Abstract
There is a growing need to develop novel strategies for the diagnosis of schizophrenia using neuroimaging biomarkers. We investigated the robustness of the diagnostic model for schizophrenia using radiomic features from T1-weighted and diffusion tensor images of the corpus callosum (CC). A total of 165 participants [86 schizophrenia and 79 healthy controls (HCs)] were allocated to training (N = 115) and test (N = 50) sets. Radiomic features of the CC subregions were extracted from T1-weighted, apparent diffusion coefficient (ADC), and fractional anisotropy (FA) images (N = 1605). Following feature selection, various combinations of classifiers were trained, and Bayesian optimization was adopted in the best performing classifier. Discrimination, calibration, and clinical utility of the model were assessed. An online calculator was constructed to offer the probability of having schizophrenia. SHapley Additive exPlanations (SHAP) was applied to explore the interpretability of the model. We identified 30 radiomic features to differentiate participants with schizophrenia from HCs. The Bayesian optimized model achieved the highest performance, with an area under the curve (AUC), accuracy, sensitivity, and specificity of 0.89 (95% confidence interval: 0.81–0.98), 80.0, 83.3, and 76.9%, respectively, in the test set. The final model offers clinical probability in an online calculator. The model explanation by SHAP suggested that second-order features from the posterior CC were highly associated with the risk of schizophrenia. The multiparametric radiomics model focusing on the CC shows its robustness for the diagnosis of schizophrenia. Radiomic features could be a potential source of biomarkers that support the biomarker-based diagnosis of schizophrenia and improve the understanding of its neurobiology.
Subject terms: Diagnostic markers, Schizophrenia
Introduction
Schizophrenia is a highly disabling psychiatric disorder with an unclear etiology and pathogenesis. A large body of evidence indicates that a constellation of cognitive, perceptual, and affective disturbances in schizophrenia originates from aberrations in neural connectivity among distinct brain regions [1]. However, despite the current notion of schizophrenia as a disorder of brain dysconnectivity, its diagnosis is exclusively based on a clinical description of the observable features of psychopathology. Therefore, it is crucial to develop novel strategies for the precise diagnosis of schizophrenia using specific and reliable biomarkers of brain connectivity.
The corpus callosum (CC) is the largest white matter structure in the brain, responsible for transferring and integrating a vast amount of multimodal information across the two hemispheres [2]. Neuroimaging abnormalities in the CC have been consistently found in patients with schizophrenia, suggesting the involvement of aberrant interhemispheric communication via the CC in the pathogenesis of schizophrenia. Structural magnetic resonance imaging (MRI) studies have reported morphological alterations in callosal shape [3, 4] and smaller size of the CC and its subregions [5, 6] in patients with schizophrenia compared to that of healthy controls (HCs). Diffusion tensor imaging (DTI), which allows for the in vivo assessment of microstructural features of the white matter, revealed myelin and axonal alterations of the CC in patients with schizophrenia, which cannot be captured by standard MRI [7]. The latest large-scale collaborative study on white matter abnormalities in schizophrenia showed that a lower fractional anisotropy (FA) and higher apparent diffusion coefficient (ADC) in the CC are among the most reliable findings with a large effect size that differentiated patients with schizophrenia from HCs [8]. However, none of the neuroimaging measures of the CC are diagnostic of schizophrenia because similar alterations are found in various psychiatric disorders, including autism [9, 10] and bipolar disorder [11]. Furthermore, single-value parameters derived from neuroimaging data, which measure the volume, area, or fiber integrity, do not fully capture the subtle and complex neuropathological changes in the CC, underlying the development of schizophrenia. The pathogenesis of schizophrenia involves microscopic alterations in tissue characteristics of the brain, resulting from multiple genetic, molecular, and cytoarchitectural factors. Therefore, an approach that quantifies the spatial distribution of microscopic tissue heterogeneity, which cannot be assessed with conventional imaging parameters, would be a promising alternative to improve the diagnosis of schizophrenia on the basis of neurobiology.
Radiomics is a new, advanced analytic technique that quantifies and extracts high-dimensional imaging features to aid clinical decision-making using medical image-based biomarkers [12]. Radiomics aims to discover meaningful “hidden” information within radiological images, which is inaccessible with single-value approaches. Radiomics relies on computational techniques to translate radiologic images into quantitative image descriptors pertaining to the shape and texture information of a region of interest (ROI) [13]. Radiomic features include shape, first-order features, and second-order features. Shape descriptors are calculated based on the two- and three-dimensional size and shape of the ROI. On the other hand, first-order and second-order features are calculated based on signal intensity values within the ROI by mathematical equations. First-order features reflect the intensity distribution of an ROI, while second-order features reflect spatial heterogeneity [14]. Radiomics has a wide potential as a diagnostic and prognostic biomarker in brain tumors [15] as well as neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [16, 17]. Recent studies on schizophrenia have also shown promising results in differentiating patients with schizophrenia from HCs using a radiomics model with structural MRI of the hippocampal subfields [18] and resting-state functional connectivity [19] and in predicting short-term treatment responses after electroconvulsive therapy in patients with treatment-resistant schizophrenia, using radiomic features extracted from structural and diffusion MRI [20]. The clinical utility of these radiomics models built through machine learning may be limited due to the lack of interpretability of the “black-box” system. However, this problem could be addressed using explanatory techniques, such as SHapley Additive exPlanations (SHAP) that estimate a value of importance for each radiomic feature in the built model and facilitate informed clinical decision-making [21].
