Abstract
Background and Objectives:
Human Enterovirus 71 (EV-A71) is the causative agent for many dermal to neurological diseases especially polio-like paralysis outbreaks around the world. This study, the first of this kind in Iran, aimed to find neutralizing antibodies against EV-A71 in serum of healthy individuals in different age groups based on neutralization test (NT).
Materials and Methods:
In this cross-sectional study, 547 serum samples were collected from healthy individuals who were referring for routine checkup tests (aged from under 6 months to over 31 years old) to Imam-Khomeini Hospital in Tehran during January-December 2015. Serum samples were examined by NT in cell culture to detect neutralizing antibodies against EV-A71. In the next step, some of the positive samples were subjected to complete titration to determine the exact titer of anti-EV-A71 antibodies.
Results:
Of 547 samples, 310 (56.7%) were positive for EV-A71 neutralizing antibody. The presence of the antibody increased with age (p<0.001), and there was a significant statistical relationship between sex and the presence of antibody (p=0.009).
Conclusion:
Our results demonstrated an apparent but limited circulation of EV-A71 in our society. After the worldwide eradication of poliovirus, EV-A71 which can cause polio-likes syndrome, might be the new challenge for our health care system as regard more in depth research is however needed.
Keywords: Iran, Human enterovirus 71, Seroepidemiological investigation, Neutralization test
INTRODUCTION
Human Enterovirus 71 (EV-A71) has been responsible for several large-scale outbreaks worldwide since its first detection in California in 1969 (1, 2). It is a member of the Picornaviridae family, Enterovirus A species, with a positive single-stranded RNA genome and icosahedral capsid (3). EV-A71 can be considered as an endemic pathogen in south-east Asia (4). Its infection manifestations may vary from asymptomatic to dermal symptoms and neurological complications. EV-A71 is one of the primary etiologic agents of Hand Foot and Mouth Disease (HFMD). Some common neurological diseases caused by EV-A71 include: aseptic meningitis, flaccid paralysis (polio-like paralysis), and meningoencephalitis. The most critical consequence of EV-A71 neurological infection is cardiorespiratory failure which may be the leading cause of death in such patients (1, 5–7).
In recent years, scientists have emphasized the ability of EV-A71 in generating polio-like flaccid paralysis, which can be a potential risk factor after eradication of the poliovirus (8, 9). Studies show that the type of illness and its symptoms are likely to have some correlations with race and geographical region. Based on documented epidemiological studies, European and Americans, unlike Asian races, are more likely to develop neurological complications other than HFMD (1, 4, 6, 9–12). It can be inferred that Iran may also be placed in a neurological group, since in all available Iranian information about EV-A71, patients had neurological complications (13–16).
Previous studies indicate a pattern for EV-A71 epidemics in different countries, which can put these countries in a health crisis situation (1, 4, 11). Since there is no exact cure for EV-A71 infection, scientists have attempted to develop a vaccine to immunize individuals against EV-A71 and eliminate transmission risk. Therefore, investigation on protective anti-EV-A71 neutralizing antibodies seems to be the critical first step for monitoring the presence of the virus in a region and public immunity against it (7, 17).
Although EV-A71 seroepidemiological investigations have done in several countries, in Iran, limited information about EV-A71 is mainly based on virus detection by RT-PCR (13, 14, 16, 18). Therefore, this study was designed as the first seroepidemiological investigation for EV-A71 in Iran. Our findings indirectly reveal first-hand information about EV-A71 frequency in Iran.
The “Gold Standard” method for detecting antibody against enteroviruses in individuals is neutralization (19–21). Furthermore, neutralizing antibody responses induced by different EV-A71 strains seem to be primarily protective against other genogroups (4, 19, 22, 23); so we conducted our research based on investigating anti-EV-A71 neutralizing antibody in serum samples of individuals in different genders and age groups.
MATERIALS AND METHODS
Patients.
