Abstract
Background:
Acute myocardial infarction (AMI) complicated by cardiogenic shock (CS) is associated with significant morbidity and mortality.
Methods:
We provide an overview of previously conducted studies on the use of mechanical circulatory support (MCS) devices in the treatment of AMI-CS and difficulties which may be encountered in conducting such trials in the United States.
Results:
Well powered randomized control trials are difficult to conduct in a critically ill patient population due to physician preferences, perceived lack of equipoise and challenges obtaining informed consent.
Conclusions:
With growth in utilization of MCS devices in patients with AMI-CS, efforts to perform well-powered, randomized control trials must be undertaken.
Keywords: acute myocardial infarction/STEMI, cardiogenic shock, clinical trials, ECMO/IABP/Tandem/Impella, mechanical circulatory support
1 |. INTRODUCTION
Cardiogenic shock (CS) is a frequent and deadly complication of acute myocardial infarction (AMI).1–3 With advancements in medical therapy, early revascularization and regional systems of care, the risk of death from AMI without CS is ~2%.4 However, in the 4–8% of patients who develop CS, the risk of in-hospital death is higher, 33–50%.1–4 With the seminal publication of the “Shock Trial,” early revascularization demonstrated improved survival in patients with AMI and cardiogenic shock (AMICS) in a randomized control trial (RCT).5 Despite two decades of medical advancements little gains have been made in improving the morbidity and mortality associated with AMICS.6–10
Mechanical circulatory support (MCS) devices improve hemodynamics in patients with AMICS and use of such devices as adjunctive therapy is supported in US guidelines (Class II a/b recommendations).11,12 The most widely available and utilized MCS device is intra-aortic balloon pump counter-pulsation (IABP). Despite several observational studies suggesting the benefit of IABP, RCTs have failed to demonstrate significant mortality benefit when compared to medical therapy.7–9 Veno-arterial extracorporeal membrane oxygenation (ECMO) has been available for many decades, however, widespread adoption has been limited given the high level of expertise needed, typically requiring dedicated perfusionists. ECMO is utilized at select centers despite little evidence of improved outcomes. Meta-analyses of observational studies demonstrate survival to discharge rates below 50% as well as frequent complications including high rates of stroke and vascular access complications.13–19 Technological advancements over the past decade have led to the development of several commercially available percutaneous, temporary, MCS devices to serve as adjunctive therapies to revascularization. Current MCS technologies include Impella (Abiomed, Danvers, MA), Tandem Heart (LivaNova, London, UK), Heartmate PHP (Abbott, Abbott Park, IL), and smaller, mobile, ECMO circuits such as CardioHelp (Maquet, Wayne, NJ). These devices differ in their methods of use, ease of placement, cannula size, flow capacity, effect on intra-cardiac hemodynamics and complication rates (Table 1).
TABLE 1.
Temporary mechanical circulatory support devices and effect
| Device | Pump inflow | Pump outflow | Device options | Oxygenation | Aortic flow | RV support | LV support | LV load | RV load |
|---|---|---|---|---|---|---|---|---|---|
| Right sided support | |||||||||
| VV ECMO | Ra,IVC,svc | Ra,IVC,svc | Centrimag, CardioHelp, TandemHeart | Y | N/A | N | N | ↔ | ↔ |
| Centrifugal RVAD | RA | PA | Centrimag, CardioHelp, TandemHeart | Y | N/A | Y | N | ↔ | ↓ |
| Axial RVAD | IVC | PA | Impella RP | N | N/A | Y | N | ↔ | ↓ |
| Left sided support | |||||||||
| Centrifugal LVAD | LA | IF | Centrimag, CardioHelp, TandemHeart | Y | Retrograde | N | Y | ↓ | ↑ |
| Axial LVAD | LV | AO | Impella 2.5, Impella CP, Impella 5.0/5.5, PHP | N | Antegrade | N | Y | ↓ | ↑ |
| Combination support | |||||||||
| VA ECMO | RA | IF | Centrimag, CardioHelp, or TandemHeart | Y | Retrograde | Y | Y | ↑ | ↓ |
| VAV ECMO | RA, IVC, SCV | IVC/SFC & IF | Centrimag, CardioHelp, or TandemHeart | Y | Retrograde | Y | Y | ↑ | ↓↔ |
Note: A combination of isolated right sided support and left sided support can be combined to provide biventricular support (BiPella, EcPella, Bi-Tandem, etc.). Similarly, ECMO cannulas can be configured in numerous configurations to provide biventricular support and can be cannulated percutaneous or centrally.
Abbreviations: AO, aorta; ECMO, extracorporeal membrane oxygenation; IF, iliofemoral artery; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; LVAD, percutaneous left ventricular assist device; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVAD, right ventricular assist device; SVC, superior vena cava.