To the best of our knowledge, there is no study to date that tests a potential diagnostic model of schizophrenia using radiomics based on multiparametric DTI and T1-weighted images (T1) focusing on the interhemispheric connectivity of the brain. Herein, we aimed to investigate the ability of a multiparametric radiomics model to diagnose schizophrenia using MRI of the CC. We hypothesized that radiomic analysis of the CC would reveal distinct combinations of neuroimaging features reflecting subtle and complex alterations in tissue characteristics of the CC, which would differentiate patients with schizophrenia from HCs. Furthermore, the interpretability of the diagnostic prediction made by the multiparametric radiomics model was explored by the application of SHAP.
Materials and methods
Participants
The participants with schizophrenia were recruited from among those receiving psychiatric treatment at the Department of Psychiatry, CHA Bundang Medical Center (Seongnam, Republic of Korea). They were diagnosed with schizophrenia based on the DSM-IV-TR or DSM-5 criteria [22, 23]. We only included patients who had no history of other psychiatric disorders and were antipsychotic-naïve or free of antipsychotics for at least 6 months. HCs were recruited from the local community through online and print advertisements, and we ensured that they had no personal or first-relative family history of psychiatric disorders. The exclusion criteria were (1) a current or past history of neurological or neurodevelopmental disorders, substance-related problems, or head trauma with loss of consciousness, (2) left-handedness, and (3) any other contraindications for undergoing MRI. A total of 165 participants (86 with schizophrenia and 79 HCs) were finally enrolled in this study. The study participants of both groups were allocated to training (N = 115; 60 with schizophrenia and 55 HCs) and test (N = 50; 26 with schizophrenia and 24 HCs) sets (Table 1).
Table 1.
Demographic and clinical profiles of the study participants in the training and test sets.
| Training set (N = 115) | Test set (N = 50) | |||
|---|---|---|---|---|
| Schizophrenia (N = 60) | HCs (N = 55) | Schizophrenia (N = 26) | HCs (N = 24) | |
| Age (y) | 34.3 (9.5) | 37.1 (8.3) | 37.0 (11.2) | 40.6 (12.4) |
| Sex | ||||
| Male | 22 (36.7%) | 25 (45.5%) | 9 (34.6%) | 7 (29.2%) |
| Female | 38 (63.3%) | 30 (54.5%) | 17 (65.4%) | 17 (70.8%) |
| Number of episodes | ||||
| First episode | 49 (81.7%) | 17 (65.4%) | ||
| Second episode | 6 (10.0%) | 4 (15.4%) | ||
| Third episodes | 5 (8.3%) | 5 (19.2%) | ||
| Duration of illness (mo) | 15.6 (23.7) | 25.0 (33.7) | ||
| Antipsychotic exposure | ||||
| Naïve | 53 (88.3%) | 23 (88.5%) | ||
| >6 months free | 7 (11.7%) | 3 (11.5%) | ||
| Duration of antipsychotics before MRI scan (d) | 6.2 (6.0) | 4.7 (4.9) | ||
| Dose of antipsychotics at MRI scan (mg/d)a | 504.3 (301.8) | 429.5 (220.7) | ||
| PANSS | ||||
| Positive symptoms | 29.5 (7.7) | 30.4 (5.6) | ||
| Negative symptoms | 20.9 (7.0) | 20.4 (6.3) | ||
| General psychopathology | 59.6 (14.2) | 57.8 (13.5) | ||
There were no significant differences between groups and between sets on any variable.
HCs healthy controls, MRI magnetic resonance imaging, PANSS positive and negative syndrome scale.
aAll participants with schizophrenia were taking atypical antipsychotics and chlorpromazine equivalent doses were calculated.
The study protocol was reviewed and approved by the Institutional Review Board of CHA Bundang Medical Center, in accordance with the latest version of the Declaration of Helsinki and principles of Good Clinical Practice. Written informed consent was obtained from all study participants after the study procedures had been fully explained.
MRI data acquisition and preprocessing
T1 and diffusion-weighted images were acquired using a 3.0-Tesla scanner (GE Signa HDxt; GE Healthcare, Milwaukee, WI, USA) equipped with an eight-channel phased-array head coil, at CHA Bundang Medical Center (Seongnam, Republic of Korea). Detailed information on the MRI protocol is presented in Supplementary Material S1.
