Abstract
Extreme cold environments are potential reservoirs of microorganisms producing unique and novel enzymes in response to environmental stress conditions. Such cold-adapted enzymes prove to be valuable tools in industrial biotechnology to meet the increasing demand for efficient biocatalysts. The inherent properties like high catalytic activity at low temperature, high specific activity and low activation energy make the cold-adapted enzymes well suited for application in various industries. The interest in this group of enzymes is expanding as they are the preferred alternatives to harsh chemical synthesis owing to their biodegradable and non-toxic nature. Irrespective of the multitude of applications, the use of cold-adapted enzymes at the industrial level is still limited. The current review presents the unique adaptive features and the role of cold-adapted enzymes in major industries like food, detergents, molecular biology and bioremediation. The review highlights the significance of omics technology i.e., metagenomics, metatranscriptomics and metaproteomics in enzyme bioprospection from extreme environments. It further points out the challenges in using cold-adapted enzymes at the industrial level and the innovations associated with novel enzyme prospection strategies. Documentations on cold-adapted enzymes and their applications are abundant; however, reports on the role of omics tools in exploring cold-adapted enzymes are still scarce. So, the review covers the aspect concerning the novel techniques for enzyme discovery from nature.
Keywords: Psychrophiles, Cold-adapted enzymes, Flexibility, Industrial applications, Omics tools
Introduction
Temperature is one of the major criteria for an organism's survival as it affects the cell structure and function. The previously assumed lifeless extreme cold ecosystems are now known to harbor rich and diverse microbial communities (Anesio et al. 2017). Life in an icy habitat is full of challenges; consequently, microorganisms thriving in harsh environments have suitably gained efficient survival strategies. A key factor for adaptation undoubtedly lies in the protein functionality, which is a major driver of metabolism and cell cycle. Cold-adapted enzymes (CAEs) are considered crucial for a psychrophilic lifestyle as the high specific activity of such enzymes compensate for the detrimental effects of low temperature. Their significance in comprehending the molecular basis of cold adaptation and a humongous biotechnological potential have aptly grasped the focus of modern researchers on cold-adapted bacteria known as psychrophilic/psychrotrophic bacteria (Ghosh and Pulicherla 2021). Much interest has developed in the isolation and identification of this group of bacteria as they are the potent sources of biocatalysts that remain functional under low to moderate temperatures. Microbial enzymes offer better alternatives to chemical synthesis with respect to their biodegradability, non-toxic nature, high selectivity, and high yields (Bruno et al. 2019). The global enzyme market value was estimated to be $9.9 billion in 2019 and is estimated to grow at a growth rate of 7.1% in the year 2020–2027 (GrandViewResearch 2020). Furthermore, it is projected that enzymatic reactions can replace up to 40% of the industrial chemical reactions that utilize toxic solvents (Bruno et al. 2019). In the quest of meeting the growing demands for novel biocatalysts with unique and special properties, many extreme environments are being explored for microorganisms as efficient sources. Amid the extremophilic microbial groups, psychrophilic microbes are major sought-after sources for industrial applications (Al-Ghanayem and Joseph 2020). Psychrophiles have successfully colonized extremely low-temperature environments, frequently experiencing temperatures close to or below 0 °C, extending from the Arctic and Antarctic, polar and Himalayan glaciers, sea ice, cold deserts, permafrosts to the deep sea and ocean bottoms (Bhatia et al. 2021). The unprecedented applications of cold-adapted enzymes in several industries provide a huge potential for the enzyme market in the near future.
Recent reports on cold-adapted enzymes have described their targeted applications in particular industries. The review article by Al-Ghanayem and Joseph (2020) summarised and emphasized the role of cold-active enzymes in the detergent industry, where they have outlined the different enzymes used as detergent additives. Further, they have discussed the methods used for their commercial development and the associated scope and challenges in eco-friendly and sustainable product development. The other article focused on the applications of cold-active enzymes in food processing and molecular biology and briefly described the structural features of these enzymes (Mangiagalli et al. 2020). Another recent review discussed the different psychrophilic microorganisms as a source of cold-adapted enzymes, their mechanisms of adaptation in a cold environment, and their application in different industries (Bhatia et al. 2021). In addition to the existing literature, the current review article highlights the role of different omics techniques in novel enzyme discovery. Comparison of the activity and kinetic parameters among mesophilic and psychrophilic enzymes to understand the advantages and disadvantages of the use of cold-adapted enzymes as industrial biocatalysts have also been outlined.
Overall, the current article thoroughly reviewed the recently reported cold-adapted enzymes, focusing on their chief adaptive features in response to cold and their applications in diverse industries. Additionally, the limitations in using cold-adapted enzymes at an industrial scale and future directions are discussed to fuel the ongoing psychrophilic enzyme research.
Adaptive features of cold-adapted enzymes
The surrounding temperature has a direct effect on the activity of an enzyme. The concept of the inherent flexibility in CAEs and high specific activity at lowered temperatures has long been introduced. Multiple techniques have been employed to assess the structural flexibility of psychrophilic enzymes, including molecular dynamics simulations (MDS), neutron scattering, EPR spectroscopy, X-ray crystallography, time-resolved fluorescence, and fluorescence quenching (Margesin 2017). These studies have specified that the increased flexibility of catalytic regions results in higher catalytic efficiency and lower thermostability. Current investigations further add to the elucidation of CAE functioning at low temperatures. For instance, recent computational data obtained from MDS and other experimental data suggest that the flexibility of the psychrophilic enzymes exists at the periphery rather than the active site of the enzyme (Arcus et al. 2020). The flexibility is mainly enhanced by the weakening of intramolecular bonds involved in protein folding and stabilizing the nascent polypeptide chain (Margesin 2017). Furthermore, it has been observed that the reduction in the number of hydrophobic and electrostatic interactions, hydrogen bonds, and disulfide bridges favour the flexibility in a cold-adapted enzyme (Al-Ghanayem and Joseph 2020; Mandelman et al. 2019). Besides, modifications in amino acid composition, including lowered arginine/lysine ratio, increased glycine residues, fewer proline residues in the loops, more alpha-helices, and more non-polar residues on the protein surface, contribute to the conformational flexibility (Al-Ghanayem and Joseph 2020). It is suggested that the formation of salt bridges among amino acids that leads to weak electrostatic interactions also contributes significantly to cold adaptation (Muñoz et al. 2017). Moreover, the prevalence of H-bonds is also responsible for maintaining protein stability (Casillo et al. 2017).
Additionally, the cold adaptation of proteins is favoured by a higher number of large loops with lesser secondary structures causing higher entropy (Al-Ghanayem and Joseph 2020). A comparative study of mesophilic and psychrophilic pectate lyase has suggested a similar mechanism of higher flexibility and some loop regions helping cold adaptation and thermal instability (Tang et al. 2019). At low-temperature conditions, a reduced proline content in cold-adapted proteins is accompanied by an increase in the number of prolyl isomerases crucial for the most rate-limiting step of protein folding, i.e., prolyl isomerization (Feller 2018). A recent study on DNA ligase derived from a psychrophilic Aliivibrio salmonicida, based on sequence comparison and homology modeling, showed greater hydrophobicity on the surface than its mesophilic homologs and indicated surface charge modifications, not flexibility, as the basis of cold adaptation (Berg et al. 2019). Crystal structure of a cold-adapted protease from a psychrophilic bacterium Pseudoalteromonas arctica PAMC 21717 indicated a wide substrate pocket size, a conserved subtilisin-like fold with a distinct amino acid triad (asp, his, and ser) in the catalytic site might account for the cold adaptation of the protein (Park et al. 2018). Similarly, MDS-based study of cold-adapted elastase revealed the presence of amino acids valine and isoleucine in the catalytic site accounting for the cold adaptation (Sočan et al. 2018). In a case study of β-galactosidase from the Antarctic archaeon Halorubrum lacusprofundi, the X-ray crystallography and MDS studies showed the mutation of just six amino acid residues could be responsible for cold adaptation (Karan et al. 2020). Another report on psychrophilic β-glucosidase from Exiguobacterium antarcticum B7 indicated oligomerization of the enzyme as a structural basis of cold adaptation, as concluded from the X-ray crystallography, in solution and molecular dynamics simulation studies (Zanphorlin et al. 2016). One unique strategy was observed in a bacterial acyl aminoacyl peptidase, whereby the tunnel joining the active site and outer protein surface was found to be widened. This possibly allowed easier substrate accessibility, enhancing the activity in cold (Brocca et al. 2016). Likewise, a dimerization of enzyme by domain swapping mechanism was reported to be responsible for the activity and stability of a cold-active acyl aminoacyl peptidase (Mangiagalli et al. 2021). Hence, cold-adapted enzymes confer a strategy to psychrophilic/psychrotrophic microorganisms to successfully colonize the cold habitats. A summary of the adaptive features embodied by the cold-adapted enzymes is shown in Fig. 1.
Fig. 1.
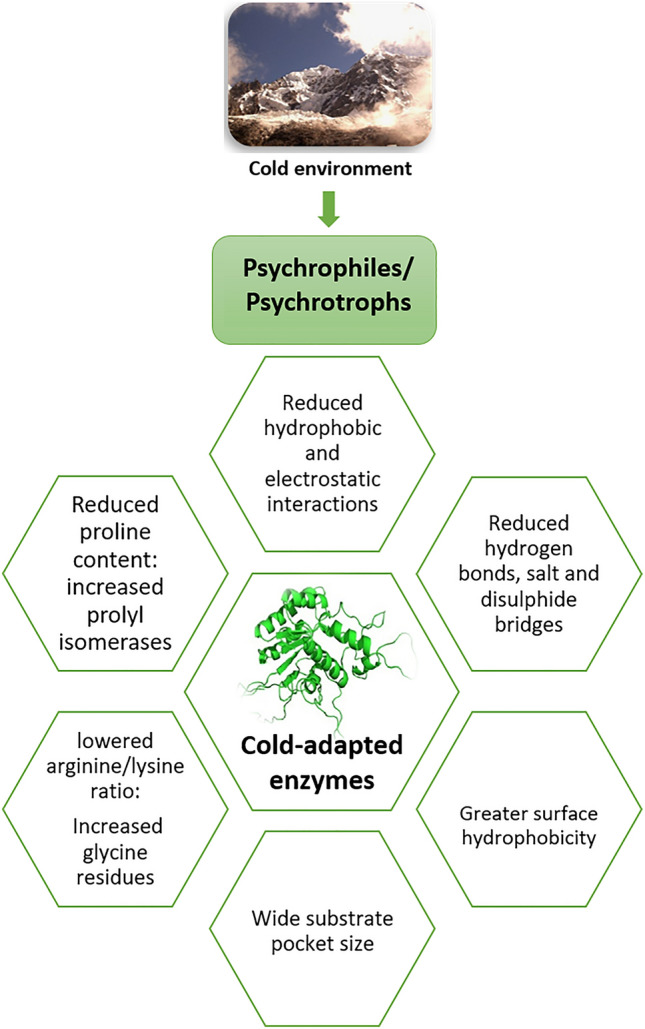
Some major structural adaptive features for the increased flexibility of cold-adapted enzymes
Applications of cold-adapted enzymes
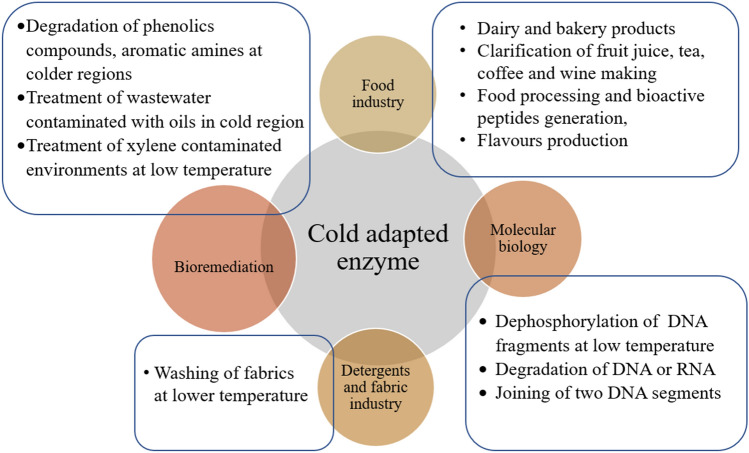
The ultimate goal of biotechnology is the delivery and usage of developed processes or technology in different industries. CAEs are well suited for various industries due to their natural properties like high catalytic activity at low temperatures, cost efficiency, limited undesirable product formation, and many more (Bruno et al. 2019). Primarily, they require lesser energy for activity as compared to their mesophilic and thermophilic counterparts. This property makes them well suited in current biotechnology industries where the prime goal is reducing the energy needs of a process. The applications of cold-adapted enzymes in various industries are discussed below and given briefly in Table 1. A brief overview of the application of cold-adapted enzymes in various industries is also shown in Fig. 2.
