Abstract
Background
Exposure of aspirin has been associated with reduced risk of colorectal cancer (CRC) incidence, but aspirin use in relation to CRC patients’ mortality remains undetermined. It is necessary to quantify the association between aspirin use and CRC mortality.
Methods
Two authors independently searched the electronic databases (PubMed, Embase, and the Cochrane Library) from 1947 through April 25, 2020. All observational studies assessing the association between different timing of aspirin use and CRC mortality were included. The effect size on study outcomes was calculated using random-effect model and presented as risk ratio (RR) with 95% confidence interval (CI). Heterogeneity, publication bias, and quality of included studies were also assessed.
Results
A total of 34 studies were included in this systematic review and meta-analysis. Prediagnosis aspirin use was not associated with CRC-specific mortality (RR = 0.91, 95% CI = 0.79 to 1.05) and all-cause mortality (RR = 0.87, 95% CI = 0.57 to 1.31). A statistically significant association between continued aspirin use and improvement in both CRC-specific mortality (RR = 0.76, 95% CI = 0.70 to 0.81) and all-cause mortality (RR = 0.83, 95% CI = 0.74 to 0.93) was observed. Postdiagnosis use of aspirin was associated only with reduced all-cause mortality (RR = 0.80, 95% CI = 0.69 to 0.94).
Conclusions
Continued aspirin use before and after CRC diagnosis has the most advantage regarding the improvement of CRC mortality. Nevertheless, further prospective trials and mechanistic studies are highly warranted.
Colorectal cancer (CRC) remains the second leading cause of cancer-related death worldwide (1), and the incidence rate in young adults (aged younger than 50 years) is increasing in recent years (2). Numerous evidence has demonstrated the protective role of aspirin on colorectal neoplasia among general populations (3) and even among high-risk populations (4). Low-dose aspirin also seems to be equally effective as colonscopy or fecal occult blood testing to reduce CRC incidence and even mortality (5). Regarding its potential biological mechanism for tumor suppression, aspirin has been identified to inhibit cyclooxygenase 2 (COX2 ) and related eicosanoids that promote malignant transformation (6,7), induce apoptosis via COX-dependent or -independent pathway (8), and modulate gut microbiota (9).
Nevertheless, the regular use of aspirin in the prevention of cancers is still a debated subject, because aspirin-induced bleeding, especially gastrointestinal bleeding, affects the risk-benefit assessment (10). In this way, secondary prevention in patients already diagnosed with CRC may offer a different risk-benefit profile. In support of this, some prospective studies designed for cardiovascular diseases prevention showed that aspirin use reduced the risk of metastasis and improved prognosis of patients with CRC (11-13). Recently, an increasing number of population-based observational studies have assessed the association between aspirin use and CRC patients’ survivorship, but inconsistent conclusions were reported regarding the difference in starting time of aspirin use (14‐17), as well as different subtypes of CRC (18‐22). Nevertheless, these publications also formed the driving force for the conduct of several ongoing clinical trials assessing the efficacy of aspirin as an adjuvant agent in CRC treatment (summarized in Supplementary Table 1, available online), though relevant data or papers are not published yet. On the other hand, given that it is at least 5 years since the publication of 2 meta-analyses concerning this controversy (23,24), the fact that there will now be more published studies leads us to reexamine this issue.
Therefore, the present systematic review and meta-analysis was performed to provide up-to-date and comprehensive estimates for the association between aspirin use and CRC survival. We investigated the different starting time to aspirin use in relation to CRC survival among total CRC patients, and subgroup analysis was further explored regarding different anatomical sites or molecular signs whenever sufficient data were available.
Methods
Search Strategy and Selection Criteria
A systematic search of PubMed, Embase, and Cochrane Library was performed from 1947 to April 25, 2020, to identify potential studies, without language restriction. Reference lists of retrieved articles and previous systematic reviews were checked for further eligible publications. Abstracts published from American Society of Clinical Oncology, European Society for Medical Oncology, the American Digestive Disease Week, and the United European Gastroenterology Week were also searched manually.
Studies were eligible for inclusion if all the following criteria were fulfilled: 1) the study type was restricted to observational study; 2) the study assessed the association between aspirin use and CRC mortality (mainly including all-cause mortality and cancer-specific mortality); 3) effect estimates (the hazard ratio [HR], risk ratio [RR], odds ratio [OR]) and 95% confidence interval (CI) were available; 4) if datasets overlapped, the recent information was extracted.
Two investigators (SYX and WHX) conducted the literature search, independent of each other. Search terms used in the search strategy were colorectal neoplasms, colorectal cancer, colorectal carcinoma, colorectal adenocarcinoma, colon cancer, colonic neoplasms, rectal neoplasms, rectal cancer, rectum cancer, aspirin, acetylsalicylic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), nonsteroidal anti-inflammatory drugs, survival, death, and mortality. The search strategy is detailed in the Supplementary Methods (available online). SYX and WHX then independently evaluated all abstracts identified by the search for eligibility and further evaluated all potentially relevant papers in more detail according to predesigned criteria. Disagreements between the 2 investigators were resolved by discussion.
Data Analysis
A data extraction form was used to finish data collection. Extracted data mainly include author, publication year, country, study design, cancer type, number of participants, sex , age at cancer diagnosis, stage, follow-up duration, assessment of outcome, dose and duration-based response, and estimates in each study. The primary outcome is the impact of aspirin use in different timing (timing 1 = ever-use; timing 2 = prediagnosis use; timing 3 = aspirin use only before diagnosis; timing 4 = continued use; timing 5 = postdiagnosis use; timing 6 = aspirin use after diagnosis regardless of its usage before diagnosis) on the mortality of CRC patients (assessed by CRC-specific mortality, all-cause mortality). A graphical illustration of the timing categories are depicted in Supplementary Figure 1 (available online). Ever-users are those who have a history of aspirin use at any time in the context of established CRC. Prediagnosis use refers to the usage of aspirin prior to CRC diagnosis with or without aspirin use after diagnosis. Postdiagnosis aspirin users are defined as those who initiate aspirin use only after the diagnosis of CRC. Continued aspirin users refer to those who initiate aspirin use prior to CRC diagnosis and continue to use after diagnosis. The secondary outcome is whether the effect size of aspirin is different regarding the clinical stage, anatomical site of tumor (colon vs rectum), and molecular marker (PIK3CA mutation status and COX2 expression). It should be noted that the same study simultaneously reported multiple outcomes regarding different timing of aspirin use. Quality assessment was carried out using the Newcastle-Ottawa scale.
