Abstract
This report describes local administration of submicron particle paclitaxel (SPP) (NanoPac®: ~ 800-nm-sized particles with high relative surface area with each particle containing ~ 2 billion molecules of paclitaxel) in preclinical models and clinical trials evaluating treatment of carcinomas. Paclitaxel is active in the treatment of epithelial solid tumors including ovarian, peritoneal, pancreatic, breast, esophageal, prostate, and non-small cell lung cancer. SPP has been delivered directly to solid tumors, where the particles are retained and continuously release the drug, exposing primary tumors to high, therapeutic levels of paclitaxel for several weeks. As a result, tumor cell death shifts from primarily apoptosis to both apoptosis and necroptosis. Direct local tumoricidal effects of paclitaxel, as well as stimulation of innate and adaptive immune responses, contribute to antineoplastic effects. Local administration of SPP may facilitate tumor response to systemically administered chemotherapy, targeted therapy, or immunotherapy without contributing to systemic toxicity. Results of preclinical and clinical investigations described here suggest that local administration of SPP achieves clinical benefit with negligible toxicity and may complement standard treatments for metastatic disease.
Graphical abstract
Keyword: NanoDoce, NanoPac, Ovarian cancer, Pancreatic cancer, Pancreatic cysts, Prostate cancer, Lung cancer
Introduction
Paclitaxel is a high molecular weight, highly protein bound, hydrophobic molecule, which limits its access to tumor cells. IV administration of paclitaxel achieves relatively low levels of drug at the tumor for a short period of time, resulting in intermittent tumor cell exposure to drug resulting in death that is typically apoptotic and may allow for some tumor cell survival, division, and/or mutation potentially resulting in paclitaxel resistance.
Local treatment of solid tumors by systemic therapies has the potential to increase tumoricidal effects without increasing systemic toxicity. It has long been hypothesized that local, sustained tumor treatment would make the drug available to tumor cells over multiple cell-division cycles, resulting in tumoricidal benefits without compromising the patient’s quality of life [1]. Various particle-based drug delivery systems are being developed for treatment of cancer [2, 3]. However, success of many systems is limited by ineffective delivery to tumor sites as well as abbreviated drug residence due, in part, to the clearance of particles by the immune system [4].
In order to address the limitations of IV treatment modalities, submicron particles of paclitaxel (SPP, NanoPac®, NanOlogy, Ft Worth, TX), with a mean particle size of ~ 800 nm (containing approximately 2 billion paclitaxel molecules each) with a high surface area to facilitate molecule release, were developed to allow for suspension in an aqueous media [5]. These aqueous suspensions of SPP allow for various routes of local administration including inhalation (IH) via nebulizer, intraperitoneal (IP) delivery, or intratumoral (IT) injection.
This review will describe the therapeutic potential of SPP for treatment of various solid tumors and associated clinical trials, all of which demonstrate clinical utility in small Phase 1/2 studies (Table 1). We will provide early preclinical and clinical evidence that tumor and immune responses to paclitaxel released from locally administered SPP are different than responses to paclitaxel delivered intravenously (IV). These findings support the conclusion that SPP may offer an important addition to cancer therapy without adding clinically significant local or systemic toxicity.
Table 1.
Clinical trials of NanoPac®
| Study ID | NCT number | Study title | Study start | Study statusa |
|---|---|---|---|---|
| HSC#1114 | NCT00666991 | Pharmacokinetic, safety and efficacy study of nanoparticle paclitaxel in patients with peritoneal cancers | July 2008 | Completed |
| NANOPAC-2016-01 | NCT03029585 | Phase II study of four dose levels of intraperitoneal NanoPac Plus IV carboplatin and paclitaxel in patients with epithelial ovarian cancer undergoing cytoreductive surgery | April 2017 | Completed |
| NANOPAC-2016-02 | NCT03077659 | Phase IIa dose escalation trial of NanoPac focal therapy for prostate cancer in subjects undergoing radical prostatectomy | September 2017 | Completed |
| NANOPAC-2017-01 | NCT03188991 | A trial evaluating escalating doses and the safety of intracystic injection of NanoPac in subjects with mucinous cystic pancreatic neoplasms | September 2017 | Completed |
| NANOPAC-2016-05 | NCT03077685 | Phase IIa trial evaluating the safety of intratumoral injection of NanoPac in subjects with locally advanced pancreatic adenocarcinoma | December 2017 | Ongoing |
| NANOPAC-2019-01 | NCT04221828 | Phase 2 trial of NanoPac focal therapy for prostate cancer in subjects undergoing radical prostatectomy | July 2020 | Recruiting |
| NANOPAC-2020-01 | NCT04314895 | Phase 2 trial evaluating the safety and tolerability of intratumoral injections of NanoPac® with standard of care therapy in subjects with lung cancer | September 2020 | Recruiting |
a = Study status current as of 5 October 2020
Preparation of submicron taxane particles
Precipitation with compressed antisolvents (PCA) is a non-solvent process by which a drug substance dissolved in an organic solvent is precipitated in a supercritical antisolvent. Baltezor et al. [5] described a method by which paclitaxel dissolved in acetone processed with supercritical CO2 created SPP with a number-weighted mean particle size ranging from 0.670 to 0.861 μm with a specific surface area greater than 22 m2/g and a bulk density between 0.05 and 0.15 g/cm3 (Fig. 1; CritiTech, Lawrence, KS). The volume-weighted mean particle size distribution for this product ranged from 2.8 to 3.5 μm and matches the size of the particles found by scanning electron microscopy (SEM). Figure 2 a and b is SEM photomicrographs of the unprocessed and PCA processed paclitaxel. They reveal morphological differences between unprocessed and PCA-processed particles. Unprocessed paclitaxel crystals were rod-shaped, thicker, and clumped together resulting in a large size variability. The PCA-processed SPP were much thinner, highly irregular shaped particles with large open areas between particles.
Fig. 1.