Table 1.
Applications of cold-adapted enzymes in various industries
| Industry | Cold-adaptive enzyme | Application | References |
|---|---|---|---|
| Food | α-Amylase | Beverages, bread, and various dairy products | Arabacı and Arıkan (2018), Sanchez et al. (2019), Rathour et al. (2020), Ottoni et al. (2020) |
| β-Galactosidase | Production of lactose-free milk products, increasing sweetness of milk products | Liu et al. (2021), Mulualem et al. (2021) | |
| Pectinase | Clarification of fruit juice, natural oils extraction, wine, coffee, and tea making | Cavello et al. (2017), Carrasco et al. (2019), Tang et al. (2019), Merín and Morata de Ambrosini (2020) | |
| Protease | Food processing (tenderizing meat at low temperatures), bioactive peptides generation | Mageswari et al. (2017), Mukhia et al. (2021a, b), Nascimento et al. (2021) | |
| Xylanase | Bread and baking industry to help in increasing softness of the bread | Qiu et al. (2017), Han et al. (2018), Liu et al. (2019), Li et al. (2020), He et al. (2020), Zang et al. (2020) | |
| Lipase | Improving flavours of various food products | De Souza et al. (2017), Musa et al. (2018), Cong et al. (2019) | |
| Detergent and fabric | Lipase | Washing of fabrics at lower temperature | Kumar et al. (2020), Sahoo et al. (2020), Phukon et al. (2020) |
| Amylase | Arabacı and Arıkan (2018), Sanchez et al. (2019), Ottoni et al. (2020) | ||
| Protease | Furhan et al. (2019), Salwan et al. (2020) | ||
| Molecular biology | Alkaline phosphatase | Dephosphorylation of the 5′ end of linearized DNA fragments at reduced temperature | Rina et al. (2000), Nandanwar et al. (2020) |
| Nucleases | Degradation of DNA or RNA to remove contaminating nucleic acids | Maciejewska et al. (2019), Wang et al. (2019) | |
| DNA ligase | Joining of two DNA segments | Berg et al. (2019) | |
| Bioremediation | Laccase | Degradation of phenolics compounds, aromatic amines, and other organic compounds at colder regions | Yang et al. (2018), Sahay et al. (2020) |
| Lipase | Bioremediation of wastewater contaminated with oils in cold regions | Fan et al. (2017), Das and Chakrabarti (2018), Miri et al. (2019), Bhandari et al. (2021) | |
| Xylene monooxygenase, Catechol 2,3-dioxygenase | Bioremediation of xylene contaminated environments at low temperature | Miri et al. (2021) | |
| Mics | Endo-β-1,4-glucanase | Biofuel production from seaweeds, cellulosic bio-ethanol production at low temperature | Song et al. (2017), Chen et al. (2020) |
| Cellulase | Cellulose processing industry requiring low temperature, saccharification of switchgrass and coffee grounds | Li et al. (2019), Ma et al. (2020), Sun et al. (2020) | |
| β-Glucosidase | Hydrolysis of cellobiose to ethanol | Wu et al. (2018), Yin et al. (2020) | |
| Lipase | Biodiesel production at low temperature | Karakaş and Arslanoğlu (2020) | |
| Mannanase | Hydrolysis of mannobiose from the non-reducing end | Xie et al. (2020) | |
| Superoxide dismutase | Antioxidative and antiapoptosis | Wang et al. (2017), Ruan et al. (2020) | |
| Cephalosporin C acylase | Low-temperature biosynthesis of 7-aminocephalosporanic acid | Tang et al. (2018) | |
| N-Acetylneuraminate lyase | Synthesis of sialic acids | Gurung et al. (2019) | |
| S-Formylglutathione hydrolase | Synthesis of glutathione and formate | Lee et al. (2019) | |
| Transaminase | Development of stereoselective amination at low temperatures | Bezsudnova et al. (2020) |
Fig. 2.
A summary of the applications of cold-adapted enzymes in various industries
Food industry
Product formation in food industries is generally carried out at low temperatures as there are possibilities of food spoilage by microbial action at higher temperatures. High-temperature treatments often lead to the alteration of nutritional value and the development of undesirable taste of the product. In this aspect, CAEs are valuable assets in food industries due to their unique properties. The functionality of these enzymes at low temperatures is favorable to minimize food spoilage. Moreover, CAEs are thermolabile and can be inactivated by a mild increase in temperature, which is further desirable in the food industry, where enzyme deactivation is needed after a specified time (Joseph et al. 2018). The application of different enzyme classes in the food industry is discussed below.
α-Amylase
Amylase helps in the degradation of complex sugars into simpler monomers and is applied in the food industry to make beverages, bread and various dairy products (Joseph et al. 2018). Cold adapted amylases are considered advantageous as they can be inactivated by a mild increase in temperature, making the process simpler (Mangiagalli et al. 2020). Recently, an amylase enzyme isolated from a cold-adapted Antarctic bacterium was found to be optimally active at 20 ℃ and suggested to be valuable for food industries (Ottoni et al. 2020). Similarly, cold-adapted amylases isolated from psychrophilic/psychrotrophic microbes having potential application in the food industry have been reported from time to time (Arabacı and Arıkan 2018; Sanchez et al. 2019; Rathour et al. 2020).
β-Galactosidase
Galactosidases hydrolyze lactose into sugar monomers, i.e., glucose and galactose, reducing the lactose content in milk products. This is essential for the alleviation of lactose intolerance and the production of more sweetened milk products (Mangiagalli et al. 2020). The hydrolysis of milk to get lactose-free products is generally carried out at lower temperatures to avoid raw milk spoilage at a higher temperature (Czyzewska and Trusek 2021). The use of cold-adapted β-galactosidases for milk hydrolysis at refrigerated temperatures to get lactose-free milk products could benefit the industry (Horner et al. 2011). Recently a cold-adapted phospho-β-galactosidase enzyme cloned from Bacillus velezensis in E. coli BL21(DE3) was capable of hydrolyzing the milk lactose at 4 ℃ that could be beneficial in the food industry (Liu et al. 2021). Similarly, in another study, the β-galactosidase gene cloned from the metagenomic library was active in a temperature range of 0–40 ℃ and had trans-glycosylation activity, which helps in the production of galacto-oligosaccharides from lactose (Mulualem et al. 2021). A detailed study on the application of cold-adapted β-galactosidases in the food industry has been discussed in a previous review (Mangiagalli and Lotti 2021).
Pectinase
Pectinase enzyme hydrolyzes pectin, thereby helping in the reduction of viscosity and clarification of a fruit juice. Pectinases are also employed in the food industry in natural oil extraction and purification, as well as in wine, coffee and tea making (Mangiagalli et al. 2020). The activity of cold-adapted pectinases at lower temperatures is beneficial in the food industry as they prevent the contamination of products at a reduced temperature which further helps in the preservation of volatile aromatic compounds in the products. In winemaking, cold-adapted pectinases are found advantageous as they help in the clarification of juices at a lower temperature, thereby improving the release of aroma and polyphenols from the raw product (Mangiagalli et al. 2020). Recently, several cold-adapted pectinases are employed for fruit juice clarification (Cavello et al. 2017; Carrasco et al. 2019; Tang et al. 2019). In another study, a cold-adapted pectinase-producing microorganism was employed for red winemaking, and the produced wine was found to possess better chromatic properties (Merín and Morata de Ambrosini 2020).
Protease
In the food industry, cold-adapted proteases have a wide range of applications. This class of cold-adapted enzymes helps in the transformation of heat-labile products at a lower temperature and maintains the flavour and nutritional values of the products by lowering the possibilities of food spoilage (Hamid and Mohiddin 2018). Cold-adapted protease is also used in meat tenderization at a lower temperature (Mageswari et al. 2017). Besides, it is employed in bioactive peptide generation from various food sources (Mukhia et al. 2021b; Nascimento et al. 2021).
Xylanase
This enzyme helps in the degradation of hemicellulose present in all flours to get simple soluble sugars. This makes the dough fluffy, voluminous and also increases its softness and elasticity (Joseph et al. 2018). This process is carried out before baking and performed at a lower temperature (Hamid and Mohiddin 2018), whereby cold-adapted xylanases have been used in the process (Dornez et al. 2011). Recently, many cold-adapted xylanases were isolated from diverse microorganisms and explored for their application in the food industry (Qiu et al. 2017; Han et al. 2018; Liu et al. 2019; He et al. 2020; Zang et al. 2020).
Lipase
Lipases are used in the food industry to improve the flavours of various food products. The lipase action commonly synthesizes various flavouring esters like ethyl caproate, ethyl lactate, and butyl butyrate. Due to the inherent properties of cold-adapted lipases, such as faster reaction time, increased flexibility and stability, low activation energy, they are useful in preparing food products (Mhetras et al. 2021). Cold-adapted lipases have been used for the synthesis of ethyl hexanoate (Musa et al. 2018), methyl and ethyl butyrate (De Souza et al. 2017), ethyl lactate, butyl butyrate, and ethyl caprylate (Cong et al. 2019), which act as flavouring agents in the food industry.
Besides, a cold-adapted 1,4-α-glucan branching enzyme isolated from a probiotic strain Bifidobacterium longum was used to convert amylopectin/amylose to linear malto-oligosaccharides, which can aid in enhancing the slow digestibility of wheat starch (Li et al. 2020). The enzyme also has a potential application in the production of low-glycaemic index soft cereal foods.
Detergent and fabric industry
The dirt in the clothes mainly consists of lipids, polysaccharides, and proteins. The removal of these stains from the fabrics requires manual heating and beating of the cloths. This, in turn, reduces the life of the fabrics and also leads to decolorization. Additionally, removing the dirt from the cloths in colder environments requires hot water, increasing energy consumption. To address these problems, enzymes are used in the detergents to improve the washing performance. Generally, the washing process is done at harsh conditions like alkaline pH and low temperature, so cold-adapted alkaline enzymes are beneficial in detergent formulations (Al-Ghanayem and Joseph 2020). In the detergent industry, commonly used cold-adapted enzymes are lipases, proteases, and amylases. Cold-adapted lipases isolated from different microorganisms have been explored for their detergent compatibility and suggested for detergent formulations (Kumar et al. 2020; Sahoo et al. 2020; Phukon et al. 2020). Amylases help in the removal of polysaccharides containing stains from the fabrics. Recently cold-adapted amylases isolated from different sources have been suggested for use as a detergent additive (Arabacı and Arıkan 2018; Sanchez et al. 2019; Rathour et al. 2020; Ottoni et al. 2020). Likewise, the protein stains present in the fabrics are removed by proteases, and cold-adapted proteases have been demonstrated as potential detergent additives (Furhan et al. 2019; Salwan et al. 2020). A detailed study on cold-adapted enzymes as detergent additives has been reviewed recently (Al-Ghanayem and Joseph 2020).