To enroll in a cross-sectional study, serum samples were collected from people who were referring for routine checkup tests to Imam-Khomeini hospital in Tehran during January-December 2015. After the checkup tests, the rest of the serums of 547 healthy individuals were collected. Samples were categorized into 5 age groups: 0–4, 5–9, 10–14, 15–30, and >31 years, with an overall male count 282 and female 265. The first age group (0–4 years) then re-categorized into 3 sub-groups: 0–6 months, 7–12 months, and 13–48 months (complementary information is available in the results and discussion part). The sera were stored at −20°C until examination (Table 1).
Table 1.
Demographic information of the individuals whose serum sample was used in this study
| Sex | Age groups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 0–4 Years | ||||||||||||||||
|
| ||||||||||||||||
| Male | Female | Total | 0–6 months | 7–12 months | 13–48 months | 5–9 Years | 10–15 Years | 16–30 Years | >31 Years | Total | ||||||
| 265 (48.4%) | 282 (51.6%) | 547 (100%) | 64 (11.7%) | 15 (2.7%) | 42 (7.7%) | 97 (17.7%) | 112 (20.5%) | 85 (15.5%) | 132 (24.1) | 547 (100%) | ||||||
Neutralization assay and titration.
The working procedure was consistent with the WHO protocol for Poliovirus and non-polio enteroviruses (24). EV-A71 (BrCr strain, GenBank: U22521.1) provided kindly by the Netherland National Institute for Public Health and the Environment, RIVM (Rijksinstituut voor Volksgezondheid en Milieu) inoculated into Rhabdomyosarcoma (RD) cell lines, which can support the replication of EV-A71. The neutralization test was performed in two steps:
In duplicate for all serum samples with 1:8 and 1:16 diluted serums;
Thirty-five percent of samples with positive 1:16 titer were serially 2-fold diluted up to 1:2048 and tested again with NT.
Tests were performed in 96 well plates by applying diluted serum samples, 100 TCID50 EV-A71, and RD cells in every well. Plates were then incubated in a 36°C incubator and checked for five days by an inverted microscope. Every well without CPE was considered as positive for anti-EV-A71 antibodies.
Statistical analysis.
Statistical analysis was performed using SPSS, version 22. Chi-square test and 95% confidence intervals were used.
RESULTS
In this study, 547 serum samples were collected, in which 51.6% belonged to males, and 48.4% belonged to female, healthy individuals. The samples were grouped into 5 age groups: 0–4, 5–9, 10–15, 16–30, and >31 years old. For better analysis of the results in newborns and young children, as mentioned before, the first group (0–4 years) was divided into three smaller groups: 0–6 months, 7–12 months, 13–48 months. Anti-EV-A71 titer 1:8 considered as positive and cut-off (19, 20).
Neutralization results.
CPE occurred in the RD cell culture as shown in Fig. 1A, indicating the absence of anti-EV-A71 antibodies and the absence of CPE shown in Fig. 1B, indicates the presence of anti-EV-A71 neutralizing antibodies.
Fig. 1.
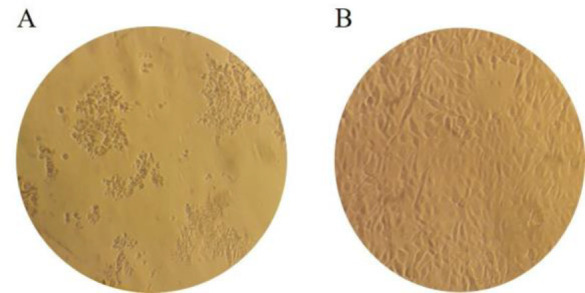
- Cytopathic Effect positive, absence of anti-EV-A71 neutralizing antibodies;
- Cytopathic Effect negative, presence of anti-EV-A71 neutralizing antibodies.
