Abstract
Background
To evaluate the safety and efficacy of single incision plus one (SI+1) port three-dimensional (3D) laparoscopic minimally invasive esophagectomy (MIE).
Methods
Clinical data of patients who underwent 3D thoracic laparoscopic MIE in our department from September 2020 to March 2021 were analyzed retrospectively. According to the different methods of laparoscopic surgery, the patients were divided into 2 groups: SI+1 port 3D laparoscopy group and multiportal 3D laparoscopy group. The operation time of the 3D laparoscopy component, amount of intraoperative blood loss, number of celiac lymph node dissections, postoperative abdominal drainage days, postoperative total abdominal drainage, postoperative complications, and length of hospital stay were analyzed.
Results
There was no significant difference between the 2 methods in laparoscopic operation time (30.11±5.86 vs. 28.45±4.72 min, P=0.49), intraoperative blood loss (34.44±9.82 vs. 35.91±6.25 mL, P=0.69), number of celiac lymph node dissections (8.44±3.13 vs. 7.09±2.12, P=0.27), postoperative abdominal drainage days (3.11±0.33 vs. 3.00±0.00 days, P=0.28), postoperative total abdominal drainage (95.00±23.33 vs. 92.27±11.26 mL, P=0.74), postoperative complications (22.2% vs. 27.3%, P=0.33), and hospital stay (9.67±0.71 vs. 10.18±0.87 days, P=0.17). None of the patients enrolled in the study had recurrence or death to date.
Conclusions
The application of SI+1 port 3D laparoscopy in minimally invasive resection of esophageal carcinoma is safe and feasible.
Keywords: Single incision plus one port (SI+1 port), multiportal, three-dimensional (3D), minimally invasive esophagectomy (MIE)
Introduction
Although esophagectomy plus lymph node dissection is considered one of the most traumatic gastrointestinal operations (1,2), subtotal esophagectomy combined with 2 or 3 field lymph node dissection is still the main treatment for local esophageal cancer (EC) (3-5). Since 1992, when Cuschieri (6) first reported the use of endoscopic resection of EC to provide treatment for EC patients, minimally invasive surgery of EC has been gradually developed. In recent years, as a more minimally invasive surgical method, minimally invasive esophagectomy (MIE) combined with thoracoscopy and laparoscopy has been shown to provide better surgical quality by less trauma and more elaborate manipulation and less postoperative complications by reducing the incidence of postoperative pulmonary infection and relieve pain (7), thus it has been increasingly widely used in the treatment of EC.
Traditional MIE requires 3 to 4 incisions in the chest and 4 to 5 incisions in the abdomen for surgical operation. With the improvement of thoracic surgeons’ operative skills and patients’ demands for “minimally invasive” procedures, single incision surgery has gradually developed. At present, the SI+1 port operation method has been applied in gastrointestinal surgery in general surgery (8-11), so we trialed the use of SI+1 port 3D laparoscopy for MIE the abdominal surgical component, with our report as detailed below.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-441).
Methods
Clinical data
We retrospectively analyzed the clinical data of patients who underwent 3D thoracoscopic laparoscopic MIE from September 2020 to March 2021. The inclusion criteria were as follows: (I) pathological diagnosis of esophageal squamous cell carcinoma, (II) 3D thoracoscopic and laparoscopic MIE surgery; The exclusion criteria were as follows: (I) preoperative neoadjuvant chemoradiotherapy, (II) history of chest or abdominal surgery, (III) incomplete case information. A total of 20 patients were included in this study, with 9 participants in the SI+1 port 3D group (7 males, 2 females, 60.22±8.57 years), and 11 in multiportal 3D group (8 males, 3 females, 62.27±0.09 years).
Operation process
Under general anesthesia, a single lumen endotracheal intubation was performed. The operation of the chest and neck was the same in the 2 groups.