Molecular biology
Enzymes are the fundamental tools in several molecular biology applications. In general, mesophilic or sometimes thermophilic enzymes are used in molecular biology. CAEs are less explored for their use in molecular biology. However, CAEs could benefit certain techniques where thermolability is needed to denature the used enzyme after a specified time. Moreover, CAEs in molecular biology could be beneficial where the reaction proceeds at a lower temperature. Various CAEs which have applications in this field are mentioned below.
Alkaline phosphatase
The enzyme helps catalyse the dephosphorylation of the 5′ end of linearized DNA fragments, thereby preventing the dsDNA self-ligation of plasmid vectors (Zappa et al. 2001). Generally, commercially available mesophilic alkaline phosphatase isolated from the calf intestine is used for this purpose (Sambrook et al. 1989). However, the used enzyme has some drawbacks as it requires some time in inactivation owing to its thermostability (Nandanwar et al. 2020). In this regard, the use of cold-adapted alkaline phosphatase could be beneficial. Cold-adapted alkaline phosphatases from psychrophilic organisms Pandalus borealis and Alteromonas undina P2 are available commercially (Nandanwar et al. 2020). Moreover, in another study, a cold-adapted and heat‐labile alkaline phosphatase isolated from an Antarctic strain was suggested for its application in dephosphorylation of nucleic acids that could be a valuable asset in molecular biology (Lee et al. 2015).
Nucleases
Nucleases aid in DNA or RNA degradation and help remove contaminating nucleic acids from the reaction mixture. After their action, the used nucleases have to be removed from the reaction mixture, and this is generally done by degrading the enzyme mixture through an increase in the reaction temperature. Here, the use of cold-adapted nucleases would be beneficial as they can be easily degraded and inactivated by minor heat treatments. Cold-adapted nucleases isolated from psychrophilic microbes Shewanella sp. and Pandalus borealis are available commercially (Nandanwar et al. 2020). Similarly, cold-adapted nuclease isolated from a psychrophilic Psychromonas ingrahamii can be explored for its application in molecular biology (Maciejewska et al. 2019). In another study, a cold-adapted RNase enzyme was isolated from the Antarctic sea ice-inhabiting bacterium Psychrobacter that could be further studied for its application in molecular biology (Wang et al. 2019).
DNA ligase
The enzyme helps in forming phosphodiester bonds between two DNA fragments leading to the joining of those two DNA segments. The most commonly used ligase for the said purpose is isolated from bacteriophages. Frequently the optimum temperature for the action of DNA ligases in molecular biology is low, so the use of cold-adapted ligases would be beneficial (Nandanwar et al. 2020). Recently three cold-adapted DNA ligases isolated from psychrophilic organisms were characterized for their temperature optima and thermal stabilities and can be explored further for their application in molecular biology (Berg et al. 2019).
Bioremediation
There is a continuous increase in environmental pollution because of human activities. To remove the pollution from the environment, bioremediation of the pollutants is necessary where pollution from the environment is removed by the action of organisms or their products like enzymes (Okino-Delgado et al. 2019). Microbial enzymes efficiently remove pollutants from the environment and are considered inexpensive in bioremediation (Bhandari et al. 2021). However, bioremediation in a cold environment by mesophilic/thermophilic enzymes is ineffective because of the low activity of mesophilic/thermophilic enzymes in these regions (Miri et al. 2019). In this regard, CAEs, due to their inherent property, are valuable tools for bioremediation. Some CAEs valuable in bioremediations are mentioned below:
Laccase
This class of enzyme catalyses the oxidation of various phenolic compounds, aromatic amines, and other organic compounds (Bhandari et al. 2021). They help in the degradation of various xenobiotic compounds and further aid in the bioremediation of polluted water. In a study, the laccase enzyme isolated from Aspergillus nidulans was active in a temperature range of 10–80 °C and helped decolorize various dyes and be useful for environmental bioremediation (Sahay et al. 2020). A cold-adapted laccase enzyme cloned from a metagenomic library showed potential in the bioremediation of dyes and prove beneficial in polluted water bioremediation (Yang et al. 2018).
Lipase
Lipases catalyse the degradation of oils and help in the bioremediation of soil or water contaminated with oil residues and petroleum contaminants (Bhandari et al. 2021). Cold-adapted lipases are useful in the bioremediation of wastewater contaminated with oils in cold regions. Cold-adapted lipases are regularly explored for their application in the bioremediation of oil-contaminated environments (Fan et al. 2017; Das and Chakrabarti 2018; Miri et al. 2019).
Other enzymes
Cold-adapted amylases isolated from different microbes are explored for the bioremediation of agricultural and household waste (Arabacı and Arıkan 2018; Ottoni et al. 2020). In a study, cold-adapted endoxylanase was reported for its potential bioremediation application (He et al. 2020). Another study explored the use of cold-adapted xylene monooxygenase and catechol 2,3-dioxygenase to degrade p-xylene and could be employed for bioremediation of xylene contaminated environments at low temperatures (Miri et al. 2021).
Additional applications
CAEs have applications in other biotechnological industries besides mentioned above. One important biotechnological application of CAEs lies in the formation of biofuels. As we know, due to the continuous consumption of petroleum fuels and their limited existence, there is a need to look for an alternative energy source. Biofuels such as ethanol are considered an alternative to petroleum fuels, and they can be generated by the enzymatic hydrolysis of cellulose or related biomass. The generation of biofuels at colder regions by CAE treatment of biomass is beneficial as they are more active at such regions than their mesophilic counterparts. Additionally, the generation of cellulosic bio-ethanol or related biofuels requires low-temperature degradation of the biomass, so the use of CAEs is beneficial in this aspect (Chen et al. 2020). Recently, many CAEs isolated from different organisms were explored for biofuel generation, which can be considered for industrial-scale production (Song et al. 2017; Wu et al. 2018; Li et al. 2019; Chen et al. 2020; Karakaş and Arslanoğlu 2020; Ma et al. 2020; Sun et al. 2020; Xie et al. 2020; Yin et al. 2020).
CAEs also have potential applications in the synthesis of various pharmaceutical compounds. The synthesis of pharmaceutical compounds requires extreme reaction conditions like low temperature and solvent stability, so CAEs, due to their inherent property, are advantageous in this aspect (Noby et al. 2020). In recent times, CAEs are being studied for their application in the synthesis of various pharmaceutical compounds and further large-scale production of such compounds (Wang et al. 2017; Noby et al. 2018; Tang et al. 2018; Gurung et al. 2019; Lee et al. 2019; Bezsudnova et al. 2020; Ruan et al. 2020; Mhetras et al. 2021). A cold-adapted epoxide hydrolase gene, isolated from Sphingophyxis alaskensis, was cloned in E. coli, and the purified enzyme showed activity at 10 ℃ (Woo et al. 2007). The purified enzyme could be beneficial in preparing enantiopure epoxides, which are used in the pharmaceutical industry.
The other major classes of cold-adaptive enzymes used in the industries include oxidoreductases and lyases. Oxidoreductases are the class of enzymes that helps in the transfer of electrons between molecules. Numerous cold-adapted oxidoreductases have been isolated and purified from psychrophiles as psychrotrophic organisms having application in various industries. Cold-adapted alanine dehydrogenases are oxidoreductases that help generate optically active amino acids at cold temperature and have been isolated from microbes inhabiting cold temperature regions (Galkin et al. 1999; Irwin et al. 2001, 2003). Leucine dehydrogenase enzyme is used to synthesize l-tert-leucine, an intermediate in synthesizing several chiral drugs (Jiang et al. 2016). Several cold-adapted leucine dehydrogenases capable of functioning at low temperatures have been purified and used to synthesize chiral drugs (Zhao et al. 2012; Li et al. 2014; Wang et al. 2018). Superoxide dismutase is another sub-class of oxidoreductase which has an antioxidative property that helps in reducing the oxidative damage of cells (Ruan et al. 2020). Several cold-adapted superoxide dismutase enzymes have been explored from the psychrophiles and used to prepare food, cosmetics, and healthcare products (Zheng et al. 2006; Castellano et al. 2006; García Echauri et al. 2009; Abrashev et al. 2016; Wang et al. 2016, 2017, 2020).
Lyases are the enzyme class that includes enzymes involved in the breakage of chemical bonds in molecules through a process other than hydrolysis and oxidation. Carbonic anhydrase is one such lyase involved in the catalysing of CO2 hydration to form bicarbonates (Vullo et al. 2015). Numerous carbonic anhydrase enzymes have been isolated from cold-inhabiting microbes with potential application in the synthesis of pharmacological agents or as agents for regulating acid–base homeostasis (Vullo et al. 2015; De Luca et al. 2015, 2016a, b). Besides carbonic anhydrase, another sub-class of lyase known as pectate lyase has been isolated from psychrophilic/psychrotrophic microbes with potential application in textile industry (Laurent et al. 2001; Margesin et al. 2005; Yuan et al. 2012; Mukhopadhyay et al. 2015; Tang et al. 2019).
Comparison of cold-adapted enzymes with mesophilic enzymes
Cold-adapted enzymes have huge market potential in biotechnology industries because of their inherent properties, making them a better catalyst at low temperatures. Cold-adapted enzymes have catalytic advantages at low temperatures, and compared to their mesophilic counterparts, they can perform well in extreme conditions. Therefore, the cold-adapted enzymes have been studied for their specific activity and kinetic parameters compared to their commercial and non-commercial mesophilic counterparts. In one such study, the activity and kinetic parameters of a cold-adapted β-galactosidase enzyme isolated from Antarctic psychrophile Pseudoalteromonas haloplanktis were compared to mesophilic commercial β-galactosidase isolated from E. coli (Hoyoux et al. 2001). The study showed that the optimal temperature of activity for cold-adapted β-galactosidase was 10 ℃ lower than its mesophilic counterparts. Additionally, the P. haloplanktis enzyme showed better kcat, Km, and (kcat/Km) values than its mesophilic counterpart at lower temperatures. These kinetic parameters showed that the cold-adapted β-galactosidase is better suited to work in colder temperatures compared to its mesophilic enzyme counterpart. In another study, the activity of a cold-adapted β-galactosidase enzyme isolated from Antarctic Rahnella inusitata was compared to the commercial mesophilic enzyme of Aspergillus oryzae, and it was found that the cold-adapted enzyme retained higher relative activity at lower temperatures than the commercial enzyme (Núñez-Montero et al. 2021). In a similar study, the specific activity and kinetic parameters of cold-active protease isolated from psychrophilic bacterium were compared with the commercially available mesophilic subtilisin Carlsberg from Bacillus licheniformis (Sigma-Aldrich) (Park et al. 2018). The results showed that the cold-active protease showed a specific activity of 160.8 U/mg at 10 ℃ while the commercial protease showed an activity of 22.6 U/mg at the same temperature. Additionally, the cold-active protease showed better Kcat, Km, and Vmax values than the commercial enzyme. In another report, the influence of temperature on the activity of cold-adapted aspartate transcarbamylase enzyme isolated from psychrophilic bacterial strain TAD1 was compared with its mesophilic counterpart isolated from the mesophilic E. coli (Sun et al. 1998). The results showed that the activity of the cold-adapted enzyme was maximum in the temperature range of 0–30 ℃, while the activity of its mesophilic counterpart was maximum above 35 ℃; moreover, the substrate specificity of the cold-adapted enzyme was higher than that of the mesophilic enzyme. These studies pointed out that the cold-adapted enzyme showed better catalytic activity, substrate specificity, and turnover number at low temperatures than their mesophilic counterparts. However, there are some disadvantages of cold-adapted enzymes like low thermostability and loss of activity at a moderately higher temperature. Considering all the factors, cold-adapted enzymes can be a valuable tool in biotechnology, where low-temperature conditions are required for a chemical reaction. A detailed comparison of activity and kinetic parameters of cold-adapted enzymes with their commercial and non-commercial mesophilic enzymes is presented in Table 2.