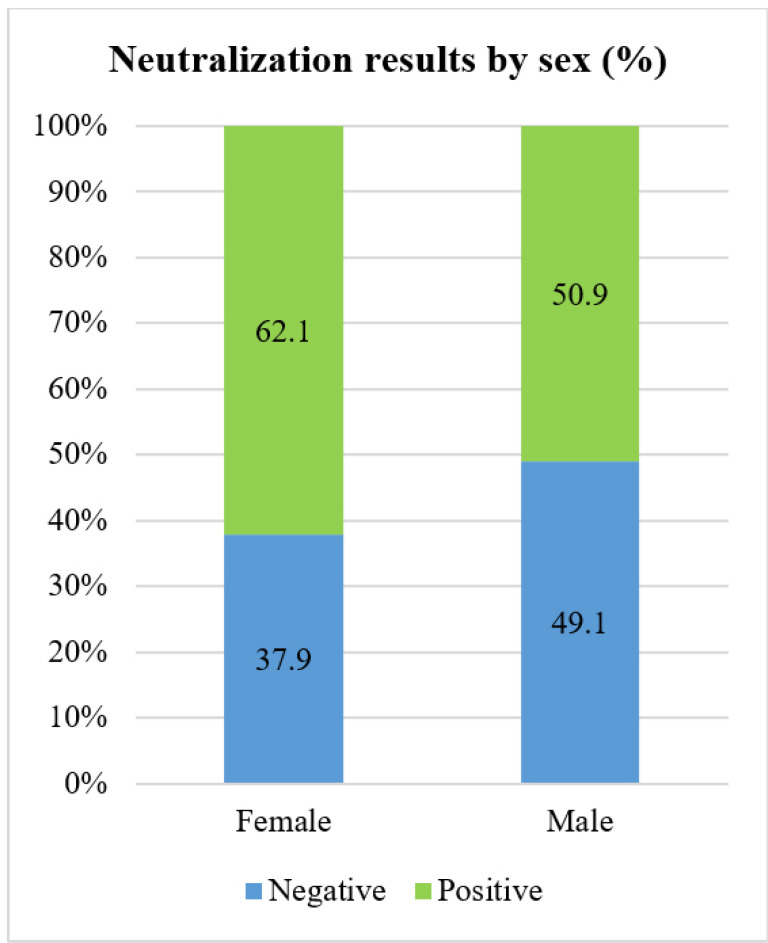
For 1:8 titer, 310 (56.7%) specimens were positive. According to Fig. 2, the female/male ratio for positive samples was statistically significant (p value=0.009). Unlike female samples, there was no significant difference between positive and negative samples proportion in the male group.
Fig. 2.
Anti EV-A71 antibodies detected more in females (1:8 dilution)
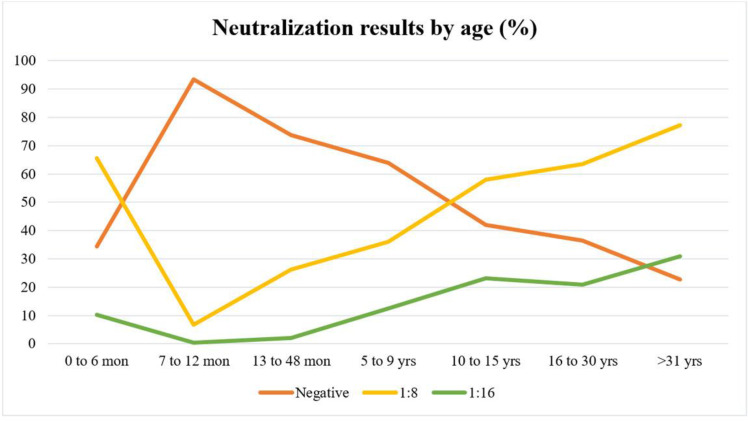
The age group >31 years old contained the most significant number of positive samples (77.3%). In the age group, 0–6 months, the antibody for EV-A71 was detected more frequently than 7–12 month age group. Data showed that after one year of age, antibody presence in serum samples is gradually rising by age (p-value <0.001) (Fig. 3).
Fig. 3.
Anti EV-A71 Neutralization results showed remarkable correlation with age.
Titration results.