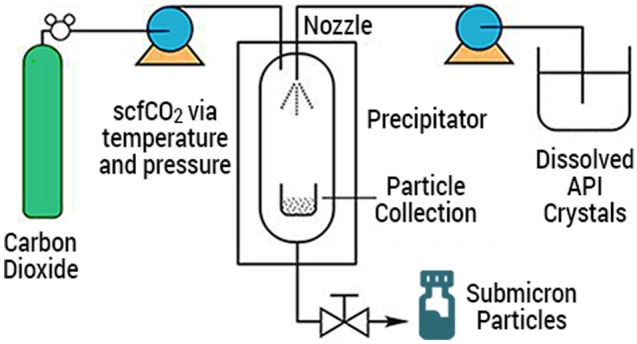
Schematic of continuous manufacturing process for submicron particle paclitaxel. Supercritical carbon dioxide (> 72.8 bar, > 31 °C) (ScCO2) is freely soluble with many organic solvents but insoluble with paclitaxel. Paclitaxel dissolved in organic solvent is sonicated into small droplets and rapidly exposed to scCO2, which strips away the solvent and precipitates the paclitaxel as small particles. These small, stable, flowable particles are captured in a collection chamber and later filled in powder form into vials
Fig. 2.
(a) Paclitaxel drug substance and (b) Paclitaxel drug substance precipitated with compressed antisolvents results in particles of paclitaxel with a specific surface area > 22 m2/gm with a bulk density between 0.05 and 0.15 gm/cm3
The SPP particles had an increased specific surface area (SSA) which is equivalent to much smaller particles. The increased SSA enhances the release rate of drug from the particles. The irregular shape of the particles is thought to result in interlocking between particles, causing them to function as if they were even larger. We theorize these particles are large enough to avoid being removed by blood flow or by phagocytosis and are retained in tumors, creating a sustained drug release depot while minimally contributing to systemic paclitaxel levels. Parallel PCA processes can also be employed to generate submicron particle docetaxel (SPD). When administered to tumors as an aqueous suspension, the PCA particles release paclitaxel [6, 7] or docetaxel [8] into the surrounding tumor microenvironment (TME) at tumoricidal levels (Fig. 3).
Fig. 3.
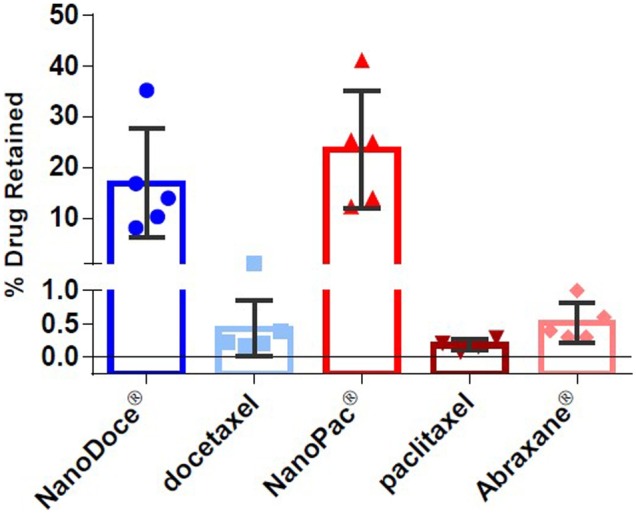
Percent drug retained in MDA-MB-231 tumors. Formulations (5 mg/mL each) were administered as 0.05 mL direct injections into MDA-MB-231 tumors implanted on the flanks of female BALB/c mice (n = 4 or 5 per group), and tumor tissues were collected 5 days later. The percent of drug retained was calculated based on the concentration of drug detected (ng/g of tumor) and tumor weight. Group mean docetaxel (blue bars) and paclitaxel percent (red bars) as well as individual tumor percent (symbols) are plotted; error bars = ± 1 SD
These large agglomerated particles with the high SSA and the low bulk density are also excellent candidates for enhancing pulmonary drug delivery. The mass median aerodynamic diameter (MMAD) of PCA particles is typically in the range of 2 to 4 μm. However, the physical size of the particles or agglomerates of the particles is > 5 μm which is large enough to inhibit removal from the lungs by phagocytosis. We have observed that PCA-produced particles provide much longer lung residence times [6].
The PCA process is adjustable to allow for particle engineering of a specific drug to create particles with the most desirable characteristics for an intended delivery method. Processing variables of the solvent, the concentration of drug in the solvent, the spray rate of the drug solution, the intensity of the sonic energy as well as the flow rate, and pressure of the supercritical CO2 are examples. Once defined and controlled, the PCA process is reproducible with high yields (> 95%) and low residual solvent.
Prior to the start of the Phase 2 clinical trials, 3 batches of SPP were manufactured under full GMP conditions using continuous process of approximately 36 h each. The batches shown in Table 2 were used for the Phase 2 clinical supplies and for providing ICH compliant stability data for SPP. Table 2 shows the results at T0 and after 48 months storage at 25°C 60%RH in 60 ml sterile glass vials. The data demonstrates the consistency and excellent stability of the 3 batches. The batches have been monitored with Xray powder diffraction (XPRD) and differential scanning calorimetry (DSC) to confirm that there have been no changes in the crystalline form of the particles. All three batches had an untapped bulk density of ~ 0.08 g/cm3. The particle size by volume (Dv50) shown in Table 2 was found to be a critical parameter in the understanding of the relationship between the particle size, the bulk density and the high specific surface area of the particles. We are still in the process of finalizing an in vitro dissolution test, but we have found that the specific surface area of the particles has a direct linear correlation with the dissolution results for the particles.
Table 2.
