Abstract
A 69-year-old man was clinically diagnosed as stage IV gastric cancer with peritoneal dissemination. We performed systemic chemotherapy consisting of S-1 plus oxaliplatin as a first line, and ramucirumab plus nab-paclitaxel as a second line. However, CT and EGD revealed growth of the primary tumor and the lymph nodes along the lesser curvature and adjacent to the cardia. In addition, CT revealed ascites in the rectovesical pouch. Therefore, treatment was switched to nivolumab. After 3 treatment courses, CT revealed shrinkage of lymph nodes and disappearance of ascites. After 12 courses of nivolumab, however, EGD revealed growth of the tumors in the stomach with minor hemorrhage, prompting the consideration of gastrectomy. At the time of laparotomy, the peritoneal dissemination had completely disappeared, and peritoneal cytology was negative. Therefore, total gastrectomy with D2 and paraaortic lymphadenectomy was performed, after 21 months following the initial diagnosis. To our knowledge, there are no previous reports that have demonstrated the disappearance of peritoneal dissemination and ascites in response to nivolumab, resulting in curative gastrectomy.
Keywords: Gastric cancer, Conversion surgery, Nivolumab
Introduction
Gastric cancer remains an important cancer worldwide and is responsible for over one million new cases in 2020 and the fourth leading cause of cancer-related deaths worldwide, with 769,000 deaths (equating to one in every 13 deaths globally) recorded in 2020 [1]. Furthermore, the prognosis of patients with stage IV gastric cancer is poor despite the remarkable development of multidisciplinary treatments that include chemotherapy: the median overall survival (OS) of patients with stage IV gastric cancer is 6–14 months [2]. On the other hand, immune checkpoint blockade has emerged as a novel immune therapy for patients with various malignant neoplasms, including gastric cancer [3, 4].
Case report
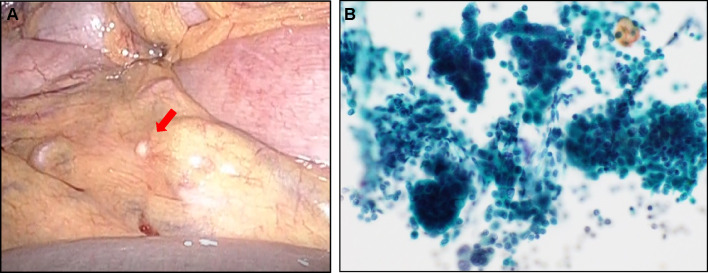
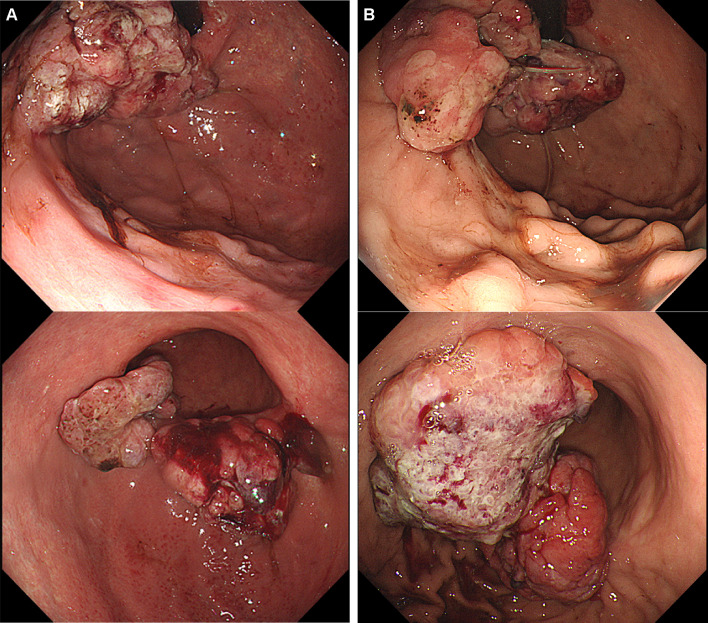
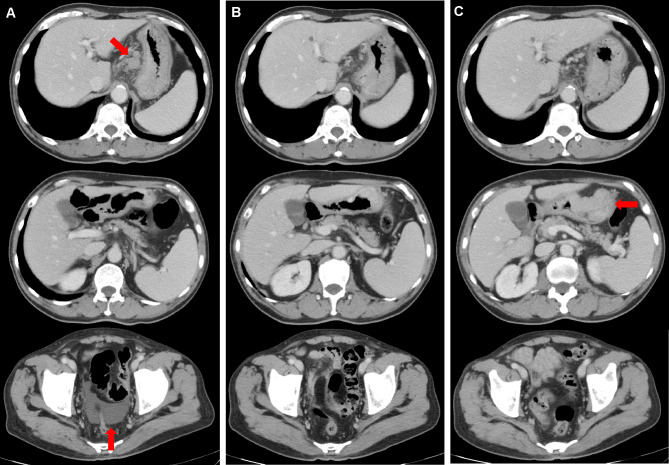
A 69-year-old man complaining of abdominal pain was diagnosed with advanced gastric cancer at his local hospital and was referred to our hospital for further examination. Esophagogastroduodenoscopy (EGD) revealed two ulcerated tumor lesions in the cardia and body of the stomach. Biopsy confirmed signet ring cell carcinoma from both lesions, which were negative for HER2 (immunohistochemistry, 2+; fluorescence in situ hybridization, negative). The serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) was not elevated at diagnosis. Enhanced computed tomography (CT) showed a thickening of the gastric wall at the tumor site, multiple lymph node metastasis, and no distant metastasis. Staging laparoscopy revealed multiple white nodules on the mesenterium, abdominal wall below left diaphragm, and rectovesical pouch, which was confirmed to be metastasis from gastric adenocarcinoma (Fig. 1A). Peritoneal lavage cytology was positive for adenocarcinoma (Fig. 1B). Consequently, the patient was clinically diagnosed T4aN3bM1, stage IV, according to the