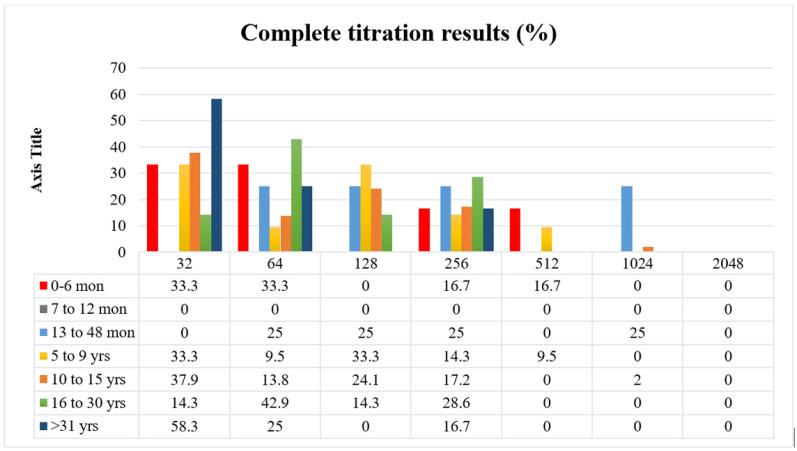
Among 310 positive samples, 234 were positive for 1:16 titer as well. To determine the exact antibody titer, 35% of 234 samples were randomly selected for additional neutralization tests with serially 2-fold dilution from 1:32 to 1:2048.
As shown in Fig. 4, the greater the serum dilution, the fewer anti-EV-A71 antibodies detected, to the extent that no samples were positive in 1:2048 dilution. This result was similar for both sexes in all age groups; however, sex or age and antibody titer increase/decrease relations were not statistically significant (p value=0.7 and 0.2 for sex and age, respectively).
Fig. 4.
Serum titration to 1:2048 in 35% of positive samples (at least 1:16 dilution) showed few number of samples with high titer of Neutralizing antibodies against EV-A71
DISCUSSION
Following the isolation of EV-A71 from the first patient in 1969, scientists noticed the importance of the newly emerging virus to produce severe dermal to neurological epidemics worldwide and might even cause death (1).
There are few studies on EV-A71 in countries without any EV-A71 epidemics. In Iran, there have been very few reports of EV-A71 related diseases, and data for protective antibody in the society has not been available. This study was conducted to give preliminary data on EV-A71 protective antibodies in Iran, and so indirectly EV-A71 frequency/circulation in our society.
Even though since 2001 Iran has achieved polio-free conditions, cases with neurological symptoms including acute flaccid paralysis (AFP), have been increasing. This might be due to either improvement of surveillance or neurological complications related to non-polio enteroviruses, especially EV-A71 (15, 16, 18, 25). The ability to cause the neurological disease like poliovirus, makes EV-A71 an important agent soon after eradication of poliovirus (26).
Different studies show the impact of age, race, season and gender in EV-A71 viral tropism, infection, type of symptoms, and also further complications. Children aged under five years of age are more vulnerable to EV-A71 infection, although other age groups might be infected as well. This study investigated serum anti-EV-A71 antibodies in almost all ages from newborn to adults. There are a few documented studies that have been designed for all age groups with a wide range of serum dilution from 1:8 to 1:2048 considering patients’ sex. In 2016 in China, similar to this study, scientists investigated 1378 healthy serum samples in all age groups for anti-EV-A71 neutralizing antibodies. They assessed antibodies in 1:8 to 1:2048 serum dilution (19).
In European and Mediterranean races (like Iran), the virus is mainly neurotropic, but in Asians, it is more likely to develop HFMD. Up until now, all reported EV-A71 cases in Iran have been Neurologic (13, 14, 16), and regarding the recent and only molecular study on HFMD cases documented in 2019 in Ahvaz, that no EV-A71 was isolated from 16 HFMD patients, there has been no reported case of HFMD in Iran so far (27).
Correlation of EV-A71 or neutralizing antibodies against it in serum samples and gender is not significant in most studies, or in some of them, EV-A71 infection or antibody is higher in females and others in males. For instance, in the seroepidemiological study conducted by Wang et al. prevalence of positive samples among females was higher than male samples (19); while in another study by Lerdsamran et al. in Thailand, no difference in seropositivity to EV-A71 by gender was reported (28), although Liu et al. in China have reported a significant higher prevalence of positive samples among males in 2015 (29). In this study, female positivity versus male positivity was higher with a ratio of 1.3 to 1.