Results of 3 GMP NanoPac® batches used for Phase 2 clinical studies and ICH compliant stability
| NanoPac® | Assay (%) | Particle size (Dn50) (µ) | Particle size (Dv50) (µ) | Surface area (m2/g) | |||
|---|---|---|---|---|---|---|---|
| Lot number | T0 | 48 months | T0 | 48 months | 48 months | T0 | 48 months |
| STV090915 | 100.4 | 98.2 | 0.79 | 0.89 | 2.86 | 29.9 | 28.4 |
| STV093015 | 98.2 | 98.2 | 0.76 | 0.89 | 3.22 | 29.1 | 26.1 |
| STV100715 | 98 | 98 | 0.83 | 0.85 | 3.2 | 27.9 | 25.9 |
Preclinical studies
Lung cancer
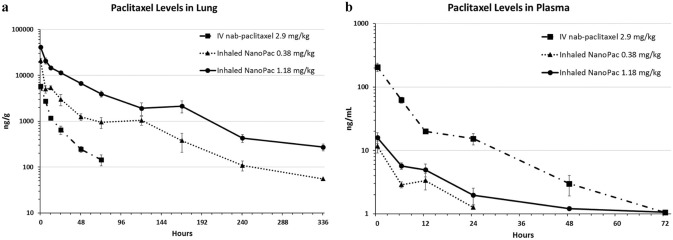
Efficacy of IV paclitaxel in the treatment of lung cancer is limited by its concentration and duration of tumor exposure. Alternate routes of administration have been evaluated [9] including IH and IT injections by bronchoscopy [10]. Studies of IH paclitaxel demonstrated preliminary proof-of-efficacy in non-small cell lung cancer (NSCLC) preclinical models [11]. Inhaled chemotherapy could theoretically deliver substantial doses directly to the pulmonary parenchyma and adjoining airways while avoiding additive toxic exposure to nontarget organs. Historically, however, achieving sustained pulmonary exposure through IH was limited by poor retention of drug within the pulmonary tissues due to clearance mechanisms such as diffusion across the alveolar–capillary membranes, the mucociliary “escalator” removing material to the gastrointestinal tract, and phagocytosis by alveolar macrophages and dendritic cells, as well as lymphatic drainage. To address these challenges, IH of nebulized SPP was evaluated. Following IH of nebulized SPP, substantial levels of paclitaxel in the lung were achieved for at least 2-week post-administration (Fig. 4), confirming the increased local retention and efficacy of IH SPP [6, 7].
Fig. 4.
Paclitaxel levels in lung (a) and plasma (b) after inhaled NanoPac treatment. Male Sprague–Dawley rats (6–8 weeks old) were administered paclitaxel on a single occasion in one of three treatment arms (n = 30 each): inhaled submicron particle paclitaxel in a nose-only exposure at a low-dose of 0.38 mg/kg or a high dose of 1.18 mg/kg, or intravenous nab-paclitaxel administered via tail vein injection at 2.9 mg/kg. Three animals from each arm were sacrificed at 0.5, 6, 12, 24, 48, 72, 120, 168, 240, and 336 h post exposure for lung tissue and plasma collections. Lung tissue (a) and plasma (b) were assayed via ultra-performance liquid chromatography tandem mass spectrometry to quantify paclitaxel concentration as a function of time with a lower level of quantification of 50 ng/g and 1 ng/mL, respectively (mean ± 1SEM) [6]. Reprinted by permission from Mary Ann Liebert, Inc.: [Mary Ann Liebert] [Journal of Aersol Medicine and Pulmonary] [Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac) in a rodent model, James Verco et al.] [2019]
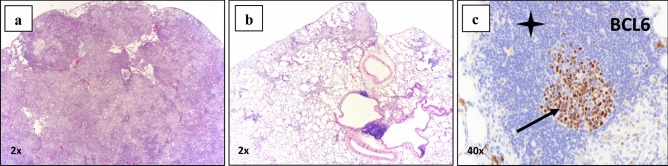
In NIH-nru nude rats orthotopically implanted with Calu-3 cells (human lung adenocarcinoma) treatment with inhaled SPP resulted in tumor growth inhibition, regression, and infiltration [7]. In some lung samples, no residual tumor was detected upon histopathological examination; scattered, small fibrotic nodules, and stroma replacing areas presumed to have once contained tumor was observed. Tumor regression and eradication were accompanied by a robust immune cell infiltrate of lymphocytes and macrophages into the tumor spaces. Since these animals were athymic and thus deficient in T cells, any immune-mediated tumoricidal affect would have been due to macrophage infiltration and primarily antibody-dependent, cell-mediated cytotoxicity (ADCC) or antibody dependent, and cell-mediated phagocytosis (ADCP). Paclitaxel is known to enhance ADCC [12]. These two anti-tumor responses depend on both the presence of antibodies against the tumor and the presence of immune effector cells. Immunohistochemical staining (IHC) of lung tissue showed an increase in CD11b + immune cell infiltration in lung samples following IH SPP. Some of the bronchus associated lymphoid tissue (BALT) exhibited architecture consistent with active germinal centers within lymphoid follicles (Fig. 5). These findings suggest that persistent paclitaxel release at high concentrations facilitates antigen presentation to the host immune system and that antigen availability enhances immune cell infiltration into tumor sites.
Fig. 5.
In an orthotopic nude NIH male rat lung cancer model (Calu-3) was treated with inhaled submicron particle paclitaxel twice weekly for 4 weeks at 0.5 and 1.0 mg/kg. All animals survived to their scheduled necropsy and exhibited no adverse clinical observations from treatment. Left lungs were paraffin embedded, serially sectioned and stained for histopathological and immunohistochemical examination. (a) Control group treated with vehicle exhibited unabated tumor growth; (b) inhaled submicron particle paclitaxel groups presented a reduced or absent tumor presence and contained occasional areas of residual fibrosis; (c) BCL-6 stained follicles revealed localized B-cells at the germinal center (black arrow) and B-cells in the submicron particle inhaled groups (black star) which reside in the surrounding mantle zone [7]. Reprinted by permission from Mary Ann Liebert, Inc.: [Mary Ann Liebert] [Journal of Aersol Medicine and Pulmonary Delivery] [Inhaled submicron particle paclitaxel (NanoPac) induces tumor regression and immune cell infiltration in an orthotopic athymic nude rat model of non-small cell lung cancer, James Verco et al.] [2019]
Genitourinary neoplasms
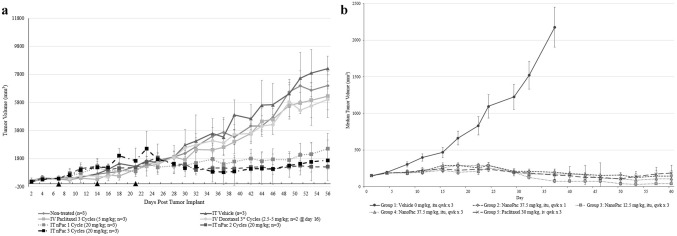
Direct IT injection of SPP into xenograft models of human prostate and renal cell carcinoma lines produced regression or eradication of tumors (Fig. 6a,b). Formation of fibrin deposits, presumably from tumor debris, was associated with a robust immune cell infiltrate at the tumor site. In contrast, animals that received IV taxane showed continued tumor growth and modest to no immune cell infiltration [8, 13, 14]. These observations suggest that the response of carcinomas to prolonged exposure to tumoricidal levels of taxanes may involve at least two different mechanisms. First, direct tumor kill by the taxane-induced disruption of mitosis is followed by tumor cell disruption, making neoantigens available and causing antigen spread within the TME. Second, these conditions stimulate immune effector cells to further the local tumoricidal response.