3D thoracoscopy
The patient was placed the left prone position with both upper limbs raised. The operator and lens holder were stationed on the ventral side of the patient, and the assistant was located on the dorsal side of the patient. A 1 cm incision between the 7th intercostal space of the right anterior axillary line was taken as the observation port, a 30° 10 mm lens was inserted with artificial pneumoperitoneum, and the CO2 pressure of pneumoperitoneum machine was 8 mmHg (1 mmHg =0.133 kPa). Otherwise, incisions made at 1 cm of the 4th intercostal space at the anterior axillary line, 0.5 cm of the 7th intercostal space, and 1 cm of the 9th intercostal space at the lower scapular line were taken as the operation ports. First, the mediastinum pleura of superior triangular esophagus was dissected, and the lymph nodes of the right recurrent laryngeal nerve were removed. Then, the azygos vein arch was dissociated, and the distal end of azygos vein was cut off with an endovascular gastrointestinal anastomosis stapler (ENDO-GIA). The esophagus was dissociated systematically, from the thoracic inlet to the esophageal hiatus of diaphragm, and the paraesophageal lymph nodes were concurrently removed. The esophagus was pulled anteriorly and the subcarinal lymph nodes were removed, then the lymph nodes of the left recurrent laryngeal nerve were removed. At the end of thoracic dissociation, a 28 Fr drainage tube was placed in the observation port and the incision was sutured.
3D laparoscopy
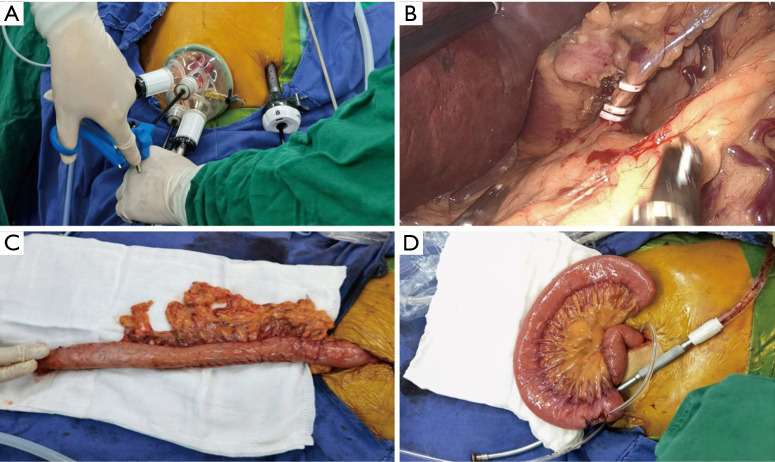
In the SI+1 port 3D group, the patient was placed in the right prone position, with the head high and the feet low, and the right upper limb abducted. The operator and the lens holder situated on the right side of the patient, and the assistant was on the left side. A longitudinal incision of 3−4 cm was made in the umbilical midline, into which a TriPortTM incision protective sleeve was inserted (Figure 1) (the 30° 10 mm lens was inserted in the lowest channel with artificial pneumoperitoneum, the CO2 pressure of pneumoperitoneum machine was 12 mmHg (1 mmHg =0.133 kPa), the other 3 channels were operation ports) (Figure 2A), and a 10 mm trocar was inserted through a 1 cm incision 2 cm below the costal edge of the left midclavicular line. The greater curvature of the stomach was dissociated with an ultrasonic scalper system, with the vascular arch of arteriae gastroepiploica dextra protected, and then the lesser omentum sac was dissected to dissociate the left gastric artery and vein (Figure 2B). Then, the lesser curvature of the stomach to the hiatus of the diaphragmatic esophagus was dissociated. Then the tubular stomach was made through the single incision (Figure 2C).
Figure 1.
Incision of SI+1 port vs. 5 traditional incisions. (A) Incision of SI+1 port, a longitudinal incision of 3–4 cm was made in the umbilical midline, a TriPortTM incision protective sleeve was installed in it, and a 10 mm trocar was inserted through a 1 cm incision at 2 cm below the costal edge of the left midclavicular line. (B) A 1 cm incision above the umbilicus was used as the observation port, another 4 incisions under the costal margin of the right midclavicular line, the junction point between the left and right midclavicular line and the 2 cm horizontal line above the umbilicus, and the subxiphoid process were used as operating ports.