EV-A71 studies are still young in Iran, and most of them are based on molecular methods like PCR. Iran National Polio Laboratory, located in Tehran University of Medical Sciences (TUMS), reported the first detection of EV-A71 in an AFP case with residual paralysis in 2008 (16). The detected strain showed 96% similarity to Norwegian strain 804/NO/03, genotype C1 reported in 2007.
According to a study accomplished in 2013, 14 patients of 100 confirmed aseptic meningitis in Tehran were diagnosed with EV-A71 infection (15). In a study conducted by the Pasteur Institute of Iran in 2014, the EV-A71 genome was detected in 21.3% of patients with meningoencephalitis (14). In another report by Pasteur Institute of Iran in 2014–2015, out of 366 children under eight years of age with aseptic meningitis, 94 samples were positive for EV-A71 (13). In a recent study in Kermanshah province, 120 CSF samples were collected from suspected aseptic meningitis cases, 4 of which were reported as non-polio enteroviruses, which were all EV-A71 (18). This is the first seroepidemiological study on EV-A71 to gain preliminary epidemiological data of EV-A71 in Iran. Investigating anti-EV-A71 antibodies in almost all age groups (from infant to over 31 years of age) and assessing the exact titer of the antibody were positive points of this study.
Based on our study, 56.7% of the specimens were positive for anti-EV-A71 antibodies, which means that EV-A71 has had a circulation in our society. Our finding is consistent with few reports of EV-A71 in neurological diseases and no reports of HFMD cases with EV-A71 (30, 31).
A notable finding in this study was a significant correlation between age and antibody titer (p-value<0.001). The number of positive samples in children was few compared to adults, which suggests the prevalence of EV-A71 is not high among children, and individuals will be exposed to the virus during their lifetime. After analyzing the first age group (0–4 years) precisely, we confronted inconsistencies, which made us to divide this group into three smaller groups: 0–6 months, 7–12 months and 13–48 months. Following this division, it was found that the majority (65.6%) of positive samples belonged to the 0–6 month age group. It can be attributed to the maternal antibodies or the child’s direct exposure to the virus, which is unlikely. A sharp drop in positive samples in the 7–12 month age group (0.4%) can be attributable to the fade of maternal immunity. Positive results increase gradually and slowly after one year of age, which is evidence for the low prevalence of EV-A71 in Iran. A study in Taiwan by Chang et al. with 539 samples shows similar results for infants under six months of age: 36% positive samples in under six months infants compared to 4% positive samples for 6–12 months age group (17). Consistent with other studies, in another research conducted by Luo et al. 459 serum samples were obtained from pregnant women immediately before delivery and their neonates’ cord blood and at 6, 12, 24, 36, and 48 months of age. Their findings showed a 99% decline in neutralizing antibodies by six months of age (32).
In this study, 35% of specimens with 1:16 positive titer underwent complete titration to 1:2048. None of the examined samples were positive in 1:2048, and 1:32 was the most abundant positive titer (35.4%). The only positive sample of 7–12 months age group with 1:16 titer which underwent complete titration was also negative for higher titers. Low titers of EV-A71 antibody might be due to low circulation of EV-A71, which is needed to boost the antibody.
For the positive samples, “Mode” and “Mean” were calculated for age, which was 21 and 24 years, respectively. This suggests that in our society, the average age to have neutralizing antibodies for EV-A71 is about 20–25 years of age. This result emphasizes the low prevalence of EV-A71 in Iran. Therefore, first exposure to this virus mostly does not happen in younger ages in Iran. Reporting just one EV-A71 AFP case from 2008 to 2019 in Iran National Polio Laboratory and few reports of non-paralytic neurological cases for EV-A71 are confirmations for limited circulation of EV-A71 in Iran (13, 14, 16, 25).
