Abstract
Leptin is an adipocyte-derived hormone that regulates appetite and energy expenditure via the hypothalamus. Since the majority of obese subjects are leptin resistant, leptin sensitizers, rather than leptin itself, are expected to be anti-obesity drugs. Endoplasmic reticulum (ER) stress in the hypothalamus plays a key role in the pathogenesis of leptin resistance. ATP-deficient cells are vulnerable to ER stress and ATP treatment protects cells against ER stress. Thus, we investigated the therapeutic effects of oral 1,3-butanediol (BD) administration, which increases plasma β-hydroxybutyrate and hypothalamic ATP concentrations, in diet induced obese (DIO) mice with leptin resistance. BD treatment effectively decreased food intake and body weight in DIO mice. In contrast, BD treatment had no effect in leptin deficient ob/ob mice. Co-administration experiment demonstrated that BD treatment sensitizes leptin action in both DIO and ob/ob mice. We also demonstrated that BD treatment attenuates ER stress and leptin resistance at the hypothalamus level. This is the first report to confirm the leptin sensitizing effect of BD treatment in leptin resistant DIO mice. The present study provides collateral evidence suggesting that the effect of BD treatment is mediated by the elevation of hypothalamic ATP concentration. Ketone bodies and hypothalamic ATP are the potential target for the treatment of obesity and its complications.
Subject terms: Endocrine system and metabolic diseases, Obesity
Introduction
Leptin is an adipocyte-derived hormone that regulates energy homeostasis mainly via the hypothalamus1–3. Leptin enters the brain from the circulatory system through the blood–brain barrier4 and binds to receptors expressed in the hypothalamus, particularly in the arcuate nucleus5, and reduces food intake and body weight6. Circulating leptin level increases in proportion to body fat content7. Although most obese subjects have high serum leptin levels, increased leptin fails to suppress the progression of obesity. Indeed, it has been demonstrated that obese humans are weakly responsive to exogenously administered leptin in terms of body weight reduction8. This pathological condition is called leptin resistance. Today, leptin sensitizers rather than leptin itself are expected to be anti-obesity drugs.
Although the precise mechanism underlying leptin resistance has not been elucidated, it has been reported that endoplasmic reticulum (ER) stress in the hypothalamus plays a key role in the pathogenesis of leptin resistance9,10. It has also been demonstrated that chemical chaperones such as 4-phenyl butyric acid and tauroursodeoxycholic acid, which have the ability to decrease ER stress, act as leptin-sensitizing agents10. Under ER stress, the accumulation of misfolded proteins facilitates the unfolded protein response (UPR) which requires appreciable amounts of ATP11,12. Indeed, ATP-deficient cells are vulnerable to ER stress, and ATP treatment protects cells against ER stress13. For these reasons, increased hypothalamic ATP concentration might have a protective effect against ER stress and leptin resistance in the hypothalamus.
Ketone bodies have been demonstrated to increase ATP concentrations in the central nervous system14–16. A simple way to increase the circulating concentration of ketone bodies is through a ketogenic diet17. Ketogenic diet is a low carbohydrate and high fat diet. Lack of carbohydrate induces the conversion of fat to fatty acids and ketone bodies in the liver. However, the effects of ketogenic diets on body weight and food intake are contradictory18,19. Ketosis induced by ketogenic diet has been reported to act both orexigenically and anorexigenically in animal studies18,19. One of the reasons why the ketogenic diet has two conflicting actions is that it is both ketogenic and low carbohydrate. Low carbohydrate diet can act similarly to calorie restriction, increasing food intake20,21. On the other hand, ketone bodies have been demonstrated to increase ATP concentrations in the central nervous system14–16. If the elevation of hypothalamic ATP concentration has a protective effect against ER stress and leptin resistance in the hypothalamus, then ketone bodies should reduce food intake and body weight in obese subjects with leptin resistance. However, the effects of ketone bodies on hypothalamic ER stress and leptin resistance have not been reported.
The aim of this study is to investigate the effect of ketone bodies on the leptin sensitivity in mice. 1,3-butanediol (BD), a precursor of β-hydroxybutyrate (βHB), was used to increase plasma ketone body concentrations. BD is an ethanol dimer (CH3–CHOH–CH2–CH2OH), one of four stable structural isomers of butanediol22. It is converted to βHB in the liver23–25. Oral BD administration has been demonstrated to increase plasma βHB concentration26,27. In addition, it was also demonstrated that oral administration of esterified BD reduces body weight in diet induced obese (DIO) mice although the involvement of leptin sensitization was not investigated28. In this study, instead of mixing BD into a diet, BD was administered to mice by oral gavage to avoid the change of the diet composition. BD treatment effectively reduced food intake and body weight in diet induced obese (DIO) mice which were leptin resistant. In contrast, no effect of BD treatment was observed in leptin deficient ob/ob mice. Co-administration experiment of BD and leptin demonstrated that BD treatment sensitizes leptin action in both DIO and ob/ob mice. These results indicate that leptin is necessary for BD treatment to exert its effects, and that leptin sensitization is the primary effect of BD treatment on body weight. It was also confirmed that BD treatment attenuated ER stress and leptin resistance at the hypothalamic level in DIO mice. This is the first report demonstrating the leptin sensitizing effect of BD treatment in DIO mice with leptin resistance. The present study suggests the possibility that the effect of BD treatment is mediated by the elevation of hypothalamic ATP concentration. Ketone bodies and hypothalamic ATP are potential targets for the treatment of obesity and its complications.