The hazard ratio, risk ratio, or odds ratio with 95% confidence interval from maximally adjusted models whenever possible were extracted to estimate the summary effect. The pooled effect was calculated with a random-effect model. Heterogeneity of included studies was assessed with I2, whereby a value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (25). Publication bias was examined by Egger and Begg test (26,27). Sensitivity analysis was also performed to evaluate whether any study had excessive influence on the results of pooled analysis. All analyses were conducted using the statistical software package Stata13.0. Two-sided P values were calculated, with a P value less than .05 considered statistically significant for all tests. Data were reported in accordance to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) reporting guidelines (Supplementary Methods, available online) (28).
Results
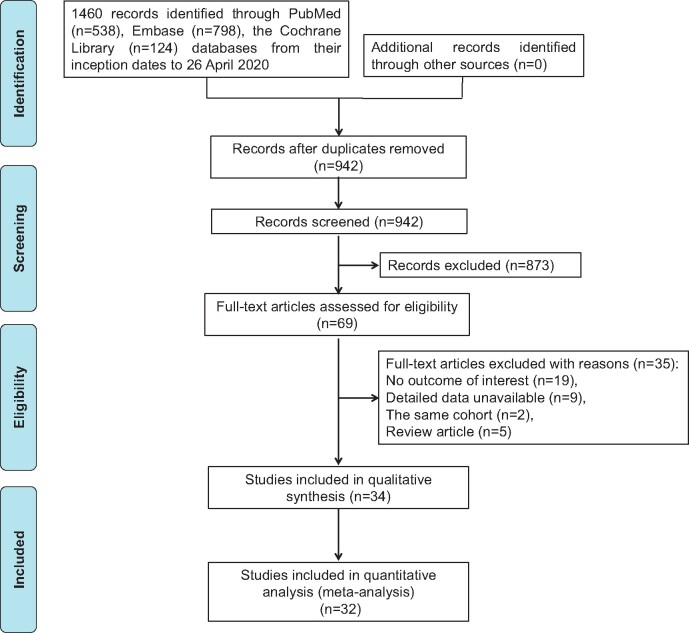
Using our search strategy, 942 potentially relevant articles were identified. Finally, 34 studies [31 in full-text (14‐22,29‐50) and 3 in conference abstract (51‐53)] were included in this systematic review and meta-analysis. The detailed literature screening process is shown in Figure 1. Most of the studies were conducted in the United States and European countries. Except for 3 case-control studies (16,31,48), the remaining studies adopted a cohort design. Among the included studies, 5 (20,21,37,38,46) enrolled patients with colon cancer or rectal cancer only, and the remaining studies included patients with both colon and rectal cancer; 10 studies also provided detailed information on site-specific outcomes (colon vs rectum). In addition, 3 (29,33,47) and 4 (16,17,30,33) studies, respectively, reported the dose- and duration-dependent association between aspirin use and CRC survival. In terms of molecular markers, 9 studies reported this association (18,19,22,29,36,40,44,51,52). The general characteristics of included studies are summarized in Table 1, and the estimated hazard ratio, risk ratio, or odds ratio with 95% confidence interval and adjustment factors for each study are listed in Supplementary Table 2 (available online). According to the Newcastle-Ottawa scale, the methodology of these included studies was generally moderate to good, as shown in Supplementary Table 3 (available online; for cohort study) and Supplementary Table 4 (available online; for case-control study).
Figure 1.
Flow diagram of included studies.
Table 1.
Baseline characteristics of included studies
| Study | Country | Study design | Cancer type | Sample size | Age at CRC diagnosis, y | Stage (AJCC/Duke) | Follow-up duration, y | Outcome indicators | Dose-response | Duration-response |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan AT et al. 2009 (29) | US | Cohort study (NHS/HPFS) | CRC | 1279a | NA | I-III | Median = 11.8 | CRC-specific mortality, overall mortality | 0.5-5 tablets/wk, ≥6 tablets/wk | — |
| Zell JA et al. 2009 (30) | US | Cohort study (CTS) | CRC | 621b | NA | I-IV | Median = 2.8; mean = 3.4 | CRC-specific mortality, overall mortality | — | <5 y, ≥ 5 y |
| Din FV et al. 2010 (31) | UK | Case-control study (SOCCS) | CRC | 2063a | Mean = 62.2 | NA | NA | CRC-specific mortality, all-cause mortality | — | — |
| Coghill AE et al. 2011 (14) | US | Cohort study | CRCd | 1737a | NA | I-IV | Mean = 8 | CRC-specific mortality | — | — |
| Bastiaannet E et al. 2012 (15) | Netherland | Cohort study | CRCd | 4481a | Median = 69 | I-IV | Median = 3.5 | Overall survival | — | — |
| Liao X et al. 2012 (18) | US | Cohort study (NHS/HPFS) | CRC | 964a | Mean = 68 | I-IV | Median = 12.7 | CRC-specific mortality, overall mortality | — | — |
| Reimers MS et al. 2012 (32) | Netherland | Cohort study | CRC | 536a | Median = 77.6 | I-IV | NA | Overall survival | — | — |
| Walker AJ et al. 2012 (33) | UK | Cohort study | CRC | 13 944a | User, mean = 68.3; Nonuser, mean = 74.5 | I-IV | NA | All-cause mortality | Prophylaxis dose, high dose | 0-5 y, 5-10 y, >10 y |
| Chae YK et al. 2013 (51) c | US | Cohort study | CRC | 243a | Mean = 58 | NA | NA | All-cause mortality | — | — |
| Domingo E et al. 2013 (19) | UK | Cohort study (VICTOR trial) | CRC | 896a | Median = 64.6 | II-III | Median = 5.1 | Overall survival, RFS | — | — |
| McCowan C et al. 2013 (34) | UK | Cohort study | CRCd | 2990a | Median = 73 | Duke A-D | NA | CRC-specific mortality, all-cause mortality | — | — |
| Sun R et al. 2013 (52)c | US | Cohort study (NHS/HPFS) | CRC | 931a | NA | I-IV | NA | CRC-specific mortality | — | — |
| Cardwell CR et al. 2014 (16) | UK | Nested case-control study | CRCd | 4794a | NA | I-IV | NA | CRC-specific mortality, all-cause mortality | — | <1 y, ≥1 y |
| Goh CH et al. 2014 (35) | Singapore | Cohort study | CRC | 726a | Median = 65 | I-III | NA | CRC-specific mortality, RFS | — | — |