The results of this study provided first-hand preliminary information on EV-A71 neutralizing antibody and circulation in Iran. Although the number of EV-A71 positive cases with neurological complications is still few in Iran, the circulation of this virus cannot be ignorable. EV-A71 is one of the most important causative agents in neurological diseases like aseptic meningitis, meningoencephalitis, or even paralysis.
To improve and expand the data on EV-A71 in Iran, we suggest: 1) Design complementary studies with more samples obtaining from different provinces to achieve a better view of the circulation of the virus, comparing these data with global and regional data, 2) Establishment of EV-A71 surveillance in AFP cases as well as healthy individuals for annual genotyping of EV-A71 cases to collect data which will be helpful for future vaccine/immunization strategies. 3) Design studies concerning HFMD cases to determine if EV-A71 is a causative agent in any or not.
ACKNOWLEDGEMENTS
This work was supported financially by Tehran University of Medical Sciences. We are grateful to RIVM, the Netherlands, for providing EV-A71. We wish to thank Dr. Hamideh Tabatabaie, Mr. Mohammad Farahmand, Dr. Abdollahi head of Vali-e-Asr Hospital laboratory and Dr. Nikmanesh executive manager of Children’s Medical Center Laboratory for their support.
REFERENCES
- 1.Yip CC, Lau SK, Woo PC, Yuen KY. Human enterovirus 71 epidemics: what’s next? Emerg Health Threats J 2013; 6:19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu WS, Kang B, Hong J, Hwang S, Kim J, Cheon DS. Clinical and etiological characteristics of enterovirus 71-related diseases during a recent 2-year period in Korea. J Clin Microbiol 2010; 48:2490–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knipe DM, Howley P. (2013). Fields Virology. 6th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins (LWW). Philadelphia. [Google Scholar]
- 4.Sabanathan S, Tan le V, Thwaites L, Wills B, Qui PT, Rogier van Doorn H. Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: current situation and ongoing challenges. J Epidemiol Community Health 2014; 68:500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi EJ, Shin YJ, Kim JH, Kim TG, Chang SY. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res 2017; 6:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Sanden S, Koopmans M, Uslu G, van der Avoort H, Dutch working group for clinical virology . epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008. J Clin Microbiol 2009; 47:2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li HG, Lao Q. The pulmonary complications associated with EV71-infected hand–foot–mouth disease. Radiol Infect Dis 2017; 4:137–142. [Google Scholar]
- 8.Eggertson L. Infectious disease experts monitor outbreaks of enterovirus 71 in Asia. CMAJ 2012; 184(15):E781–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang PC, Chen SC, Chen KT. The current status of the disease caused by enterovirus 71 infections: epidemiology, pathogenesis, molecular epidemiology, and vaccine development. Int J Environ Res Public Health 2016; 13:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Ju Y, Chen M, Xie Z, Zhou K, Tan Y, Mo J. Epidemiological and genetic characteristics of EV71 in hand, foot, and mouth disease in Guangxi, southern China, from 2010 to 2015. PLoS One 2017; 12(12):e0188640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Hyeon JY, Hwang S, Lee YP, Lee SW, Yoo JS, et al. Epidemiology and virologic investigation of human enterovirus 71 infection in the Republic of Korea from 2007 to 2012: a nationwide cross-sectional study. BMC Infect Dis 2016; 16:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CY, Gonfrier G, Ninove L, Zandotti C, Dubot-Pérès A, de Lamballerie X, et al. Screening and detection of human enterovirus 71 infection by a real‐time RT‐PCR assay in Marseille, France, 2009–2011. Clin Microbiol Infect 2012; 18(4):E77–E80. [DOI] [PubMed] [Google Scholar]
- 13.Rahimi P, Mahdian Naser H, Sohrabi A. The association of non-polio enteroviruses with aseptic meningitis in children in Iran. J Med Microbiol Infect Dis 2014; 2:56–60. [Google Scholar]