Results
Therapeutic effect of BD treatment on DIO mice
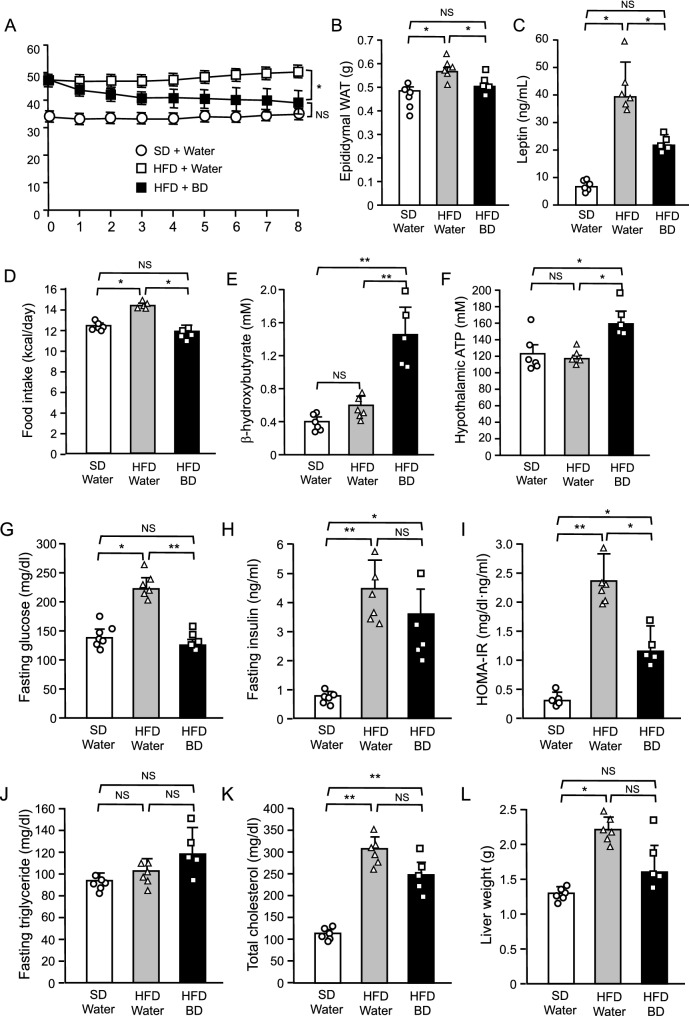
To investigate the therapeutic effect of ketone bodies on DIO, we administered BD or water orally to DIO mice twice a day for 8 weeks under HFD. As a control, we administered water to lean mice twice a day for 8 weeks under SD. Body weight in DIO mice was effectively decreased by BD treatment and was not significantly different from that in lean control mice fed SD at the end of the experiment (Fig. 1A). Consistent with the body weight change, BD treatment decreased epididymal WAT weight in DIO mice to the same level as in lean control mice (Fig. 1B). We measured plasma leptin concentrations as an indicator of total body fat mass. In DIO mice, plasma leptin concentrations were markedly elevated and were significantly reduced by BD treatment (Fig. 1C). The elevated daily food intake in DIO mice was also decreased by BD treatment to the same level as in lean control mice fed SD (Fig. 1D). We measured plasma β-hydroxybutyrate and hypothalamic ATP concentrations at the end of the experiment. Plasma β-hydroxybutyrate concentrations in DIO mice treated with water tended to be higher than in lean control mice but there was no statistically significant difference (Fig. 1E). BD treatment effectively increased plasma β-hydroxybutyrate concentrations in DIO mice. Consistent with these plasma β-hydroxybutyrate concentrations, there was no difference in hypothalamic ATP concentrations between lean control and DIO mice treated with water and BD treatment significantly increased hypothalamic ATP concentrations in DIO mice (Fig. 1F).
Figure 1.
Therapeutic effect of oral BD administration on DIO mice. (A) Body weight change, (B) epididymal WAT weight, (C) plasma leptin concentrations, (D) food intake, (E–H) plasma β-hydroxybutyrate, hypothalamic ATP, fasting plasma glucose and fasting plasma insulin concentrations, (I) HOMA-IR, (J,K) fasting plasma triglyceride and plasma total cholesterol concentrations and (L) liver weight in mice treated with water or BD under SD or HFD. Values are means ± SEM (n = 6 for SD + Water, 6 for HFD + Water, 5 for HFD + BD). NS, not significant, *P < 0.05 (Bonferroni's test) (A). NS, not significant, *P < 0.05, **P < 0.01 (Student’s t-test) (B–L).
Next, we investigated the effect of BD treatment on glucose and lipid metabolisms in DIO. In DIO mice, fasting plasma glucose and insulin concentrations and HOMA-IR were apparently elevated when compared with lean control mice (Fig. 1G–I). However, BD treatment decreased fasting glucose concentrations in DIO mice to the same level as in lean control mice. Although fasting insulin concentrations in DIO mice were not significantly decreased, HOMA-IR in DIO mice was significantly decreased by BD treatment. As to lipid metabolism, BD treatment had no effect on fasting plasma triglyceride and total cholesterol concentrations but showed a tendency to decrease the liver weight in DIO mice (Fig. 1J–L).
Effect of BD treatment in lean control mice
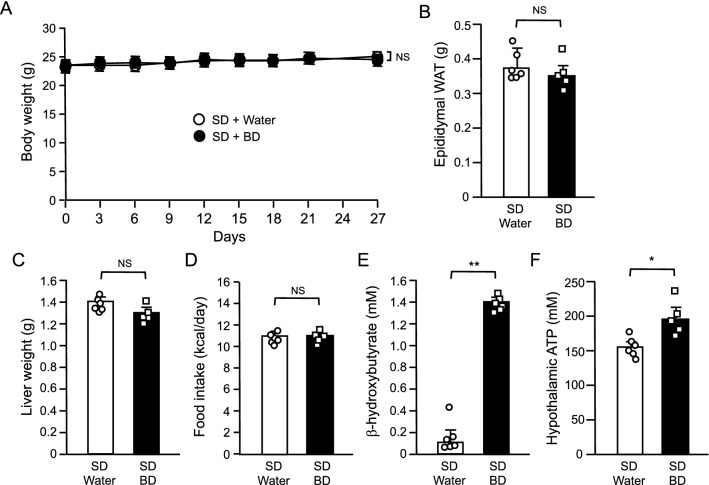
We also investigated the effect of oral BD administration in lean control mice under SD. Twice daily oral administration at the same dose (5 g/kg) used in DIO mice had any apparent effect on neither body weight nor tissue weights including epididymal WAT and liver in lean control mice (Fig. 2A–C). BD treatment also had no effect on food intake while both plasma β-hydroxybutyrate and hypothalamic ATP concentrations were significantly elevated by BD treatment (Fig. 2D–F).
