Abstract
Background and Objectives:
Non-thermal atmospheric-pressure plasma or cold plasma is defined as an ionized gas. This study aimed to investigate the effect of cold plasma on Pseudomonas aeruginosa strains. Also, the expression level of the alp virulence gene before and after treatment with cold plasma was compared with the Housekeeping gene gyrA.
Materials and Methods:
P. aeruginosa isolates recovered from hospitalized burn patients at Shahid Motahari Burns Hospital, Tehran, Iran. The Kirby Bauer disk diffusion method was used to determine the antimicrobial susceptibility test. Then, the antibacterial effect of atmospheric non-thermal plasma was evaluated on P. aeruginosa in as in vitro and in vivo studies at different times on Muller Hinton agar and in mouse model (treated by plasma every day/ 90 sec). The histopathological study was evaluated by Hematoxylin-Eosin staining. Data were analyzed using SPSS software by the Chi-square test and Pvalues less than 0.05 considered as statistically significant.
Results:
Results indicated that non-thermal atmospheric plasma inhibited the growth of P. aeruginosa. The non-thermal helium plasma accelerates wound healing for 6 days. Results showed that cold plasma decreased virulence gene expression alp after treatment. Therefore, cold plasma can be suggested as a complementary therapeutic protocol to reduce bacterial infection and accelerate wound healing and reduce the expression of virulence genes of pathogens.
Conclusion:
Cold plasma showed pathogen inhibitory properties of P. aeruginosa and virulence alkaline protease and wound healing properties in animal models, so this inexpensive and suitable method can be presented to the medical community to disinfect burn wounds and improve wound healing.
Keywords: Cold plasma, Pseudomonas aeruginosa, Burn, Wound, Alp gene, Real-time reverse transcription polymerase chain reaction
INTRODUCTION
Skin is one of the most organs of the human body, and it includes 15% of adult body weight. It is the most important defense barrier against physical, chemical, and biological invasive (1).
Wounds are hurts with a specific span caused by interior or exterior factors like disease, surgeries, traumas, or accidents on a specific organ. Skin cells can burn by direct contact with flame, hot surfaces, hot liquids or steam, electricity current, or resource. The damage intensity changes by the difference in temperature and time of exposure (2). The burn is the most prevalent and destructive kind of trauma and patient with intense burn should be hospitalized to decrease the mortality rate (3, 4).
There is a complex process to infect a wound, however, a critical wound that has signs like redness, inflation, fever, and pain are infected. Researchers believe that even if erythema, inflation or pain doesn’t appear again it is necessary to consider risk of infection (5). Infection in burn wounds is the most important problem to treat these kinds of trauma because it causes a delay in recovery and healing in wounds. Also, infection is a factor in creating skin scars. Microorganisms’ invasion into underneath layers can cause bacteremia, sepsis, or multiple organ dysfunction syndromes (4).
Infection is common in burns because the injury causes the skin to lose its natural barrier to microbes; this allows pathogens to have a direct entry route to the wound. Burn wounds usually produce high levels of exudate, which creates a suitably moist, nutrient-rich environment for bacterial growth (6).
Despite all made great progress in care and treatment of burn wound infections and improving quality of therapeutic management, and even using antimicrobial drugs, wound dressings, and the other ways of treatment, infection is one of the principal agents of mortality, exacerbation of illness, and financial difficulty in the world (3).
Many people hospitalized in the world due to burn infections because burn wounds are susceptible to infections (8). Skin burned to prepare a suitable environment for bacterial growth. Pseudomonas aeruginosa and Staphylococcus aureus is very important in terms of antibiotic resistance, so it is worthwhile to find other treatments (7).
Cold plasma is defined as an ionized gas or the fourth state of matter and researchers have been carrying out a lot of study for centuries about it. Cold atmospheric pressure plasma (CAPP), also known as non-thermal plasma, in the last decades has been attracting many people’s attention due to its special advantages (9–12).