Union for International Cancer Control (UICC 8th edition). We performed systemic chemotherapy consisting of S-1 plus oxaliplatin (G-SOX). After 3 courses of G-SOX, CT revealed that the primary tumor and lymph nodes had become smaller, suggesting that a partial response had been achieved. Although G-SOX was continued until 14 courses without severe adverse events, CT and EGD revealed the growth of the primary lesion, and chemotherapy was switched to ramucirumab plus nab-paclitaxel (nab-PTX plus RAM). After 2 courses of nab-PTX plus RAM, CT and EGD revealed growth of the primary tumor and the lymph nodes along the lesser curvature and adjacent to the cardia (Figs. 2A, 3A). In addition, CT revealed ascites in the rectovesical pouch (Fig. 3A). Therefore, treatment was switched to nivolumab (nivolumab 240 mg days 1 every 2 weeks). After 3 treatment courses, CT revealed shrinkage of lymph nodes and disappearance of ascites (Fig. 3B). After 12 courses of nivolumab, CT revealed the maintained shrinkage of the lymph nodes and disappearance of ascites (Fig. 3C). However, CT and EGD revealed growth of the tumors in the stomach with minor hemorrhage (Fig. 2B, 3C), prompting the consideration of gastrectomy. At the time of laparotomy, the peritoneal dissemination had completely disappeared, and peritoneal cytology was negative. Therefore, total gastrectomy with D2 and paraaortic lymphadenectomy was performed, after 21 months following the initial diagnosis. The pathological examination demonstrated that the histological response of the primary tumor and lymph nodes were categorized as grade 1a and 1b, respectively. The postoperative course was uneventful, and the patient was discharged 14 days after the surgery. Adjuvant treatment of nivolumab was initiated after 4 weeks from surgery. At 12 months post-surgery, he is alive with no evidence of recurrence.
Fig. 1.
Staging laparoscopy revealed peritoneal dissemination on the mesenterium (A), and peritoneal lavage cytology was positive for adenocarcinoma (×200) (B)
Fig. 2.
Primary lesions in the cardia and body of the stomach before treatment with nivolumab (A), and after 12 courses of nivolumab (B)
Fig. 3.
Enhanced CT scans of the abdomen and pelvis. Images were acquired before treatment with nivolumab (A), after 3 courses of nivolumab (B), and after 12 courses of nivolumab (C)
Discussion
To our knowledge, there are no previous reports that have demonstrated the disappearance of peritoneal dissemination and ascites in response to nivolumab, resulting in curative gastrectomy. Recently, conversion surgery has emerged as a promising therapeutic tool for providing long-term survival in responders with stage IV gastric cancer after chemotherapy [5]. Among patients undergoing conversion surgery, the presence of one non-curative factor before surgery and performing R0 resection are predictors of a favorable OS [6]. However, the clinical indication and prognostic significance of conversion surgery remain unclear in responders after immune checkpoint blockade. There are few reports regarding R0 resection of Stage IV gastric cancer after nivolumab [7–9].