Figure 2.
Effect of oral BD administration in WT mice under SD. (A) Body weight change, (B) epididymal WAT weight, (C) liver weight, (D) food intake, (E) plasma β-hydroxybutyrate and (F) hypothalamic ATP concentrations. Values are means ± SEM (n = 6 for SD + Water, 5 for SD + BD). NS, not significant (Bonferroni's test) (A). NS, not significant, *P < 0.05, **P < 0.01 (Student’s t-test) (B–F).
Effect of BD treatment in ob/ob mice with or without leptin treatment
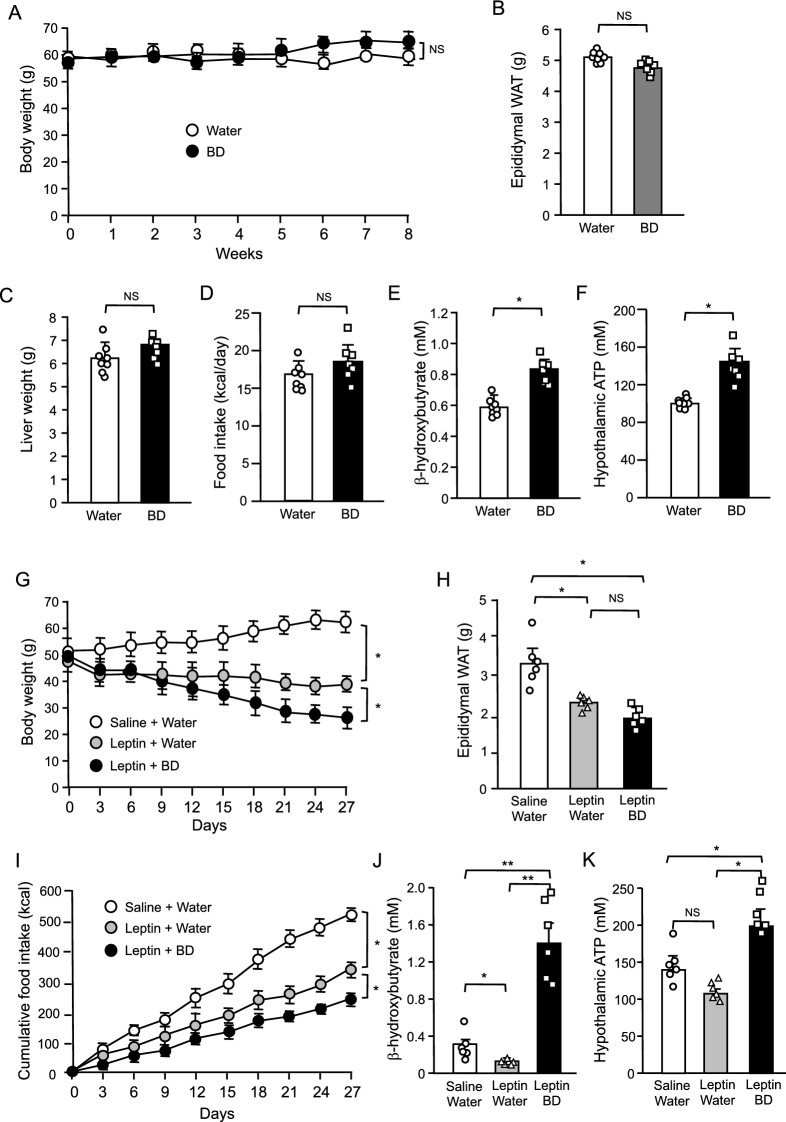
BD treatment did not significantly reduce the body weight in lean control mice at the same dose (5 g/kg) that was effective in DIO mice (Figs. 1A, 2A). Lean mice had very low levels of circulating leptin when compared with DIO mice (Fig. 1C), suggesting that the therapeutic effect of BD treatment requires a decent amount of leptin. To investigate the role of leptin in the effect of BD treatment, we examined the effect of BD treatment in ob/ob mice which genetically lack circulating leptin1. Consistent with our expectations, BD treatment did not significantly reduce the body weight in ob/ob mice at the dose (5 g/kg) that were effective in DIO mice (Fig. 3A). In addition, epididymal WAT and liver weights (Fig. 3B,C) and food intake (Fig. 3D) in these mice were unchanged by BD treatment. At this time, we confirmed the significant elevation of both plasma β-hydroxybutyrate and hypothalamic ATP concentrations by BD treatment in ob/ob mice (Fig. 3D,E).
Figure 3.
Effects of oral BD administration (A–F) and co-administration of oral BD and intraperitoneal leptin (G–J) in ob/ob mice. (A) Body weight change, (B) epididymal WAT weight, (C) liver weight, (D) food intake, (E) plasma β-hydroxybutyrate and (F) hypothalamic ATP concentrations in BD single administration experiment. (G) Body weight change, (H) epididymal WAT weight, (I) cumulative food intake, (J) plasma β-hydroxybutyrate and (K) hypothalamic ATP concentrations in leptin and BD co-administration experiment. Values are means ± SEM (A–F, n = 8 for Water, 7 for BD; G–K, n = 6 for Saline + Water, 6 for Leptin + Water, 6 for Leptin + BD). NS, not significant, *P < 0.05 (Bonferroni's test) (A,G,I). NS, not significant, *P < 0.05, **P < 0.01 (Student’s t-test) (B–F, H,J,K).
We could not deny the possibility that the dose and the administration used in this study were inadequate for ob/ob mice. Therefore, again we tested the effect of twice daily oral BD administration at the dose of 5 g/kg in ob/ob mice with leptin treatment. Once daily intraperitoneal leptin injection at the dose of 150 μg/kg effectively decreased body weight, epididymal WAT weight and food intake in ob/ob mice (Fig. 3G–I). Co-administration of BD further decreased body weight, epididymal WAT weight and food intake although the additional reduction of epididymal WAT weight was not statistically significant (Fig. 3G–I)). Leptin treatment increased neither plasma β-hydroxybutyrate nor hypothalamic ATP concentrations while BD treatment significantly increased them in ob/ob mice (Fig. 3J,K). These results indicate that BD treatment requires leptin to exert its therapeutic effect and that leptin sensitization is the main effect of BD treatment on body weight.