| Reimers MS et al. 2014 (20) | Netherland | Cohort study | CC | 999a | NA | I-IV | NA | Overall survival | — | — |
| Kothari N et al. 2015 (36) | Australia | Cohort study | CRC | 185a | Median = 72 | I-IV | Median = 4.5 | Cancer-specific survival, overall survival | — | — |
| Ng K et al. 2015 (37) | US | Cohort study (CALGB89803 trial) | CC | 799a | NA | III | Median = 6.5 | Overall survival, RFS, DFS | — | — |
| Restivo A et al. 2015 (38) | Italy | Cohort study | RC | 241a | Median = 65 | II-III | Median = 3.08 | Overall survival, PFS | — | — |
| Zanders MM et al. 2015 (39) | Netherland | Cohort study | CRCd | 1043a | Mean = 73.2 | I-IV | Mean = 3.4 | All-cause mortality | — | — |
| Babic A et al. 2016 (40) | US | Cohort study (NHS/HPFS) | CRC | 544a | NA | I-IV | NA | Overall mortality | — | — |
| Bains SJ et al. 2016 (41) | Norway | Cohort study | CRCd | 23162a | Mean = 71.5 | I-IV | Median = 3 | Cancer-specific survival, overall survival | — | — |
| Frouws MA et al. 2017 (42) | Netherland | Cohort study | CRC | 6335a | NA | I-IV | NA | Overall survival | — | — |
| Frouws MA et al. 2017 (43) | Netherland | Cohort study | CRC | 7006a | NA | I-IV | NA | Overall survival | — | — |
| Frouws MA et al. 2017 (44) | Netherland | Cohort study | CRC | 599a | NA | I-IV | NA | Overall survival | — | — |
| Giampieri R et al. 2017 (45) | Italy | Cohort study | CRC | 66a | NA | NA | NA | Overall survival, PFS, disease control rate | — | — |
| Gray RT et al. 2017 (21) | Northern Ireland | Cohort study | CC | 740a | NA | II-III | Mean = 5.7 | Cancer-specific survival, overall survival | — | — |
| Hamada T et al. 2017 (22) | US | Cohort study (NHS/HPFS) | CRC | 617a | Mean = 68.6 | I-IV | Median = 11.5 | Cancer-specific survival, overall survival | — | — |
| Hua XW et al. 2017 (17) | US | Cohort study | CRC | 2419a | Mean = 54 | I-IV | Median = 10.8 | Cancer-specific survival, overall survival | — | ≤3 y, >3 y |
| Murphy C et al. 2017 (46) | Australia | Cohort study | CC | 488a | Median = 72 | II | NA | Overall survival, RFS | — | — |
| Gray RT et al. 2018 (47) | Northern Ireland | Cohort study | CRCd | 8391a | NA | Dukes A-C | Median = 3.6 | CRC-specific survival, overall survival | 1-365 daily defined dose, >365 daily defined dose | — |
| Rouette J et al. 2018 (53)c | Canada | Cohort study | CRC | 7478a | NA | NA | NA | All-cause mortality | — | — |
| Tsoi KK et al. 2018 (48) | China | Case-control study | CRCd | 612 509a | NA | NA | NA | CRC-specific mortality, all-cause mortality, GIB/CVD/CBVD-related mortality | — | — |
| Ventura L et al. 2018 (49) | Italy | Cohort study | CRCd | 22 7011a | NA | NA | NA | CRC-specific mortality, all-cause mortality, CVD/major bleeding-related mortality | — | — |
| Sung JJY et al. 2019 (50) | China | Cohort study | CRCd | 13 528a | NA | NA | NA | CRC-specific mortality, all-cause mortality, CVD/CBVD-related mortality | — | — |
Participants were both male and female. AJCC = American Joint Committee on Cancer; CBVD = cerebrovascular diseases ; CC = colon cancer; CRC = colorectal cancer; CTS = California Teachers Study; CVD = cardiovascular diseases; DFS = disease-free survival; GIB = gastrointestinal bleeding; HPFS = Health Professionals Follow-up Study; NA = not available; NHS = Nurses’ Health Study; PFS = progression-free survival; RC = rectal cancer; RFS = recurrence-free survival; SOCCS = Study of Colorectal Cancer in Scotland .
Participants were female only.
Conference abstract only.
Studies report the subgroup result regarding the anatomical site of CRC (CC and RC).
Note: “—” represents data not available.
Six studies (21,37,39,48,49,51) reported the relation between ever-use of aspirin (timing 1) and CRC patients’ outcome. Pooled results showed a positive association between ever-use of aspirin and CRC-specific mortality (pooled RR = 0.59, 95% CI = 0.57 to 0.62; I2 = 0.0%) (Table 2;Supplementary Figure 2, A, available online) but not all-cause mortality (pooled RR = 1.10, 95% CI = 0.93 to 1.29; I2 = 94.8%) (Table 2;Supplementary Figure 2, B, available online). For CRC patients without distant metastasis (stage I-III), ever-use also did not show a positive association regarding all-cause death (pooled RR = 0.82, 95% CI = 0.66 to 1.01; I2 = 5.9%) (Figure 2, B). Stratified by tumor site, ever-use of aspirin was associated with reduced risk of both cancer-specific mortality (pooled RR = 0.60, 95% CI = 0.57 to 0.64; I2 = 0) (Figure 2, A) and all-cause mortality (pooled RR = 0.74, 95% CI = 0.60 to 0.91; I2 = 0) (Figure 2, B) among colon cancer patients and only cancer-specific mortality in rectal cancer (pooled RR = 0.56, 95% CI = 0.52 to 0.62; I2 = 0) (Figure 2, A). It should be noted, however, that the estimate for site-specific outcome from Tsoi and colleague’s study (48) was not an adjusted value which might induce bias .