Fig. 6.
(a) Mean tumor volume in 786-O renal cancer xenograft in 5–7-week-old female Sprague–Dawley Rag2; Il2rg (null) rats (n = 2–3 animals/group) following IT treatment with submicron particle paclitaxel (nPac; 20 mg/kg). Treatments were initiated seven days after tumor implant and administered weekly for one, two or three cycles (black triangles). Control groups included no treatment, IT vehicle administered on the same schedule as IT submicron particle paclitaxel, IV paclitaxel, or IV docetaxel. * = due to toxicity, following the first cycle the IV docetaxel regimen was modified from 5 to 2.5 mg/kg for one or two additional cycles. Tumors were measured with calipers three times weekly for the duration of the study [13]. (b) Median tumor volume in PC-3 prostate cancer xenograft in 11-week-old female NCr nu/nu mice (Crl:NU(NCr)-Foxn1nu; n = 10 animals/group) following IT treatment with submicron particle paclitaxel (NanoPac; 12.5 to 37.5 mg/kg) for 1 or 3 weekly cycles. Control groups included IT vehicle or IV paclitaxel. Treatments were initiated 35 days after tumor implant on Day 1 of the study. Tumors were measured with calipers twice weekly for the duration of the study. [14]. Error bars ± 1 SDEV
Clinical trials
Intraperitoneal submicron particle paclitaxel
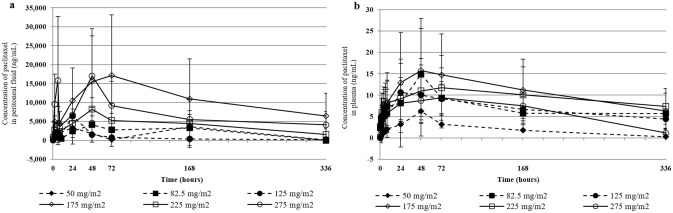
The first clinical study of SPP investigated IP administration in patients with carcinoma predominantly confined to the peritoneal cavity who failed prior therapies (NCT00666991, [15]). Twenty-one subjects received SPP via IP ports in doses ranging from 50 to 275 mg/m2. These subjects previously underwent treatments, including IV chemotherapy and debulking surgery. Treatment cycles with IP SPP were 28 days long with one to six cycles administered with the majority of subjects received two cycles. IP administration of SPP did not induce peritonitis nor systemic toxicity typically associated with IV paclitaxel. The peritoneal levels of paclitaxel rose during the 2 days after dosing to concentrations 450–2900 times the peak plasma paclitaxel concentrations and remained elevated throughout the treatment cycle (Fig. 7). Plasma paclitaxel levels remained below 10 ng/mL, well below the systemic toxicity threshold of 40 ng/mL [16]. Objective tumor response assessed by RECIST 1.0 occurred in five subjects with stable disease and 15 with progressive disease. Six of 21 subjects survived ≥ 1 year, and three survived ≥ 2 years despite their advanced disease. Compared with IP administration of nab-paclitaxel in a separate study [17], IP administration of SPP provided higher, sustained peritoneal paclitaxel levels with minimal systemic exposure and reduced toxicity [15].
Fig. 7.
Peritoneal fluid (a) and plasma (b) paclitaxel concentrations following intraperitoneal administration of submicron particle paclitaxel. Data are averaged over cycles 1 and 2 in subjects with intraperitoneal carcinomas, mostly recurrent ovarian cancer. Mean peritoneal fluid and mean plasma concentrations are presented per dose level. Error bars ± 1 SDEV [15]. Reprinted by permission from Springer Nature: [Springer] [Cancer Chemotherapy and Pharmacology] [A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax®) in patients with peritoneal malignancies, Stephen K. Williamson et al.] [2015]
A Phase 2 study in 10 subjects with primary or recurrent ovarian cancer was subsequently conducted to evaluate a single dose of SPP instilled into the peritoneal cavity at the end of debulking surgery (NCT03029585). Subjects also received standard-of-care (SOC), which consisted of IV paclitaxel and carboplatin administered once every 21 days for up to 6 cycles. In the study, 66% of subjects who received SOC plus a single dose of SPP showed progression-free survival (PFS) over the 12-month study [18].
PK sampling was conducted in seven subjects who received SPP at a dose of 100 mg/m2 and three subjects who received a dose of 200 mg/m2. Blood samples were collected immediately following SPP instillation, prior to each cycle of IV chemotherapy, and at 9- and 12-months post-administration. Plasma paclitaxel concentrations in the 100 mg/m2 dose group were quantifiable (> 25 pg/mL) in three of seven subjects prior to Cycle 6 of IV chemotherapy. Quantifiable plasma paclitaxel concentrations were detected in two of three subjects in the 200 mg/m2 dose group prior to the Cycle 3 of IV chemotherapy and in 1 subject at 12 months following SPP instillation. Given that the reported half-life for IV paclitaxel ranges from 9.9 to 16 h for a 3-h infusion and 13.1 to 24.6 h for a 24-h infusion [19], paclitaxel would have cleared systemic circulation by the sixth day following IV administration and thus, not be quantifiable prior to the next cycle of IV paclitaxel. Since plasma paclitaxel concentrations were quantifiable just prior to the 21-day dosing cycle of IV paclitaxel and at the 9- and 12-month follow-up visits, it is likely that SPP acted like a paclitaxel-depot that released drug for an extended period following IP administration. These studies support further evaluation of SPP administered into the peritoneal cavity to treat IP carcinomas.
Submicron particle paclitaxel intratumoral injection
Prostate cancer
Subjects (n = 16) with adenocarcinoma of the prostate and a Gleason score ≥ 7 were treated via transrectal ultrasound (TRUS)-guided injection of SPP into the index tumor and its lobe that contained the index tumor using multi-parametric magnetic resonance imaging (mpMRI) guidance (NCT03077659). Subjects were allocated to cohorts by a standard 3 + 3 dose rising design to receive 6, 10, or 15 mg/mL SPP in a volume equal to 20% of the lobe containing the index lesion, with additional subjects enrolled to an expansion cohort at the 15 mg/mL dose. A radical prostatectomy was performed 4 weeks after IT SPP treatment. There were no drug-related serious adverse events (SAEs), including no prostatitis, nor dose-limiting toxicities (DLTs), allowing for dose-escalation to the highest concentration (15 mg/mL). Paclitaxel was detected in all prostate tissue sampled from prostatectomy specimens and in lymph nodes of nine subjects > 30 days following intraprostatic (ITP) SPP treatment. Percentage of adenocarcinoma in core biopsies decreased between screening and prostatectomy in six subjects and remained unchanged in five. Among seven subjects that received 15 mg/mL, the mean lesion volume (as measured by mpMRI) decreased, as did the prostate specific antigen (PSA) density. ITP SPP treatment was found to be well tolerated with no safety concerns at the 15 mg/mL concentration and provided preliminary evidence of activity.