Leptin sensitizing effect of BD treatment in mice under HFD
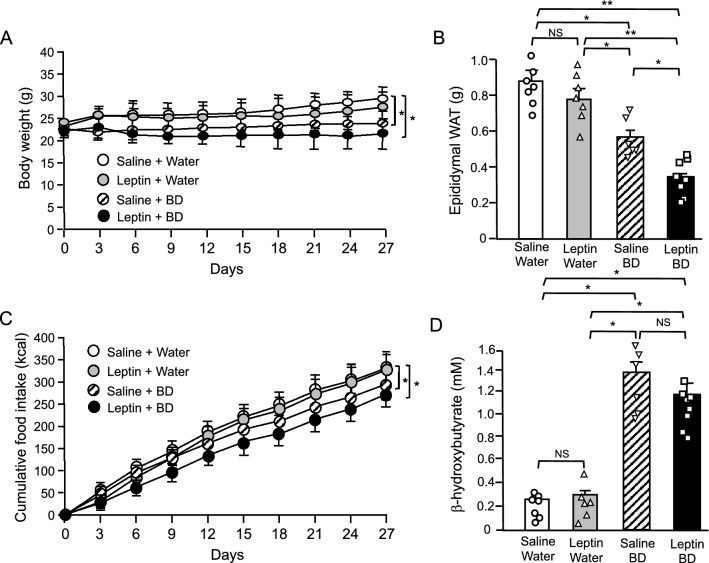
To investigate the leptin sensitizing effect of BD treatment, we treated mice with leptin and/or BD under HFD. Under HFD, once daily leptin injection at the dose of 150 μg/kg had no significant effect on body weight, epididymal WAT weight and food intake (Fig. 4A–C), indicating that HFD induced leptin resistance in mice as reported previously29,30. On the other hand, BD treatment significantly decreased body weight, epididymal WAT weight and food intake and co-administration of leptin and BD further decreased them (Fig. 4A–C). At this time, we confirmed that BD treatment effectively increased plasma β-hydroxybutyrate concentrations irrespective of the presence or absence of leptin treatment (Fig. 4D). Leptin treatment had no effect on plasma β-hydroxybutyrate concentrations. Since leptin treatment alone had no significant effect on body weight, the effect of co-administration can be described as BD treatment sensitizes leptin action.
Figure 4.
Effect of co-administration of oral BD and intraperitoneal leptin in WT mice under high fat diet. (A) Body weight change, (B) epididymal WAT weight, (C) cumulative food intake, (D) plasma β-hydroxybutyrate concentration. Values are means ± SEM (n = 7 for Saline + Water, 6 for Leptin + Water, 6 for Saline + BD, 9 for Leptin + BD). *P < 0.05, **P < 0.01 (Bonferroni's test) (A,C). NS, not significant, *P < 0.05, **P < 0.01 (Student’s t-test) (B,D).
Pair-feeding experiment in mice under HFD
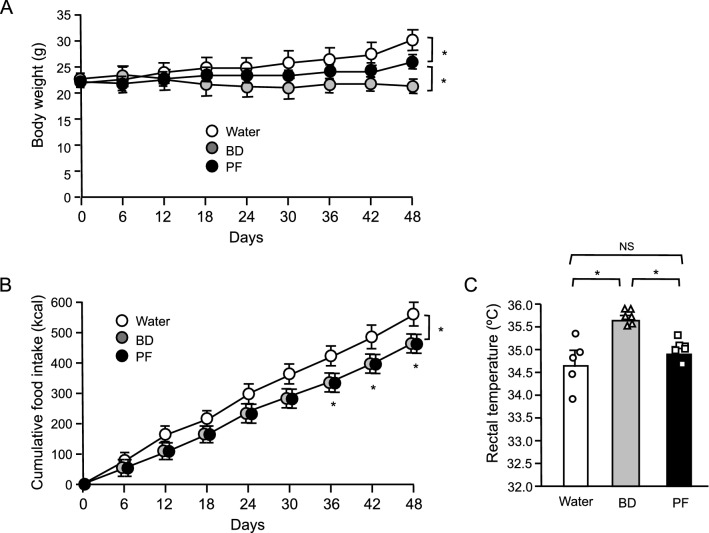
If BD treatment sensitizes leptin action, BD treatment should not only suppress food intake but also increase energy expenditure. To evaluate the influence of food intake reduction by BD treatment on body weight, we performed the pair-feeding experiment in which mice were fed the same amount of food consumed by BD administered mice. Under HFD, BD treatment significantly decreased body weight and food intake (Fig. 5A,B). Pair-feeding also significantly decreased body weight (Fig. 5A). However, Body weight reduction by BD treatment was clearly greater than that by pair-feeding, suggesting that BD treatment induced both food intake reduction and energy expenditure. As expected, while pair-feeding had no effect on rectal temperature in mice, BD treatment significantly increased rectal temperature (Fig. 5C), indicating that BD treatment increased energy expenditure at least partly by thermogenesis.
Figure 5.
Effects of oral BD administration and pair-feeding (PF) in WT mice under high fat diet. (A) Body weight change, (B) cumulative food intake, (C) rectal temperature. Values are means ± SEM (n = 5 for Water, 6 for BD, 6 for PF). *P < 0.05 (Bonferroni's test) (A,B). NS, not significant, *P < 0.05 (Student’s t-test) (C).