Table 2.
Timing of aspirin use and CRC patients’ mortalitya
| Timing of aspirin useb | CRC-specific mortality |
All-cause mortality |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Random-effect model |
Test of heterogeneity |
Test of publication bias |
No. of studies | Random-dffect model |
Test of heterogeneity |
Test of publication bias |
|||||
| RR (95% CI) | I2, % | P | Begg P | Egger P | RR (95% CI) | I2, % | P | Begg P | Egger P | |||
| Timing 1 | ||||||||||||
| All studies | 3 | 0.59 (0.57 to 0.62) | 0.0 | .38 | 1.00 | .10 | 6 | 1.10 (0.93 to 1.29) | 94.8 | <.001 | 1.00 | .09 |
| Cohort studies | 2 | 0.70 (0.55 to 0.89) | 0.0 | .91 | — | — | 5 | 0.97 (0.76 to 1.24) | 73.0 | .005 | — | — |
| Case-control studies | 1 | 0.59 (0.56 to 0.62) | — | — | — | — | 1 | 1.43 (1.42 to 1.44) | — | — | — | — |
| Timing 2 | ||||||||||||
| All studies | 6 | 0.91 (0.79 to 1.05) | 60.0 | .04 | .46 | .14 | 5 | 0.87 (0.57 to 1.31) | 96.1 | <.001 | .71 | .88 |
| Cohort studies | 4 | 0.81 (0.64 to 1.02) | 66.1 | .05 | — | — | 4 | 0.82 (0.51 to 1.33) | 96.5 | <.001 | — | — |
| Case-control studies | 2 | 1.03 (0.92 to 1.15) | 0.0 | 1.00 | — | — | 1 | 1.12 (0.90 to 1.39) | — | — | — | — |
| Timing 3 | ||||||||||||
| All studies | 1 | 1.76 (1.09 to 2.83) | — | — | — | — | 1 | 1.05 (0.63 to 1.74) | — | — | — | — |
| Cohort studies | 1 | 1.76 (1.09 to 2.83) | — | — | — | — | 1 | 1.05 (0.63 to 1.74) | — | — | — | — |
| Case-control studies | 0 | — | — | — | — | — | 0 | — | — | — | — | — |
| Timing 4 | ||||||||||||
| All studies | 6 | 0.76 (0.70 to 0.81) | 0.0 | .67 | .71 | .59 | 7 | 0.83 (0.74 to 0.93) | 83.3 | <.001 | .37 | .90 |
| Cohort studies | 5 | 0.76 (0.70 to 0.81) | 0.0 | .53 | — | — | 7 | 0.83 (0.74 to 0.93) | 83.3 | <.001 | — | — |
| Case-control studies | 1 | 0.72 (0.44 to 1.18) | — | — | — | — | 0 | — | — | — | — | — |
| Timing 5 | ||||||||||||
| All studies | 7 | 0.89 (0.73 to 1.08) | 75.0 | .001 | .55 | .63 | 11 | 0.80 (0.69 to 0.94) | 84.4 | <.001 | .16 | .10 |
| Cohort studies | 6 | 0.87 (0.70 to 1.10) | 79.1 | <.001 | — | — | 11 | 0.80 (0.69 to 0.94) | 84.4 | <.001 | — | — |
| Case-control studies | 1 | 0.95 (0.69 to 1.32) | — | — | — | — | 0 | — | — | — | — | — |
| Timing 6 | ||||||||||||
| All studies | 7 | 0.80 (0.66 to 0.97) | 84.8 | <.001 | .76 | .38 | 9 | 0.87 (0.77 to 0.98) | 85.1 | <.001 | .25 | .17 |
| Cohort studies | 6 | 0.75 (0.59 to 0.94) | 84.8 | <.001 | — | — | 8 | 0.84 (0.73 to 0.96) | 85.6 | <.001 | — | — |
| Case-control studies | 1 | 1.06 (0.92 to 1.24) | — | — | — | — | 1 | 1.06 (0.94 to 1.19) | — | — | — | — |
All statistical tests were 2-sided. CI = confidence interval; CRC= colorectal cancer; RR= risk ratio. “—” represents data not available.
Timing 1 = ever-use; timing 2 = prediagnosis use; timing 3 = aspirin use only before diagnosis; timing 4 = continued use; timing 5 = postdiagnosis use; timing 6 = aspirin use after diagnosis regardless of its usage before diagnosis.
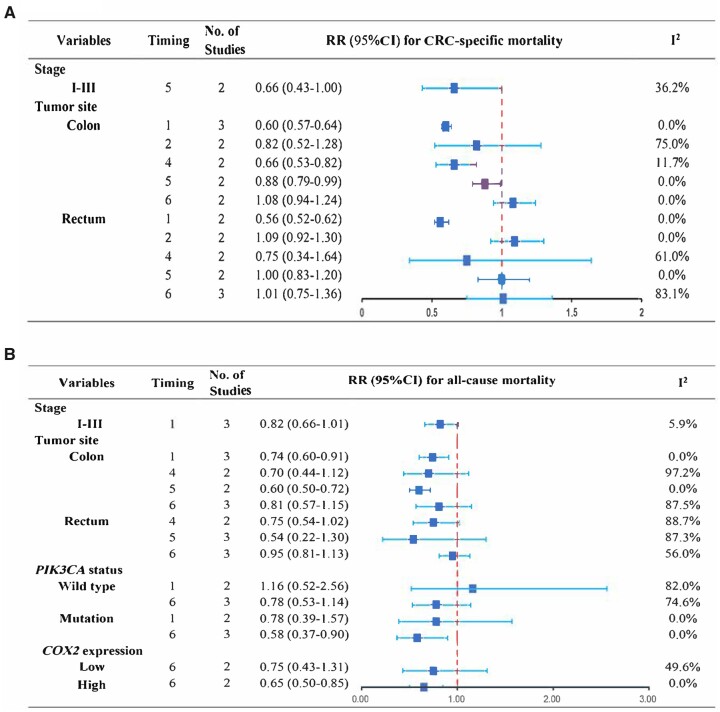
Figure 2.