Many researchers around the world, have done wide studies on cold atmospheric pressure plasma in these years and have been published their findings. These studies’ subjects are related to decontamination mechanism, plasma cell interaction, cancer treatment, skin disinfection, blood coagulation, chronic wound healing, and so on. Also, studies and researches on CAPP device development have been done to make its application easier and safer (9–16).
Plasma is containing electrons, ions, neutral particles, UV radiation (UVA, UVB, UVC), heat, reactive oxygen, nitrogen species (RONS), and so on. The type of components and their density depends on the source of plasma, in other words, the type of gas and power input (11, 12).
Plasma sterilization is the first research field of plasma medicine. Despite a lot of researches carried out on how plasma affects microorganisms but its real mechanism still is vague. Researchers believe three mechanisms are possible: electroporationand oxidation-induced cell wall/membrane dysfunction, which leads to leakage of cellular components; intracellular oxidation and nitrification causing protein damage and gene expression disorder; and direct DNA damage such as causing double-strand breaking (9–16).
The effect of cold plasma on bacterial killing has been investigated for more than 20 years. It is found that cold atmospheric pressure plasma could effectively inactivate different types of bacteria, including Gram-positive and Gram-negative, anaerobic, aerobic, or facultative anaerobic bacteria (8). According to the species of bacteria, the response to CAPP is different and actually, it’s a species-dependent process and the Gram-positive bacteria is usually more susceptible to CAPs treatment because of the difference cell-wall components, which indicates that the CAPP-induced damage to the cell membrane and cell wall may be a key factor of antibacterial effect (9–17). Bacteria cause more than 60% of infection while they become more resistant to antimicrobial drugs every day, and often develop into a chronic state (8, 18).
The interaction between plasma and human cells largely depends on the plasma source, plasma doses as well as cell type. Cell induction, proliferation and distribution, and angiogenesis are important factors in wound healing and CAPP induces them. Cells involved in wound healing are included fibroblasts and keratinocytes. Researches show that CAPP can increase their proliferation and distribution. And also, cold atmospheric pressure plasma can induce angiogenesis too (9–18).
Cold atmospheric pressure plasma could affect different stages of wound healing by deactivating and killing microorganisms in the first stage and stimulate proliferation and migration of cells which involve in wound healing in the following period. CAPP has demonstrated high wound healing abilities and it can become a promising therapy to replace or assist traditional methods in clinics for the wound healing process, especially in chronic wounds. With the certification of several CAP products, more standards and procedures for clinical treatments should be cleared in the future to guide the plasma treatment effectively and safely. The Real-time-RT-PCR technique has been used extensively for gene expression analysis (9–18).
In this study, the effect of cold atmospheric pressure plasma with helium and argon sources on microorganisms in invitro and burn wound infection in in vivo and virulence gene inhibition with Real-time PCR was investigated.
MATERIALS AND METHODS
The power supply used for plasma jet production.
To generate jet plasma; the DC power device was used. This designed device created a voltage from 0 to 10 kV with a frequency of 20 kHz and its pressure was from 6 to 8 N/m2.
Bacteria strains and culture condition.
P. aeruginosa was isolated from burn patients hospitalized at Shahid Motahari burn hospital. Isolates of P. aeruginosa cultured on the Muller Hinton Agar and incubated for 24 hours at 37°C. The identification was done by phenotypical tests (Gram staining, growth at 42°C, pigmentation, and biochemical tests including; oxidase, catalase, oxidation/fermentation, arginine dehydratase tests).
Antimicrobial susceptibility testing.
The antibiotic resistance pattern carried out according to CLSI2020 protocol. Antibiotics studied in this research include polymyxin B, amikacin, gentamicin, tobramycin, and ciprofloxacin (Merck KGaA, Darmstadt, Germany).
Antibacterial effect of atmospheric non-thermal plasma on P. aeruginosa in vitro.
CLSI pattern was used to check the inhibitory effects of cold plasma (10). To determine the antibacterial activity of non-thermal atmospheric plasma, firstly, a 0.5 Mc-Farland standard was prepared from selected strains based on CLSI2020 standards and plated on the Muller Hinton Agar medium. Then, Plates containing cultured bacteria were exposed to non-thermal atmospheric irradiation with gases (argon and helium) at intervals 5, 15, 30, 60, 90, and 120 seconds. Irradiations were placed at a distance of 2 cm from the plate, then the plates were incubated at 37°C for 24 hours, finally, the results were recorded and analyzed (8, 11). The MIC of cold plasma against P. aeruginosa determined as treatment with argon gas in different times.