Effect of short-term BD treatment on leptin sensitivity in DIO mice
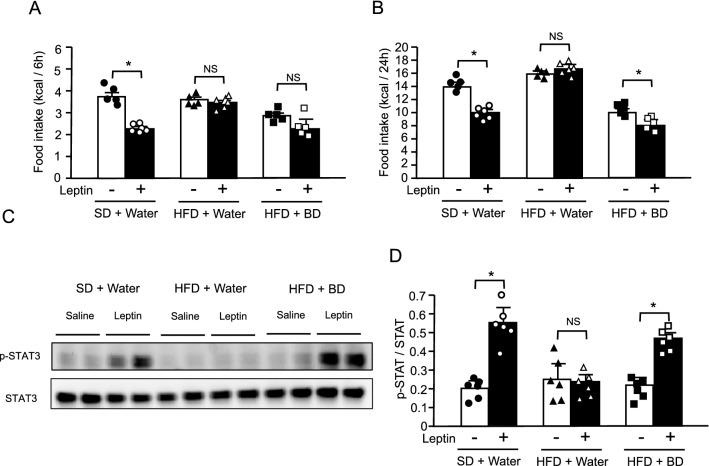
To ensure that the leptin sensitizing effect by BD treatment is not secondary to body weight reduction or metabolic improvement, we examined the effect of short-term BD treatment in DIO mice. After 3-day administration of water or BD to DIO mice, saline or leptin was injected. Three-day BD treatment did not significantly change the body weight and blood glucose concentrations (data not shown). While leptin injection effectively decreased both 6-h and 24-h food intakes after injection in lean control mice, leptin injection had no effect on both 6-h and 24-h food intakes in DIO mice pretreated with water (Fig. 6A,B). However, leptin injection significantly decreased 24-h food intake in DIO mice pretreated with BD, although the reduction of 6-h food intake was not statistically significant. We also examined the leptin receptor signaling in the hypothalamus. Leptin injection effectively increased the phosphorylation of hypothalamic STAT3 in lean control mice but had no effect in DIO mice pretreated with water (Fig. 6C,D, Supplementary Fig. 6C). On the other hand, leptin injection significantly increased the phosphorylation of hypothalamic STAT3 in DIO mice pretreated with BD. These results demonstrate that short-term BD treatment sensitizes leptin action at the level of hypothalamus.
Figure 6.
Effect of oral BD administration on leptin actions in DIO mice. (A) 6-h and (B) 24-h food intake after saline or leptin injection in lean control mice pretreated with water or DIO mice pretreated with water or BD. (C) Western blot analyses for p-STAT3 and total STAT3 protein levels 1 h after saline or leptin injection in the hypothalamus from lean control mice pretreated with water or DIO mice pretreated with water or BD. (D) Ratio of signal intensities of p-STAT3 to total STAT3 in C. Values are means ± SEM (n = 6 for each group). NS, not significant, *P < 0.05 (Student’s t-test).
Effect of BD treatment on ER stress in the hypothalamus of DIO and ob/ob mice
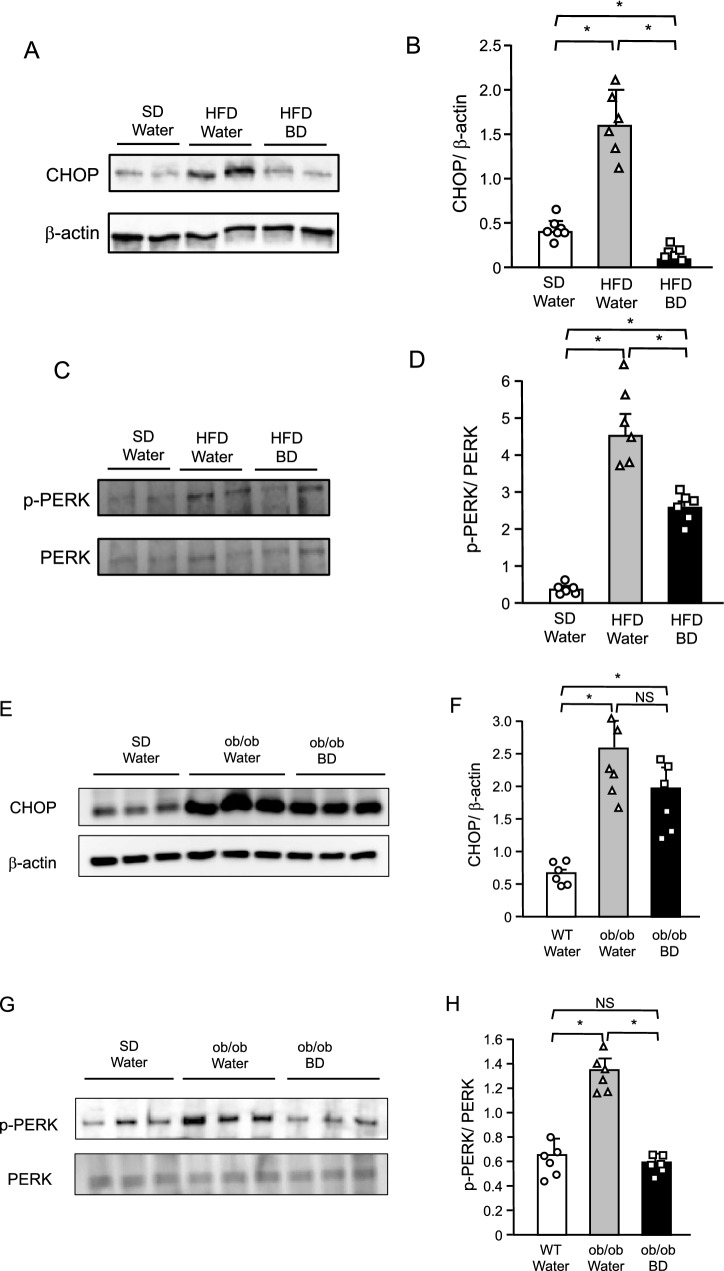
We investigated whether BD treatment attenuates ER stress in the hypothalamus of DIO mice. Water or BD were administered to DIO mice for 3 days. Expressions of ER stress markers, CHOP and the phosphorylation of PERK were markedly increased in DIO mice when compared with lean control mice (Fig. 7A–D, Supplementary Fig. 7A,C), indicating that HFD induced ER stress in the hypothalamus. However, short-term BD pretreatment effectively suppressed these increments of CHOP expression and PERK phosphorylation under HFD (Fig. 7A–D, Supplementary Fig. 7A,C), indicating that BD treatment attenuated ER stress in the hypothalamus under HFD. In this study, BD treatment sensitized leptin action in ob/ob mice (Fig. 3G–I). Therefore, we also examined expressions of ER stress markers in the hypothalamus sampled from ob/ob mice at the end of the chronic BD treatment experiment. Both expressions of CHOP and phosphorylated PERK were significantly increased in ob/ob mice treated with water when compared with lean control mice (Fig. 7E–H, Supplementary Fig. 7E,H), indicating that ER stress occurred in the hypothalamus of ob/ob mice. BD treatment did not significantly decreased CHOP expression but markedly decreased phosphorylation of PERK (Fig. 7E–H, Supplementary Fig. 7E,H), suggesting that BD treatment attenuated ER stress in the hypothalamus of ob/ob mice.