Until recently, these large bore devices had been used sporadically in states of refractory CS, in a relatively small subset of patients. Refractory CS management was largely driven by surgeons utilizing ECMO. Technological development and increasing familiarity with MCS led to a migration of shock management from the operating room into the catheterization laboratory and shock teams have expanded to include interventional and advanced heart failure specialists. This new CS treatment paradigm led to significant increases in the use of MCS with a diffusion of MCS utilization to centers without LVAD/Transplant programs.2,3,20 Variability in MCS utilization and outcomes fostered the development of multidisciplinary, intra- and inter-facility CS teams with the aims of rapid recognition and management of CS. This strategy has been comprehensively implemented in Detroit where investigators across five large health care systems created a shock protocol to share among physicians in their centers. The protocol was based on observed “best practices” and implemented in an effort to improve local outcomes and unify significant variability among physicians and health care systems. The implementation of the shock protocol resulted in a multifactorial change from recognition to treatment. The protocol emphasized the need for (1) early recognition and catheterization laboratory activation for patients who present in AMICS, (2) early use of MCS prior to a state of refractory CS and (3) use of invasive hemodynamics to guide therapies including escalation and weaning of inotropes and MCS.21 Survival in patients improved from historic rates of ~50% to >70%.22 The study was extended nationally and findings of improved survival against historical controls were replicated.23 The study, however, has significant limitations. There was no control arm and results were compared only to historical controls. There were a multitude of therapeutic changes that occurred simultaneously, and it is unclear how much effect any individual therapy made, including use of MCS. Salvage patients (unwitnessed OHCA, cardiac arrest >30 min, patients with signs of anoxic brain injury) were excluded from the protocol to limit utilization of MCS in patients who may not gain significant benefit. Other centers, including Inova Heart and Vascular Institute and the University of Utah, in observational studies, have similarly shown improved outcomes through formalized shock teams and protocols.24–25
MCS is an expensive medical intervention with inherit industry, physician and health system financial interests that incentivize utilization. Given the cost of MCS and associated care, and as we transition to value-based care, concerns about the demonstrated clinical benefits have also been magnified. MCS devices require large bore access and anticoagulation with the risk of fatal vascular complications, which may be under reported in observational studies, potentially mitigating clinical benefits. The few RCT of MCS conducted to date have not demonstrated improved survival. These concerns were readdressed by Amin et al. after they reported increasing in-hospital mortality, bleeding requiring transfusion, acute kidney injury (AKI), stroke, length of stay (LOS), and hospital costs with use of Impella when compared to IABP.26 It is important to note the significant limitations of this analysis, which consists of retrospective, claims-based data, using ICD codes (Premier Healthcare Database).