The anatomical segmentation of the CC was performed on T1 using the standard “recon-all” pipeline implemented in the FreeSurfer software (version 7.1.0; http://freesurfer.net). The CC was automatically segmented into five subregions: anterior, mid-anterior, central, mid-posterior, and posterior. Each of the subregional masks was extracted from individual T1. The preprocessing of DTI data was performed using the Functional MRI of the Brain’s (FMRIB’s) Diffusion Toolbox, which is a part of FMRIB’s Software Library (version 6.0; https://fsl.fmrib.ox.ac.uk/fsl/). The DT images underwent standard preprocessing steps, including brain extraction and correction for head motion and eddy-current distortion. ADC and FA maps were created by fitting a tensor model to the corrected diffusion data. After image resampling to 1-mm isovoxels, the nonuniformity of low-frequency intensity was corrected with the N4 bias field correction algorithm. T1 was co-registered to ADC and FA maps by the affine transformation with normalized mutual information as a cost function.
Radiomic feature extraction
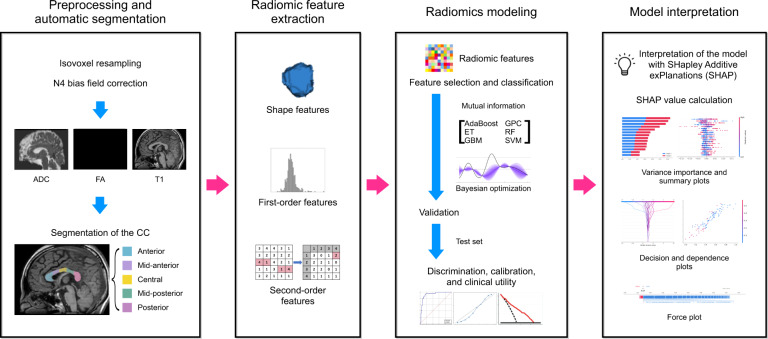
Figure 1 shows the radiomics pipeline of the study. Radiomic feature extraction from the CC subregions was performed using py-Radiomics (version 2.0; http://www.radiomics.io/pyradiomics.html) [24], which conformed to the Image Biomarker Standardization Initiative [25]. The radiomic features included 14 shape features, 18 first-order features, and 75 second-order features [such as gray-level co-occurrence matrix (GLCM; N = 24), gray-level run-length matrix (GLRLM; N = 16), gray-level size zone matrix (N = 16), gray-level dependence matrix (N = 14), and neighboring gray tone difference matrix (N = 5); Supplementary Material S3]. A total of 1605 (107 features × 5 subregions of the CC × 3 sequences) radiomic features were extracted (Supplementary Material S2).
Fig. 1. Workflow of image preprocessing, radiomics feature extraction, and machine learning.
ADC apparent diffusion coefficient, FA fractional anisotropy, T1 T1-weighted image, CC corpus callosum, ET extra trees, GBM gradient boosting machine, GPC Gaussian process classification, RF random forest, SVM support vector machine.
Construction of the radiomics model for the assessment of discrimination, calibration, and clinical utility
The feature selection and machine learning processes were performed using Python 3 with the Scikit-Learn library module (version 0.21.2). All radiomic features were Min-Max normalized. Subsequently, feature selection was performed to reduce the number of highly correlated features and avoid overfitting by applying mutual information, which measures the mutual dependence between variables. The base radiomic classifiers were constructed using classifier models, such as AdaBoost, Extra Trees, gradient boosting machine, Gaussian process classification, random forest, and support vector machine, with tenfold cross-validation. To overcome data imbalance, each machine learning model was either trained without oversampling or with random oversampling examples (ROSE) technique (Supplementary Material S3). Thus, a total of 12 combinations with different oversampling techniques were trained and validated.
The discrimination performance was evaluated in the training set and validated in the test set. The predictive performance was quantified by calculating the AUC, accuracy, sensitivity, and specificity. To improve the predictive performance and avoid potential overfitting, Bayesian optimization was applied in the best performing base classifier, which searches the hyperparameter space for optimal combinations of the hyperparameters (Supplementary Material S4).
Discrimination and calibration are two different aspects of the performance of diagnostic models. Because good discrimination does not always ensure good calibration, and vice versa, calibration performance was also assessed based on the agreement between predicted and actual rates of schizophrenia in the calibration plot [26]. Calibration plots were created to determine whether the predicted probability correlated with the actual probabilities. The Brier score, which is the mean squared prediction error, was calculated to evaluate the overall performance of the radiomics model [27]. A Brier score close to 0 indicates a better calibration and discrimination performance of the model.
Decision curve analysis (DCA) was performed by calculating clinical “net benefits” at a range of threshold probabilities to validate the clinical utility of the radiomics model (Supplementary Material S5) [28]. Net benefit differs from accuracy metrics, such as discrimination and calibration, in that it incorporates the consequences of decisions made by a model or test [29].
