Abstract
The hematopoietic proto-oncogene vav has been characterized as a Rac1-GDP/GTP exchanger protein which regulates cytoskeletal reorganization as well as signaling pathways leading to the activation of stress-activated protein kinases (SAPK/JNKs). Furthermore, vav overexpression enhances basal and T-cell receptor (TCR)-mediated stimulation of the nuclear factor of activated T cells (NFAT). We report here the interaction between Vav and hSiah2, a mammalian homolog of Drosophila Seven in absentia (Sina) that has been implicated in R7 photoreceptor cell formation during Drosophila eye development via the proteasome degradation pathway. Vav and hSiah2 interact in vitro and in vivo and colocalize in the cytoplasm of hematopoietic cells. The Src homology domain of Vav and the C-terminal region of hSiah2 are required for this interaction. We provide evidence for a negative regulation by hSiah2 of Vav-induced basal and TCR-mediated NFAT-dependent transcription. Overexpression of hSiah2 also inhibits the onco-Vav-induced JNK activation. Although the Vav-interacting domain is located in the C-terminal portion of hSiah2, the N-terminal region of hSiah2 is necessary for the inhibitory role that seems to be independent of the proteasome degradation.
The vav proto-oncogene product, p95vav, is expressed primarily in cells of hematopoietic origin (40). The oncogenic form arises from the deletion of 67 N-terminal amino acids of c-Vav and induces tumors in nude mice (15, 41). The primary structure of Vav has several structural motifs found in proteins involved in cell signaling, e.g., an N-terminal leucine-rich region, an acidic domain, a Dbl homology (DH) domain, a pleckstrin homology domain, a cysteine-rich sequence and a proline-rich sequence, two Src homology (SH3) domains flanking a single SH2 domain, and, finally, two putative nuclear localization sequences (59). Indeed, it has been demonstrated that Vav interacts, via its SH3 domains, with different cytoplasmic proteins, such as Grb2 (53, 70), heterogeneous ribonucleoprotein (hnRNP) K (11, 31), the focal adhesion protein zyxin (33), and Cbl-b (10). Moreover, we described the interactions of the C-SH3 domain of Vav with Ku-70 and hnRNP C, two predominantly nuclear proteins (58, 60).
Despite the numerous partners described, the precise function of Vav in cell-signaling pathways is unclear. Upon stimulation via immune receptors (T-cell receptor [TCR], B-cell receptor [BCR], and FcRs) or various cytokine or growth factor receptors (cKit, Epo, interleukin-2 [IL-2], IL-3, interferon, epidermal growth factor, platelet-derived growth factor, and others), Vav is rapidly and transiently tyrosine phosphorylated (46, 59). Both Src and Syk/Zap70 family kinases have been implicated in Vav phosphorylation (19, 42). Biochemical evidence has shown that activated Vav catalyzes, via its DH domain, the conversion of Rac1 protein, a member of the Rho family of GTPases, to the active GTP-bound state (18, 29, 51). Rac1 activation leads in turn to the stimulation of the JNK pathway (17). However, recent in vivo studies on vav−/− mice cast doubt on the role of Vav in JNK activation (24, 35). Previous studies on vav−/− mice also indicated impaired T-cell development and a poor proliferation of mature T cells with a reduced response to stimulation through the TCR (25, 67, 72). Overexpression of vav seems to cooperate with Syk (19) and SLP76 (69) to synergistically induce basal and TCR-activated transcription of either the IL-2 gene or reporter constructs containing binding sites for nuclear factor of activated T cells (NFAT) present in the IL-2 promoter (68). Recent findings from vav−/−-activated lymphoid cells also showed the absence of IL-2 transcription even though the JNK activity was still normal (24, 35). These experiments demonstrated that Vav is an effector molecule that functions downstream of a variety of hematopoietic cell receptors. Finally, Vav2, a protein highly homologous to Vav but with a more ubiquitous expression and poorly expressed in hematopoietic cells, has been identified. It has been proposed that this protein may have Vav-like functions in nonhematopoietic cells (30, 64).
To further investigate the role of Vav, we attempted to identify proteins that bind to its Src homology domains (SH3-SH2-SH3) by the yeast two-hybrid system. We identified hSiah2, a human homolog of Drosophila Seven in absentia (Sina) (12), a ring finger (C3HC4)-containing protein that is required for the correct integration of signal transduction downstream of the tyrosine kinase receptor Sevenless (sev) and the Ras/Raf mitogen-activated protein kinase (MAPK) pathway during Drosophila R7 photoreceptor development (9, 13, 22, 26, 43). Recently it was shown that Sina acts together with Phyllopod (PHYL), induced by the Ras pathway, to target the repressor of cell fate determination Tramtrack (TTK) for degradation by the proteasome pathway (45, 66). Three highly conserved murine sina homologs (Siah1A, Siah1B, and Siah2) and two human homologs (hSiah1 and hSiah2) have been described (1, 20, 34, 37). Although the function of the mammalian Siah proteins has not been elucidated, recent studies suggested that they might be involved in ubiquitin-mediated proteolysis of several proteins (38, 71), as well as in growth arrest and p53-induced apoptosis (2, 47, 49).
In this study, we showed that Vav and hSiah2 interact both in vitro and in coimmunoprecipitation ex vivo experiments. We determined that the C-terminal half of hSiah2 and the entire SH3-SH2-SH3 region of Vav are critical for binding. By immunofluorescence studies, we showed that the two proteins colocalize in the cytoplasm of hematopoietic cells. We further demonstrated that pathways stimulated by Vav, namely, induction of NFAT-dependent transcription and onco-Vav-mediated activation of JNK, are largely impaired in cells overexpressing hSiah2. This inhibition is modulated by the N-terminal region of hSiah2 and does not involve ubiquitin-mediated proteolysis, a function previously suggested for hSiah2.
MATERIALS AND METHODS
Plasmids.
SHVAV containing SH3-SH2-SH3 domains (residues 623 to 837 of Vav) was PCR amplified from pKLS1 (provided by M. Barbacid, Madrid, Spain, and X. R. Bustelo, New York, N.Y.) and fused to the DNA-binding domain of LexA in pVJL10 (58). pGEX-v240 and pGEX-v460 were obtained by cloning the v240 and v460 cDNA fragments, respectively, from pGAD-v240 (amino acids [aa] 13 to 324) and pGAD-v460 (aa 105 to 324) into the EcoRI-NotI sites of pGEX-4T2 (Pharmacia Biotech Inc.). Constructs encoding a truncated form of hSiah2 (pGEX-v240Δa and pGEX-v240Δb) were obtained by PCR amplification from pGAD-v240 with appropriate oligonucleotides (+39 to +555 and +39 to +475, respectively) followed by ligation into BamHI-SalI sites of pGEX-4T2. Expression plasmids of myc-tagged hSiah2 cDNA fragments were generated by cloning the corresponding EcoRI-NotI fragments from pGAD-v240 and pGAD-v460 into a pcDNA3-derived plasmid, pCAN-M2, to generate pCAN-v240 and pCAN-v460, respectively. The full-length hSiah2 was generated by replacing the SpeI-DraIII fragment of pBK-CMV v240 with a PCR fragment amplified from human genomic DNA by using a 5′ primer in the noncoding sequence of mouse Siah2 (this sequence was highly conserved among mouse Siah and hSiah1) and a 3′ internal primer of the v240 clone (nucleotides 361 to 383). pKES-Vav is a pcDNA3-derived plasmid containing a full-length mouse cDNA under the control of the cytomegalovirus promoter (1a). pEF-Myc-tagged Vav was kindly provided by A. Altman (San Diego, Calif.). pEF-Myc-tagged onco-Vav was obtained by replacing the Vav EcoRI-BstXI fragment with the corresponding sequence from pJC7 (15). The NFAT-luciferase reporter construct (kindly provided by O. Acuto, Paris, France) was derived from the pUBT-luc plasmid (21) and contained the luciferase gene under the control of the human IL-2 promoter NFAT-binding site (23). pSV-βgal vector (Promega) contained the β-galactosidase gene driven by the simian virus 40 promoter. HA-JNK and pGEX-cJun were provided by P. Crespo (Santander, Spain). pBK-CMV hSiah1 was provided by R. B. Amson (Paris, France).