Figure 7.
Effect of BD on expression of ER stress marker in the hypothalamus of DIO (A–D) and ob/ob (E–H) mice. (A,E) Western blot analyses for CHOP and β-actin protein levels in the hypothalamus. (B,F) Ratio of signal intensities of CHOP to β-actin in A and E. (C,G) Western blot analyses for p-PERK and total PERK protein levels in the hypothalamus. (D,H) Ratio of signal intensities of p-PERK to total PERK in C and G. Values are means ± SEM (A–D, n = 7 for SD + Water, 6 for HFD + Water, 6 for HFD + BD; E–H, n = 6 for WT + Water, 6 for ob/ob + Water, 6 for ob/ob + BD). NS, not significant, *P < 0.05 (Student’s t-test).
Discussion
BD treatment effectively reduced body weight and food intake in DIO mice (Fig. 1A,D). Generally, DIO mice are made obese and leptin resistant with HFD loading29,30. DIO mice used in this study were also leptin resistant, as plasma leptin concentrations were markedly elevated (Fig. 1C). Thus, it can be said that BD treatment is effective for mice with leptin resistance. On the other hand, BD treatment had no effect in lean control mice fed SD (Fig. 2). Lean mice had very low circulating leptin levels than DIO mice (Fig. 1C), suggesting that the therapeutic effect of BD treatment requires a decent amount of leptin and/or leptin resistance. To investigate the role of leptin in the therapeutic effect of BD treatment, we treated genetically leptin deficient ob/ob mice with BD. As expected, BD treatment alone had no effect, but co-administration of BD with leptin resulted in greater weight loss in ob/ob mice than leptin treatment alone (Fig. 3). These results demonstrate that leptin is necessary for BD treatment to exert its effects, and that leptin sensitization is the primary effect of BD treatment on body weight.
We verified the leptin sensitizing effect of BD treatment under HFD feeding which induces leptin resistance. In mice fed HFD, while leptin treatment had no effect, co-administration of BD and leptin resulted in a significant decrease in body weight and food intake compared to BD treatment alone (Fig. 4). This certainly indicates that BD treatment sensitized leptin action. The decrease in body weight of leptin resistant mice after BD treatment alone is thought to be due to the sensitization of endogenous leptin by BD. Furthermore, pair-feeding experiment revealed that BD treatment decreased body weight by not only suppressed food intake, but also up-regulating energy expenditure (Fig. 5). This finding supports the idea that BD treatment sensitizes endogenous leptin, which is well known to suppress appetite and increase energy expenditure31.
To ensure that the leptin sensitizing effects by BD treatment are not secondary to body weight reduction or metabolic improvement, we examined the effects of short-term BD treatment in DIO mice. Although there were no significant changes in body weight or blood glucose levels after three days of BD treatment (data not shown), apparently sensitized leptin action in DIO mice in which exogenous leptin showed no effect (Fig. 6A,B). At this time, we examined the phosphorylation of STAT3 as a leptin receptor signal in the hypothalamus of DIO mice. Three-days BD treatment also increased leptin-induced phosphorylation of STAT3 (Fig. 6C,D). These results indicate that leptin sensitization by BD treatment is the primary effect at the hypothalamic level.
The aim of this study is to investigate the effect of ketone bodies on leptin sensitivity in mice. BD was used to raise the plasma ketone body concentration. We hypothesized that ketone bodies ameliorate ER stress and leptin resistance in the hypothalamus through the elevation of hypothalamic ATP concentration. The precise mechanism underlying leptin resistance has not been clarified. However, it has been reported that ER stress in the hypothalamus plays a key role in the pathogenesis of leptin resistance9,10. Under ER stress, the accumulation of misfolded proteins facilitates UPR, which requires appreciable amounts of ATP11,12. Thus, abundant ATP supply might have protective effect against ER stress. As expected, BD treatment decreased expressions of ER stress markers, which were elevated in the hypothalamus of DIO mice (Fig. 7A–D). These results support the view that the elevation of hypothalamic ATP concentration has a protective effect against ER stress.
Since BD treatment also sensitized leptin action in ob/ob mice (Fig. 3G–I), we examined the ER stress in the hypothalamus of ob/ob mice. Compared with lean control mice, ER stress markers were elevated in the hypothalamus of ob/ob mice (Fig. 7E–H), as reported previously32. BD treatment increased plasma βHB and hypothalamic ATP concentrations (Fig. 3D,E) and decreased expressions of ER stress markers in the hypothalamus in ob/ob mice (Fig. 7E–H). These results suggest that ketone bodies ameliorate ER stress and leptin resistance in the hypothalamus by increasing hypothalamic ATP concentration in not only DIO but also in ob/ob mice.
The effects of ketogenic diets on body weight and food intake have are contradictory18. One of the reasons for this contradiction is that ketogenic diet is high fat and low carbohydrate at the same time. To test the simple effect of ketone bodies on body weight and food intake, we used BD as a ketogenic supplement to increase plasma ketone body concentrations26,27. In addition, we administered BD to mice by oral gavage to avoid the change of the diet composition. BD administration by oral gavage effectively increased plasma β-hydroxybutyrate concentrations in mice. Thus, the present study could demonstrate the effect of ketone body itself on body weight and food intake.
We further investigated the effects of BD treatment on glucose and lipid metabolism in DIO. BD treatment significantly decreased fasting glucose levels and HOMA-IR (Fig. 1G,I), indicating that BD treatment improved insulin resistance in DIO mice. On the other hand, BD treatment caused little change in lipid biomarkers such as fasting plasma triglyceride and total cholesterol concentrations (Fig. 1J,K), although showed a tendency to decrease liver weight in DIO mice (Fig. 1L). At least, there were no adverse effects of BD treatment on lipid metabolism. The sick feelings or digestive problems caused by oral BD administration may have led to decreased food intake and body weight in DIO mice. If so, oral BD administration should decrease food intake and body weight in lean control and ob/ob mice. However, it had no effect on these mice. This means that oral BD administration did not cause any major digestive problems.