Figure 2.
Operation of SI+1 3D laparoscopic MIE. (A) TriPortTM used in the operation of SI+1 port; (B) visual field exposure during dissociation and dissection of the left gastric artery; (C) a gastric tube was made through a single hole incision; (D) jejunostomy was performed through a single hole incision.
In the multiportal 3D group, the patient was placed in the right supine position, with the head high and the feet low, and the right upper limb abducted. The surgeon and the lens holder were stationed on the right side of the patient, and the assistant was located on the left side. A 1 cm incision above the umbilicus was taken as the observation hole, and a 30° 10 mm 3D lens was inserted to attach the artificial pneumoperitoneum. Another 4 incisions were made under the costal margin of the right midclavicular line, the junction point between the left and right midclavicular line, with a 2 cm horizontal line above the umbilicus and the subxiphoid process used as operating ports (Figure 1B). The greater curvature of the stomach was dissected with a harmonic ACE ultrasonic surgical devices, and the right vascular arch of the gastroepiploic artery was protected. Then, the lesser omentum sac was opened to dissect the left gastric artery and vein, which were double ligated with Hem-lock and then cut off with an ultrasonic scalpel. Next, we continued to dissociate the lesser curvature of the stomach to the diaphragmatic hiatus. After dissociation, the subxiphoid incision was extended to 3–4 cm to make the tubular stomach.
An intraperitoneal jejunostomy was performed (Figure 2D), a 12 Fr negative pressure ball was concurrently placed, then the abdominal incision was closed.
Evaluation index
The primary evaluation index was the operation time of 3D laparoscopy. The secondary evaluation indexes were intraoperative blood loss, number of abdominal lymph nodes dissected, postoperative days of abdominal drainage, postoperative total abdominal drainage volume, postoperative complications, and length of hospital stay.
Statistical analysis
The software SPSS 22.0 (IBM Corp., Chicago, IL, USA) was used for statistical analysis. Enumerate data were expressed as frequency and percentage. Chi-square test or Fisher’s exact probability test were used for comparison between groups. Measurement data were expressed as mean ± standard deviation (), and Student’s t-test was used for comparison between the 2 groups. A P value <0.05 was considered statistically significant.
Ethical statement
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Shanghai Changzheng Hospital. Individual consent for this retrospective analysis was waived.
Results
Clinical data of the 2 groups
There was no statistical difference in clinical data between the 2 groups (P>0.05), which were comparable (Table 1).
Table 1. Comparison of the clinical data of the 2 groups ().
| Clinical data | SI+1 port, n (%) | Multiport, n (%) | P value |
|---|---|---|---|
| Gender | 0.79 | ||
| Male | 7 (77.78) | 8 (72.73) | |
| Female | 2 (22.22) | 3 (27.27) | |
| Age (y) | 60.22±8.57 | 62.27±9.09 | 0.61 |
| Tumor location | 0.79 | ||
| Upper esophagus | 1 (11.11) | 1 (9.09) | |
| Middle esophagus | 6 (66.67) | 6 (54.55) | |
| Lower esophagus | 2 (22.22) | 4 (36.36) | |
| Pathological staging | 0.77 | ||
| IB | 0 | 1 (9.09) | |
| IIA | 4 (44.44) | 4 (36.36) | |
| IIB | 4 (44.44) | 4 (36.36) | |
| IIIA | 1 (11.11) | 2 (18.18) |
Evaluation indexes of the 2 groups
No participants in the 2 groups were transferred to thoracotomy, the operation was successful, and there were no deaths recorded. Laparoscopic operation time (30.11±5.86 vs. 28.45±4.72 min, P=0.49), intraperitoneal blood loss (34.44±9.82 vs. 35.91±6.25 mL, P=0.69), number of dissected abdominal lymph node (8.44±3.13 vs. 7.09±2.12, P=0.27), postoperative abdominal drainage days (3.11±0.33 vs. 3.00±0.00 days, P=0.28), total postoperative drainage volume of the abdominal cavity (95.00±23.33 vs. 92.27±11.26 mL, P=0.74), postoperative complications (22.2% vs. 27.3%, P=0.33) and the hospital days (9.67±0.71 vs. 10.18±0.87 days, P=0.17) had no statistical difference (Table 2). None of the patients enrolled in the study had recurrence or death to date.