A second clinical trial is underway in patients with localized prostate cancer (Gleason ≥ 6) utilizing multiple injections over a 16-week period prior to prostatectomy (NCT04221828) to further evaluate safety, efficacy, and immune response. The lack of systemic toxicity and minimal local irritation observed following ITP SPP treatment in the first prostate cancer study may allow for multiple staged injections in future studies, the timing of which could be triggered by rising PSA levels during routine SOC. Persistent paclitaxel within the prostate may act as an adjuvant “sensitizer” to radiation therapy or focal ablative therapies such as high-intensity focused ultrasound (HIFU).
Pancreatic cancer
Pancreatic cancer demonstrates a limited response to systemic cancer therapy. This may be due in part to the aggressive nature of tumor cells and to the desmoplastic, fibrotic stroma associated with pancreatic cancer, which blocks transport and diffusion of small molecules [20, 21]. The dense stroma also creates damaged or “leaky” blood vessels causing poor drainage of lymphatic and vascular fluids. Excess fluid increases interstitial fluid pressures, thus compressing vessels and decreasing micro-vessel density [20, 22]. The poorly vascularized stroma creates a hypoxic environment, suppressing the immune response and forming a barrier to systemic drug delivery. Surgical resection of the tumor, when possible, is currently the only curative therapy. Unfortunately, surgical resection is often not possible due to anatomical complications when the patient initially presents for medical care.
IT SPP via endoscopic ultrasound-guided fine needle injection (EUS-FNI) has shown promise in a clinical trial of patients with locally advanced pancreatic cancer (LAPC) (NCT03077685, [23]). Subjects with non-resectable LAPC lesions with a diameter of 1.5–6 cm were directly injected with SPP via EUS-FNI in a 3 + 3 dose-escalation design at 6, 10, and 15 mg/mL in volumes up to 20% of the tumor (not exceeding 5 mL). Subjects in the second phase of the study received two SPP injections 4 weeks apart at 15 mg/mL. An additional cohort of subjects is now open to recruitment, where up to four injections will be given via EUS-FNI one month apart. Injections are performed through a 22-gauge needle in a fan-like pattern to ensure drug dispersion throughout the tumor.
In the 29 subjects to date who have received one (n = 7) or 2 (n = 22) injections of SPP at 15 mg/ml, there have been no cases of pancreatitis, no SAEs definitely related to drug, and no clinically significant laboratory abnormalities. There have been no adverse events that were considered to be dose-limiting. Paclitaxel in the plasma was detected at levels below 10 ng/mL during the first 24 h after IT SPP administration, returning to undetectable levels by 4 weeks [23, 24].
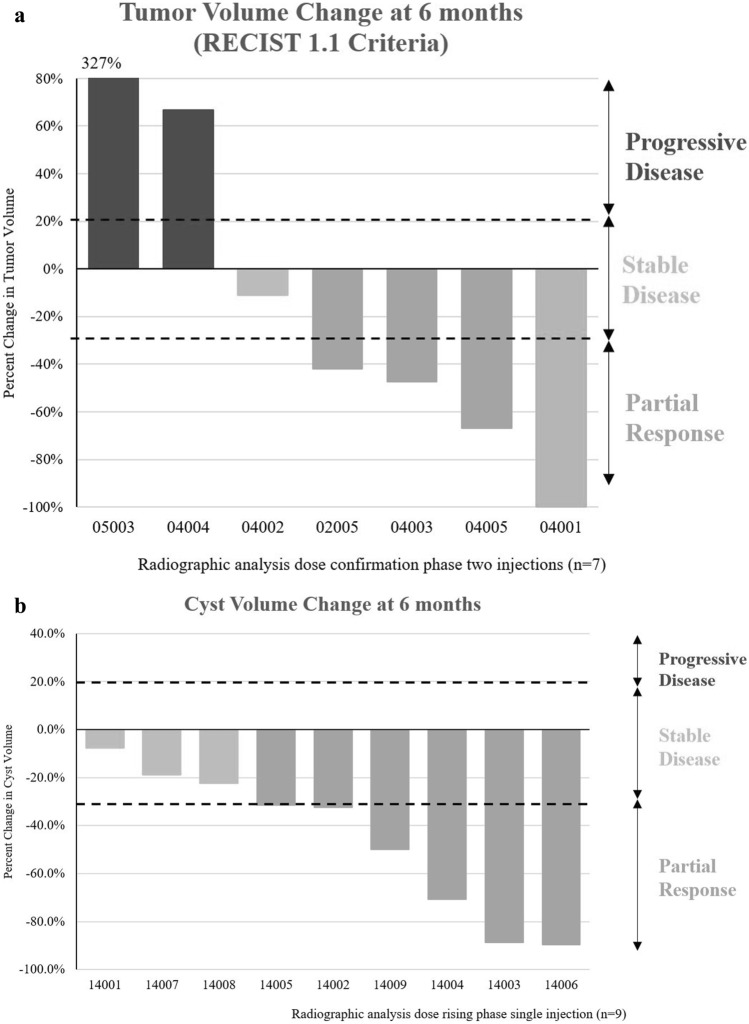
RECIST 1.1 evaluation of the tumor volumes in the two-injection group of subjects demonstrated stable disease in two of eleven subjects at 6 months (Fig. 8a), progressive disease in two subjects, and partial or full responses in seven subjects. Thus far, one subject (04001) in the second phase who received 2 injections was down-staged during study and underwent surgical resection of tumor resulting in R0 and LN0 outcomes [23], and other subjects are under evaluation for reassessment of surgical status.
Fig. 8.