In this article the authors review previously conducted studies and present the difficulties in conducting randomized clinical trials in AMICS in the United States.
2 |. PRIOR TRIALS
Over a decade of results from various trials utilizing MCS strategies for CS management have shown an absence of mortality benefit (Figure 1). Each trial, however, has been challenged by significant logistical and ethical barriers impacting patient recruitment, as well as the presence of a heterogeneous shock phenotype. While clinical trials often include hemodynamic and clinical criteria for defining CS, CS presents on a wide continuum and patient phenotypes can vary based on underlying cardiac etiology and presence or absence of preexisting systolic dysfunction. The severity of CS, duration of CS, presence of isolated versus biventricular cardiac failure, associated comorbidities and age of patients all impact CS survival. In review of the clinical trials below, we will highlight key trial characteristics (Table 2).
FIGURE 1.
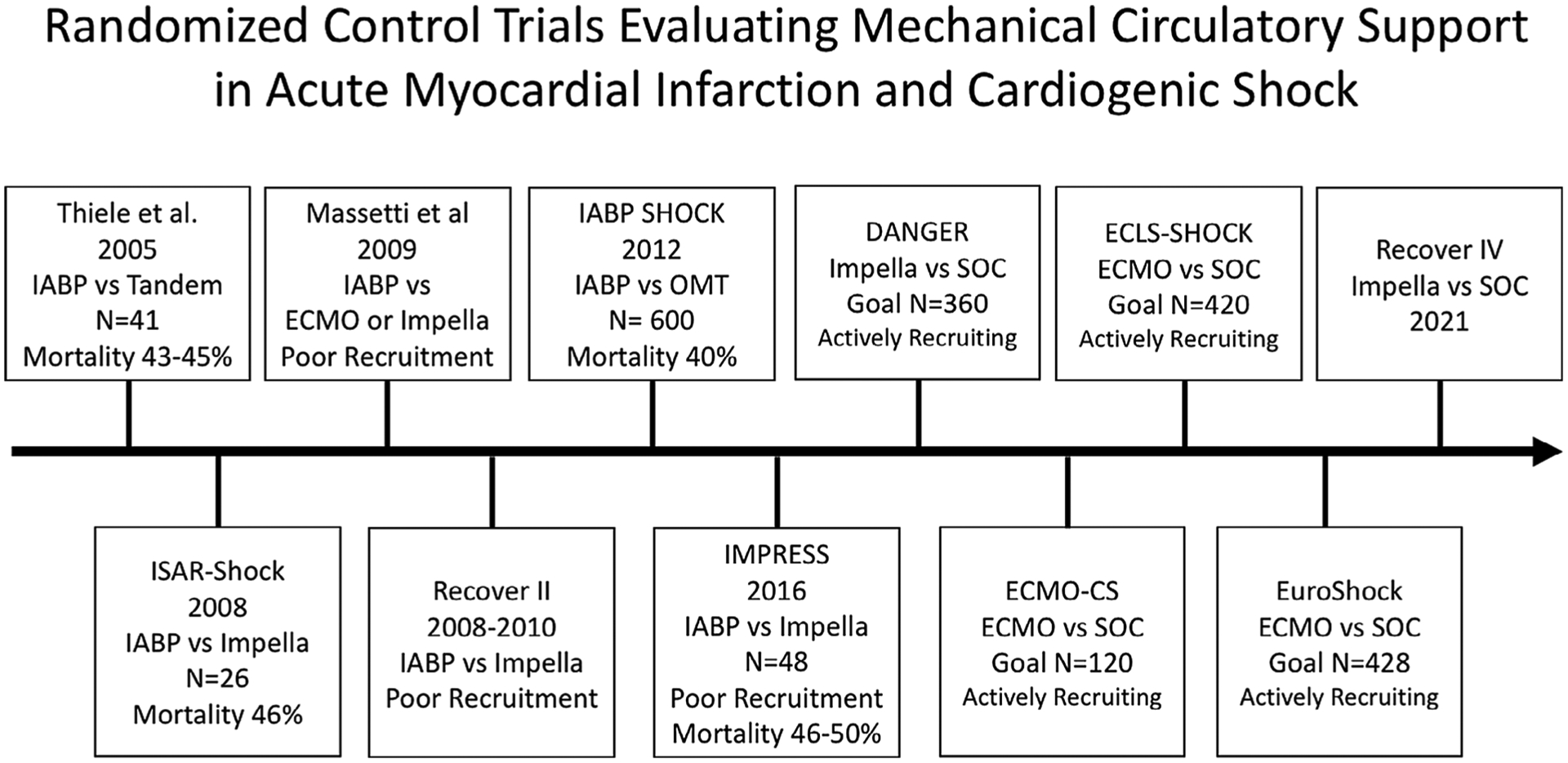
Timeline of randomized control trials performed to date and currently enrolling, evaluating the efficacy of mechanical circulatory support in acute myocardial infarction and cardiogenic shock. CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pump counter-pulsation
TABLE 2.
Key trial characteristics from RCTs evaluating MCS in AMICS
| Trial | Design/sample size | Inclusion criteria | Exclusion criteria | Outcome(s)/limitation(s) |
|---|---|---|---|---|
| Thiele et al 2005 | Single center RCT IABP vs. tandem heart 41 patients |
|
|
|
| ISAR-SHOCK trial 2008 | Multicentered RCT IABP vs. Impella 2.5 26 patients |
|
|
|
| Recover II trial 2008–10 | Multicentered RCT IABP vs. Impella 2.5 One patient |
|
|
|
| Massetti et al. 2009 | RCT ECLS (addended to Impella) vs. standard of care |
|
|
In 2007 due to slow recruitment the MCS device to be implanted was changed from ECMO to Impella but continued with low recruitment and was terminated |
| IMPRESS trial 2016 | RCT IABP vs. Impella CP 48 patients |
|
|
|
| ECMO-CS recruiting | Multi-center RCT ECMO vs. standard of care 120 patients |
Patients must fulfill criteria either:
|
|
Primary outcomes is death, resuscitated circulatory arrest and implantation of another mechanical circulatory support device at 30 days |
| ECLS-SHOCK Recruiting | Multicenter RCT ECMO vs. standard of care 420 patients |
|
|
Primary outcomes is all cause death at 30-day |
| Euro shock Recruiting | Multi-center RCT ECMO vs. standard of care 428 patients |
Altered mental status Cold and clammy skin and limbs Oliguria with a urine output of less than 30 ml per hour Elevated arterial lactate level of >2.0 mmol per liter Provision of informed assent followed by patient consent; (or relative or physician consent if the patient is unable to consent) |
|
Primary end point is 30 day all cause mortality Secondary endpoints:
|
| DANGER Recruiting | Multi-center RCT Impella CP vs. standard of care 360 patients |
|
|
Primary outcomes is all-cause death at 6 months |
Abbreviations: AMI, Acute myocardial infarction; CS, cardiogenic shock; ECLS, extracorporeal life support; IABP, intra-aortic balloon pump counter-pulsation; LV, left ventricle; MCS, mechanical circulatory support; RCT, randomized control trial; RV, right ventricle; SBP, systolic blood pressure.