Table 2. Evaluation indexes of the 2 groups ().
| Evaluation indexes | SI+1 port | Multiport | P value |
|---|---|---|---|
| Laparoscopic operation time (min) | 30.11±5.86 | 28.45±4.72 | 0.49 |
| Intraperitoneal blood loss (mL) | 34.44±9.82 | 35.91±6.25 | 0.69 |
| Number of dissected abdominal lymph nodes | 8.44±3.13 | 7.09±2.12 | 0.27 |
| Postoperative abdominal drainage days (d) | 3.11±0.33 | 3.00±0.00 | 0.28 |
| Total postoperative drainage volume of the abdominal cavity (mL) | 95.00±23.33 | 92.27±11.26 | 0.74 |
| Postoperative complications, n (%) | 0.33 | ||
| Anastomotic fistula | 1 (11.11) | 2 (9.09) | |
| Pulmonary infection | 0 | 1 (9.09) | |
| Arrhythmia | 1 (11.11) | 0 | |
| Hospital days (d) | 9.67±0.71 | 10.18±0.87 | 0.17 |
Discussion
After proposal of the concept of “minimally invasive surgery” in 1985 and the development of minimally invasive surgical techniques, MIE has been shown to provide better surgical quality and fewer postoperative complications (7). Nowadays, traditional MIE and robot-assisted MIE have been widely used in the therapy of patients with esophageal cancer. Robot-assisted MIE can provide better operational stability and convenience in suture and other aspects of the advantages. Traditional MIE has the advantage of universality and low price. Whether robot-assisted MIE or traditional MIE surgery requires 4 to 5 incisions in the abdomen, and 3–4 cm incisions in the upper abdomen are still needed for the reconstruction of the stomach tube and removal of the tumor. Repeated compression of the incision by the surgical instrument during operative procedures has been shown to result in postoperative pain at multiple incisions. Therefore, in seeking less trauma, more discreet incision, and faster recovery, thoracic surgeons have gradually introduced single incision surgery on the basis of traditional thoracoscopic surgery. At present, single incision surgery is mainly used in lung resection, mediastinal mass resection, and thoracic dissection of EC. Only a few hospitals (12-14) have applied the single incision technique to the surgical process of the thoracic component of MIE, and reports on the application of single incision technique in the abdominal part of MIE have been scarce. Therefore, we drew from the SI+1 port technique (8-11) used in gastrointestinal surgery of the general surgery department and applied it to the abdominal component of MIE. We found that on the basis of the same surgical effect, the SI+1 port technique made the incision more discreet and reduced postoperative pain.
We performed the SI+1 port abdominal part of MIE on 9 participants, and found that there were no statistically significant differences in abdominal operation time, intraoperative blood loss, number of abdominal lymph nodes dissected, postoperative days of abdominal drainage, postoperative total abdominal drainage volume, postoperative complications, and length of hospital stay between the 2 groups. Therefore, the safety and effectiveness of this surgical method are supported.