Yeast two-hybrid system.
Saccharomyces cerevisiae L40 (MATa trp1 leu2 his3 LYS::lexA-HIS3 URA3::lexA-lacZ) was grown at 30°C in YPD medium (1% yeast extract, 2% polypeptone, 2% glucose) and sequentially cotransformed by the lithium acetate method (65) with pVJL10-SHVAV and a cDNA library from Jurkat cells fused to the GAL4 activation domain in pGAD1318 (58). Double transformants were plated on yeast dropout medium lacking Trp, Leu, His, Lys, and Ura (65). After 5 days at 30°C, colonies were patched on the same medium and replica plated on Whatman 40 filters to test for β-galactosidase activity (8). Positive clones were rescued and tested for specificity by retrotransformation into L40 either with pVJL10-SHVAV or with extraneous targets (pLexA-Rasv12 or pLex-Lamin).
Sequences of cDNA inserts from positive clones of the two-hybrid screen were obtained for both strands with an automatic sequencer (Applied Biosystems model 373A) by the dideoxy-termination method of Sanger et al. (62). Sequence comparisons were done with the FASTA program.
Cell culture, transfection, and antibodies.
Jurkat cells or simian virus 40 T-antigen (T-Ag)-transfected Jurkat cells (provided by G. Baier, Innsbruck, Austria) were grown in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (Boehringer Mannheim), 2 mM l-glutamine, penicillin, and streptomycin in a 5% CO2 humidified atmosphere at 37°C. For T-Ag Jurkat cells, the medium was supplemented with 2 mg of Geneticin per ml (Gibco). Rat basophilic leukemia (RBL) and COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin, and streptomycin.
COS-7 cells were transfected by the DEAE-dextran technique (16). T-Ag Jurkat cells (106) were electroporated (at 260V and 960 μF) in 0.5 ml of RPMI 1640 supplemented with 20% fetal calf serum in a Gene Pulser cuvette (Bio-Rad).
The anti-Vav monoclonal antibody (MAb) was purchased from Upstate Biotechnology. MAbs against myc and hemagglutinin (HA) epitopes were purchased from the American Type Culture Collection (9E10-CRL 1725) and Babco (12CA5), respectively. The anti-CD3 MAb (UCHT1) was provided by G. Bismuth (Paris, France), and anti-CD28 was provided by D. Olive (Marseille, France).
Rabbit polyclonal anti-hSiah2 antibodies were generated against a peptide corresponding to aa 150 to 162 of hSiah2 (Syntem).
Immunofluorescence staining.
RBL or Jurkat cells were fixed in phosphate-buffered saline (PBS) plus 3% paraformaldehyde and permeabilized in PBS plus 0.1% Triton X-100. The coverslips were rinsed and blocked for 10 min in PBS plus 0.2% bovine serum albumin prior to incubation with antibodies. Fixed cells were incubated simultaneously with both primary antibodies (anti-Vav MAb and anti-hSiah2 sera, 1:500) for 30 min and then incubated with donkey anti-mouse antibody coupled to Texas red (1:250) and with donkey anti-rabbit antibody coupled to fluorescein isothiocyanate (1:100). For peptide-blocking experiments, the anti-hSiah2 antiserum was depleted by incubation for 1 h at 4°C with a nitrocellulose membrane saturated with 1 mg of the immunizing peptide prior to immunofluorescence staining. The coverslips were mounted in Mowiol with Dabco antifading (Hoechst, Frankfurt, Germany). The staining pattern was analyzed by confocal laser-scanning microscopy (Bio-Rad) at two different emission wavelengths (fluorescein isothiocyanate, 522/535; Texas red, 605/632), and colocalization was performed by further analysis of superposed images that were obtained as TIFF files.
In vitro binding and immunoprecipitation experiments.
Glutathione S-transferase (GST) fusion proteins were induced and purified as previously described (58). Lysates from lysis of 107 Jurkat cells in Nonidet P-40 (NP-40) buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 10% glycerol, 1% NP-40, 1 mM dithiothreitol, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mg of pepstatin per ml, 1 mg of leupeptin per ml) were incubated for 2 h with fusion protein bound to glutathione-coupled agarose beads (Pharmacia). The beads were washed five times in NP-40 lysis buffer, resuspended in Laemmli buffer and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide). The proteins were electrotransferred to nitrocellulose membranes and probed with primary antibody (MAb anti-Vav diluted 1:1,000 in Tris-buffered saline [pH 7.6]–0.05% Tween 20) followed by secondary antibody conjugated to horseradish peroxidase and then revealed with the ECL kit (Amersham).
For coimmunoprecipitation experiments, COS-7 and T-Ag Jurkat cells were lysed in NP-40 buffer approximately 48 h after transfection and lysates were incubated at 4°C with anti-myc tag MAb for 4 h followed by protein A-Sepharose (Pharmacia) for 1 h. The precipitates were analyzed as described below.
Kinase assays.
Subconfluent COS-7 or T-Ag Jurkat cells were transfected as described previously (16). After transfection, the cells were cultured for 48 h, washed with PBS, and lysed at 4°C in a buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 2 mM EDTA, 1% Triton X-100, 25 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM PMSF, 20 μg of aprotinin per ml, and 20 μg of leupeptin per ml. Clear lysates were immunoprecipitated by incubation with anti-HA antibodies (12CA5) for 2 h at 4°C. Immunocomplexes were recovered with Gamma bind Sepharose beads (Pharmacia) and washed twice in lysis buffer and once more in kinase buffer (25 mM HEPES [pH 7.5], 25 mM MgCl2, 25 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM PMSF, 20 μg of aprotinin per ml, 20 μg of leupeptin per ml). JNK activity was determined after resuspension of the immunocomplexes in 50 μl of kinase buffer containing 10 μCi of [γ-32P]ATP (3,000 Ci/mmol) per reaction and 50 μM unlabeled ATP, with 1 μg of GST–c-Jun fusion protein as a substrate (16). After 30 min at 30°C, the reactions were stopped by the addition of 50 μl of sample buffer, and the products were boiled at 95°C for 5 min and resolved by SDS-PAGE (12% polyacrylamide). Autoradiography was performed with the aid of an intensifying screen and quantitated with a PhosphorImager (Molecular Dynamics).
Luciferase assays.