In 2005 Thiele et al.27 conducted a single center RCT comparing IABP to Tandem Heart in 41 patients from 2000–2003.28 The primary outcome of this pilot trial was measured cardiac power index (CPI). CPI along with other hemodynamic and metabolic variables were improved with Tandem Heart support (from 0.22 [interquartile range (IQR) 0.19–0.30] to 0.37 W/m2 [IQR 0.30–0.47, p < .001] when compared with IABP from 0.22 [IQR 0.18–0.30] to 0.28 W/m2 [IQR 0.24–0.36, p = .02; p = .004 for intergroup comparison]). However, complications like severe bleeding (n = 19 vs. n = 8, p = .002) and limb ischemia (n = 7 vs. n = 0, p = .009) were encountered more frequently after Tandem Heart support. Overall, 30-day mortality was similar between the two groups (IABP 45% vs. Tandem Heart 43%, log-rank, p = .86).
The ISAR-SHOCK trial was a feasibility trial presented in 2008, randomizing 26 patients with AMICS who received either an IABP or Impella.28 Compared to patients on IABP support, Impella patients had higher cardiac indices and diastolic arterial pressures after 30 min of support, however, mortality was 46% (i.e., 6 of 13 patients) in both groups after 30 days. The cohort had several high-risk characteristics including mechanical ventilation on admission in 92% and CPR/VT before randomization in 69–85%. The trial was not designed nor powered to examine mortality.
The Recover II Trial was a multicenter RCT comparing IABP and Impella 2.5 in AMICS designed with the primary intent of assessing a composite endpoint of major adverse events within 30 days or at hospital discharge. The sample size needed to determine significant differences between groups was 384. Despite 58 sites with IRB approval in the United States, only one patient enrolled in the study between July 2008 and August 2010, resulting in discontinuation of the trial.
The Impella versus IABP Reduces Infarct Size in STEMI (IMPRESS) trial was a randomized, prospective, open-label, multicenter trial, with the aim to randomize 130 patients with acute anterior STEMI and clinical signs of “pre-cardiogenic” shock, defined as a heart rate > 100, systolic blood pressure (SBP) <100 mmHg and clinical signs of CS including cold extremities, cyanosis, and altered mentation.29 Between 2008 and 2011, only 21 patients (n = 12 with Impella) were enrolled and investigators cited the inclusion criteria as the primary obstacle; “Although heart rate and blood pressure are objective and easily available measures, it is less easy to define the clinical pre-shock condition within the continuum from pre-shock to severe shock.” The trial enrollment criteria were then revised to include patients with “severe shock,” defined as a SBP <90 mmHg or need for inotrope support and the need for mechanical ventilation.30 Using the broader definition, a critically ill cohort was recruited: all patients were on inotropes and 92% had a cardiac arrest (75% required hypothermia and 48% achieving ROSC after more than 20 min of CPR). In total, 48 patients with AMICS were recruited and 24 received an Impella CP and 24 an IABP. At 30 days, mortality in patients treated with either IABP or Impella CP was similar (50 and 46%, respectively, hazard ratio [HR] with Impella CP, 0.96 (95% confidence interval [CI] 0.42 to 2.18)). At 6 months, mortality for both the Impella CP and IABP was 50% (HR 1.04 (95% CI; 0.47–2.32). The main cause of death was neurologic injury and refractory CS.
ECMO has not been studied in any RCT in CS. In 2006 Massetti et al attempted the “Comparison of Standard Treatment Versus Standard Treatment Plus Extracorporeal Life Support (ECLS) in Myocardial Infarction Complicated with CS trial” which was halted in 2009 due to slow recruitment.31
3 |. ONGOING TRIALS
On the horizon RCTs have begun in Europe to help better evaluate the utility of MCS in AMICS. ECMO-CS is a multicenter RCT comparing current standard of care to ECMO in AMICS.32 The primary endpoint is a composite of death from any cause, resuscitated circulatory arrest, and implantation of another MCS device at 30 days. The sample size of 120 individuals (60 in each arm) provides 80% power to detect a 50% reduction of primary endpoint, at alpha = 0.05. Patient recruitment started in October 2014. Similarly, Thiele et al have begun the “ECLS-Shock” trial comparing ECMO with standard of care in a 420 patient multicenter study, evaluating 30-day mortality.33 The trial will only enroll patients with a lactate >3 mmoL/L, exclude patient with resuscitation >45 min or those with shock onset >12 hr. The study will use a large working group of hospitals with the goal of completing recruitment within 3 years. Banning et al. have begun the Euro Shock trial, the largest trial planned to date evaluating the use of ECMO versus standard of care; with a goal recruitment of 428 patients across 44 European centers.34
The Danish CS (DanShock) trial is a multicenter, RCT, comparing Impella CP with standard of care that is currently enrolling in Denmark.35 Due to slow recruitment, sites in Germany have been added and the trial is now called DanGer Shock. A total of 360 patients are planned to be enrolled to assess the primary outcome of death from all causes at 6 months. Inclusion criteria of study participants include: STEMI for <36 hr, CS for <24 hr, confirmed based on arterial blood lactate ≥2.5 mmoL/L and/or SvO2 < 55% with a normal PaO2 and systolic BP < 100 mmHg and/or need to vasopressor therapy, and a left ventricular ejection fraction <45%. Since the study initiation in December 2012, about 150 patients have been enrolled through the end of 2019. Lastly, planning has begun for the RECOVER IV trial, which will evaluate outcomes of Impella using best practices incorporated from the National Cardiogenic Shock Initiative (NCSI) versus standard of care. The trial is expected to include an international cohort of patients, including those from the United States with plans to start recruitment in 2021 or 2022.