- 14.Rahimi P, Roohandeh A, Sohrabi A, Mostafavi E, Bahram Ali G. Impact of human enterovirus 71 genotypes in meningoencephalitis in Iran. Jundishapur J Microbiol 2015; 8(12):e27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roohandeh A, Rahimi P, Sohrabi A, Mobasheri M, Azadmanesh K, Shahosseini Z, et al. Frequency of human enterovirus 71 in children under 8 years old with aseptic menengitis in Tehran. Clin Lab 2013; 59:915–920. [DOI] [PubMed] [Google Scholar]
- 16.Shahmahmoodi S, Mehrabi Z, Eshraghian MR, Azad TM, Tabatabaie H, Yousefi M, et al. First detection of enterovirus 71 from an acute flaccid paralysis case with residual paralysis in Iran. J Clin Virol 2008; 42:409–411. [DOI] [PubMed] [Google Scholar]
- 17.Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109(6):e88–e88. [DOI] [PubMed] [Google Scholar]
- 18.Akya A, Elahi A, Moghoofei M, Chegenelorestani R, Nejati A, Babaei F. Enterovirus detection in patients suspected aseptic meningitis by RT-PCR in Kermanshah, Iran. J Kerman Univ Med Sci 2018; 25:18–26. [Google Scholar]
- 19.Wang JX, Zhu SL, Wang J, Lin Y, Pei YW, Sun DP, et al. Seroprevalence of enterovirus A71 and coxsackievirus A16 in healthy people in Shandong province, China. PLoS One 2016; 11(9):e0162373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Deng C, Wan J, Zhu L, Leng Q. Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J 2011; 8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu R, Cheng T, Yin Z, Liu D, Xu L, Li Y, et al. Serological survey of neutralizing antibodies to eight major enteroviruses among healthy population. Emerg Microbes Infect 2018; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Sanden S, van Eek J, Martin DP, van der Avoort H, Vennema H, Koopmans M. Prediction of protection against Asian enterovirus 71 outbreak strains by cross-neutralizing capacity of serum from Dutch donors, the Netherlands. Emerg Infect Dis 2016; 22:1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuta K, Aoki Y, Suto A, Ootani K, Katsushima N, Itagaki T, et al. Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine 2009; 27:3153–3158. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . (2004). Polio laboratory manual, 4th ed. World Health Organization. Geneva, Switzerland: WHO Document Production Services. [Google Scholar]
- 25.Yousefi M, Nejati A, Zahraei SM, Mahmoudi S, Parhizgari N, Farsani SMJ, et al. Enteroviruses and adenoviruses in stool specimens of paralytic children-can they be the cause of paralysis? Iran J Microbiol 2018; 10:194–201. [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097–1105. [DOI] [PubMed] [Google Scholar]
- 27.Rasti M, Rastegarvand N, Makvandi M, Teimoori A, Azaran A. Co-circulation of echovirus 6 and 30 with coxsackievirus A6 among children with hand, foot, and mouth disease in Ahvaz, Southwest Iran. Arch Clin Infect Dis 2019; 14(3):e83522. [Google Scholar]
- 28.Lerdsamran H, Prasertsopon J, Mungaomklang A, Klinmalai C, Noisumdaeng P, Sangsiriwut K, et al. Seroprevalence of antibodies to enterovirus 71 and coxsackievirus A16 among people of various age groups in a northeast province of Thailand. Virol J 2018; 15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Luo L, Yan S, Wen T, Bai W, Li H, et al. Clinical features for mild hand, foot and mouth disease in China. PLoS One 2015; 10(8):e0135503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez-López N, Muñoz-Almagro C, Launes C, Navascués A, Imaz-Pérez M, Reina J, et al. Surveillance for enteroviruses associated with hand, foot, and mouth disease, and other mucocutaneous symptoms in spain, 2006–2020. Viruses 2021; 13:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song C, Li Y, Zhou Y, Liang L, Turtle L, Wang F, et al. Enterovirus genomic load and disease severity among children hospitalised with hand, foot and mouth disease. EBioMedicine 2020; 62:103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo ST, Chiang PS, Chao AS, Liou GY, Lin R, Lin TY, et al. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis 2009; 15:581. [DOI] [PMC free article] [PubMed] [Google Scholar]