T-Ag Jurkat cells (107) were electroporated with 5 μg of the reporter plasmid pNFAT-Luc together with 20 μg of pEF-Vav-Myc with or without 20 μg of pCAN-v240/pCAN-v460. Similar amounts of empty vector were used as a control. At 24 h after transfection, 106 cells were either left unstimulated or stimulated in growth medium containing anti-CD3 MAb (10 μg/ml) plus anti-CD28 (10 μg/ml). After 8 h at 37°C, the cells were lysed in 200 μl of buffer containing 100 mM KPO4 (pH 7.8), 1 mM dithiothreitol, and 0.5% Triton X-100. Lysate (20 μl) was mixed with 100 μl of assay buffer (200 mM KPO4 [pH 7.8], 10 mM ATP, 20 mM MgCl2) followed by 100 μl of 1 mM luciferin. Luciferase activity was determined in triplicate and expressed as arbitrary units (AU) after normalization to the β-galactosidase values to correct for variation in transfection efficiency.
RESULTS
hSiah2, a new Vav-interacting protein.
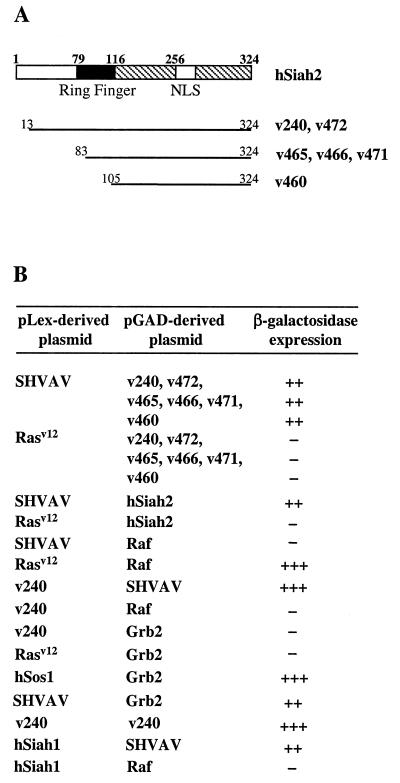
To identify proteins implicated in Vav signaling pathways, we used the yeast two-hybrid system with the SH2 and SH3 domains of Vav (SHVAV, aa 623 to 837) fused to the DNA-binding domain of LexA as a bait to screen a Jurkat T-cell cDNA library. Of approximately 4 × 106 clones screened, 450 positive clones were analyzed for their specific interaction with SHVAV. Of these, six clones from three independent LexA fusions strongly interacted with SHVAV. Sequence analysis of the yeast plasmids showed that they were identical to hSiah2 (37). The previously described Sina/Siah proteins contain an N-terminal cysteine-rich region (C3HC4) called the ring finger domain and, in the C-terminal region, two basic clusters close to a bipartite nuclear localization sequence (12, 20) (Fig. 1A). The two largest clones isolated, v240 and v472 (aa 13 to 324), contained almost the entire coding sequence of hSiah2, whereas the shortest one, v460 (aa 105 to 324), maintained only the C-terminal 11 aa of the ring finger domain of hSiah2. A strong interaction was also detected when v240 was expressed as a fusion to the LexA DNA-binding domain and SHVAV was expressed as a fusion to the Gal4 activation domain. Full-length hSiah2 was isolated and, as expected, interacted with SHVAV. As indicated in Fig. 1B, no transactivation was observed when different hSiah2 clones were coexpressed with unrelated fusion plasmids (pLexA-Rasv12 or pLexA-lamin). When hSiah2 clones were tested with Grb2, which, like Vav, has closely spaced SH3-SH2-SH3 domains (63), no reporter gene activity was detected (Fig. 1B), suggesting that the hSiah2-SHVAV interaction requires rather specific SH3-SH2-SH3 sequences. Finally, when v240 was cloned in both pLexA and pGAD, a strong self-interaction was observed in the yeast trap assay, suggesting a possible dimerization process for hSiah2 (Fig. 1B). Taking account of hSiah1/hSiah2 homology, the expected interaction between SHVAV and hSiah1 was also observed (Fig. 1B).
FIG. 1.
Vav interacts with hSiah2 in the yeast two-hybrid system. (A) Schematic representation of hSiah2 and the clones obtained from the two-hybrid screening. (B) Protein interaction in the two-hybrid system. The L40 reporter strain was cotransformed with 1 μg of the indicated pLex- and pGAD-derived plasmids, and interactions were detected as β-galactosidase activity.
hSiah2 interacts with Vav in vitro, and the proteins coimmunoprecipitate from COS-7 and Jurkat T cells.
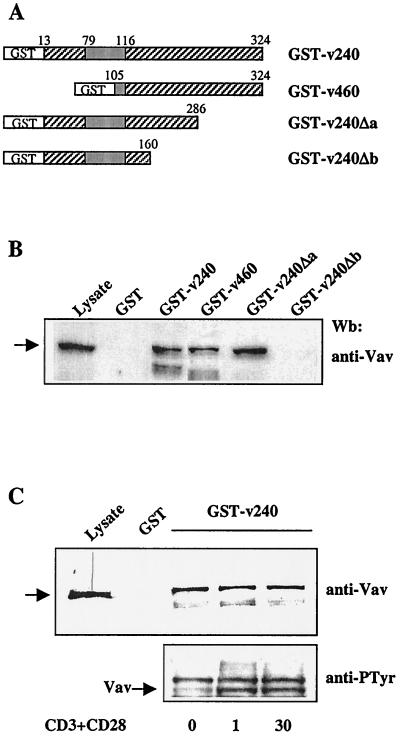
The interaction between Vav and hSiah2 was then confirmed by an in vitro binding assay. Different hSiah2 regions fused to GST (Fig. 2A) were expressed in Escherichia coli, and the affinity-purified proteins were tested for their ability to bind endogenous Vav from Jurkat T-cell lysates. Consistent with the yeast two-hybrid assay, both hSiah2 fusion proteins, containing an almost full-length hSiah2 (GST-v240) or only the C-terminal portion of hSiah2 (GST-v460), were able to interact with Vav (Fig. 2B). To delineate the region of hSiah2 responsible for this interaction, we produced C-terminal deletions of 38 aa (GST-v240Δa) or 164 aa (GST-v240Δb) of clone v240. With the latter deletion, the interaction with Vav could no longer be detected (Fig. 2B). A similar result was also obtained in the yeast two-hybrid assays (results not shown). This indicates that the domain of hSiah2 responsible for the interaction with Vav lies between aa 160 and 286 of hSiah2 and does not involve the ring finger domain, which is known to be a protein-protein interaction domain (7). We used the yeast two-hybrid assay to delineate the hSiah2-binding site in the Vav molecule. None of the individual SH3 or SH2 domains nor subdomains such as N-terminus–SH3-SH2 or SH2-SH3–C-terminus were able to interact with hSiah2 (clone v240), indicating that a conformation present in the complete SHVAV region was required for binding to hSiah2 (data not shown). The association between Vav and hSiah2 was not modulated by cellular activation. Lysates from unstimulated or anti-CD3- plus anti-CD28-stimulated Jurkat cells exhibited the same amount of Vav proteins bound to the GST-v240 fusion protein (Fig. 2C, top), despite an increased Vav tyrosine phosphorylation after activation (Fig. 2C, bottom).
FIG. 2.