4 |. CHALLENGES IN PERFORMING RCT
The current “absence of evidence” of survival benefit with MCS is not “evidence of absence” of benefit. The aforementioned trials lack the appropriate sample size to determine if a survival benefit exists or not. Possible explanations for low enrollment include physician preferences, perceived lack of equipoise and challenges in obtaining informed consent.
4.1 |. Low incidence
The principal challenge in conducting RCT in AMICS is recruitment. The incidence of AMICS and use of MCS is relatively low.36 The expertise needed in implanting and more importantly managing MCS devices such as Impella, Tandem Heart, and ECMO is challenging and currently only a small number of regional centers can perform and manage these devices safely.37 This challenge was present in the IMPRESS trial, which mandated that each center have pre-trial experience with at least 10 high-risk PCI procedures with Impella to demonstrate the ability to implant and manage this device safely. Even among centers with an expertise in the utilization of one form of MCS, one cannot have an expectation that such expertise will translate into the management of other forms of MCS and therefore centers with a high expertise in multiple MCS modalities are further limited.37 Though the use of these devices is expanding to community programs, these initiatives are usually led by a physician leader and at times do not cultivate in other operators or the institution as a whole.
4.2 |. Heterogeneous patient phenotype
CS in clinical trials is often defined using both hemodynamic and clinical signs and/or symptoms. Definitions used in trials vary and include evidence of persistent hypotension (SBP < 80–90 mmHg or a mean arterial pressure 30 mmHg below baseline) with a low-cardiac index (<1.8 L/min/m2 without support or < 2.0–2.2 L/min/m2 with support), low-cardiac power output (CPO <0.6 W) and elevated filling pressures (left ventricular end-diastolic pressure > 18 mmHg or right atrial pressure > 10–15 mmHg) along with cool extremities, lactic acidosis, and/or evidence of end-organ dysfunction. Despite the CS criteria outlined above, CS tends to present on a wide continuum and patient phenotypes are highly variable with presentations driven by underlying cardiac etiology, presence or absence of prior cardiac dysfunction and duration of CS.38 While prolonged shock is associated with worse outcomes, the onset of CS is often hard to pinpoint. Timing for enrollment into RCT is therefore critical in evaluating the efficacy of MCS in a particular stage of CS. As mentioned previously investigators in the IMPRESS trial originally intended to recruit patients in pre-shock (SCAI Shock Stage B); however, substantial difficulties were encountered that required changes to their inclusion criteria ultimately leading to recruitment of patients in deteriorating shock (SCAI Shock Stage D), exemplifying the difficulties in recruiting across the shock continuum. Thus, while a minimum level of hemodynamic compromise is necessary for an inclusion definition, trials must be similarly cognizant of the worst level of shock acceptable in a given trial, so as not to include patients who have little to gain from a given therapy (i.e., the futile patient).
4.3 |. FDA approval
Ethical concerns are an additional impediment to enrollment. In contrast to FDA requirements for new drugs, medical devices are subject to a separate approval pathway. When a device already has an indication for use, physicians charged with the care of these patients may perceive it to be unethical to randomize patients not to receive MCS. Hence, with market expansion, physicians are left under a cloud of uncertainty regarding the ethics of withholding treatment in a randomized trial. In an ideal world regulatory and clinical treatment decisions should be based on assessment of treatment effectiveness and safety based on RCT data. Approvals that are based solely on data that do not involve a well-structured RCT can result in patients and clinicians practicing with uncertainties regarding the benefits and harms associated with new medical devices and therapies.
4.4 |. Crossover
In order to most efficiently test a hypothesis that an intervention works, a RCT should minimize bail-out crossover from the medical therapy arm to MCS. Cardiologists, however, may feel that not having a bail-out option would be unethical leaving a critically ill patient to die. Even without a RCT proving mortality benefit, MCS devices are implanted in an effort to improve hemodynamics with the belief that their efforts will result in improved survival. Early adopters of MCS who have a perception of improving outcomes therefore may be less likely to participate in such trials. As utilization continues, particularly in the United States, the perception has left many to wonder if such trials can only take place outside of the United States. Trials, which will allow cross over will also need to have strict definitions and parameters when such cross over can or should occur.