Change in tumor volume of locally advanced pancreatic cancer following two IT injections of submicron particle paclitaxel (a) and mucinous pancreatic cysts in subjects following a single injection of submicron particle paclitaxel via EUS-FNI (b). No subject developed clinically significant local or systemic toxicity from the drug or injection(s) [23, 25]
Pancreatic mucinous cysts
Pancreatic mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs) have significant potential to undergo malignant transformation into pancreatic cancer [26]. These cysts are at high risk for progression and often require precautionary pancreatectomy, a procedure associated with high morbidity and potential for cyst recurrence. Ethanol ablation followed by paclitaxel administration via EUS-FNI is a minimally invasive technique that has shown benefit in the treatment of cystic lesions [27, 28].
A trial evaluating intracystic administration of SPP by EUS-FNI into mucinous pancreatic cysts has completed (NCT03188991). This trial was similar in design and dose to the pancreatic cancer trial, with a dose escalation phase (SPP at 6, 10, and 15 mg/mL at volumes sufficient to fill the cyst, at least equal to the amount of cyst fluid aspirated) followed by a second phase in which subjects received two administrations of SPP at 15 mg/mL administered 12 weeks apart. Patients were followed for 6 months after the first injection. Nineteen patients were enrolled in the study. Nine patients have completed the dose-rising phase of the study and eight have completed the second (two-injection) phase. No clinically significant local toxicity or significant laboratory abnormalities from SPP have been observed. Plasma paclitaxel concentration did not exceed 3.5 ng/mL at any timepoint measured and fell below 1 ng/mL by Week 2, supporting retention of paclitaxel particles within the pancreatic cyst [25]. Currently, cyst volumes in eight of the nine evaluable subjects in the dose escalation cohorts remain reduced at Month 6 (Fig. 8b).
Mechanism of action
Paclitaxel’s primary cytotoxic mechanism acts by inhibiting tubulin depolymerization, stalling the cell cycle in the G2/M phase, interfering with tumor cell replication and resulting in tumor cell death [29] which can produce both apoptotic as well as necroptotic cell death. Necroptosis (necrosis) is a drug-and dose-dependent mechanism of tumor cell destruction achieved by the persistence of relatively high levels of chemotherapy in the TME. In contrast to apoptosis, which often includes collapse and contraction of tumor cells, necroptosis is associated with loss of tumor cell membrane integrity, exposing tumor-specific antigens to immune surveillance. This process can stimulate a robust response of the adaptive immune system against the tumor-specific antigens that otherwise would remain “unseen” by immune effector cells, enhancing immune effector cell infiltration into the TME [30–32]. Paclitaxel was shown to induce necroptotic tumor cell death following exposure to high drug concentrations [33]. The histologic patterns of tumor cell death following local administration of SPP are reminiscent of those associated with necroptotic tumor cell death [7, 30]. Thus, SPP-induced cell death appears to be a continuum between apoptosis and necroptosis depending on drug concentration and duration of tumor cell exposure [31, 34, 35].
Summary and conclusions
Local administration of SPP continuously exposes primary tumors to therapeutic levels of paclitaxel for several weeks. Continuous exposure of solid carcinomas to tumoricidal levels of paclitaxel was shown in preclinical studies and early, limited clinical trials to provide clinical benefits with minimal local or systemic toxicity. Paclitaxel-induced neutropenia and other systemic toxicities are related to the duration and extent of systemic exposure to paclitaxel above a threshold plasma concentration of ≥ 40 ng/mL [16]. In contrast, plasma paclitaxel concentrations observed after injecting SPP into the lobe of the prostate had Cmax values of 19 to 20 ng/mL recorded at the 1-h timepoint. By comparison, a standard IV dose of paclitaxel of 175 mg/m2 administered over 3 h results in a Cmax of 3,650 ng/mL. This is approximately 192 × higher than the mean paclitaxel concentration following SPP injection in a dose of 15 mg/ml in a volume 20% of the prostate lobe 1 h after injection. In addition, measurements of plasma paclitaxel levels following IT SPP in pancreatic cancer trials and IP SPP in peritoneal cancer trials remained well below the 40 ng/mL toxicity threshold. These low levels of plasma paclitaxel following local administration of SPP may explain the absence of clinically significant toxicity seen thus far in various clinical trials (Table 1) involving more than 150 subjects.
SPP administration may complement treatment of metastatic disease with traditional therapies such as chemotherapy, targeted therapy, immunotherapy, and radiation. Paclitaxel affects many aspects of immune function, including lymphocyte recruitment and activation as well as production of immunoenhancing cytokines, including IL-12 [34–37], IFNγ and TNFα, which may augment the antitumor activity of immunotherapies [38]. Paclitaxel was shown to enhance immune responses including increased concentrations of tumor-infiltrating lymphocytes that successfully eradicate malignant cells [39–41]. Paclitaxel may be a particularly strong immunostimulant, as it is able to both activate CD8 + T cells and reduce immunosuppressive cells, such as regulatory T cells [34–36, 42–44] and myeloid-derived suppressor cells (MDSC) [30, 36, 45]. To maximize tumoricidal effects, it has been hypothesized that the immune system can be primed with systemic chemotherapy ahead of immunotherapy to re-instate or enhance immunosurveillance [37, 39]. The priming effect of locally administered SPP may not only provide tumoricidal activity but also induce immune-mediated effects [37, 46–49]. The persistence of paclitaxel in the tumor site may expose slowly replicating tumor cells to tumoricidal drug levels. This chronic state of cell death could affect the immune system by (1) increasing the opportunity for tumor-associated antigens to be released and recognized by the immune system, (2) decreasing the number of cells able to send an immunosuppressive signal, and (3) attracting immune and phagocytic cells to remove tumor cell debris. While a combination of systemic chemotherapy and immunotherapy has the potential to increase efficacy [46], these regimens are also additive to systemic toxicity. Local administration of SPP has the potential to synergize with immunotherapy without added toxic exposure to nontarget organs.