Model interpretability with SHAP
To interpret and understand the radiomic features from the radiomics model, SHAP was applied, which is a game-theoretic approach to explain the output of a tree-based machine learning model [21]. SHAP measures the contribution of each feature of a model to the increase or decrease of the probability of single output (i.e., the probability of the diagnosis of schizophrenia in this study; Supplementary Material S6).
Statistical analysis
Demographic and clinical characteristics between participants with schizophrenia and HCs and between the training and test sets were compared using the independent t-test for continuous variables and the chi-squared test for categorical variables. The diagnostic performance of the radiomics model was compared with a logistic regression model with mean ADC and FA values from the CC subregions using AUC. Statistical procedures were conducted using the Statistical Package for the Social Sciences, version 26 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05.
Results
Discrimination, calibration, and clinical utility of the radiomics model
A total of 30 radiomic features (ten from anterior, three from mid-anterior, three from central, seven from mid-posterior, and seven from posterior subregions of the CC; 16 from ADC, five from FA, and nine from T1) were identified to differentiate participants with schizophrenia from HCs in the best performing classifier (Table 2). Supplementary Fig. 1 shows the heatmap of significant radiomic features.
Table 2.
List of significant radiomic features to differentiate participants with schizophrenia from HCs.
| Subregion | Image | Feature category | Feature name |
|---|---|---|---|
| Anterior | ADC | First-order (7) | 10 percentile |
| 90 percentile | |||
| Maximum | |||
| Mean | |||
| Median | |||
| Root mean squared | |||
| Variance | |||
| FA | GLDM (1) | Large dependence emphasis | |
| T1 | GLRLM (1) | Gray-level nonuniformity | |
| GLSZM (1) | Size zone nonuniformity | ||
| Mid-anterior | ADC | First-order (3) | 90 percentile |
| Minimum | |||
| Interquartile range | |||
| Central | ADC | First-order (1) | 90 percentile |
| FA | NGTDM (1) | Complexity | |
| GLSZM (1) | Zone variance | ||
| Mid-posterior | ADC | First-order (1) | Minimum |
| FA | First-order (2) | Mean | |
| Root mean squared | |||
| T1 | First-order (3) | Mean absolute deviation | |
| Minimum | |||
| Robust mean absolute deviation | |||
| GLRLM (1) | Gray-level nonuniformity normalized | ||
| Posterior | ADC | First-order (2) | Range |
| Variance | |||
| GLCM (1) | Correlation | ||
| Information measure of correlation 1 | |||
| T1 | First-order (2) | Mean absolute deviation | |
| Skewness | |||
| GLRLM (1) | Run entropy |
HCs healthy controls, ADC apparent diffusion coefficient, FA fractional anisotropy, GLDM gray-level dependence matrix, GLRLM gray-level run-length matrix, GLSZM gray-level size zone matrix, NGTDM neighboring gray tone difference matrix, GLCM gray-level co-occurrence matrix.
The AUCs of the base classifiers ranged from 0.72 to 0.89 in the training set and 0.65 to 0.87 in the test set (Supplementary Fig. 2). The best performing base classifier in the training and test sets was achieved using the Extra Trees classifier model with ROSE technique; the AUC, accuracy, sensitivity, and specificity of the best performing base classifier in the test set were 0.87 [95% confidence interval (CI): 0.77–0.97], 78.0, 83.3, and 73.1%, respectively. After the Bayesian hyperparameter tuning, the optimized model with the best performing classifier (Extra Trees model with ROSE technique) achieved better predictive performance. In the Bayesian optimized model of the training set, the AUC, accuracy, sensitivity, and specificity were 0.90 (95% CI: 0.85–0.95), 81.7, 85.0, and 78.3%, respectively; in the test set, the AUC, accuracy, sensitivity, and specificity were 0.89 (95% CI: 0.81–0.98), 80.0, 83.3, and 76.9%, respectively (Fig. 2A). Supplementary Table 1 shows the performance of base classifiers and the optimized model in the test set.
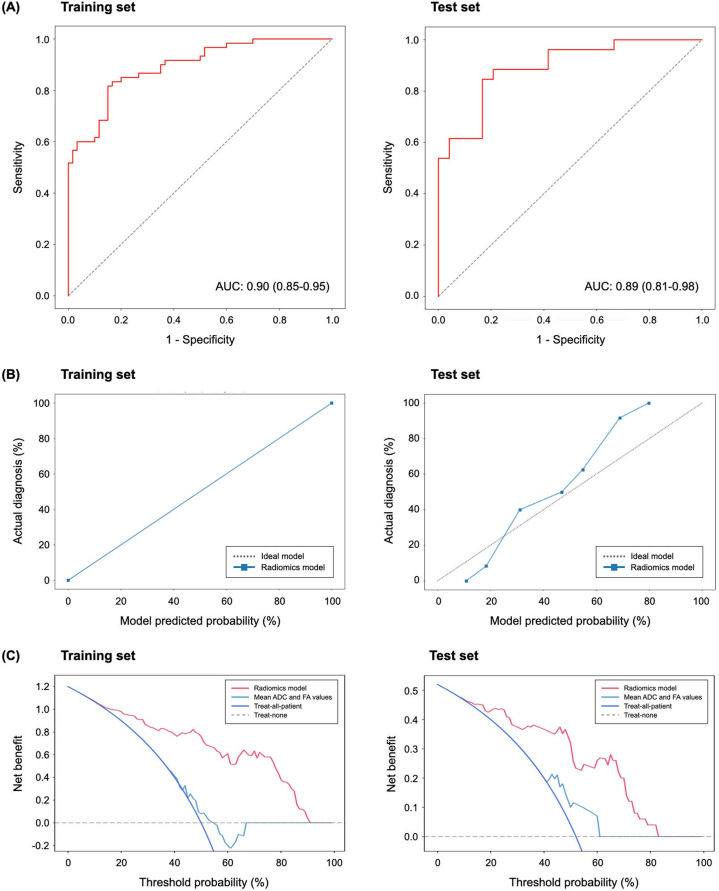
Fig. 2. Discrimination, calibration, and clinical utility of the radiomics model for the diagnosis of schizophrenia in the training and test sets.