Subgroup analysis of aspirin use in relation to CRC mortality. A) Different timing of aspirin use in relation to CRC-specific mortality regarding clinical stage and tumor site. B) Different timing of aspirin use in relation to all-cause mortality with regard to tumor stage, tumor site, and molecular markers (PIK3CA status and COX2 expression). Timing definitions: 1 = ever-use; 2 = prediagnosis use; 3 = aspirin use only before diagnosis; 4 = continued use; 5 = postdiagnosis use; 6 = aspirin use after diagnosis regardless of its use before diagnosis. The error bars represent the 95% CI of pooled effect. CI = confidence interval; CRC = colorectal cancer; RR = risk ratio.
To better characterize the association between the starting time of aspirin use relative to CRC diagnosis and patients’ survival, we divided aspirin use into prediagnosis use, postdiagnosis use, and continued use. It was found that prediagnosis aspirin use (timing 2) was not associated with CRC-specific mortality (pooled RR = 0.91, 95% CI = 0.79 to 1.05; I2 = 60.0%) (Table 2;Supplementary Figure 3, A, available online) and all-cause mortality (pooled RR = 0.87, 95% CI 0.57 to 1.31; I2 = 96.1%) (Table 2;Supplementary Figure 3, B, available online). Site-specific cancer-related mortality was 0.82 (95% CI = 0.52 to 1.28) and 1.09 (95% CI = 0.92 to 1.30) for colon cancer and rectal cancer, respectively, among these patients (Figure 2, A). Additionally, 2 studies respectively reported the CRC-specific mortality (unadjusted HR = 1.76, 95% CI = 1.09 to 2.83) (35) and overall mortality (adjusted HR = 1.05, 95% CI = 0.63 to 1.74) (17) among those taking aspirin only before CRC diagnosis (timing 3).
For continued users (timing 4) (15‐17,29,33,41,47,50), there was a statistically significant association between aspirin use and improvement in both CRC-specific mortality (pooled RR = 0.76, 95% CI = 0.70 to 0.81; I2 = 0%) and all-cause mortality (pooled RR = 0.83, 95% CI = 0.74 to 0.93; I2 = 83.3%) (Table 2;Supplementary Figure 4, available online). In subgroup analysis, continued use of aspirin was associated with lower cancer-specific mortality in colon cancer patients (pooled RR = 0.66, 95% CI = 0.53 to 0.82; I2 = 11.7%) but not rectal cancer patients (pooled RR = 0.75, 95% CI = 0.34 to 1.64; I2 = 61.0%) (Figure 2, A), whereas all-cause mortality was not modified by continued aspirin use regarding separate tumor site (colon cancer RR = 0.70, 95% CI = 0.44 to 1.12; rectal cancer RR = 0.75, 95% CI = 0.54 to 1.02) (Figure 2, B).
Postdiagnosis use of aspirin (timing 5) was associated with improved all-cause mortality (pooled RR = 0.80, 95% CI = 0.69 to 0.94; I2 = 84.4%) but not CRC-specific mortality (pooled RR = 0.89, 95% CI = 0.73 to 1.08; I2 = 75.0%) (Table 2;Supplementary Figure 5, available online). For those diagnosed in stage I-III, aspirin use also did not show an improved trend regarding cancer-specific death (pooled RR = 0.66, 95% CI = 0.43 to 1.00; I2 = 36.2%) (Figure 2, A). Stratifying by tumor anatomical site, pooled risk ratio for cancer-specific death was 0.88 (95% CI = 0.79 to 0.99) and for overall death was 0.60 (95% CI = 0.50 to 0.72) among colon cancer patients, and no statistical association was observed in patients with rectal cancer (cancer-specific mortality: 1.00, 95% CI = 0.83 to 1.20; overall mortality: 0.54, 95% CI = 0.22 to 1.30) (Figure 2). For those taking aspirin after diagnose regardless of its usage before diagnosis (timing 6), statistical association regarding CRC-specific mortality (pooled RR = 0.80, 95% CI = 0.66 to 0.97; I2 = 84.8%) or all-cause mortality (pooled RR = 0.87, 95% CI = 0.77 to 0.98; I2 = 85.1%) (Table 2;Supplementary Figure 6, available online) was observed. Further analysis of site-specific cancer outcome showed no association with aspirin use among these patients (Figure 2).
In terms of molecular biomarkers in predicting the adjunctive function of aspirin, only 4 studies (18‐20,29) targeting PIK3CA or COX2 were available to pool the estimates. Meta-analysis showed that aspirin use after diagnosis irrespective of its usage before diagnosis was associated with improved all-cause mortality among patients with tumor PIK3CA mutation (pooled RR = 0.58, 95% CI = 0.37 to 0.9) or COX2 overexpression (pooled RR = 0.65, 95% CI = 0.50 to 0.85) (Figure 2, B).
Furthermore, sensitivity analysis was performed to test the robustness of this relationship. We first assessed the effect of study design on the pooled estimate. The results revealed that pooled relative risk was not disturbed by the stratification of study design (Table 2). The influence of study design on pooled estimates was not evaluated in subgroup analysis because of the small number of included studies. Omitting each study iteratively was then performed. It was found that the stability was affected by exclusion of a particular study in assessing the relation between postdiagnosis use (timing 5) or aspirin use after diagnosis irrespective of its use before diagnosis (timing 6) and CRC survival. The detailed results are listed in Table 3. For publication bias test, it was only tested in primary outcome analysis but not in subgroup analysis because of their limited number of available studies. No small study effect existed in each analysis (Table 2).
Table 3.
Sensitivity analysis of different timing of aspirin use in relation to CRC mortality
| Timinga | Study | RR (95% CI) |
|---|---|---|
| Timing 1 | ||
| CRC-specific mortality |
|
|
| All-cause mortality |
|
|
| Timing 2 | ||
| CRC-specific mortality |
|
|
| All-cause mortality |
|
|
| Timing 4 | ||
| CRC-specific mortality |
|
|
| All-cause mortality |
|
|
| Timing 5 | ||
| CRC-specific mortality |
|
|
| All-cause mortality |
|
|
| Timing 6 | ||
| CRC-specific mortality |
|
|
| All-cause mortality |
|
Timing 1 = ever-use; timing 2 = prediagnosis use; timing 4 = continued use; timing 5 = postdiagnosis use; timing 6 = aspirin use after diagnosis regardless of its usage before diagnosis. CI = confidence interval; CRC = colorectal cancer; RR = risk ratio.