Injection of SPP into a primary tumor appears to facilitate an immune response which may improve clinical benefits reminiscent of those reported in studies where radiation and ablation were used to create an “inflamed” TME that synergizes with immune therapy [47–56]. Preliminary studies of SPD injected into syngeneic Renca tumors also produced reduction in tumor size with associated increases in effector immune cell concentrations [57]. Potential therapeutic opportunities for administration of SPP to treat carcinomas include (1) inhalation of nebulized particles and/or direct injection of tumors obstructing airways to treat pulmonary cancers; 2) IP SPP to treat peritoneal metastasis, ovarian, hepatic, and other gastrointestinal cancers; (3) direct injection via TRUS-FNI to achieve reduction of prostate cancer or delay progression, allowing for delay or prevention of prostatectomy or whole gland therapy; (4) direct injection via EUS-FNI into pancreatic cancer; and (5) intracystic injection of pancreatic mucinous cysts to prevent partial pancreatectomy. The broad antitumor effects and safety observed preclinically and clinically, and the apparent stimulation of the immune system following SPP treatment support additional preclinical and clinical investigations with this drug.
Open access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Acknowledgments
NanoPac® and NanoDoce® are registered trademarks of NanOlogy, LLC. The authors would like to thank CritiTech, Inc. for investigational product supply; NanOlogy, LLC. for funding the preclinical and clinical trials; Louis Lazo Radulovic and Mark Milad for pharmacokinetic consulting; and Samantha Mauro, Karan Dewnani, Kainoa Peterson, David Jones, Alexis Voss, Lauren Hylle, and Sara Dafoe for their assistance in document preparation.
Funding
This research was funded by NanOlogy, LLC.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The following authors report holding a consultant/advisory role, having stock ownership, or receiving funding from NanOlogy, S.V., H.M., M.B., E.W., M.I., A.W., J.V., A.M., S.C., P.D., and G.S.d.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Investigational Review Boards for all participating clinical sites provided approval for protocol and informed consents for subjects in accordance with the Code of Federal Regulations and local requirements.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldberg EP, Hadba AR, Almond BA, Marotta JS. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J Pharm Pharmacol. 2002;54(2):159–180. doi: 10.1211/0022357021778268. [DOI] [PubMed] [Google Scholar]
- 2.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 3.Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol. 2013;4(2):1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao M, Liu M. New avenues for nanoparticle-related therapies. Nanoscale Res Lett. 2018;13(1):136. doi: 10.1186/s11671-018-2548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltezor M, Farthing J, Sittenauer J, Espinosa J, Campbell S, McClorey M, Fischer J, Williams M, Clapp G. Taxane particles and their use. U.S. Patent 9,814,685;2017.
- 6.Verco J, Johnston W, Baltezor M, Kuehl PJ, Gigliotti A, Belinsky SA, Lopez A, Wolff R, Hylle L, diZerega G. Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac((R))) in a rodent model. J Aerosol Med Pulm Drug Deliv. 2019;32(2):99–109. doi: 10.1089/jamp.2018.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verco J, Johnston W, Frost M, Baltezor M, Kuehl PJ, Lopez A, Gigliotti A, Belinsky SA, Wolff R, diZerega G. Inhaled submicron particle paclitaxel (NanoPac) induces tumor regression and immune cell infiltration in an orthotopic athymic nude rat model of non-small cell lung cancer. J Aerosol Med Pulm Drug Deliv. 2019 doi: 10.1089/jamp.2018.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maulhardt HA, Hylle L, Frost MV, Tornio A, Dafoe S, Drummond L, Quinn DI, Kamat AM, diZerega GS. Local injection of submicron particle docetaxel is associated with tumor eradication, reduced systemic toxicity and an immunologic response in uro-oncologic xenografts. Cancers. 2019;11(4):577. doi: 10.3390/cancers11040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, Vogl T, Goldberg EP, Huang H, Simoff M, Li Q, Browning R, Turner FJ, Le Pivert P, Spyratos D, Zarogoulidis K, Celikoglu SI, Celikoglu F, Brachmann J. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther. 2013;7:571–583. doi: 10.2147/DDDT.S46393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Rosiere R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T, Vermeersch M, Van Antwerpen P, Amighi K, Wauthoz N. New Folate-Grafted Chitosan Derivative To Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol Pharm. 2018;15(3):899–910. doi: 10.1021/acs.molpharmaceut.7b00846. [DOI] [PubMed] [Google Scholar]
- 12.Miura D, Yoneyama K, Furuhata Y, Shimizu K. Paclitaxel enhances antibody-dependent cell-mediated cytotoxicity of trastuzumab by rapid recruitment of natural killer cells in HER2-positive breast cancer. J Nippon Med Sch. 2014;81(4):211–220. doi: 10.1272/jnms.81.211. [DOI] [PubMed] [Google Scholar]
- 13.Baltezor M, diZerega G, Decedue C, Campbell S, McClorey M, Maulhardt H. Treatment of kidney tumors by intratumoral injection of taxane particles. US 2019/0365699;2019.
- 14.Baltezor M, diZerega G, Decedue C, Campbell S, McClorey M. Methods for solid tumor treatment. U.S. Patent 10,391,090;2019.
- 15.Williamson SK, Johnson GA, Maulhardt HA, Moore KM, McMeekin DS, Schulz TK, Reed GA, Roby KF, Mackay CB, Smith HJ, Weir SJ, Wick JA, Markman M, diZerega GS, Baltezor MJ, Espinosa J, Decedue CJ. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax(R)) in patients with peritoneal malignancies. Cancer Chemother Pharmacol. 2015;75(5):1075–1087. doi: 10.1007/s00280-015-2737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowinsky EK, Jiroutek M, Bonomi P, Johnson D, Baker SD. Paclitaxel steady-state plasma concentration as a determinant of disease outcome and toxicity in lung cancer patients treated with paclitaxel and cisplatin. Clin Cancer Res. 1999;5(4):767–774. [PubMed] [Google Scholar]
- 17.Cristea MC, Frankel P, Synold T, Rivkin S, Lim D, Chung V, Chao J, Wakabayashi M, Paz B, Han E, Lin P, Leong L, Hakim A, Carroll M, Prakash N, Dellinger T, Park M, Morgan RJ. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother Pharmacol. 2019;83:589–598. doi: 10.1007/s00280-019-03767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullany S, Miller D, Robison K, Levinson K, Lee Y, Yamada SD, Walker J, Markman M, Marin A, Mast P, diZerega G. Phase II study of intraperitoneal submicron particle paclitaxel (SPP) plus IV carboplatin and paclitaxel in patients with epithelial ovarian cancersurgery. Gynecologic Oncology Reports. 2020;34:100627. doi: 10.1016/j.gore.2020.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsu T, Sasaki Y, Tamura T, Miyata Y, Nakanomyo H, Nishiwaki Y, Saijo N. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion. Clin Cancer Res. 1995;1(6):599–606. [PubMed] [Google Scholar]
- 20.Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40(1):118–128. doi: 10.1016/j.ctrv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Smyth EN, Bapat B, Ball DE, Andre T, Kaye JA. Metastatic pancreatic adenocarcinoma treatment patterns, health care resource use, and outcomes in france and the United Kingdom between 2009 and 2012: a retrospective study. Clin Ther. 2015;37(6):1301–1316. doi: 10.1016/j.clinthera.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma N, Othman M, Mendoza-Ladd A, Verco S, Verco J, diZerega G, Lo S. EUS-guided injection of intratumoral submicron particle paclitaxel (SPP) for the treatment of locally advanced pancreatic adenocarcinoma (LAPC): Phase 2 study. American Society for Gastrointestinal Endoscopy: Sa2006;2020.