The present study provides collateral evidence that ketone bodies ameliorate ER stress and leptin resistance in the hypothalamus through the elevation of hypothalamic ATP concentration. However, although it has been reported that ATP treatment protects cultured cells against ER stress13, we did not directly prove that the elevation of ATP concentrations ameliorates ER stress in the mouse hypothalamus. As a mechanism, it is speculated that abundant ATP supply reinforces the UPR, which requires appreciable amounts of ATP. However, the precise mechanism by which ATP ameliorates ER stress is also unclear.
Previous studies demonstrated that overexpression of X-box binding protein 1 (XBP1) in in vitro and in vivo settings reduces ER stress and increases leptin sensitivity10,33. It was also reported that chemical chaperones such as 4-phenyl butyrate (4-PBA) and tauroursodeoxycholic acid (TUDCA), which are agents that decrease ER stress, increase leptin sensitivity10,34. Because treatment of 4-PBA reduces ER stress even in the absence of XBP134, each manipulation regulates ER stress via independent pathways. Celastrol, which was a small molecule discovered by analyzing the effects of both XBP1 and 4-PBA, was also demonstrated to decrease ER stress and increase leptin sensitivity in the hypothalamus35. The effect of Celastrol on body weight is more potent than 4-PBA or TUDCA35. ER stress response has been shown to have multiple pathways36. Although the present study demonstrated that BD treatment decreases ER stress in the hypothalamus, the details of pathways remains unclear. More elaborate studies are needed to elucidate the pathway by which ketone bodies or ATP decreases ER stress.
In conclusion, this is the first report demonstrating the leptin sensitizing effect of BD treatment in DIO mice with leptin resistance. BD treatment effectively increased plasma βHB and hypothalamic ATP concentrations and ameliorated ER stress and leptin resistance at the hypothalamic level. The present study suggests the possibility that the effect of BD treatment is mediated by the elevation of hypothalamic ATP concentration. Ketone bodies and hypothalamic ATP are the potential targets for the treatment of obesity and its complications.
Methods
Materials
All reagents were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise mentioned.
Animals
Male C57BL/6J wild type and ob/ob mice were purchased from Japan SLC (Shizuoka, Japan). Mice were housed under conditions of controlled temperature (23 °C ± 1 °C), humidity (55% ± 5%) and lighting (light phase 7:30–19:30) and fed an ad libitum standard diet (SD) (MF; Oriental Yeast, Tokyo, Japan). For high fat diet (HFD) loading, mice were fed an ad libitum HFD containing 20% kcal for kcal (kcal/kcal) protein, 20% kcal/kcal carbohydrate, and 60% kcal/kcal fat (D12492; Research Diets, New Brunswick, NJ) from the ages of 8 weeks. To induce DIO in mice, HFD loading was conducted for 8 weeks. All animal experiments were carried out after receiving approval from the Institutional Animal Experiment Committee and in accordance with the Institutional Regulation for Animal Experiments at Jichi Medical University.
BD treatment
BD was administered twice daily by oral gavage at the dose of 5 g/kg at 8:00 and 17:00. As a control, water of the same volume was administered. The calorie of BD used for treatment was added to the amount of food intake for BD treatment groups.
Co-administration experiment of leptin and BD
Leptin (Amylin Pharmaceuticals, San Diego, CA) was intraperitoneally injected at the dose of 150 μg/kg at 17:00. As a control, saline of the same volume was injected. BD was administered as described above. For an experiment with ob/ob mice, 12 weeks old mice were divided into three treatment groups of ‘saline + water’, ‘leptin + water’ and ‘leptin + BD’ to be counterbalanced for starting body weight. For an experiment under HFD, 8 weeks old wild type mice were divided into four treatment groups of ‘saline + water’, ‘leptin + water’, ‘saline + BD’ and ‘leptin + BD’ to be counterbalanced for starting body weight. HFD feeding was continued during the experiment.
Pair-feeding experiment
Pair-feeding experiment was performed under HFD. Eight weeks old wild type mice were divided into three groups of ‘water’, ‘BD’ and ‘water + pair-fed’ to BD administered mice not to make differences in starting body weight among groups. For pair-fed mice, food of the same amount consumed by BD administered mice during light and dark phases on the previous day was placed at 8:00 and 17:00, respectively.
Biochemical assays
Blood was obtained from the retro-orbital sinus after 4 h fasting. Plasma leptin concentrations were measured by ELISA kit for rat leptin (Millipore, St. Charles, MO). Plasma glucose concentrations were measured by a glucose assay kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin concentrations were measured by an insulin-ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan). Plasma triglyceride (TG), nonesterified fatty acid (NEFA), and total cholesterol concentrations were measured by enzymatic kits (Triglyceride E-test Wako, NEFA C-test Wako, and Cholesterol E-test Wako, respectively; Wako Pure Chemical Industries). For measurement of plasma βHB concentrations, blood was obtained 4 h after water or BD administration. Plasma βHB concentrations were measured by beta Hydroxybutyrate Assay Kit (abcam, Cambridge, MA).
Calculation of HOMA of insulin resistance
The HOMA of insulin resistance (HOMA-IR) was calculated as an indicator of insulin sensitivity according to Eq. (1):
| 1 |
Measurement of hypothalamic ATP concentrations
Whole hypothalami were obtained from mice 4 h after water or BD administration. ATP levels were measured by using the luciferase-based Tissue ATP assay Kit (TOYO B-Net, Tokyo, Japan). As described in the instruction, whole hypothalami were lysed in lysis buffer. After centrifugation (4 °C 1000×g, 10 min), the supernatant was incubated with ATP extraction regent at room temperature for 30 min. The samples (100 μl) mixed with 100 μl of ATP luminescence reagent were measured by luminometer GL-200 (MICROTEC, Funabashi, Japan) and calculated.