Discussion
This updated comprehensive systematic review and meta-analysis suggests that continued aspirin use is associated with lower cancer-specific and overall mortality, and postdiagnosis use is only associated with reduced overall mortality. In addition, the association between aspirin use and lower mortality seems to be more pronounced in tumors with PIK3CA mutation or COX2 overexpression. Thus, our data support the hypothesis that aspirin might be served as an adjuvant agent to treat CRC.
Optimizing the timing of aspirin use as an adjuvant treatment is clinically important. Our data first suggest that exposure of aspirin before and after CRC diagnosis is associated with reduced 24% cancer-specific mortality and 17% all-cause mortality. One explanation may be that patients who were exposed to aspirin prior to CRC development were more likely to have CRC in a less advanced stage and with less aggressive properties (41,54). Postdiagnosis use, which is most clinically relevant when considering recommendations for CRC treatment, was associated only with reduced 20% overall mortality. Notably, the observed benefit in overall mortality may be partially due to an improvement in cardiovascular-related mortality, because cancers increase the risk of some specific cardiovascular diseases such as thromboembolism (55). Thus, the improvement in overall mortality for aspirin use is less sensitive regarding its antitumor effect. In this way, those who take aspirin for heart disease reasons or for other indications might enjoy additional benefit from adjunctive aspirin therapy once they develop CRC. Our analysis did not obtain robust results for those who take aspirin after diagnosis irrespective of its usage before diagnosis, which was not in line with previous reports presented by Ye et al. (23) and Li et al. (24). These results support that aspirin use before diagnosis might be one of the key confounders when assessing the association between aspirin use and patients’ survival. No evidence of an association between prediagnosis aspirin use and improved patients’ survival was observed in our analysis and previous study (CRC-specific mortality: pooled HR = 0.93, 95% CI = 0.82 to 1.05; overall mortality: pooled HR = 1.10, 95% CI = 0.96 to 1.06) (24).
CRC is a heterogeneous disease with different molecular characteristics that would lead to different response to therapy, and adverse events of aspirin use (such as bleeding) are concerning in clinical practice. Thus, molecular biomarkers are needed to better identify individuals deriving a benefit from aspirin. The present meta-analysis noted a protective association for aspirin with all-cause mortality in PIK3CA-mutant (reduced by 10%) or COX2-overexpressed (reduced by 15%) tumors, which is consistent with the previous studies (23,24,56). PIK3CA mutation that frequently results in activated PI3K-signaling pathway is present in about 15%-20% of CRC (57). Activation of PI3K enhances COX2 activity and prostaglandin E2 synthesis, resulting in inhibition of apoptosis in CRC. Upregulated COX2 expression is common in about 70% CRC, and high expression predicts poor prognosis of CRC (58). Thus, aspirin use can inhibit the 2 targets to induce apoptosis of CRC cells. However, it should be noted that whether the adjuvant efficacy of aspirin use after diagnosis in relation to mortality is dependent in its usage before diagnosis or is different in anatomical site of tumor for these 2 targets is not determined because of the limited information. Except for these 2 targets, studies also indicated that CTNNB1 (gene encoding β-catenin) (52), BRAF or KRAS mutation status (44), and CD724 (also known as PD-L1) expression (22) might be candidate molecular biomarkers for the personalized use of aspirin in CRC patients. Of them, KRAS and BRAF are currently used in the precision treatment (59), and a prospective study also demonstrated that regular use of aspirin was associated with risk reduction of CRC without BRAF mutation (60,61). However, among these studies, the number of participants is limited, so the results might be less definitive. Therefore, the prospective trials of aspirin as an adjuvant therapy in specific molecular subtype with large sample size are warranted.
Questions still remain about the optimal dose and duration of aspirin use for secondary prevention. Available individual studies did not suggest potential dose-dependent association of aspirin use and CRC prognosis (29,33), whereas the most recent meta-analysis has confirmed that the favorable effect of aspirin tended to increase with longer duration of use and increasing dose for primary prevention of CRC incidence (3). In addition, only 2 ongoing trials assessed dose-dependent relation: 100 mg vs 200 mg (NCT02607072) or 100 mg vs 300 mg (NCT02804815). Duration-dependent response was only reported in 4 studies with different time spans (16,17,30,33). Hua and colleagues (17) found that compared with those taking aspirin for less than 3 years, postdiagnosis or continued use for more than 3 years had lower mortality (all-cause mortality: 0.71, 95% CI = 0.52 to 0.94; CRC-specific mortality: 0.26, 95% CI = 0.11 to 0.61), whereas Walker et al. (33) suggested aspirin might be beneficial in reducing CRC mortality during the first 5 years. This might be one of the reasons that most of the current ongoing trials adopt 3 years or 5 years as the duration of adjunctive aspirin therapy (Supplementary Table 1, available online). On the other hand, CRC often recurs in the first 3 years after surgery, so there is likely to be diminishing benefit after 3 years, and at some point (maybe 5 years), the risks might exceed the benefits from aspirin. Based on this available evidence, it is insufficient to make a speculation that the adjuvant effect of aspirin use in relation to CRC mortality would be a dose- or duration-dependent manner.
In addition to the factors we discussed above, some important factors that were closely associated with CRC mortality should be considered when interpreting the results. CRC screening is considered a primary way to control this disease; numerous studies have demonstrated the significant effect of fecal occult blood test or coloscopy screening on reducing CRC mortality (62). But few observational studies considered these variables in multivariate analysis, although 1 study considering this issue showed that the reduction of CRC mortality was not attributable to a higher CRC screening participation in aspirin users (49). Moreover, regular aspirin use may increase the risk of bleeding events especially among elderly patients, which might prompt more frequent interactions with the medical system. Thus, individuals with incident CRC might be detected earlier resulting in a better prognosis. Additionally, lifestyle (such as diet preference, physical activity) and other modifiable factors (such as smoking, body mass index) during aspirin use should be taken into account, although some studies adjusted certain factors in multivariable Cox analysis as we summarized in Supplementary Table 2 (available online). Taken together, for future studies, screening and preventive health behaviors of individuals should not be neglectable in study design.