Antibacterial impact of atmospheric non-thermal (Helium) plasma on P. aeruginosa.
Ten female rats with average weights 30 ± 2 gm were categorized into separate groups and the back of the mice was shaved in a length of 3 cm then the rats were anesthetized by injecting 10 ml of ketamine substance (19).
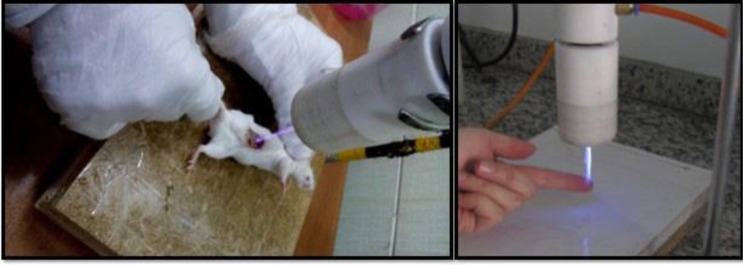
The ketamine was administered intramuscularly (10–25 mg/kg IM). Then burn wound with a diameter of 2 cm was created by the Ian Holder method (19). After that, from a 0.5 McFarland bacterial suspension with an insulin syringe inoculated on the wound, this procedure was repeated 3 days as the infection would be visible on the skin. Then, 4 mice were used as a negative control, and 6 mice were treated with identical values of Helium plasma. Argon plasma was ignored due to the high breakdown voltage that caused tissue necrosis. All 6 mice were encountered with helium plasma for 90 secs for 5 consecutive days. Next, the tissues were prepared from section exposed by plasma gas at control non exposed with plasma, and mice were treated by plasma with voltage from 0 to 10 kV with frequency 20 kHz and its pressure was from 6 to 8 N/m2 (Fig. 1).
Fig. 1.
The method of exposing BALB/c mice to non-thermal atmospheric helium plasma.
Histopathology.
To study the histological effect of atmospheric non-thermal plasma, the pathology sections were prepared and stained with Hematoxylin-Eosin dyes, the histology samples were observed under a microscope with magnifying 1000× after staining. Sydney protocol was applied for histology classification and scales of 0 to 3 were used for the activity of atrophy and inflammation (19).
Real-time PCR.
The inhibitory effect of cold plasma against the virulence alkaline protease (alp) gene of P. aeruginosa was evaluated by the Real-time PCR technique and compared with the housekeeping gene gyrA. The primer sequences of alp and DNA gyrase A are showed in the below (Table 1) (9, 13).
Table 1.
Sequences of primers used for amplification alp and gyrA
| alp gene primer | |
|---|---|
| R | ATTACTGACGCTGATTGTGC |
| F | CTGGAGAGACTAAGCCCTCC |
| gyrA gene primerAgene primer | |
| F | GGTCTGGGCATAGAGGTTGT |
| R | GGTCTGGGCATAGAGGTTGT |
RESULTS
The results of susceptibility testing reported that all strains were resistant to selected antibiotics; ceftazidime (30 μg), amikacin (30 μg), gentamicin (10 μg), and piperacillin (100 μg) with growth inhibition zone according to CLSI 2020.
Results of the effect of non-thermal atmospheric pressure argon plasma jet on P. aeruginosa in vitro.
The confirmation of the antibacterial effects of the non-thermal atmospheric argon plasma jet was accomplished by observing the diameter of the growth inhibition zone P. aeruginosa in Muller Hinton agar medium. The diameter of the inhibition zones are demonstrated in Table 2 and Fig. 2.
Table 2.