In vitro binding between hSiah2 and Vav. (A) Schematic representation of GST-hSiah2 fusion proteins. (B) Binding of Vav to GST-hSiah2 fusion proteins. Total-cell lysates from 107 Jurkat T cells were incubated at 4°C for 2 h with 1 μg of the fusion proteins or GST alone. (C) (Top) Lysates from unstimulated (0 min) and CD3-plus-CD28-stimulated (1 min and 30 min) Jurkat T cells (107) were incubated with 1 μg of GST-v240. The resulting complexes were resolved by SDS-PAGE, and the Western blot (Wb) was developed with anti-Vav MAb. The left-hand lane contains total lysate from 2 × 105 cells. (Bottom) Western blot analysis with antiphosphotyrosine antibody (anti-PTyr) of the total-cell extracts used in the top panel.
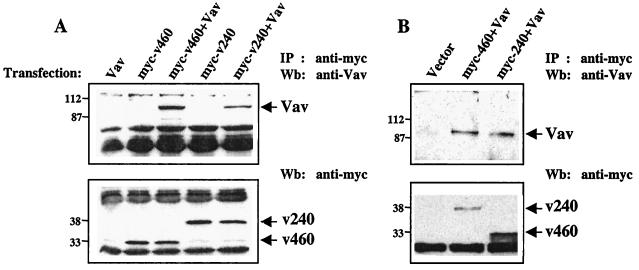
To further analyze the association between hSiah2 and Vav, both COS-7 and T-Ag Jurkat cells were transfected with expression vectors encoding Vav alone, myc epitope-tagged hSiah2 alone (v240 or v460), or myc epitope-tagged hSiah2 together with Vav. This approach was rendered necessary because the anti-hSiah2 antiserum did not allow us to immunoprecipitate the protein. The lysates were immunoprecipitated with anti-myc antibody, and the immunocomplexes were analyzed for the presence of Vav and hSiah2 proteins. As shown in Fig. 3, Vav could be detected in the immunocomplexes derived from both cell types cotransfected with Vav and either the v240 or v460 clones. As expected, Vav could not be detected in anti-myc immunoprecipitates from cells transfected with either myc-hSiah2 (v240 or v460) or Vav constructs alone or from hSiah2-plus-Vav transfectants immunoprecipitated with unrelated sera (Fig. 3A and B, top, and data not shown). We carried out reciprocal immunoprecipitation experiments (IP anti-Vav in Vav and myc-hSiah2 transfected cells), but in spite of the presence of similar levels of the proteins, the level of myc-tagged hSiah2 detected was too low to be meaningful (data not shown). This discrepancy might be because the anti-Vav antibody interfered with hSiah2-Vav interaction.
FIG. 3.
hSiah2 interacts with Vav in mammalian cells. COS-7 cells (A) or T-Ag Jurkat cells (B), transiently transfected with 4 and 20 μg, respectively, of either pKES-Vav (Vav), myc-tagged pCAN-v240 (myc-v240), or pCAN-v460 (myc-v460) alone or with a combination of the vectors (Vav+myc-v240; Vav+myc-v460), were lysed in NP-40 buffer and immunoprecipitated with anti-myc MAb and protein G-Sepharose beads. Immunoprecipitates (IP) were detected with anti-Vav MAb (top). Expression of transfected hSiah2 (clones v240 and v460) was verified by reprobing the nitrocellulose membrane with anti-myc MAb (bottom). Wb, Western blot.
Colocalization of Siah and Vav.
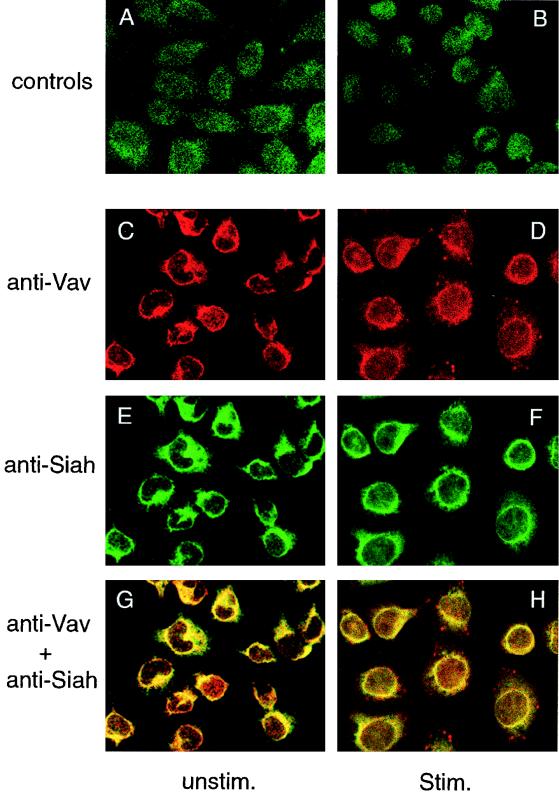
Further support for the in vivo Vav-hSiah2 interaction was obtained by studying the subcellular localization of endogenous Siah and Vav by immunofluorescence labeling. We used both Jurkat T cells and RBL cells, whose adherence properties facilitate the visualization of subcellular structures. In both cell models, Siah and Vav were detected mainly in the cytoplasm, although a weak signal was observed in the nucleus (Fig. 4C and E). No staining was detected with the preimmune Siah antiserum followed by the secondary antibody (Fig. 4A) or when anti-Siah antiserum was depleted with an excess of the immunizing peptide prior to incubation with the cells (Fig. 4B). Although previous studies indicated that Drosophila Sina was a nuclear protein (12) and that in transfected COS-7 cells hSiahs were distributed in discrete cytoplasmic particles (38), we found that endogenous Siah was evenly distributed in the cytoplasm, with a pronounced perinuclear localization. Interestingly, this region is the major site of colocalization of the two proteins (Fig. 4G). After stimulation of RBL cells via aggregation of FcɛRI, a partial nuclear translocation of Vav but not Siah could be detected (Fig. 4D and F), leaving the major colocalization site around the nucleus (Fig. 4F and H). These data provide further evidence for the existence of a cytoplasmic in vivo complex between Vav and hSiah2 and reinforce the coimmunoprecipitation results showing that the interactions were not induced during the experimental procedure, although a specific conformation was required to detect this interaction.
FIG. 4.
Immunolocalization of Vav and hSiah2 by confocal immunofluorescence microscopy. RBL cells were labeled with preimmune Siah antiserum (A), Siah antiserum depleted of the immunizing peptide (B), anti-Vav MAb (C and D), and anti-hSiah2 rabbit polyclonal antibody (E and F) as described in Materials and Methods. Colocalization of red fluorescence from Vav and green fluorescence from hSiah2 produced a yellow signal, indicating an overlap in the distribution of the two proteins (G and H). In panels D, F, and H, cells were stimulated (Stim.) by FcɛRI cross-linking. Panels A and B were obtained with a much higher transmission rate in order for the signal to be detectable.
hSiah2 inhibits Vav-mediated NFAT activation.