The molecular mechanisms underlying the synergistic anticancer effect of aspirin are still incompletely understood. Biologically, this might be attributed to the induction of apoptosis via COX-dependent or COX-independent pathway (8), reduction of metastatic risk through disrupting platelet-circulating cancer cell interaction (54,63,64) or modulation of antitumor immune response (65). Eventually, aspirin may have more than 1 target and probably acts in different ways as an adjunctive agent.
The strength of our study lies in the comprehensive inclusion of all observational studies concerning the relationship between different timing of aspirin use and the mortality of CRC patients. We also present all available evidence in a systematic and unbiased fashion; however, limitations may exist when the findings are interpreted. First, for many of the pooled estimates, there was substantial between-study heterogeneity. It was likely because of differences in study population, distribution of tumor stages at entry, aspirin dose and duration, other medications (mainly including nonaspirin NSAIDs, metformin, statin), and the adjusted covariates across individual studies (Supplementary Table 2, available online). Second, inherent biases of observational studies cannot be ignored, such as selection bias and information bias which could lead to exaggeration or underestimation of survival benefit estimates. Meanwhile, causal interpretations of the estimates measures of association cannot be made, because findings from this study are based on observational data. Third, most of the studies were conducted in the United States or in European countries. This may make generalization of the findings to the patients in other ethnicities and geographical regions uncertain. Moreover, it is noteworthy that some estimates in subgroup analysis should be cautiously interpreted, because certain subgroup analysis is based on a limited number of studies. In addition, cancer-specific mortality was lacking in certain subgroup analyses, which may weaken the conclusion, because overall survival is less sensitive regarding antitumor effect and may be subject to dilution of any real effect.
In conclusion, based on best available evidence, the updated comprehensive systematic review and meta-analysis suggests that persistent aspirin use prior to and after CRC diagnosis has the most advantage with respect to cancer-specific mortality and overall mortality. Stratifying by tumor site and certain molecular markers further demonstrates that patients with colon cancer or CRC with PIK3CA mutation and COX2 expression might be candidates for aspirin use as an adjuvant therapy. Therefore, the current ongoing randomized clinical trials are highly warranted to determine the clinical efficacy of this findings. In addition, adequately powered mechanistic research is also needed to help elucidate the mechanism underlying this correlation.
Funding
This study was supported by the special funds of Beijing Key Laboratory of Helicobacter Pylori Infection and Upper Gastrointestinal Diseases (Y57405-32).
Notes
The Role of the Funder: The sponsor of the study did not participate in the design of the study; collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors declare that they have no conflict of interest.
Author contributions: Conception and design: LYZ, SYX. Acquisition of data: SYX, WHX. Analysis and interpretation of data: SYX, WHX, and YHF. Drafting manuscript and review it: SYX and LYZ. Final approval of the version to be submitted: SYX, WHX, YHF, and LYZ.
Data Availability
The data supporting the findings of this study are available in the article and in its online supplementary materials .
Supplementary Material
Contributor Information
Shiyu Xiao, Department of Gastroenterology, Peking University Third Hospital, Beijing, China; Beijing Key Laboratory of Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Peking University Third Hospital, Beijing, China.
Wenhui Xie, Department of Rheumatology and Clinical Immunology, Peking University First Hospital, Beijing, China.
Yihan Fan, Department of Gastroenterology, Peking University Third Hospital, Beijing, China; Beijing Key Laboratory of Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Peking University Third Hospital, Beijing, China.
Liya Zhou, Department of Gastroenterology, Peking University Third Hospital, Beijing, China; Beijing Key Laboratory of Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Peking University Third Hospital, Beijing, China.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Santucci C, Gallus S, et al. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31(5):558–568. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. [DOI] [PubMed] [Google Scholar]
- 5.Emilsson L, Holme Ø, Bretthauer M, et al. Systematic review with meta-analysis: the comparative effectiveness of aspirin vs. screening for colorectal cancer prevention. Aliment Pharmacol Ther. 2017;45(2):193–204. [DOI] [PubMed] [Google Scholar]
- 6.Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–1515.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos CL, Kodach LL, van den Brink GR, et al. Effect of aspirin on the Wnt/beta-catenin pathway is mediated via protein phosphatase 2A. Oncogene. 2006;25(49):6447–6456. [DOI] [PubMed] [Google Scholar]
- 8.Langley RE, Burdett S, Tierney JF, et al. Aspirin and cancer: Has aspirin been overlooked as an adjuvant therapy? Br J Cancer. 2011;105(8):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao R, Coker OO, Wu J, et al. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology. 2020;159(3):969–983.e4. doi:1053/j.gastro.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K; for the U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836–845. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Fowkes FGR, Belch JFF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. [DOI] [PubMed] [Google Scholar]
- 12.Algra AM, Rothwell PM.. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. [DOI] [PubMed] [Google Scholar]
- 13.Veettil SK, Jinatongthai P, Nathisuwan S, et al. Efficacy and safety of chemopreventive agents on colorectal cancer incidence and mortality: systematic review and network meta-analysis. Clin Epidemiol. 2018;10:1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill AE, Newcomb PA, Campbell PT, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60(4):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastiaannet E, Sampieri K, Dekkers OM, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106(9):1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardwell CR, Kunzmann AT, Cantwell MM, et al. Low-dose aspirin use after diagnosis of colorectal cancer does not increase survival: a case-control analysis of a population-based cohort. Gastroenterology. 2014;146(3):700–708. [DOI] [PubMed] [Google Scholar]
- 17.Hua X, Phipps AI, Burnett-Hartman AN, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35(24):2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domingo E, Church DN, Sieber O, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31(34):4297–4305. [DOI] [PubMed] [Google Scholar]
- 20.Reimers MS, Bastiaannet E, Langley RE, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174(5):732–739. [DOI] [PubMed] [Google Scholar]