The diameter of the inhibition zone of a non-thermal atmospheric argon plasma jet on P. aeruginosa at different times of plasma radiation on Muller Hinton agar.
| Time strains | 5 sec | 15 sec | 30 sec | 60 sec | 90 sec | 120 sec | Std. Deviation |
|---|---|---|---|---|---|---|---|
| No 1 | 9 mm | 11 mm | 18 mm | 20 mm | 22 mm | 25 mm | 77005. |
| No 2 | 9 mm | 10 mm | 17 mm | 20 mm | 21 mm | 24 mm | 54730. |
| No 3 | 10 mm | 11 mm | 18 mm | 21 mm | 22 mm | 26 mm | 39830. |
| No 4 | 9 mm | 10 mm | 18 mm | 20 mm | 22 mm | 25 mm | 46820. |
| No 5 | 9 mm | 10 mm | 18 mm | 20 mm | 22 mm | 25 mm | 62530. |
No 1: strain number 1. No 2: strain number 2. No 3: strain number 3. No 4: strain number 4. No 5: strain number 5.
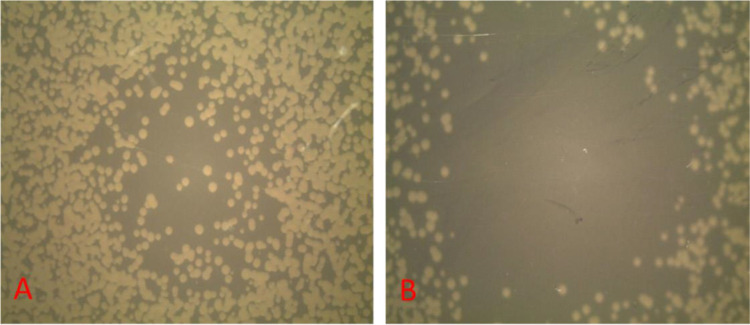
Fig. 2.
The inhibition zone diameter of a non-thermal atmospheric argon plasma jet on P. aeruginosa at different times of plasma radiation on MHA medium, A; after 5 seconds and B; after 60 seconds.
Results of the impact of non-thermal atmospheric helium on P. aeruginosa in vitro.
The confirmation of the antibacterial effects of the non-thermal atmospheric helium plasma jet was accomplished by observing the diameter of the inhibition zone on P. aeruginosa and the diameter of the inhibition zone was stated in Table 3 and Fig. 3. Results indicated MIC with argon plasma against P. aeruginosa was 15 seconds.
Table 3.
The diameter of the inhibition zone of non-thermal atmospheric helium plasma on P. aeruginosa at different times of plasma radiation
| Time strains | 5 sec | 15 sec | 30 sec | 60 sec | 90 sec | 120 sec | Std. Deviation |
|---|---|---|---|---|---|---|---|
| No 1 | 0 mm | 4 mm | 6 mm | 8 mm | 12 mm | 15 mm | 67005. |
| No 2 | 0 mm | 3 mm | 6 mm | 8 mm | 12 mm | 15 mm | 44830. |
| No 3 | 0 mm | 3 mm | 6 mm | 8 mm | 12 mm | 15 mm | 43830. |
| No 4 | 0 mm | 3 mm | 6 mm | 8 mm | 11 mm | 15 mm | 45830. |
| No 5 | 0 mm | 3 mm | 6 mm | 8 mm | 11 mm | 15 mm | 42830. |
No 1: strain number 1. No 2: strain number 2. No 3: strain number 3. No 4: strain number 4. No 5: strain number 5.
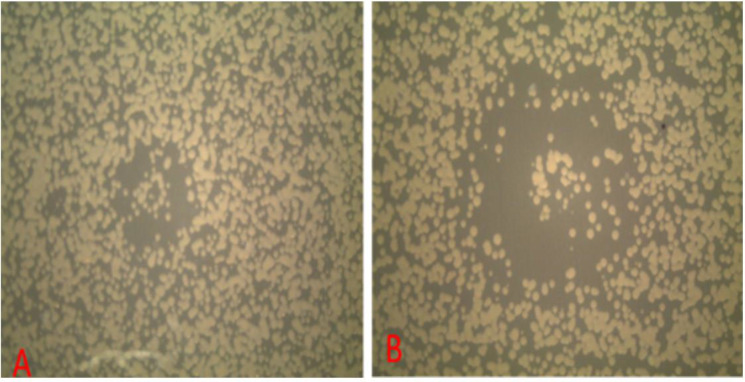
Fig. 3.