4.5 |. High reimbursement
Given the current crisis of healthcare costs, one may also question why the system is paying for expensive MCS in the absence of survival benefit from RCTs. MCS are costly and range from $10,000–30,000 with Medicare Severity Diagnosis Related Groups (MS-DRG) reimbursement reaching $100,000.
5 |. TRIAL DESIGN
Taking into account the aforementioned challenges in conducting RCTs using MCS in AMICS, investigators are left with the challenge of designing trials that will accomplish the objectives of determining efficacy while balancing issues such as low recruitment, cross over, and cost. Suggested efficacy end points are listed in Table 3.
TABLE 3.
Suggested end points in a RCT for AMICS
| Primary end point |
| Short-term (30–90 day) survival |
| Secondary end points |
| Need for MCS upgrade |
| MCS major complications (BARC 5 bleeding, amputation, CVA CPC 3–5) |
| 6-month and 1-year survival |
| 6-month and 1-year heart failure admissions |
| Other end points |
| Hemodynamic effects (RA, PA, HR, BP, CO, CI) |
| Utilization of inotropes and vasopressors |
| End-organ perfusion (GFR, Cr, AST, ALT, lactate) |
| Shock stage (A, B, C, D, E) |
| MCS complications (BARC 2–4 bleeding, surgical interventions, peripheral interventions) |
| Adverse events (CVA 1–2, transfusion, limb ischemia, MI, new ventilator/dialysis requirement at discharge) |
| Need for durable MCS or transplant |
| Discharge disposition (home, rehab, long-term acute care facility) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BARC, Bleeding Academic Research Consortium; BP, blood pressure; CI, cardiac index; CO, cardiac output; Cr, creatinine; CVA, cerebral vascular attack; GFR, glomerular filtration rate; HR, heart rate; MCS, mechanical circulatory support, MI, myocardial infarction; PA, pulmonary artery pressure; RA, right atrial pressure.
The DAWN trial in ischemic stroke provides a template for an innovative trial design that lends itself well to some of these challenges.38 Briefly, the DAWN trial randomly assigned patients with an ischemic stroke to receive late endovascular thrombectomy or standard therapy. After 206 patients were enrolled, the trial was stopped for efficacy; Bayesian posterior probabilities of >0.999 suggested strong evidence in favor of thrombectomy. The DAWN trial has two interesting design features that lend themselves nicely to a trial of MCS in AMICS.
First, this design allows frequent interim analyses for benefit and harm without compromising the validity of the final results. With a trial design based on Bayesian posterior probabilities of success, the investigators planned to conduct interim analyses after enrollment of 150 patients and again after every 50 patients thereafter up to a maximum enrollment of 500 patients; thanks to the adaptability of this design, the trial was stopped after enrolling 206 patients with strong and uniform signs of efficacy (Bayesian posterior probability >0.999 for superiority). Had there been a smaller benefit that was not conclusive at the interim analysis, the trial would have continued until sufficient data accrued to conclude that (1) there was a true benefit or (2) there was no benefit. While the specific number of patients required and interim strategy deployed for a MCS trial would be dependent on other operating characteristics, the key takeaway is that this design allows us to enroll “just as many” patients as needed to answer the question, thereby minimizing patient exposure. This provides a practical advantage as well as since it is difficult to enroll these patients into trials. It also may mitigate ethical concerns by exposing the fewest patients needed to obtain a valid answer.
Second, the adaptive-enrichment strategy allows for fine tuning of the patient population at interim analyses. The DAWN trial for example prespecified five patient subpopulations based on infarct size. At each planned interim analysis, if the highest currently open group had less than 40% probability of demonstrating an average positive treatment effect, enrollment of patients in that group would be suspended. Thereby concluding that the experimental treatment was “futile” in that population and that there was nothing to be gained by continuing to enroll those patients. The study would remain open in the other groups. A similar design could be quite useful in AMICS if prespecified subgroups were identified to eliminate patient groups where MCS was demonstrating little evidence of benefit. Subgroups for example could be based upon SCAI shock stages or a variation of the stages.39
If MCS has a strong and uniform survival benefit, the trial would terminate relatively early and have conclusive proof of efficacy with a relatively small number of patients exposed. If MCS has a strong survival benefit in some patients but not all, the trial could be designed to suspend enrollment as soon as efficacy was proven for the subgroup in which benefit has been proven while enrollment in the other subgroups remains open long enough to determine whether benefit extends to those groups. If MCS has little or no survival benefit in any patients, the trial will enroll just long enough to rule out benefit in all patients. If MCS has a strong harmful tendency in some patients, enrollment will likely be suspended in the specific subgroups in which harm has been proven very quickly while remaining open long enough to rule out benefit in the other subgroups. If MCS has a strong and uniform harmful tendency, the trial would again terminate relatively early with conclusive evidence against the use of MCS.