Table 2.
Comparison of cold-adapted enzymes with commercial and non-commercial mesophilic enzymes in terms of kinetic parameters
| Enzyme | Enzyme source | Temperature (℃) | Vmax (U/mg) | kcat (s) | kcat/Km | Km (mM) | References |
|---|---|---|---|---|---|---|---|
| β-Galactosidase | Psychrophilic Pseudoalteromonas haloplanktis | 25 | – | 33 | 13.7 (/s/mM) | 2.4 | Hoyoux et al. (2001) |
| Mesophilic E. coli | – | 2 | 0.15 (/s/mM) | 13 | |||
| Protease | Pseudoalteromonas arctica | 10 | 1.83 | 1.04 | – | 0.65 | Park et al. (2018) |
| Mesophilic subtilisin Carlsberg from Bacillus licheniformis (Sigma-Aldrich) | 0.31 | 0.14 | – | 0.02 | |||
| Alcohol dehydrogenase | Antarctic Moraxella sp. | 25 | – | – | 946.7 (/min/mM) | 0.58 | Tsigos et al. (1998) |
| Mesophilic Horse liver ADH | – | – | 100 (/min/mM) | 0.6 | |||
| Aspartate transcarbamylase | Psychrophilic bacterial strain TAD1 | – | – | – | – | 1.5 | Sun et al. (1998) |
| Mesophilic E. coli | – | – | – | 11.4 | |||
| Ornithine Transcarbamylase | Psychrophilic Moritella abyssi | 5 | – | – | – | 1.78 | Xu et al. (2003) |
| E. coli | 37 | – | – | – | 2.4 | ||
| α-Amylase | Psychrophilic Pseudoalteromonas haloplanktis | – | – | 294 | – | – | D'Amico et al. (2003) |
| Pig pancreas | – | – | 97 | – | – | ||
| Arginine kinase | Deep-sea Clam Calyptogena kaikoi | 25 | – | 3.3 | – | – | Suzuki et al. (2012) |
| Mesophilic Corbicula | 13.4 | – | – |
Role of omics in novel enzyme discovery
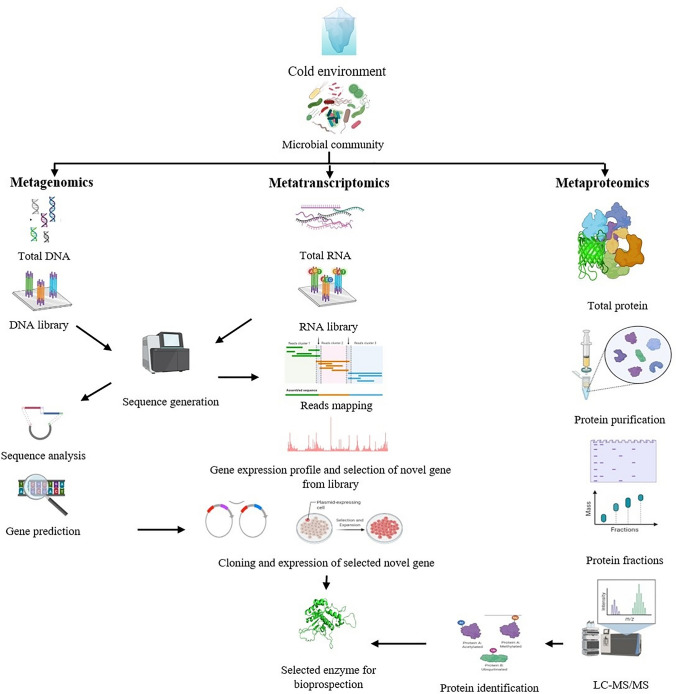
Currently, the term “omics” has become popular in the field of enzymology. The approaches of “omics” are relevant in the discovery and development of novel efficient extremozymes for industrial applications. Furthermore, the use of bioinformatics in gene annotations and comparative genome analyses offer unprecedented evidence for functional genes and enzymatic pathways that enhance our overall understanding of an organism’s metabolic and enzymatic potentials (Zhu et al. 2020). To begin with, the bacterial whole-genome sequencing has been greatly significant in deciphering the survival strategies of psychrophilic bacteria in cold environments (Kumar et al. 2018, 2019). Genome sequence data has enabled enzyme prediction in psychrophilic/psychrotrophic bacteria obtained from extreme niches of the glacier ecosystem (Kumar et al. 2015a, b, 2018, 2019; Pal et al. 2017). Comparative genomics and molecular adaptation analysis of psychrotrophic Arthrobacter strains revealed the prevalence of cold-adapted proteins, with the tryptophan amino acid being favourable over tyrosine for thermal stability of cold-adapted enzymes (Mukhia et al. 2021a). A recent study on the enzymatic potential of Psychrobacter sp. utilized comparative genomic approaches for identifying a gene for cold-active protease that can be cloned and expressed for further characterization (Perfumo et al. 2020). A similar approach can be adopted for finding other important bacterial biocatalysts useful for industrial processes. The modern genomic approaches are useful in providing relevant information on the structure and mechanisms of enzymes. This allows a deeper understanding of the cold-active enzymes regarding their compatibility as detergent additives (Al-Ghanayem and Joseph 2020). For instance, genome-based computational analysis of a psychrotrophic Chryseobacterium polytrichastri ERMR1:04 helped predict the structure of its lipase enzyme, its broad temperature activity, and substrate specificity for developing a potential detergent formulation (Kumar et al. 2020). For the maximum discovery of industrially relevant enzymes with novel properties from microbial sources, the use of the culture-free technique of metagenomics is very valuable. Metagenomics allows the isolation of genetic material directly from the environmental samples for further screening and finding novel enzymes (Madhavan et al. 2017). Function-based metagenomic screening and sequence-based metagenomic approaches are commonly used for enzyme discovery (Madhavan et al. 2017). These approaches can be adopted for the identification and isolation of new cold-adapted enzymes having novel properties. Recently, a cold-active type-I pullulanase enzyme was isolated from a hot spring metagenome library that was found to be active at low temperature and effective in starch debranching (Thakur et al. 2021). In other recent studies, the cold-active esterase gene was identified from the permafrost (Kryukova et al. 2019) and deep-sea sponge (Borchert et al. 2017) metagenomic libraries that were further cloned and expressed in the E. coli host. Similarly, functional metagenomics identified a cold-active α-amylase from a cold environment homologous to α-amylase of Clostridia, retained above 70% relative activity at 1 °C, and showed useful properties as a detergent enzyme (Vester et al. 2015). In one interesting study, functional metagenomics enabled the finding of multiple enzymes i.e., α-amylase, β-galactosidase, phosphatase, from the permanently cold Ikaite columns that showed low sequence homology but exhibited functional properties (Vester et al. 2014). The cold-adapted β-galactosidase showed lactose hydrolysis and hence potential candidature for the dairy industry. Lipase is an important industrial enzyme, and the metagenomics approach has been useful in its discovery from cold sources such as deep-sea sediment (Chan et al. 2016); marine sponge Stelletta normani (Borchert et al. 2017), Ircinia sp. (Su et al. 2015); Siberian permafrost (Petrovskaya et al. 2017) and many others. In another instance, a gene for novel cold-active and metal ion-tolerant xylanase enzyme was isolated from the metagenome of frozen soil and recombinantly expressed in E. coli (Qiu et al. 2017). One recent study reported a cold-adapted, thermostable laccase-like enzyme from a marine metagenomic library, which retained above 40% maximal activity at 10 °C. The enzyme showed superior decolorization ability as well as tolerance to salt and organic solvents and finds potential use in the bioremediation of dye wastewater (Yang et al. 2018). Alkaline phosphatase plays a pivotal role in molecular biology, particularly the one with thermolabile nature. The first metagenomic thermolabile alkaline phosphatase with efficient DNA dephosphorylating property was isolated from ocean-tidal flat sediment (Lee et al. 2015). Therefore, the metagenomic approaches have undoubtedly facilitated the screening, isolation, and bioprospection of cold-adapted enzymes from extreme niches.
In addition to metagenomics, the metatranscriptomic approach could be very advantageous in novel enzyme discovery. The metatranscriptomic approach has allowed the study of gene expression patterns of a microbial community in response to particular environmental conditions (Hu et al. 2019). Likewise, the metatranscriptomic study of the cold environments could help us understand the key genes involved in microbial cold adaptation and survival at these niches. Furthermore, the targeted metatranscriptomic study of the hydrolytic enzymes expressed in the cold habitat could help discover novel cold-adapted enzymes (Raymond-Bouchard and Whyte 2017). Besides, metatranscriptomics and recombinant DNA technology could aid in the quicker and economical discovery of cold-adapted enzymes with superior activities (Białkowska and Turkiewicz 2014). The approach has been used recently in the discovery of novel hydrolytic enzymes from various habitats like compost microbiome (Mello et al. 2017), sheep rumen microbiome (He et al. 2019), and metal-contaminated soil (Mukherjee et al. 2019). Despite the vast advantage of metatranscriptomics over the conventional approaches, the use of metatranscriptomics in the novel cold-adapted enzyme discovery is limited and could be investigated further for quicker and economic exploration.
Metaproteomics is another emerging discipline for exploring the functional capabilities of microbial populations in extreme niches (Maseh et al. 2021). This approach enables the prospecting of entire microbial enzymes present in an environment through top-down or bottom-up methods (Abiraami et al. 2020). Bottom-up or shot-gun approach is the commonly accepted one that involves protein digestion and feeding into LC–MS for protein identification. In contrast, the top-down method directly detects the intact protein by LC–MS. With recent advancements in high-throughput mass spectrometry, protein separation systems, and updated databases for documentation, the field of metaproteomics is progressing. It may provide a promising future for bioprospection of novel industrial enzymes from extreme niches. The technique has been applied for the elucidation of proteins in the microbiota of fermented food (Ji et al. 2017), anaerobic fermentation system (Jia et al. 2017), marine sponge microbial symbionts etc. (De Mares et al. 2018), yet its application in extreme cold regions like glaciers and permafrosts remains to be explored. Altogether, the research on metagenomic, metatranscriptomic and metaproteomic approaches for extremophilic enzymes is escalating and opening up new opportunities for the discovery of novel enzymes from nature. A summary of the role of omics tools in novel enzyme discovery is described in Fig. 3.
Fig. 3.