- 24.Lo S, Hendifar A, Sharma N, Othman M, Mendoza-Ladd A, Verco S, Verco J, diZerega G. A novel EUS-guided intratumoral delivery of submicron particle paclitaxel (SPP) for the treatment of locally advanced pancreatic cancer (LA-PAC): a prospective safety, tolerability and preliminary efficiency study: 6. Am J Gastroenterol. 2019;114:S3–S4. doi: 10.14309/01.ajg.0000589556.41054.b3. [DOI] [Google Scholar]
- 25.Othman M, Patel K, Krishna S, Mendoza-Ladd A, Verco S, Verco J, Wendt A, diZerega G. Intracystic Injection of Submicron Particle Paclitaxel (SPP) for the Treatment of Mucinous Pancreatic Cystic Lesions Resulted in Reduction in Cyst Volume, an Interim Report. American Society for Gastrointestinal Endoscopy: Sa1399;2020.
- 26.Tada M, Kawabe T, Arizumi M, Togawa O, Matsubara S, Yamamoto N, Nakai Y, Sasahira N, Hirano K, Tsujino T, Tateishi K, Isayama H, Toda N, Yoshida H, Omata M. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4(10):1265–1270. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Oh HC, Seo DW, Song TJ, Moon SH, Park DH, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140(1):172–179. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.DeWitt JM, Murthy SK, Ardhanari R, DuVall GA, Wallner G, Litka P, Daugherty C, Fowers K. EUS-guided paclitaxel injection as an adjunctive therapy to systemic chemotherapy and concurrent external beam radiation before surgery for localized or locoregional esophageal cancer: a multicenter prospective randomized trial. Gastrointest Endosc. 2017;86(1):140–149. doi: 10.1016/j.gie.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi: 10.1091/mbc.E14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Yu J. Zhang L (2016) Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta. 1865;2:228–236. doi: 10.1016/j.bbcan.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(7240):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang MS, Lee SJ, Kang NS, Kim E. Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to taxol triggers both apoptotic and necrotic cell death. Cancer Res. 2011;71(23):7207–7215. doi: 10.1158/0008-5472.CAN-11-0760. [DOI] [PubMed] [Google Scholar]
- 34.Soliman HH. nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther. 2017;10:101–112. doi: 10.2147/OTT.S122974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38(4):283–290. doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Zeltsman M, Zauderer MG, Eguchi T, Vaghjiani RG, Adusumilli PS. Chemotherapy-induced immunomodulation in non-small-cell lung cancer: a rationale for combination chemoimmunotherapy. Immunotherapy. 2017;9(11):913–927. doi: 10.2217/imt-2017-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, Bennewith KL. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193(2):116–130. doi: 10.1164/rccm.201508-1545CI. [DOI] [PubMed] [Google Scholar]
- 38.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49(4–5):181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7(10):3025–3030. [PubMed] [Google Scholar]
- 40.Moschetta M, Pretto F, Berndt A, Galler K, Richter P, Bassi A, Oliva P, Micotti E, Valbusa G, Schwager K, Kaspar M, Trachsel E, Kosmehl H, Bani MR, Neri D, Giavazzi R. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–1824. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 41.Pasche N, Wulhfard S, Pretto F, Carugati E, Neri D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18(15):4092–4103. doi: 10.1158/1078-0432.CCR-12-0282. [DOI] [PubMed] [Google Scholar]
- 42.Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC, Weeratna RD, Krieg AM. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58(4):615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62(2):203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11(3):215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 46.Champiat S, Tselikas L, Farhane S, Raoult T, Texier M, Lanoy E, Massard C, Robert C, Ammari S, De Baere T, Marabelle A. Intratumoral immunotherapy: from trial design to clinical practice. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-20-0473. [DOI] [PubMed] [Google Scholar]
- 47.Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, Allison JP. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72(2):430–439. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, Mansfield AS, Furutani KM, Olivier KR, Kwon ED. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3(6):610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, Younes AI, Cortez MA, Erasmus JJ, de Groot P, Carter BW, Hong DS, Glitza IC, Ferrarotto R, Altan M, Diab A, Chun SG, Heymach JV, Tang C, Nguyen QN, Welsh JW. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer. 2019;7(1):237. doi: 10.1186/s40425-019-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin AJ, Roach M, Bradley J, Robinson C. Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res. 2019;8(1):107–115. doi: 10.21037/tlcr.2018.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walshaw RC, Honeychurch J, Illidge TM. Stereotactic ablative radiotherapy and immunotherapy combinations: turning the future into systemic therapy? Br J Radiol. 2016;89(1066):20160472. doi: 10.1259/bjr.20160472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, Zafra-Martin J, Kannemann A, Blanco J, Lloret M, Lara PC. Stereotactic ablative radiotherapy combined with immune checkpoint inhibitors reboots the immune response assisted by immunotherapy in metastatic lung cancer: a systematic review. Int J Mol Sci. 2019;20(9):2173. doi: 10.3390/ijms20092173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britschgi C, Riesterer O, Burger IA, Guckenberger M, Curioni-Fontecedro A. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non-small cell lung cancer. Radiat Oncol. 2018;13(1):102. doi: 10.1186/s13014-018-1049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maulhardt HA, Marin AM, diZerega GS. Intratumoral submicron particle docetaxel inhibits syngeneic Renca renal cancer growth and increases CD4+, CD8+, and Treg levels in peripheral blood. Invest New Drugs. 2020 doi: 10.1007/s10637-020-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.