Measurement of rectal temperature
The rectal temperature was measured by a digital thermometer (BAT-7001H, Physitemp Instruments, Inc., Clifton, NJ) in animal rooms at 9:00.
Assessment of leptin sensitivity
Water or BD (5 g/kg) was administered at 8:00 and 17:00 twice daily for three days to 16-week-old lean or DIO mice in which had been fed HFD for 8 weeks. Water and BD groups were divided into two sub-groups and mice were intraperitoneally injected either with saline or leptin (150 μg/kg) at 10:00 on the third day. Food intake were monitored for 24 h after saline or leptin injection. For western blot analysis for phosphorylation of STAT3, whole hypothalami were obtained from mice 1 h after saline or leptin injection.
Western blot analysis
Whole hypothalami were homogenized and lysed in a solution containing 20 mM Tris (pH 7.5) 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate and 1 mM sodium orthovanadate; the lysates were analyzed for protein content before SDS-PAGE analysis. For the analysis of ER stress markers, whole hypothalamic were obtained from mice on the last day of each experiment. Membranes were immunoblotted with each antibody to phospho-STAT3 (Tyr705), STAT3, CHOP, β-actin, phospho-PERK (Thr980) and PERK. All antibodies were purchased from Cell Signaling Technology (Beverley, MA). Amersham ECL prime (GE Healthcare Life Sciences, Pittsburgh, PA) and ImageQuant LAS 4000mini (GE Healthcare Life Sciences) were used for the detection.
Statistics
Data are expressed as means ± SEM. Comparison between or among groups was assessed by Student’s t test or Bonferroni's multiple comparison test. P < 0.05 was considered statistically significant.
Supplementary Information
Author contributions
M.I. and K.E. designed research. M.I., N.S., A.M., and C.E. performed research. M.I., N.S., A.M., K.S., C.E., A.T., S.T., D.Y., T.W., and K.E. analyzed the data. M.I., C.E., and K.E. wrote the paper. M.T., S.N., and S.I. supervised the research. All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96460-y.
References
- 1.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Halaas JL, Boozer C, Blair-West J, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 7.Hosoda K, Masuzaki H, Ogawa Y, et al. Development of radioimmunoassay for human leptin. Biochem. Biophys. Res. Commun. 1996;221:234–239. doi: 10.1006/bbrc.1996.0579. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 9.Hosoi T, Sasaki M, Miyahara T, et al. Endoplasmic reticulum stress induces leptin resistance. Mol. Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol. Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Depaoli MR, Hay JC, Graier WF, Malli R. The enigmatic ATP supply of the endoplasmic reticulum. Biol. Rev. Camb. Philos. Soc. 2019;94:610–628. doi: 10.1111/brv.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohashi Ikeda H, Sasaoka N, Koike M, et al. Novel VCP modulators mitigate major pathologies of rd10, a mouse model of retinitis pigmentosa. Sci. Rep. 2014;4:5970. doi: 10.1038/srep05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 15.Maalouf M, Sullivan PG, Davis L, et al. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrett SG, Milder JB, Liang L-P, et al. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 17.Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: One decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- 18.Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015;6:27. doi: 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Kim DS, Daily JW. Central infusion of ketone bodies modulates body weight and hepatic insulin sensitivity by modifying hypothalamic leptin and insulin signaling pathways in type 2 diabetic rats. Brain Res. 2011;1401:95–103. doi: 10.1016/j.brainres.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie C, Bralet A-M, Laboratoire JB. Protective action of 1,3-butanediol in cerebral ischemia. A neurologic, histologic, and metabolic study. J. Cereb. Blood Flow Metabol. 1987;7:794–800. doi: 10.1038/jcbfm.1987.136. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Isoda F, Belsham DD, Mobbs CV. Inhibition of agouti-related peptide expression by glucose in a clonal hypothalamic neuronal cell line is mediated by glycolysis, not oxidative phosphorylation. Endocrinology. 2008;149:703–710. doi: 10.1210/en.2007-0772. [DOI] [PubMed] [Google Scholar]
- 24.Kies C, Tobin RB, Fox HM, Mehlman MA. Utilization of 1,3-butanediol and nonspecific nitrogen in human adults. J. Nutr. 1973;103:1155–1163. doi: 10.1093/jn/103.8.1155. [DOI] [PubMed] [Google Scholar]
- 25.Mehlman MA, Veech RL. Redox and phosphorylation states and metabolite concentrations in frozen clamped livers of rats fed diets containing 1,3-butanediol and DL-carnitine. J. Nutr. 1972;102:45–51. doi: 10.1093/jn/102.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in the rat: Conversion to β-hydroxybutyrate. J. Nutr. 1971;101:1719–1726. doi: 10.1093/jn/101.12.1719. [DOI] [PubMed] [Google Scholar]
- 27.Kesl SL, Poff AM, Ward NP, et al. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague–Dawley rats. Nutr. Metab. (Lond.) 2016;13:9. doi: 10.1186/s12986-016-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RAH, Deemer SE, Bergeron JM, et al. Dietary R, S-1,3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. FASEB J. 2019;33:2409–2421. doi: 10.1096/fj.201800821RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Git KC, Adan RA. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. 2016;16:207–224. doi: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Hidaka S, Masuzaki H, Yasue S, Minokoshi Y, Ebihara K, Chusho H, Ogawa Y, Toyoda T, Sato K, Miyanaga F, Fujimoto M, Tomita T, Kusakabe T, Kobayashi N, Tanioka H, Hayashi T, Hosoda K, Yoshimatsu H, Sakata T, Nakao K. Skeletal muscle AMP-activated protein kinase phosphorylation parallels metabolic phenotype in leptin transgenic mice under dietary modification. Diabetes. 2005;54:2365–2374. doi: 10.2337/diabetes.54.8.2365. [DOI] [PubMed] [Google Scholar]
- 31.Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S, Aintablian A, Coupe B, Bouret SG. The endoplasmic reticulum stress-autophagy pathway controls hypothalamic development and energy balance regulation in leptin-deficient neonates. Nat. Commun. 2020;11:1914. doi: 10.1038/s41467-020-15624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams KW, Liu T, Kong X, et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–482. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.