The role of omics tools in the discovery of novel enzymes. Figure created using BioRender (https://biorender.com/)
Challenges and future directions
Regardless of the enthralling aspects and humongous applications of psychrophilic enzymes, there still lie limitations in the industrial applications of these enzymes on a large scale. To begin with, the sampling and cultivation of microorganisms from extreme environments in itself are challenging. Although low-temperature habitats are known to dominate the earth's biosphere, which makes psychrophiles/psychrotrophs to be one of the most abundant extremophiles to exist (Collins and Margesin 2019), their cultivation is limited to a very low number. The unique and fascinating features of these organisms that confer biotechnological significance pose complications in handling and cultivation. Traditional methods of enrichment and isolation may support the growth and proliferation of fast-growing organisms while subduing the growth of cold-adapted microbes with slow metabolic rates (Cario et al. 2019). Adequate and efficient strategies are necessary to improve the existing cultivation techniques to make them appropriate for psychrophiles. Innovations in terms of improved and extended in situ cultivation techniques that resemble the natural environmental conditions can be targeted for the better recovery of novel cold-loving microorganisms. Furthermore, finding the desired enzyme for targeted application is challenging as it requires precise methods for selection from huge natural biodiversity. The persistent implementation of omics approaches could fill the gaps associated with inefficient culturing techniques and advanced enzymatic pathway predictions (Usmani et al. 2021). The dearth of appropriate genetic tools for metabolic engineering of psychrophilic enzymes to enhance the catalytic efficiency and product yield is a limiting factor (Zhu et al. 2020). Likewise, the equipment and instruments tend to show low tolerance to extreme conditions operative during enzyme processing which reduces their efficiency and lifespan (Bhatia et al. 2021). The development of molecular tools and bioprocessing equipment that will endure extreme conditions would help overcome some industrial limitations. Low-temperature catalysis is particularly significant in food industries; however, its use is still limited by many factors, such as costly enzyme isolation and purification procedure, low stability of the enzymes, and minimal exploration of cold niches for novel cold-adapted microbial sources (Kuddus 2018). The approaches of genetic engineering in the expression of psychrophilic enzymes in mesophilic hosts using efficient systems will lead to enhanced production of recombinant enzymes. This may provide solutions to the existing bottlenecks associated with large-scale downstream processing to meet the commercial requirements. More efforts are required to identify inexpensive nutrient sources for achieving large-scale economic production of cold-adapted enzymes through targeted strain improvement. Further, extensive in-depth study of enzyme crystal structures can contribute significantly to deciphering structure–function relationships that will ultimately aid industrial applications (Furhan 2020). Additionally, the omics approaches could offer useful perspectives for developing cost-effective and economically viable biocatalysts. Upcoming challenges for the industrial use of cold-adapted enzymes will include meeting the demand for tailored and customised, stable biocatalysts from the massive pool of uncultivable microorganisms by means of next generation “omics” techniques. Nonetheless, many practical challenges are associated with implementing metagenomic analysis (Sarmiento et al. 2015). The extraction of high-quality DNA and cell recovery from extremophiles of environmental samples is difficult, and suitable host–vector combinations are required for the expression of recovered metagenomic genes, which restricts the potential of functional metagenomics (Ahmad et al. 2019). Moreover, metagenomic approaches for enzyme discovery rely on the gene sequence homology to enzymes in the database, which might impede the finding of very novel enzymes, particularly in relatively obscure psychrophiles. One major challenge for psychrophilic enzymes lies in the vast technological gap between laboratory-based enzyme production and the accomplishment of an ultimate commercial product ready for marketing (Zhu et al. 2020; Bhatia et al. 2021). One key factor can be the extensive time frame taken from the discovery to the commercialization of a bacterial enzyme (Ferrer et al. 2019). The entire process is time-consuming as it includes multiple steps of finding the efficient enzyme through approaches of bioinformatics, systems biology, genome engineering, cloning and expression, and functional metagenomics that fit the industrial requirements. Enzymes must undergo a series of processing through mutagenesis and protein engineering to be industry-ready (Bhatia et al. 2021). Therefore, the development of appropriate host–vector expression systems for metagenomics libraries and more refined methods for protein engineering, and highly efficient high-throughput technologies are crucial for encountering the current challenges. Finally, the technological gap can be effectively addressed by innovations through the integration of academic or research institutes and industrial partners for streamlined and shortened technical and pipeline developments (Ferrer et al. 2019).
Conclusion
Exploring the microbial communities of extremely cold environments has contributed significantly to understanding psychrophilic lifestyle, their physiological and molecular mechanisms of adaptation. This, in turn, would be relevant for further applications of psychrophilic enzymes through protein engineering and evolution. With an increasing demand for cheap, renewable, and eco-friendly alternatives to the wide utilization of harsh chemicals in industrial processes, as well as rising concerns over environmental and economic issues, extreme environments have been targeted as sources of efficient biocatalysts. Despite the enormous benefits, cold-adapted enzyme technology faces setbacks for the lab-scale journey to the industry. In the coming years, the untapped microbial resources may be exploited by implementing structure-based protein engineering and directed evolution to develop broad substrate-specific cold-adapted enzymes (Nandanwar et al. 2020). Further understanding of in-depth enzymatic machinery would promote innovations in these biocatalysts’ novel applications, further promoting the ‘white biotechnology.'
Acknowledgements
AK acknowledges the Department of Biotechnology (DBT), Ministry of Science and Technology Government of India, for the PhD studentship award (DBT/JRF/BET-17/I/2017/AL/367). SM acknowledges the Indian Council of Medical Research (ICMR), Government of India, for Senior Research Fellowship (SRF) award (No. 45/17/2020-/BIO/BMS). RK acknowledges the Department of Science and Technology (DST), Government of India, for the DST INSPIRE faculty award (DST/INSPIRE/04/2014/001280). Authors acknowledge the financial support by the CSIR in-house project MLP00201, NMHS project of MoEF&CC (NMHS sanction no. GBPNI/NMHS-2018-19/SG/178), Science and Engineering Research Board Start-up research Grant no. SRG/2019/001071) and DST-TDT project no. DST/TDT/WM/2019/43. This manuscript represents CSIR-IHBT communication no. 4838.
Author contributions
AK, SM: conceptualization, writing—original draft. RK: conceptualization, writing—review and editing, supervision, project administration, funding acquisition.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Anil Kumar and Srijana Mukhia contributed equally.
References
- Abiraami TV, Singh S, Nain L. Soil metaproteomics as a tool for monitoring functional microbial communities: promises and challenges. Rev Environ Sci Bio/technol. 2020;19:73–102. doi: 10.1007/s11157-019-09519-8. [DOI] [Google Scholar]
- Abrashev R, Feller G, Kostadinova N, et al. Production, purification, and characterization of a novel cold-active superoxide dismutase from the Antarctic strain Aspergillus glaucus 363. Fungal Biol. 2016;120:679–689. doi: 10.1016/j.funbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Singh RS, Gupta G et al (2019) Metagenomics in the search for industrial enzymes. In: Singh RS, Singhania RR, Pandey A, Larroche C (eds) Biomass, biofuels, biochemicals: advances in enzyme technology. Elsevier, pp 419–451. 10.1016/B978-0-444-64114-4.00015-7
- Al-Ghanayem AA, Joseph B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl Microbiol Biotechnol. 2020;104:2871–2882. doi: 10.1007/s00253-020-10429-x. [DOI] [PubMed] [Google Scholar]
- Anesio AM, Lutz S, Chrismas NAM, Benning LG. The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes. 2017;3:1–11. doi: 10.1038/s41522-017-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabacı N, Arıkan B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep Biochem Biotechnol. 2018;48:419–426. doi: 10.1080/10826068.2018.1452256. [DOI] [PubMed] [Google Scholar]
- Arcus VL, van der Kamp MW, Pudney CR, Mulholland AJ. Enzyme evolution and the temperature dependence of enzyme catalysis. Curr Opin Struct Biol. 2020;65:96–101. doi: 10.1016/j.sbi.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Berg K, Leiros I, Williamson A. Temperature adaptation of DNA ligases from psychrophilic organisms. Extremophiles. 2019;23:305–317. doi: 10.1007/s00792-019-01082-y. [DOI] [PubMed] [Google Scholar]
- Bezsudnova EY, Stekhanova TN, Ruzhitskiy AO, Popov VO. Effects of pH and temperature on (S)-amine activity of transaminase from the cold-adapted bacterium Psychrobacter cryohalolentis. Extremophiles. 2020;24:537–549. doi: 10.1007/s00792-020-01174-0. [DOI] [PubMed] [Google Scholar]
- Bhandari S, Poudel DK, Marahatha R, et al. Microbial enzymes used in bioremediation. J Chem. 2021;2021:1–17. doi: 10.1155/2021/8849512. [DOI] [Google Scholar]
- Bhatia RK, Ullah S, Hoque MZ, et al. Psychrophiles: a source of cold-adapted enzymes for energy efficient biotechnological industrial processes. J Environ Chem Eng. 2021;9:104607. doi: 10.1016/j.jece.2020.104607. [DOI] [Google Scholar]
- Białkowska A, Turkiewicz M. In: Miscellaneous cold-active yeast enzymes of industrial importance. Buzzini P, Margesin R, editors. Berlin, Heidelberg: Cold-adapted yeasts. Springer; 2014. [Google Scholar]
- Borchert E, Selvin J, Kiran SG, et al. A novel cold active esterase from a deep sea sponge Stelletta normani metagenomic library. Front Mar Sci. 2017;4:287. doi: 10.3389/fmars.2017.00287. [DOI] [Google Scholar]
- Brocca S, Ferrari C, Barbiroli A, et al. A bacterial acyl aminoacyl peptidase couples flexibility and stability as a result of cold adaptation. FEBS J. 2016;283:4310–4324. doi: 10.1111/febs.13925. [DOI] [PubMed] [Google Scholar]
- Bruno S, Coppola D, Di Prisco G, et al. Enzymes from marine polar regions and their biotechnological applications. Mar Drugs. 2019;17:1–36. doi: 10.3390/md17100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario A, Oliver GC, Rogers KL. Exploring the deep marine biosphere: challenges, innovations, and opportunities. Front Earth Sci. 2019;7:1–9. doi: 10.3389/feart.2019.00225. [DOI] [Google Scholar]