Another potential model is the recently published ARREST trial of reperfusion strategies in patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation. Patients were randomly assigned to ECMO-facilitated resuscitation versus initial standard advanced cardiac life support (ACLS) treatment. Like the DAWN trial, ARREST was designed using Bayesian group sequential monitoring in efforts to maximize efficiency, with planned response adaptive randomization if the trial continued past the first interim analysis. The design planned for interim analyses after every 30 participants followed-up for the primary endpoint, potentially enrolling up to 150 total participants. If strong evidence was found of an effect on survival to hospital discharge (posterior probability of 0.986 or higher) the DSMB was obliged to provide a formal recommendation on whether to stop the trial. For the first group of 30 patients, patients were randomized in a 1:1 allocation ratio; if the trial continued, randomization to the subsequent group of participants was to be weighted in proportion to the posterior probability of the superior treatment at the most recent analysis. Like DAWN, the study was terminated at the first interim analysis (30 patients) because the posterior probability of superiority exceeded the prespecified monitoring boundary; six patients that had been randomly assigned to early ECMO had survived versus just one in the standard ACLS group for a posterior probability of benefit of 0.986 with ECMO versus ACLS. These trial designs are potentially attractive in the setting of CS research, a high-mortality population where treatments may have very large treatment effects. By performing frequent interim analyses that allow for stopping once the data are sufficiently convincing to meet a prespecified threshold for success, the trial can be “rightly-sized” to enroll just as many participants as needed to establish therapeutic efficacy without going further and randomizing participants beyond the point where the data are sufficient to prove that the therapy is effective. Such trials may also specify a maximum sample size; if a stopping rule is not met at any of the previously conducted interim analysis, the trial will cease and provide a final estimate of treatment based on the observed data.
Planning this trial would require outlining the important trial operating characteristics including: estimated mortality in control arm, possible effect sizes, definition of subgroups, agreed-upon Bayesian probability thresholds for futility, and so on. Similarly, we would need to conduct extensive simulations to ensure that the design would function well under variations of these parameters. The NCSI network along with other collaborative networks could come together to perform such a study.
The NCSI enrolled patients with similar inclusion and exclusion criteria when compared to the Shock and IABP-Shock Trials. If we assume 41% mortality in the control arm, similar to the outcomes seen the IABP-Shock trial and a 28% mortality in the intervention arm, similar to the outcomes seen in the NCSI, a traditional trial design planning to enroll 500 patients would have approximately 87% power.
An adaptive trial design in the model of DAWN or ARREST would have potential to conduct this trial even more efficiently. As an example: suppose that the trial is designed to enroll up to a maximum of 500 patients (using 1:1 randomization throughout), with planned interim analysis after each group of 50 patients enrolled, allowing for efficacy termination if the posterior probability of superiority exceeds 0.99 for one treatment group. Under the same assumptions used above, in 100,000 simulations we demonstrate that the trial would have comparable power to the traditional design (about 86%) with an additional benefit that the majority of such trials would stop before enrolling 500 participants (mean number of about 296 participants required) while controlling the Type I error rate at about 5% overall. The ability for trials to terminate early in the setting of a very large observed mortality benefit is an attractive feature of the adaptive design in high-mortality populations.
6 |. CONCLUSIONS
AMI complicated by CS is associated with substantial morbidity and mortality. There has been an increasing utilization of MCS devices for management of such patients to improve hemodynamics, facilitate revascularization, and preserve end organ function. MCS devices are expensive and invasive interventions with inherent industry, physician, and health system financial interests that may increase utilization. Well-powered RCTs are difficult to conduct in a critically ill patient population due to physician practice preferences, perceived lack of equipoise, and challenges in obtaining informed consent. Despite these challenges it is imperative to guide physicians with the most compelling level of evidence. Given uncertainty stemming from observational studies suggesting both benefit and harm when utilizing MCS, physician leaders and regulatory bodies must come together to ensure trials are conducted to provide the safest, most evidence-based care for our patients.
Footnotes
CONFLICT OF INTEREST
Mir B Basir is a consultant for Abbott Vascular, Abiomed, Cardiovascular Systems, Chiesi, Procyrion, and Zoll. Duane S. Pinto is a consultant for Abbott Vascular, Abiomed, Boston Scientific, Medtronic, NuPulse CV, and Telefelex. Jennifer Cowger is a consultant for Abbott Vascular and Medtronic. William Suh is a consultant for Edwards. Boback Ziaeian, Akshay Khandelwal, and Andrew Althouse report no relevant discloses.