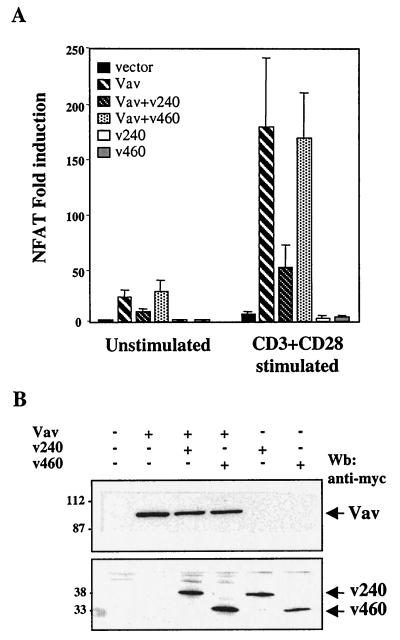
It has been reported that TCR stimulation contributes to IL-2 production through activation of different transcription factors, including NFAT and AP1 (55). Overexpression of Vav leads to an increased basal NFAT transcriptional activity, which is further enhanced by TCR stimulation (36, 68). We tested whether hSiah2 may also be involved in Vav-mediated NFAT activation. To this end, we overexpressed Vav either alone or in combination with myc-tagged hSiah2 (clone v240 or v460) in T-Ag Jurkat cells and examined their relative effects on the activity of a NFAT-luciferase reporter construct (NFAT-luc) containing a trimer of an IL-2-derived cis-acting element cloned upstream of the luciferase reporter gene. The activation of this promoter requires cooperative binding of NFAT proteins and AP1 transcription factors (55). As expected, Vav overexpression led to a significant induction of the basal transcriptional activity of the NFAT reporter construct relative to the control vector, which was further increased by CD3 plus CD28 stimulation (Fig. 5A). Coexpression of hSiah2 (clone v240) with Vav inhibited the Vav-induced NFAT activity in unstimulated cells, and a consistent reduction was observed in CD3- plus CD28-stimulated cells. On the other hand, no inhibition was observed when Vav was coexpressed with clone v460. Expression of either v240 or v460 alone did not significantly modify basal and CD3- plus CD28-stimulated NFAT activity (Fig. 5A).
FIG. 5.
Vav-mediated NFAT activation is inhibited by hSiah2. (A) T-Ag Jurkat cells (107) were transfected with NFAT and pSVβ-galactosidase reporter plasmids (5 and 1 μg, respectively) and 20 μg of either an empty vector (vector), pEF-Vav (Vav), pCAN-v240 (v240), pCAN-v460 (v460), or a combination of the vectors as indicated. A total of 106 cells were either left unstimulated or stimulated after 24 h with anti-CD3 plus anti-CD28 for 8 h. Luciferase activity was measured and corrected for β-galactosidase activity, and the results were expressed as average fold induction relative to unstimulated cells transfected with the empty vector. The data are representative of four independent experiments. The basal activity and the maximum NFAT responses were approximately 600 and 2 × 105 AU, respectively. (B) T-Ag Jurkat cell lysates from panel A were analyzed by immunoblotting for expression of Vav and hSiah2 (v240 and v460). Wb, Western blot.
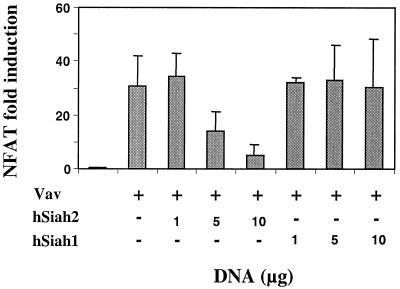
A similar result was also observed with the full-length hSiah2. As reported in Fig. 6, hSiah2 inhibited Vav-mediated NFAT induction in a dose-dependent manner. Interestingly, hSiah1, which is highly homologous to hSiah2 apart from the N-terminal region, did not exhibit any significant inhibition of Vav-mediated NFAT activity (Fig. 6). Since an intact N-terminal region of hSiah2 is required for the inhibition of Vav-induced NFAT activity, this inhibitory effect may reflect an association of hSiah2 and Vav in a complex involving the N-terminal region of hSiah2 with an as yet uncharacterized protein. The N-terminal region of hSiah1, which showed only 53% homology to its counterpart in hSiah2, could not mediate such an interaction and hence could not have any inhibitory role.
FIG. 6.
Overexpression of hSiah2 inhibits Vav-induced NFAT activity in a dose-dependent manner. T-Ag Jurkat cells (107) were transfected with NFAT and pSVβ-galactosidase reporter plasmids (5 and 1 μg, respectively), 10 μg of pEF-Vav (Vav), and increasing concentrations of pBK-CMV hSiah2 or pBK-CMV hSiah1 as indicated. Luciferase activity was determined and normalized to β-galactosidase activity to correct for transfection efficiency.
hSiah2 inhibits onco-Vav-mediated JNK activation.
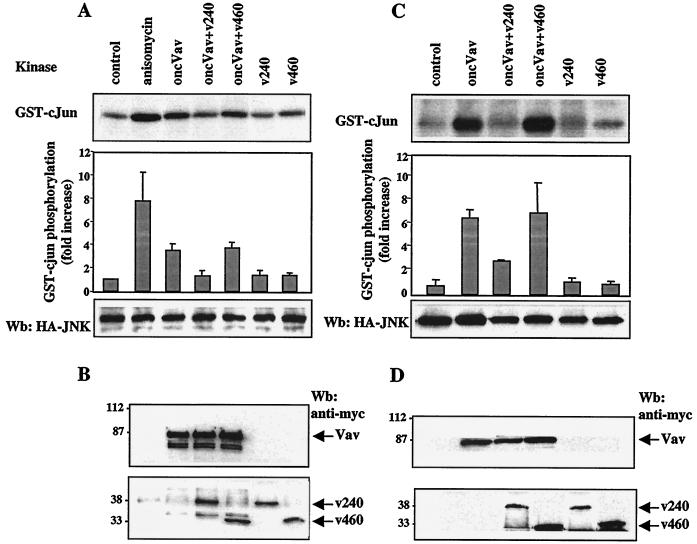
Recent evidence showed that activated Vav catalyzes GDP-GTP exchange on Rac1 and that Rac1-GTP stimulates the kinase activity of JNK (17, 18). The finding that hSiah2 interacted with Vav led us to investigate whether hSiah2 may modulate Vav-induced JNK activity. To this end an HA-tagged JNK and the oncogenic form of Vav (onco-Vav), which constitutively activates JNK, were cotransfected in COS-7 or T-Ag Jurkat cells, alone or together with myc epitope-tagged hSiah2 (v240 or v460 clones). As shown in Fig. 7A and C, coexpression of v240 with onco-Vav and HA-JNK abrogated almost completely the onco-Vav-induced JNK activation (three- to fourfold), which returned to levels found in cells transfected only with JNK. In contrast, cotransfection of the N-terminally deleted hSiah2 (v460), which still binds to Vav, did not modify the activation of JNK by onco-Vav despite similar levels of expression of the different proteins (Fig. 7B and D). These results favor an inhibitory role for hSiah2 in the onco-Vav-induced JNK activation and agree with the inhibitory effect observed with clone v240 but not v460 in Vav-induced NFAT activity.
FIG. 7.