- Carrasco M, Rozas JM, Alcaíno J, et al. Pectinase secreted by psychrotolerant fungi: identification, molecular characterization and heterologous expression of a cold-active polygalacturonase from Tetracladium sp. Microb Cell Fact. 2019;18:1–11. doi: 10.1186/s12934-019-1092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillo A, Parrilli E, Sannino F, et al. Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: a strategy for cryoprotection. Carbohydr Polym. 2017;156:364–371. doi: 10.1016/j.carbpol.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano I, Di Maro A, Ruocco MR, et al. Psychrophilic superoxide dismutase from Pseudoalteromonas haloplanktis: biochemical characterization and identification of a highly reactive cysteine residue. Biochimie. 2006;88:1377–1389. doi: 10.1016/j.biochi.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Cavello I, Albanesi A, Fratebianchi D, et al. Pectinolytic yeasts from cold environments: novel findings of Guehomyces pullulans, Cystofilobasidium infirmominiatum and Cryptococcus adeliensis producing pectinases. Extremophiles. 2017;21:319–329. doi: 10.1007/s00792-016-0904-0. [DOI] [PubMed] [Google Scholar]
- Chan Z, Wang R, Yang F, Zeng R. Enhanced cold active lipase production by metagenomic library recombinant clone CALIP3 with a step-wise temperature and dissolved oxygen level control strategy. Chin J Chem Eng. 2016;24:1263–1269. doi: 10.1016/j.cjche.2016.04.033. [DOI] [Google Scholar]
- Chen F, Ye J, Sista Kameshwar AK, et al. A novel cold-adaptive endo-1,4-β-glucanase From Burkholderia pyrrocinia JK-SH007: gene expression and characterization of the enzyme and mode of action. Front Microbiol. 2020;10:3137. doi: 10.3389/fmicb.2019.03137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Margesin R. Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Appl Microbiol Biotechnol. 2019;103:2857–2871. doi: 10.1007/s00253-019-09659-5. [DOI] [PubMed] [Google Scholar]
- Cong S, Tian K, Zhang X, et al. Synthesis of flavor esters by a novel lipase from Aspergillus niger in a soybean-solvent system. 3 Biotech. 2019;9:244. doi: 10.1007/s13205-019-1778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzewska K, Trusek A. Encapsulated nolaTM fit 5500 lactase—an economically beneficial way to obtain lactose-free milk at low temperature. Catalysts. 2021;11:527. doi: 10.3390/catal11050527. [DOI] [Google Scholar]
- D’Amico S, Marx JC, Gerday C, Feller G. Activity-stability relationships in extremophilic enzymes. J Biol Chem. 2003;278:7891–7896. doi: 10.1074/jbc.M212508200. [DOI] [PubMed] [Google Scholar]
- Das A, Chakrabarti K. A cold tolerant lipase develops enhanced activity, thermal tolerance and solvent stability in the presence of calcium nanoparticles: an alternative approach to genetic modulation. Biocatal Agric Biotechnol. 2018;15:1–10. doi: 10.1016/j.bcab.2018.05.002. [DOI] [Google Scholar]
- De Luca V, Del Prete S, Carginale V, et al. Cloning, characterization and anion inhibition studies of a γ-carbonic anhydrase from the Antarctic cyanobacterium Nostoc commune. Bioorg Med Chem Lett. 2015;25:4970–4975. doi: 10.1016/j.bmcl.2015.03.010. [DOI] [PubMed] [Google Scholar]
- De Luca V, Del Prete S, Vullo D, et al. Expression and characterization of a recombinant psychrophilic γ-carbonic anhydrase (NcoCA) identified in the genome of the Antarctic cyanobacteria belonging to the genus Nostoc. J Enzyme Inhib Med Chem. 2016;31:810–817. doi: 10.3109/14756366.2015.1069289. [DOI] [PubMed] [Google Scholar]
- De Luca V, Vullo D, Del Prete S, et al. Cloning, characterization and anion inhibition studies of a γ-carbonic anhydrase from the Antarctic bacterium Colwellia psychrerythraea. Bioorg Med Chem. 2016;24:835–840. doi: 10.1016/j.bmc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- De Mares MC, Jiménez DJ, Palladino G, et al. Expressed protein profile of a Tectomicrobium and other microbial symbionts in the marine sponge Aplysina aerophoba as evidenced by metaproteomics. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-30134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza MCM, Dos Santos KP, Freire RM, et al. Production of flavor esters catalyzed by Lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz J Chem Eng. 2017;34:681–690. doi: 10.1590/0104-6632.20170343s20150575. [DOI] [Google Scholar]
- Dornez E, Verjans P, Arnaut F, et al. Use of psychrophilic xylanases provides insight into the xylanase functionality in bread making. J Agric Food Chem. 2011;59:9553–9562. doi: 10.1021/jf201752g. [DOI] [PubMed] [Google Scholar]
- Fan X, Liang W, Li Y, et al. Identification and immobilization of a novel cold-adapted esterase, and its potential for bioremediation of pyrethroid-contaminated vegetables. Microb Cell Fact. 2017;16:1–12. doi: 10.1186/s12934-017-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller G. Protein folding at extreme temperatures: current issues. Semin Cell Dev Biol. 2018;84:129–137. doi: 10.1016/j.semcdb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Méndez-García C, Bargiela R, et al. Decoding the ocean’s microbiological secrets for marine enzyme biodiscovery. FEMS Microbiol Lett. 2019;366:fny285. doi: 10.1093/femsle/fny285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furhan J. Adaptation, production, and biotechnological potential of cold-adapted proteases from psychrophiles and psychrotrophs: recent overview. J. Genet. Eng. Biotechnol. 2020;18:1–13. doi: 10.1186/s43141-020-00053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furhan J, Awasthi P, Sharma S. Biochemical characterization and homology modelling of cold-active alkophilic protease from Northwestern Himalayas and its application in detergent industry. Biocatal Agric Biotechnol. 2019;17:726–735. doi: 10.1016/j.bcab.2019.01.028. [DOI] [Google Scholar]
- Galkin A, Kulakova L, Ashida H, et al. Cold-adapted alanine dehydrogenases from two Antarctic bacterial strains: gene cloning, protein characterization, and comparison with mesophilic and thermophilic counterparts. Appl Environ Microbiol. 1999;65:4014–4020. doi: 10.1128/aem.65.9.4014-4020.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Echauri SA, Gidekel M, Moraga AG, et al. Heterologous expression of a novel psychrophilic Cu/Zn superoxide dismutase from Deschampsia antarctica. Process Biochem. 2009;44:969–974. doi: 10.1016/j.procbio.2009.04.021. [DOI] [Google Scholar]
- Ghosh M, Pulicherla KK. Psychrophiles as the source for potential industrial psychrozymes. In: Prasad R, Kumar V, Singh J, Upadhyaya CP, editors. Recent developments in microbial technologies. Environmental and microbial biotechnology. Singapore: Springer; 2021. [Google Scholar]
- GrandViewResearch (2020) Enzymes market size, share & trends analysis report by application (industrial enzymes, specialty enzymes), by product (carbohydrase, proteases, lipases), by source, by region, and segment forecasts, 2020–2027. In: Gd. View Res. https://www.grandviewresearch.com/industry-analysis/enzymes-industry. Accessed 1 Apr 2021
- Gurung MK, Altermark B, Helland R, et al. Features and structure of a cold active N-acetylneuraminate lyase. PLoS ONE. 2019;14:1–27. doi: 10.1371/journal.pone.0217713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid B, Mohiddin FA. Cold-active enzymes in food processing. Enzym Food Technol Improv Innov. 2018 doi: 10.1007/978-981-13-1933-4_19. [DOI] [Google Scholar]
- Han Z, Shang-guan F, Yang J. Characterization of a novel cold-active xylanase from Luteimonas species. World J Microbiol Biotechnol. 2018;34:1–13. doi: 10.1007/s11274-018-2505-9. [DOI] [PubMed] [Google Scholar]
- He B, Jin S, Cao J, et al. Metatranscriptomics of the Hu sheep rumen microbiome reveals novel cellulases. Biotechnol Biofuels. 2019;12:153. doi: 10.1186/s13068-019-1498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Liu L, Liu X, Tang K. Isolation and characterization of a novel cold-active, halotolerant endoxylanase from Echinicola rosea Sp. Nov. JL3085T. Mar Drugs. 2020;18:245. doi: 10.3390/md18050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner TW, Dunn ML, Eggett DL, Ogden LV. β-Galactosidase activity of commercial lactase samples in raw and pasteurized milk at refrigerated temperatures. J Dairy Sci. 2011;94:3242–3249. doi: 10.3168/jds.2010-3742. [DOI] [PubMed] [Google Scholar]
- Hoyoux A, Jennes I, Dubois P, et al. Cold-adapted β-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl Environ Microbiol. 2001;67:1529–1535. doi: 10.1128/AEM.67.4.1529-1535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A, Lu Y, Hernández García M, Dumont MG. Targeted metatranscriptomics of soil microbial communities with stable isotope probing. In: Dumont M, Hernández García M, editors. Stable isotope probing. Methods in molecular biology. New York, NY: Humana; 2019. [DOI] [PubMed] [Google Scholar]
- Irwin JA, Gudmundsson HM, Marteinsson VT, et al. Characterization of alanine and malate dehydrogenases from a marine psychrophile strain PA-43. Extremophiles. 2001;5:199–211. doi: 10.1007/s007920100191. [DOI] [PubMed] [Google Scholar]
- Irwin JA, Lynch SV, Coughlan S, et al. Alanine dehydrogenase from the psychrophilic bacterium strain PA-43: overexpression, molecular characterization, and sequence analysis. Extremophiles. 2003;7:135–143. doi: 10.1007/s00792-002-0305-4. [DOI] [PubMed] [Google Scholar]
- Ji C, Zhang J, Lin X, et al. Metaproteomic analysis of microbiota in the fermented fish, Siniperca chuatsi. LWT. 2017;80:479–484. doi: 10.1016/j.lwt.2017.03.022. [DOI] [Google Scholar]
- Jia X, Xi B-D, Li M-X, et al. Metaproteomics analysis of the functional insights into microbial communities of combined hydrogen and methane production by anaerobic fermentation from reed straw. PLoS ONE. 2017;12:e0183158. doi: 10.1371/journal.pone.0183158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Sun D, Lu J, et al. A cold-adapted leucine dehydrogenase from marine bacterium Alcanivorax dieselolei: characterization and l-tert-leucine production. Eng Life Sci. 2016;16:283–289. doi: 10.1002/elsc.201500092. [DOI] [Google Scholar]
- Joseph B, Kumar V, Ramteke PW. Psychrophilic enzymes: potential biocatalysts for food processing. New York: Elsevier Inc.; 2018. [Google Scholar]
- Karakaş F, Arslanoğlu A. Gene cloning, heterologous expression, and partial characterization of a novel cold-adapted subfamily I.3 lipase from Pseudomonas fluorescence KE38. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-79199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan R, Mathew S, Muhammad R, et al. Understanding high-salt and cold adaptation of a polyextremophilic enzyme. Microorganisms. 2020;8:1–19. doi: 10.3390/microorganisms8101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryukova MV, Petrovskaya LE, Kryukova EA, et al. Thermal inactivation of a cold-active esterase PMGL3 isolated from the permafrost metagenomic library. Biomolecules. 2019 doi: 10.3390/biom9120880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuddus M. Cold-active enzymes in food biotechnology: an updated mini review. J Appl Biol Biotechnol. 2018;6:58–63. doi: 10.7324/jabb.2018.60310. [DOI] [Google Scholar]