- 21.Gray RT, Cantwell MM, Coleman HG, et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol. 2017;8(4):e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada T, Cao Y, Qian ZR, et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J Clin Oncol. 2017;35(16):1836–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye XF, Wang J, Shi WT, et al. Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br J Cancer. 2014;111(11):2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64(9):1419–1425. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 29.Chan AT, Ogino S, Fuchs CS.. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zell JA, Ziogas A, Bernstein L, et al. Nonsteroidal anti-inflammatory drugs: effects on mortality after colorectal cancer diagnosis. Cancer. 2009;115(24):5662–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Din FV, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59(12):1670–1679. [DOI] [PubMed] [Google Scholar]
- 32.Reimers MS, Bastiaannet E, van Herk-Sukel MP, et al. Aspirin use after diagnosis improves survival in older adults with colon cancer: a retrospective cohort study. J Am Geriatr Soc. 2012;60(12):2232–2236. [DOI] [PubMed] [Google Scholar]
- 33.Walker AJ, Grainge MJ, Card TR.. Aspirin and other non-steroidal anti-inflammatory drug use and colorectal cancer survival: a cohort study. Br J Cancer. 2012;107(9):1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCowan C, Munro AJ, Donnan PT, et al. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2013;49(5):1049–1057. [DOI] [PubMed] [Google Scholar]
- 35.Goh CH, Leong WQ, Chew MH, et al. Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Res. 2014;34(12):7407–7414. [PubMed] [Google Scholar]
- 36.Kothari N, Kim R, Jorissen RN, et al. Impact of regular aspirin use on overall and cancer-specific survival in patients with colorectal cancer harboring a PIK3CA mutation. Acta Oncol. 2015;54(4):487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng K, Meyerhardt JA, Chan AT, et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2015;107(1):345–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Restivo A, Cocco IM, Casula G, et al. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113(8):1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanders MMJ, van Herk-Sukel MPP, Vissers PAJ, et al. Are metformin, statin and aspirin use still associated with overall mortality among colorectal cancer patients with diabetes if adjusted for one another? Br J Cancer. 2015;113(3):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babic A, Shah SM, Song M, et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br J Cancer. 2016;114(9):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bains SJ, Mahic M, Myklebust TÅ, et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. J Clin Oncol. 2016;34(21):2501–2508. [DOI] [PubMed] [Google Scholar]
- 42.Frouws MA, Bastiaannet E, Langley RE, et al. Effect of low-dose aspirin use on survival of patients with gastrointestinal malignancies; an observational study. Br J Cancer. 2017;116(3):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frouws MA, Rademaker E, Bastiaannet E, et al. The difference in association between aspirin use and other thrombocyte aggregation inhibitors and survival in patients with colorectal cancer. Eur J Cancer. 2017;77:24–30. [DOI] [PubMed] [Google Scholar]
- 44.Frouws MA, Reimers MS, Swets M, et al. The influence of BRAF and KRAS mutation status on the association between aspirin use and survival after colon cancer diagnosis. PLoS One. 2017;12(1):e0170775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giampieri R, Restivo A, Pusceddu V, et al. The role of aspirin as antitumoral agent for heavily pretreated patients with metastatic colorectal cancer receiving capecitabine monotherapy. Clin Colorectal Cancer. 2017;16(1):38–43. [DOI] [PubMed] [Google Scholar]
- 46.Murphy C, Turner N, Wong HL, et al. Examining the impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern Med J. 2017;47(1):88–98. [DOI] [PubMed] [Google Scholar]
- 47.Gray RT, Coleman HG, Hughes C, et al. Low-dose aspirin use and survival in colorectal cancer: results from a population-based cohort study. BMC Cancer. 2018;18(1):228–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsoi KK, Chan FC, Hirai HW, et al. Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: a retrospective study of 612 509 patients. J Gastroenterol Hepatol. 2018;33(10):1728–1736. [DOI] [PubMed] [Google Scholar]
- 49.Ventura L, Miccinesi G, Barchielli A, et al. Does low-dose aspirin use for cardiovascular disease prevention reduce colorectal cancer deaths? A comparison of two cohorts in the Florence district, Italy. Eur J Cancer Prev. 2018;27(2):134–139. [DOI] [PubMed] [Google Scholar]
- 50.Sung JJY, Ho JMW, Chan FCH, et al. Low-dose aspirin can reduce colorectal cancer mortality after surgery: a 10-year follow-up of 13 528 colorectal cancer patients. J Gastroenterol Hepatol. 2019;34(6):1027–1034. [DOI] [PubMed] [Google Scholar]
- 51.Chae YK, Kim K, Hong DS, et al. PIK3CA mutation, aspirin use and mortality in patients with metastatic colorectal cancer participating in early-phase clinical trials. Cancer Res. 2013;73(suppl 8):164-164. [Google Scholar]
- 52.Sun R, Nishihara R, Qian ZR, et al. Aspirin and colorectal cancer incidence and mortality by CTNNB1 expression: a molecular pathological epidemiology (MPE) study. Cancer Epidemiol Biomark Prev. 2013;22(3):472–473. [Google Scholar]
- 53.Giorli G, Rouette J, Yin H, et al. Pre-diagnostic use of low-dose aspirin and risk of incident metastasis and mortality in patients with colorectal cancer. Pharmacoepidemiol Drug Saf. 2018;27(11):462–463. [Google Scholar]
- 54.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. [DOI] [PubMed] [Google Scholar]
- 55.Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paleari L, Puntoni M, Clavarezza M, et al. PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer. a systematic review and meta-analysis of epidemiological studies. Clin Oncol (R Coll Radiol). 2016;28(5):317–326. [DOI] [PubMed] [Google Scholar]
- 57.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. [DOI] [PubMed] [Google Scholar]
- 58.Soumaoro LT, Uetake H, Higuchi T, et al. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. [DOI] [PubMed] [Google Scholar]
- 59.Sinicrope FA, Okamoto K, Kasi PM, et al. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol. 2016;14(5):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishihara R, Lochhead P, Kuchiba A, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309(24):2563–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amitay EL, Carr PR, Jansen L, et al. Association of aspirin and nonsteroidal anti-inflammatory drugs with colorectal cancer risk by molecular subtypes. J Natl Cancer Inst. 2019;111(5):475–483. [DOI] [PubMed] [Google Scholar]
- 62.Lin JS, Perdue LA, Henrikson NB, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978–1997. [DOI] [PubMed] [Google Scholar]
- 63.Gay LJ, Felding-Habermann B.. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu XR, Yousef GM, Ni H.. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131(16):1777–1789. [DOI] [PubMed] [Google Scholar]
- 65.Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the article and in its online supplementary materials .