A Receiver operating characteristics curves in the training and test sets. B Calibration plots of the radiomics model in the training and test sets. The x-axis represents the model predicted risk of schizophrenia. The y-axis represents the actual diagnosed proportion of schizophrenia. The gray line represents a perfect prediction by an ideal model, and the blue line represents the performance of the radiomics model. The closer fit between the diagonal dotted lines and solid lines shows a good prediction of schizophrenia. C Decision curves for the radiomics model in the training and test sets. The x-axis represents the threshold probability, which refers to a minimum probability of having a disease that patients require to justify treatment. The y-axis represents the net benefit, determined based on the difference between the expected benefit and harm associated with the diagnosis and treatment decision. The radiomics model shows superior net benefit in both the training and test sets within most of the range of threshold probability compared to either default strategies (treat-all or -none) or the model with mean ADC and FAs. AUC area under the curve, ADC apparent diffusion coefficient, FA fractional anisotropy.
The calibration plot showed that the predicted outcomes closely approximated the observed outcomes, indicating a good agreement between the predicted and actual rates of schizophrenia in the training and test sets (Fig. 2B). The Brier score was 0 and 0.16 in the training and test sets, respectively. The DCA result showed that the radiomics model added more net benefit compared to the default strategies of treating all or no subjects or the model with mean ADC and FA values when the threshold probability was within the range of 0–0.91 in the training set and 0–0.83 in the test set (Fig. 2C). Since the radiomics model had a higher net benefit than the strategy using mean ADC and FA values of the CC subregions within a reasonable range of threshold probability, it was considered clinically useful.
An online site has been made available to provide a free calculator based on the presented radiomics model (https://nhimc.shinyapps.io/sirano-schizoml/), which enables clinicians to access and estimate the probability of having schizophrenia. Representative results of the prediction of five participants (three patients with schizophrenia and two HCs) from the radiomics calculator are shown in Supplementary Fig. 3.
Comparison of performance between the radiomics model and model with mean ADC and FA values
In the training set, the model with mean ADC and FA values from CC subregions showed an AUC, accuracy, sensitivity, and specificity of 0.63 (95% CI: 0.55–0.70), 60, 53.3, and 66.7%, respectively. In the test set, the model showed an AUC, accuracy, sensitivity, and specificity of 0.66 (95% CI: 0.50–0.82), 70.0, 54.2, and 84.6%, respectively. The radiomics model showed a significantly higher performance than the model with mean ADC and FA values from CC subregions, in both the training and test sets (p < 0.001 and p = 0.010, respectively).
Model interpretability with SHAP
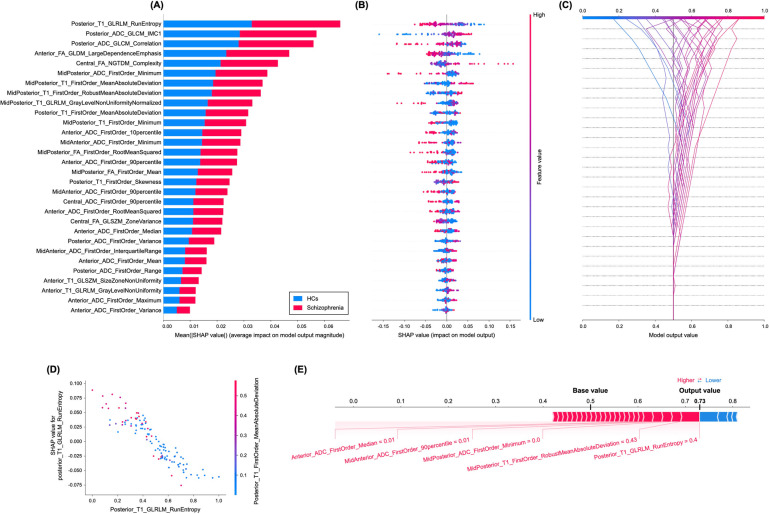
The SHAP values for each radiomic feature in the radiomics model were calculated. The variance importance plot, summary plot, decision plot, dependence plot, and force plot are shown in Fig. 3. For each prediction, a positive SHAP value indicates an increase in the risk of schizophrenia and vice versa. As observed in the plot, posterior_T1_GLRLM_run entropy, followed by posterior_ADC_GLCM_informational measure of correlation 1 (IMC1) and posterior_ADC_GLCM_correlation features, were the three most important risk factors.