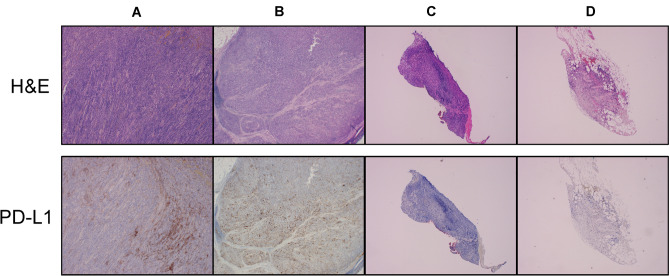
In this patient, nivolumab resulted in complete response of the peritoneal dissemination, partial response of the lymph node metastasis, and progression disease of the primary tumors. The Cancer Genomic Atras (TCGA) classified gastric cancer into four molecular subtypes: Epstein–Barr Virus (EBV) positive, MSI, genomic stability, and chromosomal instability [10]. Tumors categorized by EBV-positive or microsatellite instability (MSI) high status, called MSI, have a promising response to immune checkpoint inhibitors. In this case, however, EBV status was negative as the result of the EBV-encoded small RNA in situ hybridization, and the MSI test was also stable. Although the correlation with the effect of immune check-point inhibitor, including nivolumab, to the expression of Programmed cell Death Ligand 1 (PD-L1) is unclear in gastric cancer [11], we evaluate the expression of PD-L1 by immunohistochemistry using the 22C3 antibody. As shown in Fig. 4, PD-L1 combined positive score (CPS) was highly expressed in the primary tumor (CPS = 15–20, Fig. 4-A) and lymph node (CPS = 20–30, Fig. 4-B) at the time of laparotomy than the endoscopic biopsy sample (CPS = 1–5, Fig. 4-C) and peritoneal dissemination (CPS = 1–5, Fig. 4-D) at diagnosis. Although there was an increase in CPS with the use of nivolumab in the primary tumor, which would be expected to show an effect, nivolumab actually had a poor effect on the primary tumor. In gastric cancer, Th17 and Treg cells in the tumor microenvironment were gradually increased according to disease progression has been reported [12]. Furthermore, it has been reported that PD-1 blockade may facilitate e the proliferation of highly suppressed PD-1 + eTreg cells in hyper progressive disease (HPD), resulting in the inhibition of anti-tumor immunity [13]. These results suggest that gastric cancer, especially primary tumors, may be less likely to respond to nivolumab.
Fig. 4.
Upper images: Hematoxylin and eosin (H&E) staining of the tumor tissues (×40). Lower images: PD-L1 expression determined by an immunohistochemistry assay using the PD-L1 IHC 22C3 pharmDx kit. PD-L1 protein expression was determined by combined positive score (CPS). A Primary tumor, CPS 15–20%; B lymph node, CPS 20–30%; C endoscopic biopsy samples, CPS 1–5%; D peritoneal dissemination, 1–5% (×40)
With increasing use of nivolumab, we should have a high level of suspicion for the disappearance of peritoneal disease so as not to miss the window of opportunity of performing surgery. Further evidence from prospective clinical trials is required to assess the results of conversion surgery for gastric cancer following immune checkpoint blockade.
Conflict of interest
The author(s) indicated no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyuna S, Jacques F, Rebecca LS, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018, 5th edn. Gastric Cancer 2020; 24: 1–21. [DOI] [PMC free article] [PubMed]
- 3.Cohen NA, Strong VE, Janjigian YY. Checkpoint blockade in esophagogastric cancer. J Surg Oncol. 2018;118:77–85. doi: 10.1002/jso.25116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beom SH, Choi YY, Baek SE, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116. doi: 10.1186/s12885-018-4998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22:3618–3624. doi: 10.1245/s10434-015-4422-6. [DOI] [PubMed] [Google Scholar]
- 7.Toyota S, Orita H, Fukuyama Y, Motoyoshi S, et al. Successful conversion surgery following Chylous ascites after nivolumab for advances gastric cancer. Vivo. 2020;34:583–585. doi: 10.21873/invivo.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraishi T, Kano M, Sakata H, et al. Successful treatment of gastric cancer with conversion surgery after nivolumab treatment-a case report. Gan To Kagaku Ryoho. 2019;46:1614–1616. [PubMed] [Google Scholar]
- 9.Matsumoto R, Arigami T, Matsushita D, et al. Conversion surgery for stage IV gastric cancer with a complete pathological response to nivolumab: a case report. World J Surg Oncol. 2020;18:179. doi: 10.1186/s12957-020-01954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. [DOI] [PMC free article] [PubMed]
- 11.Shitara K, Mustafa Özgüroğlu, Yung-Jue Bang, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial: Lancet. 392(10142): 123–133, 2018, [DOI] [PubMed]
- 12.Li Q, Li Q, Chen J, et al. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013;2:1215–1222. doi: 10.3892/or.2013.2570. [DOI] [PubMed] [Google Scholar]
- 13.Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. PNAS. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]