REFERENCES
- 1.Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. [DOI] [PubMed] [Google Scholar]
- 3.Strom JB, Zhao Y, Shen C, et al. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention. 2018;13(18):e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 4.Anderson ML, Peterson ED, Peng SA, et al. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: a report from NCDR. Circ Cardiovasc Qual Outcomes. 2013;6(6):708–715. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic SHOCK. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634. [DOI] [PubMed] [Google Scholar]
- 6.Scholz KH, Maier SKG, Maier LS, et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39(13):1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367(14):1287–1296. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad Y, Sen S, Shun-Shin MJ, et al. Intra-aortic balloon pump therapy for acute myocardial infarction: a meta-analysis. JAMA Intern Med. 2015;175(6):931–939. [DOI] [PubMed] [Google Scholar]
- 9.Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;27:CD007398.(3): 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele H, Akin I, Sandri M, et al. CULPRIT-SHOCK investigators. One year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379(18):1699–1710. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):e240–e327. [DOI] [PubMed] [Google Scholar]
- 12.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013; 127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 13.Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015;61(1):31–36. [DOI] [PubMed] [Google Scholar]
- 15.Brugts JJ, Caliskan K. Short-term mechanical circulatory support by veno-arterial extracorporeal membrane oxygenation in the management of cardiogenic shock and end- stage heart failure. Expert Rev Cardiovasc Ther. 2014;12(2):145–153. 10.1586/14779072.2014.880051. [DOI] [PubMed] [Google Scholar]
- 16.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–616. [DOI] [PubMed] [Google Scholar]
- 17.Wilson-Smith AR, Bogdanova Y, Roydhouse S, et al. Outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock: systematic review and meta-analysis. Ann Cardiothorac Surg. 2019;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamogeorgakis T, Rafael A, Shafii AE, Nagpal D, Pokersnik JA, Gonzalez-Stawinski GV. Which is better: a miniaturized percutaneous ventricular assist device or extracorporeal membrane oxygenation for patients with cardiogenic shock? ASAIO J. 2013;59(6):607–611. [DOI] [PubMed] [Google Scholar]
- 19.Vallabhajosyula S, Prasad A, Bell MR, et al. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail. 2019;12(12):e005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stretch R, Sauer C, Yuh D, Bonde P. National trends in the utilization of short-term mechanical circulatory support incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64(14):1407–1415. [DOI] [PubMed] [Google Scholar]
- 21.Basir MB, Schreiber TL, Grines CL, et al. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119(6):845–851. [DOI] [PubMed] [Google Scholar]
- 22.Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91(3):454–461. [DOI] [PubMed] [Google Scholar]
- 23.Basir MB, Kapur NK, Patel K, et al. National Cardiogenic Shock Initiative Investigators. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. [DOI] [PubMed] [Google Scholar]
- 24.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based Care for Cardiogenic Shock. J Am Coll Cardiol. 2019;73(13): 1659–1669. [DOI] [PubMed] [Google Scholar]
- 25.Taleb I, Koliopoulou AG, Tandar A, et al. Drakos SG shock team approach in refractory cardiogenic shock requiring short-term mechanical circulatory support. Circulation. 2019;140:98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2019;141(4):273–284. [DOI] [PubMed] [Google Scholar]
- 27.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intraaortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. [DOI] [PubMed] [Google Scholar]
- 28.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. [DOI] [PubMed] [Google Scholar]
- 29.Ouweneel DM, Engstrom AE, Sjauw KD, et al. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int J Cardiol. 2016;202:894–896. [DOI] [PubMed] [Google Scholar]
- 30.Ouweneel DM, Engstrom AE, Sjauw KD, et al. Experience from a randomized controlled trial with impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Int J Cardiol. 2016;202:894–896. [DOI] [PubMed] [Google Scholar]
- 31.Massetti M. Comparison of Standard Treatment Versus Standard Treatment Plus Extracorporeal Life Support (ECLS) in Myocardial Infarction Complicated With Cardiogenic Shock 2006. [https://clinicaltrials.gov/ct2/show/NCT00314847]
- 32.Ostadal P. ExtraCorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS) [https://clinicaltrials.gov/ct2/show/NCT02301819]
- 33.Thiele H. Extracorporeal Life Support in Cardiogenic Shock (ECLS-SHOCK) Trial. [https://clinicaltrials.gov/ct2/show/NCT03637205]
- 34.Banning AS, Adriaenssens T, Berry C, et al. The EURO SHOCK Trial: design, aims and objectives randomised comparison of extra corporeal membrane oxygenation (ECMO) delivered after acute-PCI plus standard of care versus standard of care alone after acute PCI, in patients presenting with acute coronary syndrome and cardiogenic shock. EuroInterventions 2021;16(15):e1227–e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udesen NJ, Møller JE, Lindholm MG, et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. 2019;214:60–68. [DOI] [PubMed] [Google Scholar]
- 36.Vallabhajosyula S, Prasad A, Sandhu GS, et al. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes. EuroIntervention. 2019;16(5): e1254–e1261. 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg DD, Barnett CF, Kenigsberg BB, et al. Clinical practice patterns in temporary mechanical circulatory support for shock in the critical care cardiology trials network (CCCTN) registry. Circ Heart Fail. 2019;12(11):e006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 39.Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128. [DOI] [PubMed] [Google Scholar]