Fig. 3. Model interpretability of the radiomics model for the diagnosis of schizophrenia with SHAP.
A Variance importance plot listing the most significant variables in descending order. The top variables (such as posterior_T1_GLRLM_run entropy, posterior_ADC_GLCM_IMC1, and posterior_ADC_GLCM_correlation features) contribute more to the model than the bottom ones and thus have higher predictive power. B Summary plot of feature impact on the decision of the radiomics model and interaction between radiomics features in the model. A positive SHAP value indicates an increase in the risk of predicting schizophrenia and vice versa. The high value corresponds to a higher risk of schizophrenia. Each point corresponds to a prediction in each participant. C Decision plot showing how the radiomics model predicts schizophrenia. Moving from the bottom of the plot to the top, SHAP values for each feature are added to the model’s base value showing how each feature contributes to the overall prediction of schizophrenia. D An example of the dependence plot of the posterior_T1_GLRLM_run entropy feature, which is the top-most important feature. The plot shows how there is an approximately linear and negative trend between “posterior_T1_GLRLM_RunEntropy” and schizophrenia prediction, and that the “posterior_T1_GLRLM_RunEntropy” interacts with “posterior_T1_FirstOrder_MeanAbsoluteDeviation” frequently. E Force plot of a representative case of participants with schizophrenia from the test set, showing local interpretability. Note that the posterior_T1_GLRLM_RunEntropy largely pushes the model prediction score into the higher direction from the base value.
Discussion
In the present study, we assessed the diagnostic value of radiomic features extracted from multiparametric MRI of the CC to differentiate participants with schizophrenia from HCs. The radiomics model with Bayesian optimization, constructed with 30 significant features from the CC subregions, showed a robust performance with an AUC of 0.89 in the test set. Moreover, the model showed good calibration and had a greater net benefit for clinical decision-making in the diagnosis of schizophrenia. The finalized model was then implemented in an online interface. The robustness of the radiomics model was further supported by its significantly higher performance compared to the model with mean ADC and FA values from the CC subregions. The model explanation by SHAP suggested that radiomic features reflecting aberrations in tissue heterogeneity in the posterior CC were particularly associated with a higher risk of schizophrenia.
The neuropathology of schizophrenia involves alterations in the cytoarchitectural and synaptic organizations of the brain, including a loss and disarray of neurons. Previous postmortem studies found impaired myelinations of the CC and reduced numbers of lamina III pyramidal cells from which most CC axons originate in patients with schizophrenia [30, 31]. In vivo MRI data may contain biological information reflecting histological features of the CC in patients with schizophrenia, resulting in changes in signal intensity on DTI and T1. While T1 relaxation time is a direct reflection of tissue composition and cytoarchitectural organization [32], DTI represents white matter microstructure, including axonal coherence, fiber density, and myelin integrity [33]. The radiomics approach enables the comprehensive characterization of microscopic tissue heterogeneity and extraction of meaningful biomarkers from both modalities, providing different aspects of biological information. We found that selected radiomic features were distributed throughout the CC subregions, with distinct tissue characteristics in each of them. The majority of selected radiomic features were first-order features, followed by second-order features; none of the shape features, such as volume, area, and length, were included. This indicates that changes in interrelationships among voxel-based signal intensity as well as their distributions within the CC subregions, which are reflected in the first- and second-order features, could aid in differentiating participants with schizophrenia from HCs. Our findings of radiomic feature selection demonstrate the high potential of radiomics in capturing specific information regarding the neuropathological changes underlying the pathogenesis of schizophrenia, which cannot be quantified by a single parameter based on morphometry.
Radiomic analysis is based on modeling, and it offers an improved discriminatory power by utilizing supervised machine learning with binary classification. To establish the generalizability of the radiomics model, it is crucial to test its performance in a separate validation set [34]. Our radiomics model built with 30 selected radiomic features demonstrated its robustness by showing a significantly better performance compared to the model with mean ADC and FA values from the CC subregions, in the test set. Furthermore, the clinical usefulness of the radiomics model for the diagnosis of schizophrenia was demonstrated by the calibration plot and DCA. The calibration plot of the model showed a good agreement between the predicted and actual diagnoses of schizophrenia in the test set. The DCA result showed that the clinical application of the radiomics model for the diagnosis of schizophrenia is more beneficial than default strategies or the model using mean ADC and FA values within a reasonable range of threshold probability. Our result implies that the radiomics model has a substantial potential to support evidence-based decision-making in clinical practice by using objective and reliable neuroimaging biomarkers in the diagnosis of schizophrenia.