Inhibitory effect of hSiah2 on onco-Vav-mediated JNK activation. COS-7 (A) or T-Ag Jurkat (C) cells were transfected with 1 μg (A) or 5 μg (C) of pcDNA3-HA-JNK together with 3 μg (A) or 15 μg (C) of expression vectors containing cDNA for the indicated plasmids. The total amount of transfected DNA was kept constant by using empty pcDNA3 vector. COS-7 cells treated with 1 μg of anisomycin per ml for 20 min were used as a control. The kinase reaction was performed in anti-HA immunoprecipitates from the corresponding cellular lysates with purified GST–c-Jun as a substrate (A and C, top panels). The levels of HA-JNK protein were confirmed by Western blot (Wb) analysis with anti-HA antibody (bottom panels). Values in the histogram represent the means and the standard errors of four independent experiments. COS-7 (B) and T-Ag Jurkat (D) cell lysates from panels A and C were analyzed by immunoblotting for expression of Vav and myc-epitope-tagged hSiah2 (v240 and v460). The additional bands in these blots could be due to degradative events caused by onco-Vav overexpression.
hSiah2 does not decrease the stability of Vav protein.
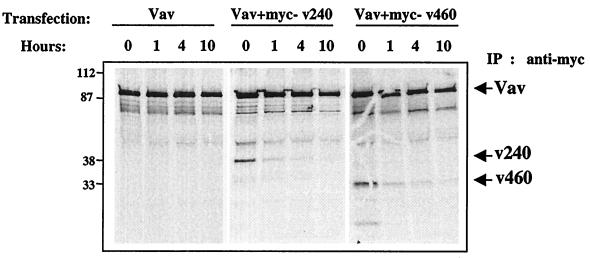
Both Sina and murine Siah2 have been implicated in regulating the proteasomal degradation of diverse proteins to which they bind (38, 45, 66). This function seems to be associated with the amino-terminal 114 aa of the Sina and Siah proteins, including the ring finger domain, which interacts in a yeast trap assay with ubiquitin-conjugating enzymes and Ubc9 homologs (38, 45, 66). To investigate whether hSiah2 expression affects Vav protein stability, we performed a pulse-chase experiment (Fig. 8). COS-7 cells were transiently transfected with myc epitope-tagged Vav alone or together with myc epitope-tagged hSiah2 (v240 or v460). Cotransfection of Vav with either v240 or v460 did not accelerate Vav degradation (Fig. 8). We also observed that the half-life of Siah was somehow much shorter than that of Vav. Taken together, these findings exclude the notion that hSiah2 could alter Vav stability. The evidence that the N-terminal deletion mutant of hSiah2 fails to inhibit Vav-induced NFAT and JNK activity prompted us to ask whether the inhibitory mechanism of hSiah2 could be mediated by the degradation of one or more components of the signal transduction pathways induced by Vav. JNK assays with COS-7 cells cotransfected with hSiah2 and Vav showed that JNK inhibition did not occur via the degradation of either JNK, Rac1, or c-Jun (data not shown). Additionally, treatment of the cells with the proteasome inhibitor LLnL (N-acetyl-Leu-Leu-norleucinal) or lactacystin at 50 μM did not modify the inhibitory effect of hSiah2 in NFAT or JNK assays (data not shown). Together, these data demonstrate that the inhibitory mechanism of hSiah2 might not involve a proteasome degradation pathway.
FIG. 8.
hSiah2 does not decrease the half-life of Vav. COS-7 cells were cotransfected with 4 μg of myc-tagged pEF-Vav (myc-Vav) and pCAN-v240 (myc-v240) or pCAN-v460 (myc-v460). At 48 h after transfection, the cells were pulse-labeled for 1 h with [35S]methionine, chased with cold methionine for the indicated times, and then lysed as described in Materials and Methods. Vav and hSiah2 proteins were immunoprecipitated (IP) with anti-myc antibody and analyzed by SDS-PAGE and autoradiography.
DISCUSSION
We report here the identification of a new Vav-associated protein, hSiah2, a human homolog of Drosophila Seven in absentia (Sina). Sina was initially described as a nuclear protein implicated in the specification of the R7 photoreceptor cell through the Ras1-MAPK signaling cascade (4, 52), comprising the tyrosine kinase Sevenless, Grb2, and Raf (26, 28, 44, 50, 61). Sina interacts with ubiquitin-conjugating enzymes that are able to target TTK, a transcriptional repressor of neuronal cell fate, to the degradative pathway (45, 66). Recently, Siah proteins were found to bind either to the cytoplasmic domain of the receptor for netrin 1 (DCC, deleted in colorectal cancer) or to the nuclear receptor corepressor (N-CoR) and to mediate their degradation via a proteasome-dependent mechanism (38, 72). Additionally, it has been reported that the p53-inducible hSiah1 is a negative regulator of cell proliferation and that its interaction with BAG1, a ubiquitin-like Hsp70-Hsc70-regulating protein, abrogated this antiproliferative role (47). The high level of evolutionary conservation of the Siah and Sina proteins between humans, mice, and Drosophila (76%) suggests that these proteins may play a crucial role in cellular processes such as proliferation, differentiation, and survival.
Through a two-hybrid screen in yeast, we detected an interaction between Vav and hSiah2 (Fig. 1), which was confirmed by using hSiah2 GST fusion proteins (Fig. 2) and also by performing coimmunoprecipitation experiments with cotransfected COS-7 and Jurkat T cells (Fig. 3). We also found, by immunofluorescence microscopy, a colocalization of Siah and Vav in the cytoplasm of hematopoietic cells, where Siah is predominantly observed (Fig. 4). Although the original studies reported a nuclear localization signal for Sina, a somehow controversial distribution in cytoplasmic particles has been reported for transfected hSiah proteins (12, 38). Both Vav and hSiah2 contain nuclear localization sequences which could mediate the translocation of one or both proteins to the nucleus. Several Vav-interacting nuclear proteins, such as Ku70 (58), ENX-1 (32) and hnRNP C (60), have been reported, and the presence of Vav in the nucleus has already been documented (14). In the present study, we showed some nuclear accumulation of Vav in RBL cells after FcɛRI stimulation that did not correlate with a delocalization of hSiah2, indicating that the functional relevance of the Vav-hSiah2 interaction should be cytoplasmic rather than nucleoplasmic. On the other hand, we reported that tyrosine phosphorylation of Vav did not influence the Vav-hSiah2 interaction (Fig. 2). Nevertheless, the mechanism by which Vav might link receptor-mediated signals to the nucleus is not yet established, and the interaction between Siah and Vav could be regulated within the cell by an as yet uncharacterized stimulus, offering to Siah the possibility of modulating some Vav functions.
The Vav-hSiah2 interaction requires a specific conformational structure of Vav present in the complete SH3-SH2-SH3 domain (Fig. 2). Residues 160 to 286 in the C-terminal region of hSiah2 are also involved in binding to Vav but do not contain a proline-rich sequence expected to interact with Vav SH3 domains. Therefore, the interaction between Vav and hSiah2 probably corresponds to a different protein-protein interaction. Interestingly, Ku70 protein does not use its proline-rich motif in binding to the carboxy-terminal SH3 domain of Vav (58), and Cbl-b needs the complete SH3-SH2-SH3 domain of Vav for correct binding (10). We also have evidence, obtained with the two-hybrid system, indicating that hSiah2 could dimerize through a region (aa 105 to 160) adjacent to the Vav-interacting domain (data not shown). It will be interesting to investigate whether the two interactions Vav-hSiah2 and hSiah2-hSiah2 are simultaneous or competitive. In this way, it is plausible that the formation of the complexes is controlled by substrate availability. Although the ring finger domains are generally involved in protein-protein interactions, neither Vav-hSiah2 nor hSiah2-hSiah2 interactions require this domain (Fig. 2 and data not shown).