The diameter of the inhibition zone of non-thermal atmospheric Helium plasma on P. aeruginosa at different times of plasma radiation, A; after 5 seconds and B; after 1 minute.
Table 4.
The concentrations of compounds for Real-Time -PCR
| Compound | Concentration |
|---|---|
| qPCR SYBR Green Master Mix | 12.5 μl (1 x) |
| Template | 1 μl (1 μg) |
| Primer Forward | 1 μl (10 μm) |
| Primer Reverse | 1 μl (10 μm) |
| D.D.W | 9.5 μl |
| Total Volume | 25 μl |
Negative control samples of helium and argon gas were non-plasma, which had no effect with a growth inhibition zone of 0 mm.
Results of confirmation of the inhibition of P. aeruginosa gorwth by non-thermal atmospheric plasma.
In the growth inhibition zone, to ensure the absence of bacterium was prepared a culture and results showed no growth. This showed that the atmospheric non-thermal plasma was fully appropriate for the inhibition of P. aeruginosa.
Results of the impact of non-thermal atmospheric Helium plasma in vivo.
After 5 days of treatment of mice with cold plasma, the results showed that the burn wounds infection was completely treated (Fig. 4).
Fig. 4.
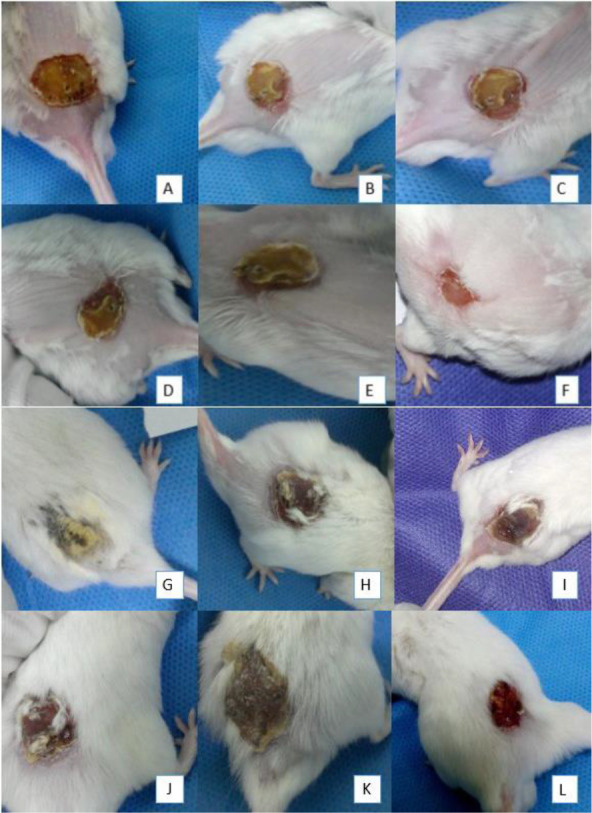
The images of Mice treated with non-thermal atmospheric plasma, A to F, respectively, from the first day of treatment until the 6th day. A (First day), B (second day), C (Third day), D (Fourth day), E (Fifth day) and (Six days), and the image of the control group are from G to L respectively, G (First day), H (second day), I (Third day), J (Fourth day), K (Fifth day) and L (Sixth day).
Histopathology.
Results were interpreted based on the Sydney protocol for classification inflammation degree. In control samples, chemotaxis, and the number of macrophages and neutrophils in the tissue were less than treated mice and the wound healing was also slower and the number of bacteria reported more. In the treated sample, chemotaxis and plenty of macrophages and neutrophils were observed. By increasing the number of defensive cells and the inflammation rate, the number of bacteria was decreased (Fig. 5).