- Kumar R, Singh D, Swarnkar MK, et al. Complete genome sequence of Arthrobacter sp. ERGS1:01, a putative novel bacterium with prospective cold active industrial enzymes, isolated from East Rathong glacier in India. J Biotechnol. 2015;214:139–140. doi: 10.1016/j.jbiotec.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Kumar R, Singh D, Swarnkar MK, et al. Genome assembly of Chryseobacterium polytrichastri ERMR1:04, a psychrotolerant bacterium with cold active proteases, isolated from east rathong glacier in India. Genome Announc. 2015 doi: 10.1128/genomeA.01305-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Acharya V, Singh D, Kumar S. Strategies for high-altitude adaptation revealed from high-quality draft genome of non-violacein producing Janthinobacterium lividum ERGS5:01. Stand Genomic Sci. 2018 doi: 10.1186/s40793-018-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Acharya V, Mukhia S, et al. Complete genome sequence of Pseudomonas frederiksbergensis ERDD5:01 revealed genetic bases for survivability at high altitude ecosystem and bioprospection potential. Genomics. 2019;111:492–499. doi: 10.1016/j.ygeno.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mukhia S, Kumar N, et al. A broad temperature active lipase purified from a psychrotrophic bacterium of sikkim himalaya with potential application in detergent formulation. Front Bioeng Biotechnol. 2020 doi: 10.3389/fbioe.2020.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Buchon L, Burini JF, Orange N. Low pH and cold temperature combine to limit growth and pectate lyase production by the psychrotrophic bacterium Erwinia carotovora ssp. carotovora MFCL0. Biotechnol Lett. 2001;23:753–756. doi: 10.1023/A:1010392624650. [DOI] [Google Scholar]
- Lee DH, Choi SL, Rha E, et al. A novel psychrophilic alkaline phosphatase from the metagenome of tidal flat sediments. BMC Biotechnol. 2015 doi: 10.1186/s12896-015-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Yoo W, Park SH, et al. Structural and functional characterization of a novel cold-active S-formylglutathione hydrolase (SfSFGH) homolog from Shewanella frigidimarina, a psychrophilic bacterium. Microb Cell Fact. 2019;18:1–13. doi: 10.1186/s12934-019-1190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pan J, Zhang J, Xu JH. Stereoselective synthesis of l-tert-leucine by a newly cloned leucine dehydrogenase from Exiguobacterium sibiricum. J Mol Catal B Enzym. 2014;105:11–17. doi: 10.1016/j.molcatb.2014.03.010. [DOI] [Google Scholar]
- Li Y, Wang Z, Zhou Y, et al. Enzymatic identification and functional sites study of a novel cold-active cellulase (MkCel5) from Microbacterium kitamiensea. Biotechnol Biotechnol Equip. 2019;33:739–747. doi: 10.1080/13102818.2019.1612278. [DOI] [Google Scholar]
- Li D, Fei T, Wang Y, et al. A cold-active 1,4-α-glucan branching enzyme from Bifidobacterium longum reduces the retrogradation and enhances the slow digestibility of wheat starch. Food Chem. 2020;324:126855. doi: 10.1016/j.foodchem.2020.126855. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang L, Zheng D, et al. Biochemical characterization of a novel GH43 family β-xylosidase from Bacillus pumilus. Food Chem. 2019;295:653–661. doi: 10.1016/j.foodchem.2019.05.163. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Z, Zeng X, et al. A novel cold-adapted phospho-beta-galactosidase from Bacillus velezensis and its potential application for lactose hydrolysis in milk. Int J Biol Macromol. 2021;166:760–770. doi: 10.1016/j.ijbiomac.2020.10.233. [DOI] [PubMed] [Google Scholar]
- Ma L, Aizhan R, Wang X, et al. Cloning and characterization of low-temperature adapted GH5-CBM3 endo-cellulase from Bacillus subtilis 1AJ3 and their application in the saccharification of switchgrass and coffee grounds. AMB Express. 2020;10:1–11. doi: 10.1186/s13568-020-00975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewska N, Walkusz R, Olszewski M, Szymańska A. New nuclease from extremely psychrophilic microorganism Psychromonas ingrahamii 37: identification and characterization. Mol Biotechnol. 2019;61:122–133. doi: 10.1007/s12033-018-0142-z. [DOI] [PubMed] [Google Scholar]
- Madhavan A, Sindhu R, Parameswaran B, et al. Metagenome analysis: a powerful tool for enzyme bioprospecting. Appl Biochem Biotechnol. 2017;183:636–651. doi: 10.1007/s12010-017-2568-3. [DOI] [PubMed] [Google Scholar]
- Mageswari A, Subramanian P, Chandrasekaran S, et al. Systematic functional analysis and application of a cold-active serine protease from a novel Chryseobacterium sp. Food Chem. 2017;217:18–27. doi: 10.1016/j.foodchem.2016.08.064. [DOI] [PubMed] [Google Scholar]
- Mandelman D, Ballut L, Wolff DA, et al. Structural determinants increasing flexibility confer cold adaptation in psychrophilic phosphoglycerate kinase. Extremophiles. 2019;23:495–506. doi: 10.1007/s00792-019-01102-x. [DOI] [PubMed] [Google Scholar]
- Mangiagalli M, Lotti M. Cold-active β-galactosidases: insight into cold adaption mechanisms and biotechnological exploitation. Mar Drugs. 2021;19:43. doi: 10.3390/md19010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiagalli M, Brocca S, Orlando M, Lotti M. The “cold revolution”. Present and future applications of cold-active enzymes and ice-binding proteins. N Biotechnol. 2020;55:5–11. doi: 10.1016/j.nbt.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Mangiagalli M, Barbiroli A, Santambrogio C, et al. The activity and stability of a cold-active acylaminoacyl peptidase rely on its dimerization by domain swapping. Int J Biol Macromol. 2021;181:263–274. doi: 10.1016/j.ijbiomac.2021.03.150. [DOI] [PubMed] [Google Scholar]
- Margesin R, Fauster V, Fonteyne PA. Characterization of cold-active pectate lyases from psychrophilic Mrakia frigida. Lett Appl Microbiol. 2005;40:453–459. doi: 10.1111/j.1472-765X.2005.01704.x. [DOI] [PubMed] [Google Scholar]
- Margesin R. Psychrophiles: from biodiversity to biotechnology. 2. Berlin: Springer; 2017. [Google Scholar]
- Maseh K, Ehsan N, Mukhtar S, et al. Metaproteomics: an emerging tool for the identification of proteins from extreme environments. Environ Sustain. 2021;4:39–50. doi: 10.1007/s42398-020-00158-2. [DOI] [Google Scholar]
- Mello BL, Alessi AM, Riaño-Pachón DM, et al. Targeted metatranscriptomics of compost-derived consortia reveals a GH11 exerting an unusual exo-1,4-β-xylanase activity. Biotechnol Biofuels. 2017;10:1–17. doi: 10.1186/s13068-017-0944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merín MG, Morata de Ambrosini VI. Application of a grape surface majority pectinolytic species, Aureobasidium pullulans, to low-temperature red winemaking: development and stability of wine colour. J Wine Res. 2020;31:218–239. doi: 10.1080/09571264.2020.1816534. [DOI] [Google Scholar]
- Mhetras N, Mapare V, Gokhale D. Cold active lipases: biocatalytic tools for greener technology. Appl. Biochem. Biotechnol. 2021;193:2245–2266. doi: 10.1007/s12010-021-03516-w. [DOI] [PubMed] [Google Scholar]
- Miri S, Naghdi M, Rouissi T, et al. Recent biotechnological advances in petroleum hydrocarbons degradation under cold climate conditions: a review. Crit Rev Environ Sci Technol. 2019;49:553–586. doi: 10.1080/10643389.2018.1552070. [DOI] [Google Scholar]
- Miri S, Davoodi SM, Brar SK, et al. Psychrozymes as novel tools to biodegrade p-xylene and potential use for contaminated groundwater in the cold climate. Bioresour Technol. 2021;321:124464. doi: 10.1016/j.biortech.2020.124464. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Yadav R, Marmeisse R, et al. Heavy metal hypertolerant eukaryotic aldehyde dehydrogenase isolated from metal contaminated soil by metatranscriptomics approach. Biochimie. 2019;160:183–192. doi: 10.1016/j.biochi.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Mukhia S, Khatri A, Acharya V, Kumar R. Comparative genomics and molecular adaptational analysis of Arthrobacter from Sikkim Himalaya provided insights into its survivability under multiple high-altitude stress. Genomics. 2021;113:151–158. doi: 10.1016/j.ygeno.2020.12.001. [DOI] [PubMed] [Google Scholar]
- Mukhia S, Kumar A, Kumar R. Generation of antioxidant peptides from soy protein isolate through psychrotrophic Chryseobacterium sp. derived alkaline broad temperature active protease. LWT. 2021;143:111152. doi: 10.1016/j.lwt.2021.111152. [DOI] [Google Scholar]
- Mukhopadhyay A, Bhattacharyya T, Dasgupta AK, Chakrabarti K. Nanotechnology based activation-immobilization of psychrophilic pectate lyase: a novel approach towards enzyme stabilization and enhanced activity. J Mol Catal B Enzym. 2015;119:54–63. doi: 10.1016/j.molcatb.2015.05.017. [DOI] [Google Scholar]
- Mulualem DM, Agbavwe C, Ogilvie LA, et al. Metagenomic identification, purification and characterisation of the Bifidobacterium adolescentis BgaC β-galactosidase. Appl Microbiol Biotechnol. 2021;105:1063–1078. doi: 10.1007/s00253-020-11084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz PA, Márquez SL, González-Nilo FD, et al. Structure and application of antifreeze proteins from Antarctic bacteria. Microb Cell Fact. 2017 doi: 10.1186/s12934-017-0737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa N, Latip W, Abd Rahman RNZ, et al. Immobilization of an antarctic Pseudomonas AMS8 lipase for low temperature ethyl hexanoate synthesis. Catalysts. 2018;8:234. doi: 10.3390/catal8060234. [DOI] [Google Scholar]
- Nandanwar SK, Borkar SB, Lee JH, Kim HJ. Taking advantage of promiscuity of cold-active enzymes. Appl Sci. 2020;10:1–18. doi: 10.3390/app10228128. [DOI] [Google Scholar]
- Nascimento TCES, Molino JVD, Donado PRS, et al. Antarctic fungus proteases generate bioactive peptides from caseinate. Food Res Int. 2021;139:109944. doi: 10.1016/j.foodres.2020.109944. [DOI] [PubMed] [Google Scholar]
- Noby N, Saeed H, Embaby AM, et al. Cloning, expression and characterization of cold active esterase (EstN7) from Bacillus cohnii strain N1: a novel member of family IV. Int J Biol Macromol. 2018;120:1247–1255. doi: 10.1016/j.ijbiomac.2018.07.169. [DOI] [PubMed] [Google Scholar]
- Noby N, Hussein A, Saeed H, Embaby AM. Recombinant cold-adapted halotolerant, organic solvent-stable esterase (estHIJ) from Bacillus halodurans. Anal Biochem. 2020;591:113554. doi: 10.1016/j.ab.2019.113554. [DOI] [PubMed] [Google Scholar]
- Núñez-Montero K, Salazar R, Santos A, et al. Antarctic Rahnella inusitata: a producer of cold-stable β-galactosidase enzymes. Int J Mol Sci. 2021;22:4144. doi: 10.3390/ijms22084144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino-Delgado CH, Zanutto-Elgui MR, do Prado DZ, et al. Enzymatic bioremediation: current status, challenges of obtaining process, and applications. In: Arora P, et al., editors. Microbial metabolism of xenobiotic compounds. Microorganisms for sustainability. Singapore: Springer; 2019. [Google Scholar]
- Ottoni JR, RodrigueseSilva T, MaiadeOliveira V, ZambranoPassarini MR. Characterization of amylase produced by cold-adapted bacteria from Antarctic samples. Biocatal Agric Biotechnol. 2020;23:101452. doi: 10.1016/j.bcab.2019.101452. [DOI] [Google Scholar]
- Pal M, Swarnkar MK, Dhar H, et al. Genome assembly of Chryseobacterium sp. strain IHBB 10212 from glacier top-surface soil in the Indian trans-Himalayas with potential for hydrolytic enzymes. Genom Data. 2017;13:46–49. doi: 10.1016/j.gdata.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee CW, Kim D, et al. Crystal structure of a cold-active protease (Pro21717) from the psychrophilic bacterium, Pseudoalteromonas arctica PAMC 21717, at 1.4 Å resolution: Structural adaptations to cold and functional analysis of a laundry detergent enzyme. PLoS ONE. 2018;13:1–20. doi: 10.1371/journal.pone.0191740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfumo A, Freiherr von Sass GJ, Nordmann EL, et al. Discovery and characterization of a new cold-active protease from an extremophilic bacterium via comparative genome analysis and in vitro expression. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskaya LE, Novototskaya-Vlasova KA, Gapizov SS, et al. New member of the hormone-sensitive lipase family from the permafrost microbial community. Bioengineered. 2017;8:420–423. doi: 10.1080/21655979.2016.1230571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phukon LC, Chourasia R, Kumari M, et al. Production and characterisation of lipase for application in detergent industry from a novel Pseudomonas helmanticensis HS6. Bioresour Technol. 2020;309:123352. doi: 10.1016/j.biortech.2020.123352. [DOI] [PubMed] [Google Scholar]