The expression of hSiah2 impaired the transcriptional activity of NFAT reporter gene expression induced by Vav in Jurkat T cells (Fig. 5). NFAT is an important activator of gene transcription of cytokines, such as IL-2, IL-3, IL-4, and tumor necrosis factor alpha (55). This activation is mediated by cooperative binding of NFAT proteins with transcription factors of the AP-1 family (Jun-Fos complex) (54). It has been shown that Vav activates IL-2 gene expression and synergizes with the TCR stimulation in inducing NFAT-dependent transcription (36, 68). Our results confirm these findings and further demonstrate that coexpression of hSiah2 and Vav causes a significant decrease of NFAT reporter activity that is more pronounced in TCR-stimulated cells. Interestingly, neither the N-terminal deletion mutant of hSiah2 nor a member of the Siah family, hSiah1, is able to inhibit Vav-induced NFAT activity (Fig. 5 and 6). It is known that hSiah1 and hSiah2 proteins exhibit striking homology, diverging significantly only at their N termini. Together, these results favor a model in which hSiah2 modulation of Vav activation might be dependent on the formation of a specific signaling complex with other effectors, probably through the N-terminal region of hSiah2, but not of hSiah1, which in turn functions cooperatively to influence the downstream events leading to IL-2 gene regulation. We also observed that hSiah2 was able to block the onco-Vav-induced JNK stimulation in cotransfected COS-7 and T-Ag Jurkat cells (Fig. 7). It has been reported that onco-Vav leads to the stimulation of GDP-GTP exchange in Rac1, a protein implicated in cell proliferation and cytoskeletal organization (5, 56, 57) as well as in the activation of the JNK cascade (16). This inhibition could not be obtained with the N-terminal deletion mutant of hSiah2 (aa 105 to 324, clone v460). Although there are controversial data reporting, on the one hand, onco-Vav constitutive activation of Rac proteins and sequential JNK activation after transfection in T-cell lines (17, 18) and, on the other hand, unimpaired JNK activity in T cells isolated from vav−/− animals (24, 35), the implication of Sina in the Drosophila Ras-MAPK-induced signaling and the presence of Vav in both pathways involving Grb2 (53, 70) and Rac1 (18, 29, 51) suggest that hSiah2 interaction with Vav might also impair diverse signal transduction pathways. This correlates with recent findings demonstrating that several pathways could be implicated in JNK activation in T cells (39, 56).
Additionally, the inhibitory activity of hSiah2 seems to be independent of the proteolytic mechanism previously described for Sina and mSiah2 (38, 45, 66, 71). Our own experiments showed that hSiah2 did not modify the half-life of Vav, and the use of proteasomal blocking agents showed that the inhibitory effect of hSiah2 on NFAT activity and JNK pathways does not seem to be mediated via ubiquitin-proteasome-dependent degradation (data not shown). Studies to identify hSiah2-interacting proteins are under way and will be helpful to clarify its inhibitory role.
ACKNOWLEDGMENTS
We thank G. Baier for T-Ag Jurkat cells, M. Barbacid and X. Bustelo for plasmid pKLS1, A. Altman for pEF-Myc-tagged Vav and pKES-Vav plasmids, O. Acuto for the NFAT reporter construct, P. Crespo for HA-JNK and pGEX-cJun plasmids, R. B. Amson for plasmid pBK-hSiah1, G. Bismuth for anti-CD3 antibody, D. Olive for anti-CD28 antibody, I. Bouchaert for technical assistance in confocal microscopy, and D. Littman and I. Dusanter for critical reading of the manuscript.
This work was supported by the Ligue Nationale Contre le Cancer—Axe oncogénèse and Fondation pour le Recherche Medicale. A.G. was the recipient of a Poste Vert from INSERM (France).
REFERENCES
- 1.Adams M D, Dubnick M, Kerlavage A R, Moreno R, Kelley J M, Utterback T R, Nagle J W, Fields C, Venter J C. Sequence identification of 2,375 human brain genes. Nature. 1992;355:632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- 1a.Altman, M. Personal communication.
- 2.Amson R B, Nemani M, Roperch J P, Israeli D, Bougueleret L, Le Gall I, Medhioub M, Linares-Cruz G, Lethrosne F, Pasturaud P, Piouffre L, Prieur S, Susini L, Alvaro V, Millasseau P, Guidicelli C, Bui H, Massart C, Cazes L, Dufour F, Bruzzoni-Giovanelli H, Owadi H, Hennion C, Charpak G, Telerman A, et al. Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: activation of the vertebrate homologue of the Drosophila seven in absentia gene. Proc Natl Acad Sci USA. 1996;93:3953–3957. doi: 10.1073/pnas.93.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel P, Allegretto E A, Okino S T, Hattori K, Boyle W J, Hunter T, Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988;332:166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- 4.Biggs W H D, Zipursky S L. Primary structure, expression, and signal-dependent tyrosine phosphorylation of a Drosophila homolog of extracellular signal-regulated kinase. Proc Natl Acad Sci USA. 1992;89:6295–6299. doi: 10.1073/pnas.89.14.6295. . (Erratum, 90:6377, 1993.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 6.Bohmann D, Bos T J, Admon A, Nishimura T, Vogt P K, Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987;238:1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- 7.Borden K L, Freemont P S. The RING finger domain: a recent example of a sequence-structure family. Curr Opin Struct Biol. 1996;6:395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 8.Breeden L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Brunner D, Oellers N, Szabad J, Biggs W H R, Zipursky S L, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 10.Bustelo X R, Crespo P, Lopez-Barahona M, Gutkind J S, Barbacid M. Cbl-b, a member of the Sli-1/c-Cbl protein family, inhibits Vav-mediated c-Jun N-terminal kinase activation. Oncogene. 1997;15:2511–2520. doi: 10.1038/sj.onc.1201430. [DOI] [PubMed] [Google Scholar]
- 11.Bustelo X R, Suen K L, Michael W M, Dreyfuss G, Barbacid M. Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol Cell Biol. 1995;15:1324–1332. doi: 10.1128/mcb.15.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carthew R W, Rubin G M. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- 13.Chang H C, Solomon N M, Wassarman D A, Karim F D, Therrien M, Rubin G M, Wolff T. phyllopod functions in the fate determination of a subset of photoreceptors in Drosophila. Cell. 1995;80:463–472. doi: 10.1016/0092-8674(95)90497-2. [DOI] [PubMed] [Google Scholar]
- 14.Clevenger C V, Ngo W, Sokol D L, Luger S M, Gewirtz A M. Vav is necessary for prolactin-stimulated proliferation and is translocated into the nucleus of a T-cell line. J Biol Chem. 1995;270:13246–13253. doi: 10.1074/jbc.270.22.13246. [DOI] [PubMed] [Google Scholar]
- 15.Coppola J, Bryant S, Koda T, Conway D, Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991;2:95–105. [PubMed] [Google Scholar]
- 16.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 17.Crespo P, Bustelo X R, Aaronson D S, Coso O A, Lopez-Barahona M, Barbacid M, Gutkind J S. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 18.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 19.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 20.Della N G, Senior P V, Bowtell D D. Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina) Development. 1993;117:1333–1343. doi: 10.1242/dev.117.4.1333. [DOI] [PubMed] [Google Scholar]
- 21.de Martin R, Strasswimmer J, Philipson L. A new luciferase promoter insertion vector for the analysis of weak transcriptional activities. Gene. 1993;124:137–138. doi: 10.1016/0378-1119(93)90776-y. [DOI] [PubMed] [Google Scholar]