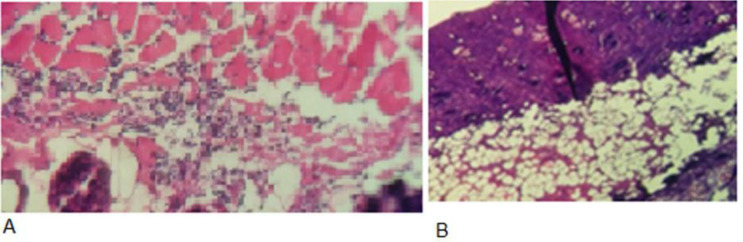
Fig. 5.
Impact of atmospheric non-thermal plasma after staining with Hematoxylin Eosin and observing by light microscopy. (A) Pathology image of the control group and (B) Pathology image of the plasma-treated group.
Real-time RT-PCR.
The concentrations of compounds for Real-Time -PCR reactions and temperature protocols are demonstrated in Tables 3 and 4.
Table 5.
Temperature protocol required for Real-Time -PCR reaction
| Step | Temp | Time | Cycle No. |
|---|---|---|---|
| Optional: UDG pre-treatment | 50°C | 2 min | 1 |
| Initial Denaturation | 95°C | 10 min | 1 |
| Denaturation | 95°C | 15 Second | 40 |
| Annealing | 60°C | 60 Second | 40 |
After preparation of the reaction mixture, these samples were placed in the Applied Biosystem (AB) and the reactions were performed according to Tables 3 and 4. Reactions were repeated twice with negative control (NTC) for further ensure and lasted for 100 minutes.
The alp gene, which is one of the virulence factors of P. aeruginosa, plays an important role in inhibiting the immune system. The results showed that the expression of the alp gene decreased to 1.33-fold after treatment with cold plasma (Fig. 6).
Fig. 6.
alp gene expression diagram before and after treatment with cold plasma.
Column A; before treatment, column B after treatment, and NTC no template sample.
DISCUSSION
The burn is an acute medical situation that provides a good condition for infections caused by opportunistic pathogens. P. aeruginosais causes 10–20% of hospital infections, mainly, in the form of septicemia, and burn wound infections (20). The burn ward is known as an ideal harbor for Multi-Drug Resistance (MDR) strains of P. aeruginosa, which can colonize the wounds and cause infection (15, 16). This pathogen is resistant to most commonly used antibiotics (8, 21), therefore, monitoring and control of the infection caused by this bacterium in the burn ward are vital. For this reason, there are several strategies for control of burn wound infections, such as cold plasma (10, 11). In this study surveyed the impact of non-thermal atmospheric-pressure plasma on P. aeruginosa isolated from burn wound infections in vitro and in vivo. Research findings of research showed no bacterial growth, that indicating the atmospheric non-thermal plasma is completely effective in inhibition of P. aeruginosa isolates. Also, histological analysis of the impact of non-thermal atmospheric helium plasma in vivo and food showed that the healing in the mice wound, the burn wound infections were appropriatelytreated by the treatment of by cold plasma (13, 17, 19).
In a study conducted by Pei et al. the effect of atmospheric plasma temperature treatment on the biofilm of Enterococcus showed that the reactive plasma species penetrate to the bottom thick layer of E. faecalis biofilm and create a robust bactericidal effect. Some studies have believed that the use of atmospheric cold plasma is safe because the antimicrobial resistance less occurs (22). Also, another research evaluated the effect of cold plasma treatment on pathogenic oral biofilms and in-vitro reconstituted oral epithelium, that showed no sign of significant damage after the use of cold plasma, and another advantage was its low cytotoxicity (19). Similar to their results, as mentioned in our results, in the control sample, chemotaxis and number of macrophages and neutrophils in the tissue were less and the wound healing was also slower and the number of bacteria was increased, but in the treated sample, chemotaxis and increase of macrophages and neutrophils were observed, indicating the recovery and healing the wounds. By increasing the number of defensive cells and the inflammation rate, the number of bacteria was decreased. In the present study, all five strains were resistant to all selected antibiotics (gentamicin, ceftazidime, amikacin, and piperacillin), but cold atmospheric plasma inhibited the growth of all bacteria. In line with the present study, another literature reported that in interval 2–5 minutes cold plasma deactivated ocular pathogens without tissue damage (19) as well as, presented that the treatment of human corneas ex vivo with cold plasma for 2 minutes meaningfully reduced colony counts of bacteria.