- Qiu H, Li Z, Wang H, et al. Molecular and biochemical characterization of a novel cold-active and metal ion-tolerant GH10 xylanase from frozen soil. Biotechnol Biotechnol Equip. 2017;31:955–963. doi: 10.1080/13102818.2017.1359667. [DOI] [Google Scholar]
- Rathour R, Gupta J, Tyagi B, Thakur IS. Production and characterization of psychrophilic α-amylase from a psychrophilic bacterium, Shewanella sp. ISTPL2. Amylase. 2020;4:1–10. doi: 10.1515/amylase-2020-0001. [DOI] [Google Scholar]
- Raymond-Bouchard I, Whyte LG. From transcriptomes to metatranscriptomes: cold adaptation and active metabolisms of psychrophiles from cold environments. In: Margesin R, editor. Psychrophiles: from biodiversity to biotechnology. Cham: Springer; 2017. [Google Scholar]
- Rina M, Pozidis C, Mavromatis K, et al. Alkaline phosphatase from the Antarctic strain TAB5 properties and psychrophilic adaptations. Eur J Biochem. 2000;267:1230–1238. doi: 10.1046/j.1432-1327.2000.01127.x. [DOI] [PubMed] [Google Scholar]
- Ruan L, Lin W, Shi H, et al. Characterization of a novel extracellular Cu[sbnd]Zn superoxide dismutase from Rimicaris exoculata living around deep-sea hydrothermal vent. Int J Biol Macromol. 2020;163:2346–2356. doi: 10.1016/j.ijbiomac.2020.09.128. [DOI] [PubMed] [Google Scholar]
- Sahay S, Chaurse V, Chauhan D. Laccase from Aspergillus nidulans TTF6 showing Pb activation for smaller substrates and dyes remediation in all climates. Proc Natl Acad Sci India Sect B Biol Sci. 2020;90:143–150. doi: 10.1007/s40011-019-01092-y. [DOI] [Google Scholar]
- Sahoo RK, Das A, Gaur M, et al. Parameter optimization for thermostable lipase production and performance evaluation as prospective detergent additive. Prep Biochem Biotechnol. 2020;50:578–584. doi: 10.1080/10826068.2020.1719513. [DOI] [PubMed] [Google Scholar]
- Salwan R, Sharma V, Kasana RC, Gulati A. Bioprospecting psychrotrophic bacteria for serine-type proteases from the cold areas of Western Himalayas. Curr Microbiol. 2020;77:795–806. doi: 10.1007/s00284-020-01876-w. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold spring harbor laboratory press
- Sanchez AC, Ravanal MC, Andrews BA, Asenjo JA. Heterologous expression and biochemical characterization of a novel cold-active α-amylase from the Antarctic bacteria Pseudoalteromonas sp. 2–3. Protein Expr Purif. 2019;155:78–85. doi: 10.1016/j.pep.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Sarmiento F, Peralta R, Blamey JM. Cold and hot extremozymes: industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015;3:148. doi: 10.3389/fbioe.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sočan J, Kazemi M, Isaksen GV, et al. Catalytic adaptation of psychrophilic elastase. Biochemistry. 2018;57:2984–2993. doi: 10.1021/acs.biochem.8b00078. [DOI] [PubMed] [Google Scholar]
- Song JM, Hong SK, An YJ, et al. Genetic and structural characterization of a thermo-tolerant, cold-active, and acidic endo-β-1,4-glucanase from antarctic springtail, Cryptopygus antarcticus. J Agric Food Chem. 2017;65:1630–1640. doi: 10.1021/acs.jafc.6b05037. [DOI] [PubMed] [Google Scholar]
- Su J, Zhang F, Sun W, et al. A new alkaline lipase obtained from the metagenome of marine sponge Ircinia sp. World J Microbiol Biotechnol. 2015;31:1093–1102. doi: 10.1007/s11274-015-1859-5. [DOI] [PubMed] [Google Scholar]
- Sun K, Camardella L, Prisco G, Hervé G. Properties of aspartate transcarbamylase from TAD1, a psychrophilic bacterial strain isolated from Antarctica. FEMS Microbiol Lett. 1998;164:375–382. doi: 10.1111/j.1574-6968.1998.tb13112.x. [DOI] [PubMed] [Google Scholar]
- Sun S, Zhang Y, Liu K, et al. Insight into biodegradation of cellulose by psychrotrophic bacterium Pseudomonas sp. LKR-1 from the cold region of China: optimization of cold-active cellulase production and the associated degradation pathways. Cellulose. 2020;27:315–333. doi: 10.1007/s10570-019-02798-y. [DOI] [Google Scholar]
- Suzuki T, Yamamoto K, Tada H, Uda K. Cold-adapted features of arginine kinase from the deep-sea CLAM Calyptogena kaikoi. Mar Biotechnol. 2012;14:294–303. doi: 10.1007/s10126-011-9411-6. [DOI] [PubMed] [Google Scholar]
- Tang CD, Shi HL, Jiao ZJ, et al. Exploitation of cold-active cephalosporin C acylase by computer-aided directed evolution and its potential application in low-temperature biosynthesis of 7-aminocephalosporanic acid. J Chem Technol Biotechnol. 2018;93:2925–2930. doi: 10.1002/jctb.5647. [DOI] [Google Scholar]
- Tang Y, Wu P, Jiang S, et al. A new cold-active and alkaline pectate lyase from Antarctic bacterium with high catalytic efficiency. Appl Microbiol Biotechnol. 2019;103:5231–5241. doi: 10.1007/s00253-019-09803-1. [DOI] [PubMed] [Google Scholar]
- Thakur M, Sharma N, Rai AK, Singh SP. A novel cold-active type I pullulanase from a hot-spring metagenome for effective debranching and production of resistant starch. Bioresour Technol. 2021;320:124288. doi: 10.1016/j.biortech.2020.124288. [DOI] [PubMed] [Google Scholar]
- Tsigos I, Velonia K, Smonou I, Bouriotis V. Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. TAE123. Eur J Biochem. 1998;254:356–362. doi: 10.1046/j.1432-1327.1998.2540356.x. [DOI] [PubMed] [Google Scholar]
- Usmani Z, Sharma M, Awasthi AK, et al. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour Technol. 2021;322:124548. doi: 10.1016/j.biortech.2020.124548. [DOI] [PubMed] [Google Scholar]
- Vester JK, Glaring MA, Stougaard P. Discovery of novel enzymes with industrial potential from a cold and alkaline environment by a combination of functional metagenomics and culturing. Microb Cell Fact. 2014 doi: 10.1186/1475-2859-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester JK, Glaring MA, Stougaard P. An exceptionally cold-adapted alpha-amylase from a metagenomic library of a cold and alkaline environment. Appl Microbiol Biotechnol. 2015;99:717–727. doi: 10.1007/s00253-014-5931-0. [DOI] [PubMed] [Google Scholar]
- Vullo D, De Luca V, Del Prete S, et al. Sulfonamide inhibition studies of the γ-carbonic anhydrase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Bioorganic Med Chem Lett. 2015;25:3550–3555. doi: 10.1016/j.bmcl.2015.06.079. [DOI] [PubMed] [Google Scholar]
- Wang QF, Wang YF, Hou YH, et al. Cloning, expression and biochemical characterization of recombinant superoxide dismutase from Antarctic psychrophilic bacterium Pseudoalteromonas sp. ANT506. J Basic Microbiol. 2016;56:753–761. doi: 10.1002/jobm.201500444. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Wang Y, et al. Statistical optimization for the production of recombinant cold-adapted superoxide dismutase in E. coli using response surface methodology. Bioengineered. 2017;8:693–699. doi: 10.1080/21655979.2017.1303589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hou Y, Wang Y, et al. A novel cold-adapted leucine dehydrogenase from antarctic sea-ice bacterium Pseudoalteromonas sp. ANT178. Mar Drugs. 2018;16:359. doi: 10.3390/md16100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hou Y, Nie P, et al. A novel cold-adapted and salt-tolerant RNase R from Antarctic sea-ice bacterium Psychrobacter sp. Ant206. Molecules. 2019;24:2229. doi: 10.3390/molecules24122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Nie P, Hou Y, Wang Y. Purification, biochemical characterization and DNA protection against oxidative damage of a novel recombinant superoxide dismutase from psychrophilic bacterium Halomonas sp. ANT108. Protein Expr Purif. 2020;173:105661. doi: 10.1016/j.pep.2020.105661. [DOI] [PubMed] [Google Scholar]
- Woo JH, Hwang YO, Kang SG, et al. Cloning and characterization of three epoxide hydrolases from a marine bacterium, Erythrobacter litoralis HTCC2594. Appl Microbiol Biotechnol. 2007;76:365–375. doi: 10.1007/s00253-007-1011-z. [DOI] [PubMed] [Google Scholar]
- Wu J, Geng A, Xie R, et al. Characterization of cold adapted and ethanol tolerant β-glucosidase from Bacillus cellulosilyticus and its application for directed hydrolysis of cellobiose to ethanol. Int J Biol Macromol. 2018;109:872–879. doi: 10.1016/j.ijbiomac.2017.11.072. [DOI] [PubMed] [Google Scholar]
- Xie H, Poon CKK, Liu H, et al. Molecular and biochemical characterizations of a new cold-active and mildly alkaline β-Mannanase from Verrucomicrobiae DG1235. Prep Biochem Biotechnol. 2020 doi: 10.1080/10826068.2020.1870235. [DOI] [PubMed] [Google Scholar]
- Xu Y, Feller G, Gerday C, Glansdorff N. Metabolic enzymes from psychrophilic bacteria: challenge of adaptation to low temperatures in ornithine carbamoyltransferase from Moritella abyssi. J Bacteriol. 2003;185:2161–2168. doi: 10.1128/JB.185.7.2161-2168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhang M, Zhang M, et al. Characterization of a novel, cold-adapted, and thermostable laccase-like enzyme with high tolerance for organic solvents and salt and potent dye decolorization ability, derived from a marine metagenomic library. Front Microbiol. 2018;9:2998. doi: 10.3389/fmicb.2018.02998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YR, Sang P, Xiao M, et al. Expression and characterization of a cold-adapted, salt- and glucose-tolerant GH1 β-glucosidase obtained from Thermobifida halotolerans and its use in sugarcane bagasse hydrolysis. Biomass Convers Biorefinery. 2020 doi: 10.1007/s13399-019-00556-5. [DOI] [Google Scholar]
- Yuan P, Meng K, Wang Y, et al. A low-temperature-active alkaline pectate lyase from Xanthomonas campestris aCCC 10048 with high activity over a wide ph range. Appl Biochem Biotechnol. 2012;168:1489–1500. doi: 10.1007/s12010-012-9872-8. [DOI] [PubMed] [Google Scholar]
- Zang H, Du X, Wang J, et al. Insight into cold-active xylanase production and xylan degradation pathways in psychrotrophic Acinetobacter sp. HC4 from the cold region of China. Cellulose. 2020;27:7575–7589. doi: 10.1007/s10570-020-03286-4. [DOI] [Google Scholar]
- Zanphorlin LM, De Giuseppe PO, Honorato RV, et al. Oligomerization as a strategy for cold adaptation: structure and dynamics of the GH1 β-glucosidase from Exiguobacterium antarcticum B7. Sci Rep. 2016 doi: 10.1038/srep23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa S, Rolland JL, Flament D, et al. Characterization of a highly thermostable alkaline phosphatase from the Euryarchaeon Pyrococcus abyssi. Appl Environ Microbiol. 2001;67:4504–4511. doi: 10.1128/AEM.67.10.4504-4511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wakamatsu T, Doi K, et al. A psychrophilic leucine dehydrogenase from Sporosarcina psychrophila: purification, characterization, gene sequencing and crystal structure analysis. J Mol Catal B Enzym. 2012;83:65–72. doi: 10.1016/j.molcatb.2012.06.018. [DOI] [Google Scholar]
- Zheng Z, Jiang YH, Miao JL, et al. Purification and characterization of a cold-active iron superoxide dismutase from a psychrophilic bacterium, Marinomonas sp. NJ522. Biotechnol Lett. 2006;28:85–88. doi: 10.1007/s10529-005-4951-3. [DOI] [PubMed] [Google Scholar]
- Zhu D, Adebisi WA, Ahmad F, et al. Recent development of extremophilic bacteria and their application in biorefinery. Front Bioeng Biotechnol. 2020;8:1–18. doi: 10.3389/fbioe.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]