- 22.Dickson B. Nuclear factors in sevenless signalling. Trends Genet. 1995;11:106–111. doi: 10.1016/S0168-9525(00)89011-3. [DOI] [PubMed] [Google Scholar]
- 23.Emmel E A, Verweij C L, Durand D B, Higgins K M, Lacy E, Crabtree G R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K D, Kong Y Y, Nishina H, Tedford K, Marengere L E, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem M P, Bouchard D, Barbacid M, Bernstein A, Penninger J M. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 25.Fischer K D, Zmuldzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 26.Fortini M E, Simon M A, Rubin G M. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992;355:559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs S Y, Xie B, Adler V, Fried V A, Davis R J, Ronai Z. c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J Biol Chem. 1997;272:32163–32168. doi: 10.1074/jbc.272.51.32163. [DOI] [PubMed] [Google Scholar]
- 28.Gale N W, Kaplan S, Lowenstein E J, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Das B, Wei W, Van Aelst L, Mosteller R D, Khosravi-Far R, Westwick J K, Der C J, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henske E P, Short M P, Jozwiak S, Bovey C M, Ramlakhan S, Haines J L, Kwiatkowski D J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann Hum Genet. 1995;59:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 31.Hobert O, Jallal B, Schlessinger J, Ullrich A. Novel signaling pathway suggested by SH3 domain-mediated p95vav/heterogeneous ribonucleoprotein K interaction. J Biol Chem. 1994;269:20225–20228. [PubMed] [Google Scholar]
- 32.Hobert O, Jallal B, Ullrich A. Interaction of Vav with ENX-1, a putative transcriptional regulator of homeobox gene expression. Mol Cell Biol. 1996;16:3066–3073. doi: 10.1128/mcb.16.6.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobert O, Schilling J W, Beckerle M C, Ullrich A, Jallal B. SH3 domain-dependent interaction of the proto-oncogene product Vav with the focal contact protein zyxin. Oncogene. 1996;12:1577–1581. [PubMed] [Google Scholar]
- 34.Holloway A J, Della N G, Fletcher C F, Largespada D A, Copeland N G, Jenkins N A, Bowtell D D. Chromosomal mapping of five highly conserved murine homologues of the Drosophila RING finger gene seven-in-absentia. Genomics. 1997;41:160–168. doi: 10.1006/geno.1997.4642. [DOI] [PubMed] [Google Scholar]
- 35.Holsinger L J, Graef I A, Swat W, Chi T, Bautista D M, Davidson L, Lewis R S, Alt F W, Crabtree G R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 36.Holsinger L J, Spencer D M, Austin D J, Schreiber S L, Crabtree G R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci USA. 1995;92:9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Chung Y L, Glover T, Valentine V, Look A T, Fearon E R. Characterization of human homologs of the Drosophila seven in absentia (sina) gene. Genomics. 1997;46:103–111. doi: 10.1006/geno.1997.4997. [DOI] [PubMed] [Google Scholar]
- 38.Hu G, Zhang S, Vidal M, Baer J L, Xu T, Fearon E R. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:2701–2714. doi: 10.1101/gad.11.20.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- 40.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katzav S, Packham G, Sutherland M, Aroca P, Santos E, Cleveland J L. Vav and Ras induce fibroblast transformation by overlapping signaling pathways which require c-Myc function. Oncogene. 1995;11:1079–1088. [PubMed] [Google Scholar]
- 42.Katzav S, Sutherland M, Packham G, Yi T, Weiss A. The protein tyrosine kinase ZAP-70 can associate with the SH2 domain of proto-Vav. J Biol Chem. 1994;269:32579–32585. [PubMed] [Google Scholar]
- 43.Lai Z C, Rubin G M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992;70:609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Li Y, Carthew R W, Lai Z C. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- 46.Margolis B, Hu P, Katzav S, Li W, Oliver J M, Ullrich A, Weiss A, Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 47.Matsuzawa S, Takayama S, Froesch B A, Zapata J M, Reed J C. p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musti A M, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 49.Nemani M, Linares-Cruz G, Bruzzoni-Giovanelli H, Roperch J P, Tuynder M, Bougueleret L, Cherif D, Medhioub M, Pasturaud P, Alvaro V, der Sarkissan H, Cazes L, Le Paslier D, Le Gall I, Israeli D, Dausset J, Sigaux F, Chumakov I, Oren M, Calvo F, Amson R B, Cohen D, Telerman A. Activation of the human homologue of the Drosophila sina gene in apoptosis and tumor suppression. Proc Natl Acad Sci USA. 1996;93:9039–9042. doi: 10.1073/pnas.93.17.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olivier J P, Raabe T, Henkemeyer M, Dickson B, Mbamalu G, Margolis B, Schlessinger J, Hafen E, Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993;73:179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- 51.Olson M F, Pasteris N G, Gorski J L, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 52.Pelech S L, Sanghera J S. MAP kinases: charting the regulatory pathways. Science. 1992;257:1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- 53.Ramos-Morales F, Romero F, Schweighoffer F, Bismuth G, Camonis J, Tortolero M, Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3-3. Oncogene. 1995;11:1665–1669. [PubMed] [Google Scholar]
- 54.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 55.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 56.Reif K, Cantrell D A. Networking Rho family GTPases in lymphocytes. Immunity. 1998;8:395–401. doi: 10.1016/s1074-7613(00)80545-2. [DOI] [PubMed] [Google Scholar]
- 57.Ridley A J. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev. 1995;5:24–30. doi: 10.1016/s0959-437x(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 58.Romero F, Dargemont C, Pozo F, Reeves W H, Camonis J, Gisselbrecht S, Fischer S. p95vav associates with the nuclear protein Ku-70. Mol Cell Biol. 1996;16:37–44. doi: 10.1128/mcb.16.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero F, Fischer S. Structure and function of vav. Cell Signal. 1996;8:545–553. doi: 10.1016/s0898-6568(96)00118-0. [DOI] [PubMed] [Google Scholar]
- 60.Romero F, Germani A, Puvion E, Camonis J, Varin-Blank N, Gisselbrecht S, Fischer S. Vav binding to heterogeneous nuclear ribonucleoprotein (hnRNP) C. Evidence for Vav-hnRNP interactions in an RNA-dependent manner. J Biol Chem. 1998;273:5923–5931. doi: 10.1074/jbc.273.10.5923. [DOI] [PubMed] [Google Scholar]
- 61.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 62.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 64.Schuebel K E, Bustelo X R, Nielsen D A, Song B J, Barbacid M, Goldman D, Lee I J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- 65.Shermann F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 66.Tang A H, Neufeld T P, Kwan E, Rubin G M. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 67.Tarakhovsky A, Turner M, Schaal S, Mee P J, Duddy L P, Rajewsky K, Tybulewicz V L. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Katzav S, Weiss A. A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol. 1995;15:4337–4346. doi: 10.1128/mcb.15.8.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, Motto D G, Koretzky G A, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 70.Ye Z S, Baltimore D. Binding of Vav to Grb2 through dimerization of Src homology 3 domains. Proc Natl Acad Sci USA. 1994;91:12629–12633. doi: 10.1073/pnas.91.26.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Guenther M G, Carthew R W, Lazar M A. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]