One major pitfall of the radiomics model is the lack of explainability in the “black-box” system, which limits its use in clinical practice. It is challenging for the clinicians to determine how the model arrives at a conclusion and to identify radiomic features that are critical in decision-making [35]. The advantage of SHAP is that it identifies certain patterns of complex machine learning algorithms and provides both global and local interpretability for the radiomics model [21]. We found that run entropy of GLRLM from T1 of the posterior CC was the most important factor in differentiating participants with schizophrenia from HCs, followed by IMC1 and correlation of GLCM from the ADC maps of the posterior CC. The GLRLM and GLCM are second-order radiomic features reflecting tissue heterogeneity by calculating the frequency of occurrence of pairs of voxels with specific values and a specified spatial relationship in an image [36]. The GLRLM describes heterogeneity in the distribution of run lengths, whereas GLCM describes heterogeneity in the distribution of co-occurring voxel values. Although the neurobiological underpinnings of radiomic features remain to be elucidated, microscopic aberrations in complex neuronal and glial organizations within the CC may alter the voxel signal intensities in DTI and T1 [37]. The posterior CC contains white matter tracts connecting the bilateral temporal, parietal, and occipital lobes, including multimodal sensory association cortices [38]. Our findings from SHAP imply that the disease-defining feature of schizophrenia may be associated with the “bottom-up” dysfunction in the integration of multisensory information, leading to cognitive and perceptual disturbances in patients with schizophrenia [39]. Future research is warranted to understand the neurobiological meaning of radiomic features in the pathogenesis of schizophrenia.
This study has a few limitations. First, this is a retrospective study from a single institution, and the sample size was relatively small. However, our study is the first to demonstrate the usefulness of the radiomics model with multiparametric MRI of the CC in diagnosing schizophrenia. Further studies with a larger dataset and external validation are needed. Second, although our participants with schizophrenia were initially antipsychotic-naïve or free of antipsychotics for at least 6 months, some MRI data were acquired after the administration of atypical antipsychotics. This may raise a concern regarding the confounding effect of antipsychotic medication. However, the influence of antipsychotics on the brain is controversial, and the mean duration of antipsychotic medication is very short in this study (5.7 ± 5.7 days). Third, the causality of changes in radiomic features of the CC subregions cannot be determined due to the cross-sectional nature of this study. However, given that the majority of participants with schizophrenia were in the early phase of their disorder, our findings may provide some clues to understand the early pathogenesis of schizophrenia.
In conclusion, the multiparametric radiomics model using selected features from the CC may offer a novel approach to improve the diagnosis of schizophrenia with good clinical applicability. Radiomic features extracted from neuroimaging data could be a potential source of biomarkers that support the objective diagnosis of schizophrenia and improve the understanding of its neurobiology. We expect that the estimation of microscopic tissue characteristics using radiomics and diagnostic modeling will promote the quality of clinical practice in managing patients with schizophrenia.
Supplementary information
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education and Ministry of Science & ICT (Republic of Korea; grant numbers: NRF-2020R1I1A1A0107164811 to Y.W.P. and NRF-2021R1C1C1012901 to M.B.) and the Bio & Medical Technology Development Program through the NRF, funded by the Ministry of Science & ICT (Republic of Korea; grant number: NRF-2019M3C7A1032262 to S.-H.L.).
Author contributions
Y.W.P. and S.-H.L. designed the study. M.B. and S.-H.L. managed the participant recruitment and data acquisition; compiled the database; and conducted the data preprocessing. S.S.A., J.K., and S.-K.L. designed the radiomics pipeline, and JE performed the radiomics analyses. S.K. and Y.W.P. performed the statistical analysis. M.B. and Y.W.P. wrote the first draft of the manuscript and S.-H.L. supervised the manuscript. All authors contributed to and approved the final manuscript.
Data availability
The data supporting the findings of this study are not publicly available due to ethical restrictions for protecting participants’ confidentiality and privacy but are accessible from the corresponding author on reasonable request with the approval of the Institutional Review Board of CHA Bundang Medical Center.
Code availability
The codes used in the radiomics analysis are made publicly available at https://github.com/AIM-Harvard/pyradiomics and https://github.com/scikit-learn/scikit-learn. The code used for SHapley Additive exPlanations (SHAP) analysis is available at https://github.com/slundberg/shap.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yae Won Park, Email: yaewonpark@yuhs.ac.
Sang-Hyuk Lee, Email: drshlee@cha.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01586-2.