Like our study, Isbary et al. showed the acceleration in wound healing by plasma predominantly for chronic venous ulcers (23). Study conducted by Brehmer et al. showed the PlasmaDerm® VU-2010 device can reduce the bacterial load in patients with chronic venous leg ulcers with reduction of more than 50% in ulcer size (24).
Overall, our study together with other studies showed that non-thermal atmospheric-pressure plasma can cause wound healing by its antiseptic effects, but with further studies, this scientific finding should be investigated on the human skin cells (11). Atmospheric cold plasma is a promising non-thermal technology and effective against pathogenic microorganisms (25–29). Mechanism of Atmospheric-Pressure is release of enzymes and destruction of the cell membrane and cause pore in the cell wall (14, 15, 20).
Argon plasma is one of the most common types of plasma gas used for wound cleaning because of its low cost, ability to inhibition of oxidation, and wide availability (19). Real-time -RT PCR technique so far used to evaluate the gene expression inhibition. In the present study, the expression levels of the alp gene studied in this research was different before and after treatment in Pseudomonas aeruginosa and decreased expression was observed (3). Alkaline protease is one of the virulence factors of P. aeruginosa in inhibiting cytokines and immune cells. The results showed that this system can reduce alp gene expression up to 1.5 folds, so in addition to inhibiting bacterial growth, this system can also reduce bacterial pathogenicity.
CONCLUSION
The results of this study showed that cold atmospheric-pressure plasma can be a promising technique in controlling the infections caused by P. aeruginosa in various wounds by inhibiting the growth and expression of P. aeruginosa virulence genes and accelerating the healing of burn wounds. It is also an inexpensive promising way to reduce antibiotic use and prevent the development and distribution of antibiotic resistance.
REFERENCES
- 1.Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003; 426:306–310. [DOI] [PubMed] [Google Scholar]
- 2.Klausen M, Heydorn A, Ragas P, Lambertsen L, Ages‐ J, ørgensen A, Molin S, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella, and type IV pili mutants. Mol Microbiol 2003; 48:1511–1524. [DOI] [PubMed] [Google Scholar]
- 3.Meskini M, Esmaeili D. The study of formulated Zoush ointment against wound infection and gene expression of virulence factors Pseudomonas aeruginosa. BMC Complement Altern Med 2018; 18:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 2000; 16:749–767. [DOI] [PubMed] [Google Scholar]
- 5.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 2006; 103:8487–8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeghi-Nejad B, Shiravi F, Ghanbari S, Alinejadi M, Zarin M. Antifungal activity of Satureja khuzestanica (Jamzad) leaves extracts. Jundishapur J Microbiol 2010; 3:36–40. [Google Scholar]
- 7.Yang Y, Guo J, Zhou X, Liu Z, Wang C, Wang K, et al. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: an in vitro study. Dent Mater J 2018; 37:157–166. [DOI] [PubMed] [Google Scholar]
- 8.Church D, Elsayed S, Reid O, Winston B, Lindsay A. Burn wound infection. Clin Microbiol Rev 2006; 19:403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipić A, Primc G, Zaplotnik R, Mehle N, Gutierrez-Aguirre I, Ravnikar M, et al. Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ Virol 2019; 11:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratmann B, Costea TC, Nolte C, Hiller J, Schmidt J, Reindel J, et al. Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: arandomized clinical trial. JAMA Netw Open 2020; 3(7):e2010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedzwieds I, Wasko A, Pawlat J, Polak-Berecka M. The State of research on antimicrobial activity of cold plasma. Pol J Microbiol 2019; 68:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keidar M, Shashurin A, Volotskova O, Stepp MA, Srinivasan P, Sandler A, Trink B. Cold atmospheric plasma in cancer therapy. Phys Plasma 2013; 20:057101. [Google Scholar]
