. 2020;27(8):1625–1637. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29(3):464–479. doi: 10.1038/jcbfm.2008.141 [DOI] [PubMed] [Google Scholar]
- 15.Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5(3–4):143–159. doi: 10.1159/000026331 [DOI] [PubMed] [Google Scholar]
- 16.Seners P, Hurford R, Tisserand M, et al. Is unexplained early neurological deterioration after intravenous thrombolysis associated with thrombus extension? Stroke. 2017;48(2):348–352. doi: 10.1161/STROKEAHA.116.015414 [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 18.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37(2):291–293. doi: 10.1161/01.STR.0000200561.69611.f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12(10):594–604. doi: 10.1038/nrneurol.2016.125 [DOI] [PubMed] [Google Scholar]
- 20.Chamorro A, Amaro S, Vargas M, et al. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252(1):29–35. doi: 10.1016/j.jns.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Bae JH, Park KY, et al. Incidence and mechanism of early neurological deterioration after endovascular thrombectomy. J Neurol. 2019;266(3):609–615. doi: 10.1007/s00415-018-09173-0 [DOI] [PubMed] [Google Scholar]
- 22.Dziedzic T, Slowik A, Szczudlik A. Interleukin-6 and stroke: cerebral ischemia versus nonspecific factors influencing interleukin-6.. Stroke. 2003;34(12):e229–e230. doi: 10.1161/01.STR.0000103350.88094.5B [DOI] [PubMed] [Google Scholar]
- 23.Acalovschi D, Wiest T, Hartmann M, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34(8):1864–1869. doi: 10.1161/01.STR.0000079815.38626.44 [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Kim J, Kim OJ, et al. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Critical Care. 2013;17(2):R45. doi: 10.1186/cc12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechtouff L, Bochaton T, Paccalet A, et al. Association of Interleukin-6 levels and futile reperfusion after mechanical thrombectomy. Neurology. 2020;96(5):e752–e757. doi: 10.1212/WNL.0000000000011268 [DOI] [PubMed] [Google Scholar]
- 26.Hotter B, Hoffmann S, Ulm L, Meisel C, Fiebach JB, Meisel A. IL-6 plasma levels correlate with cerebral perfusion deficits and infarct sizes in stroke patients without associated infections. Front Neurol. 2019;10:83. doi: 10.3389/fneur.2019.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenny NS, Callas PW, Judd SE, et al. Inflammatory cytokines and ischemic stroke risk: the REGARDS cohort. Neurology. 2019;92(20):e2375–e2384. doi: 10.1212/WNL.0000000000007416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotgiu S, Zanda B, Marchetti B, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. 2006;13(5):505–513. doi: 10.1111/j.1468-1331.2006.01280.x [DOI] [PubMed] [Google Scholar]
- 29.Perini F, Morra M, Alecci M, Galloni E, Marchi M, Toso V. Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurol Sci. 2001;22(4):289–296. doi: 10.1007/s10072-001-8170-y [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann S, Harms H, Ulm L, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671–3682. doi: 10.1177/0271678X16671964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzotta G, Sarchielli P, Caso V, et al. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol. 2004;11(6):377–381. doi: 10.1111/j.1468-1331.2004.00798.x [DOI] [PubMed] [Google Scholar]
- 32.Smith CJ, Emsley HC, Gavin CM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4(1):2. doi: 10.1186/1471-2377-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liesz A, Bauer A, Hoheisel JD, Veltkamp R. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci Lett. 2014;579:18–23. doi: 10.1016/j.neulet.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 35.Protti GG, Gagliardi RJ, Forte WC, Sprovieri SR. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arq Neuropsiquiatr. 2013;71(11):846–851. doi: 10.1590/0004-282X20130168 [DOI] [PubMed] [Google Scholar]
- 36.Singh HV, Pandey A, Shrivastava AK, Raizada A, Singh SK, Singh N. Prognostic value of neuron specific enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Clin Chim Acta. 2013;419:136–138. doi: 10.1016/j.cca.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 37.van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frolich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33(4):1135–1138. doi: 10.1161/01.STR.0000014206.05597.9E [DOI] [PubMed] [Google Scholar]
- 38.Xie G, Myint PK, Zaman MJ, et al. Relationship of serum interleukin-10 and its genetic variations with ischemic stroke in a Chinese general population. PLoS One. 2013;8(9):e74126. doi: 10.1371/journal.pone.0074126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Lu G, Wang D, et al. MicroRNA-4443 regulates monocyte activation by targeting TRAF4 in stroke-induced immunosuppression. Eur J Neurol. 2020;27(8):1625–1637. doi: 10.1111/ene.14282 [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Deng QW, Peng AN, et al. beta-arrestin2 functions as a key regulator in the sympathetic-triggered immunodepression after stroke. J Neuroinflammation. 2018;15(1):102. doi: 10.1186/s12974-018-1142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42(7):2026–2032. doi: 10.1161/STROKEAHA.110.593772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, Roitbak T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation. 2016;13(1):287. doi: 10.1186/s12974-016-0753-x [DOI] [PMC free article] [PubMed] [Google Scholar]