References
- 1.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–24. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 3.Narr KL, Thompson PM, Sharma T, Moussai J, Cannestra AF, Toga AW. Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex. 2000;10:40–9. doi: 10.1093/cercor/10.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Walterfang M, Wood AG, Reutens DC, Wood SJ, Chen J, Velakoulis D, et al. Corpus callosum size and shape in first-episode affective and schizophrenia-spectrum psychosis. Psychiatry Res. 2009;173:77–82. doi: 10.1016/j.pscychresns.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2008;101:124–32. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Goghari VM, Lang DJ, Flynn SW, Mackay AL, Honer WG. Smaller corpus callosum subregions containing motor fibers in schizophrenia. Schizophr Res. 2005;73:59–68. doi: 10.1016/j.schres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2011;129:149–55. doi: 10.1016/j.schres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261–9. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66:935–41. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37:1096–8. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- 12.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Scott J, Chaudhury B, Hall L, Goldgof D, Yeom KW, et al. Radiomics in brain tumor: image assessment, quantitative feature descriptors, and machine-learning approaches. Am J Neuroradiol. 2018;39:208–16. doi: 10.3174/ajnr.A5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YW, Han K, Ahn SS, Choi YS, Chang JH, Kim SH, et al. Whole-tumor histogram and texture analyses of DTI for evaluation of IDH1-mutation and 1p/19q-codeletion status in World Health Organization grade II gliomas. Am J Neuroradiol. 2018;39:693–8. doi: 10.3174/ajnr.A5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YW, Oh J, You SC, Han K, Ahn SS, Choi YS, et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radio. 2019;29:4068–76. doi: 10.1007/s00330-018-5830-3. [DOI] [PubMed] [Google Scholar]
- 16.Rahmim A, Huang P, Shenkov N, Fotouhi S, Davoodi-Bojd E, Lu L, et al. Improved prediction of outcome in Parkinson’s disease using radiomics analysis of longitudinal DAT SPECT images. Neuroimage Clin. 2017;16:539–44. doi: 10.1016/j.nicl.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Won SY, Park YW, Park M, Ahn SS, Kim J, Lee SK. Quality reporting of radiomics analysis in mild cognitive impairment and Alzheimer’s disease: a roadmap for moving forward. Korean J Radio. 2020;21:1345–54. doi: 10.3348/kjr.2020.0715.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park YW, Choi D, Lee J, Ahn SS, Lee SK, Lee SH, et al. Differentiating patients with schizophrenia from healthy controls by hippocampal subfields using radiomics. Schizophr Res. 2020;223:337–44. doi: 10.1016/j.schres.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Cui LB, Liu L, Wang HN, Wang LX, Guo F, Xi YB, et al. Disease definition for schizophrenia by functional connectivity using radiomics strategy. Schizophr Bull. 2018;44:1053–9. doi: 10.1093/schbul/sby007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J, Cui LB, Xi YB, Zhao YS, Yang XJ, Xu ZL, et al. Predicting response to electroconvulsive therapy combined with antipsychotics in schizophrenia using multi-parametric magnetic resonance imaging. Schizophr Res. 2020;216:262–71. doi: 10.1016/j.schres.2019.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg SM, Lee S-I. A unified approach to interpreting model predictions. Proceedings of the 31st international conference on neural information processing systems. Long Beach, CA: Neural Information Processing Systems Foundation, Inc. (NIPS); 2017. p. 4768–77.
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn., text rev. ed. Washington, DC: American Psychiatric Association; 2000.
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Washington, DC: American Psychiatric Association; 2013.
- 24.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e7. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwanenburg A, Vallières M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38. doi: 10.1148/radiol.2020191145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Han K. Methodologic guide for evaluating clinical performance and effect of artificial intelligence technology for medical diagnosis and prediction. Radiology. 2018;286:800–9. doi: 10.1148/radiol.2017171920. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. doi: 10.1136/bmj.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–20. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 31.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–73. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 32.Goubran M, Hammond RR, de Ribaupierre S, Burneo JG, Mirsattari S, Steven DA, et al. Magnetic resonance imaging and histology correlation in the neocortex in temporal lobe epilepsy. Ann Neurol. 2015;77:237–50. doi: 10.1002/ana.24318. [DOI] [PubMed] [Google Scholar]
- 33.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 34.Park JE, Kim HS. Radiomics as a quantitative imaging biomarker: practical considerations and the current standpoint in neuro-oncologic studies. Nucl Med Mol Imaging. 2018;52:99–108. doi: 10.1007/s13139-017-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500–10. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Materka A, Strzelecki M. Texture analysis methods–a review. Tech Univ lodz, Inst Electron, COST B11 Rep., Bruss. 1998;10:4968. [Google Scholar]
- 37.Tomaszewski MR, Gillies RJ. The biological meaning of radiomic features. Radiology. 2021;298:505–16. doi: 10.1148/radiol.2021202553.202553.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, et al. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are not publicly available due to ethical restrictions for protecting participants’ confidentiality and privacy but are accessible from the corresponding author on reasonable request with the approval of the Institutional Review Board of CHA Bundang Medical Center.
The codes used in the radiomics analysis are made publicly available at https://github.com/AIM-Harvard/pyradiomics and https://github.com/scikit-learn/scikit-learn. The code used for SHapley Additive exPlanations (SHAP) analysis is available at https://github.com/slundberg/shap.