- 13.Cordaro L, de Masi G, Fassina A, Gareri C, Pimazzoni A, Desideri D, et al. The role of thermal effects in plasma medical applications: biological and calorimetric analysis. Appl Sci 2019; 9:5560. [Google Scholar]
- 14.Heslin C, Boehm D, Milosavljevic V, Laycock M, Cullen PJ, Bourke P. Quantitative assessment of blood coagulation by cold atmospheric plasma. Plasma Med 2014; 4:153–163. [Google Scholar]
- 15.Semmler ML, Bekeschus S, Schäfer M, Bernhardt T, Fischer T, Witzke K, et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers (Basel) 2020; 12:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X, Bazaka K, Thompson EW, Ostrikov KK. Cold atmospheric plasma: apromising controller of cancer cell states. Cancers (Basel) 2020; 12:3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pankaj SK, Wan Z, Keener KM. Effects of cold plasma on food quality: areview. Foods 2018; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terefinko D, Dzimitrowicz A, Bielawska-Pohl A, Klimczak A, Pohl P, Jamroz P. The influence of cold atmospheric pressure plasma-treated media on the cell viability, motility, and induction of apoptosis in human non-metastatic (MCF7) and metastatic (MDA-MB-231) breast cancer cell lines. Int J Mol Sci 2021; 22:3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazar Namini Y, Heidarzadeh S, Khaledi A, Abbasi E, Abbasi A, Esmaeili D. Study on the killing effect of cold atmospheric pressure plasma on MRSA Staphylococcus aureus in vitro and in vivo infection model. Malaysian J Microbiol 2019; 15:394–399. [Google Scholar]
- 20.Tajadod Y, Jangravi Z, Mahmoudabadi AZ, Esmaeili D, Dadseresht S, Bahadoran H, Korani M. Comparative study of the effects of Zoush ointment as a natural product and sliver sulfadiazine on the second-degree burn wounds healing in mice: role of antioxidants and the gene expression of matrix metalloproteinase-9. Bull Pharm Sci 2021; 44:149–160. [Google Scholar]
- 21.Betancourt-Ángeles M, Peña-Eguiluz R, López-Callejas R, Domínguez-Cadena NA, Mercado-Cabrera A, Muñoz-Infante J, et al. Treatment in the healing of burns with a cold plasma source. Int J Burns Trauma 2017; 7:142–146. [PMC free article] [PubMed] [Google Scholar]
- 22.Pei X, Lu X, Liu J, Liu D, Yang Y, Ostrikov K, et al. Inactivation of a 25.5 µm Enterococcus faecalis biofilm by a room-temperature, battery-operated, handheld air plasma jet. J Phys D: Appl Phys 2012; 45:165205. [Google Scholar]
- 23.Isbary G, Zimmermann JL, Shimizu T, Li YF, Morfill GE, Thomas HM, et al. Non-thermal plasma—more than five years of clinical experience. Clin Plasma Med 2013; 1:19–23. [Google Scholar]
- 24.Brehmer F, Haenssle H, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, et al. Alleviation of chronic venous leg ulcers with a hand‐held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU‐2010): results of a monocentric, two‐armed, open, prospective, randomized and controlled trial (NCT01415622). J Eur Acad Dermatol Venereol 2015; 29:148–155. [DOI] [PubMed] [Google Scholar]
- 25.Alkawareek MY, Algwari QT, Gorman SP, Graham WG, O’Connell D, Gilmore BF. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol Med Microbiol 2012; 65:381–384. [DOI] [PubMed] [Google Scholar]
- 26.Mai-Prochnow A, Murphy AB, McLean KM, Kong MG, Ostrikov KK. Atmospheric pressure plasmas: infection control and bacterial responses. Int J Antimicrob Agents 2014; 43:508–517. [DOI] [PubMed] [Google Scholar]
- 27.Deben JA, Zago CE, Tyhovych N, Duarte S, Vergani CE. Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One 2016;11(5):e0155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep 2018; 8:5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haertel B, von Woedtke T, Weltmann KD, Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther (Seoul) 2014; 22:477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]