For the SI+1 port 3D laparoscopic operation, we used the right-inclined supine position with the head high and the feet low to better reveal the abdominal field of view with the help of gravity. At the same time, we only used an incision of 3–4 cm on the midline of the abdomen above the umbilicus and a 1 cm incision at 2 cm below the costal edge of the left midclavicular line, which was a reduction of 3 incisions compared with the traditional multiportal 3D laparoscopy. Reducing the number of incisions effectively alleviates the postoperative pain of patients, and it is more esthetically appealing. Lee et al. (15) pointed out that the main difficulty in the single incision operation of EC was the challenge of exposing the visual field of deep anatomy. We used 3D thoracoscopy to address the problem of poor visual field of deep anatomy in combination with its advantages of 24 times enlarged visual field and providing actual depth. In addition, because the operation hole is closer to the foot than in the original procedure, it can better expose the gastric pylorus and the greater and lesser curvature of the stomach. The operator can disentangle from the pylorus to the cardia along the greater and lesser curvatures of the stomach, respectively, which is more in line with the anatomical direction and a smoother trajectory. At the same time, due to the downward movement of the incision, the operation space is virtually increased, so that the visual field is more fully exposed. In addition, our study found that there was no significant difference in the number of abdominal lymph nodes dissected by SI+1 port 3D laparoscopy compared with multiportal 3D laparoscopy, so the reduction of abdominal incisions did not come at the cost of surgical quality, such as reduced number of lymph nodes dissected. However, due to the downward movement of surgical incision, a greater length of surgical instruments is required. Therefore, for taller patients, the current length of surgical instruments may not sufficiently meet the requirements of surgery. In addition, considering the need for extended operation time and the use of a stapler, we still employed the method of extraluminal tube making, which is more flexible than intraabdominal tube making.
During the operation, we found that the 4 channels of the TriPortTM incisional protective sleeve should be used flexibly. Lee et al. (15), Zheng et al. (16), and Wang et al. (17) used suture suspension to pull the liver during the abdominal part of single incision laparoscopic surgery. During the actual operation, when the surgeon is in the process of dissociating the lesser curvature of the stomach, the assistant can pull the liver up by inserting the head of a cavity mirror clamp into the diaphragmatic esophageal hiatus from the right channel of the TriPortTM to expose the lesser curvature of the stomach. Meanwhile, with the aid of the left midclavicular incision, the left gastric dynamic retinal vein can be sufficiently exposed. After the surgeon has dissociated the greater curvature of the stomach, the assistant can use the patient’s left channel or the incision of the left midclavicular line to help the surgeon better expose the greater curvature of the omentum and the vascular arch of the right gastroepiploic artery. In addition, because the incision is located in the middle of the abdominal cavity, the surgeon can better search for the location of the torsion ligament when performing jejunostomy, which simplifies the jejunostomy. We also tried to use only one umbilical incision for single incision abdominal dissection, but as the lens and all the instruments were almost parallel, the “chopstick effect” caused by the collision between the instruments presented some difficulties to the operation, especially when the left gastric artery was dissected, it was difficult to fully expose the left gastric artery and vein via single incision operation. The increase of the auxiliary operation port of the left midclavicular line greatly simplified the operation and reduced the difficulty of the operation. Moreover, the use of this port to place the jejunostomy fistula did not actually increase the number of incisions.
However, SI+1 port 3D laparoscopic operation also resulted in a certain degree of interference between instruments and lens which may make it difficult for the thoracic surgeon to use the technique. Meanwhile, the complexity of surgery of MIE leads to less use of SI+1 compared to other operations. So reasonable arrangement of the instrument placement presents a certain challenge to the surgeon. Lerut (18) highlighted that the arrangement of instruments is more complex in single incision resection of EC than that in single incision lung surgery. We found that the lens should keep a certain distance from the surgical area before the surgical instruments enter the operative field of vision. When the surgical instruments have been appropriately positioned, the lens should be moved closer to the surgical area to avoid the interaction between the lens and the surgical instruments. In addition, we also found that this operation method can be adopted in a very short time by surgeons with experience in single incision lung surgery and the tacit cooperation of the assistant and the lens holder.
There were still many limitations to our study. For example, our study was retrospective, with a small number of cases, and could not provide a higher level of evidence. Therefore, further clinical randomized controlled trials are needed to verify the feasibility and rationality of SI+1 port 3D laparoscopic surgery for EC. In conclusion, we believe that the use of SI+1 port 3D laparoscopy for the abdominal part of MIE is safe and effective and worthy of further promotion. At the same time, we are also conducting research on minimally invasive surgery for EC with single incision 3D thoracoscopy plus laparoscopy, and we believe that the single incision minimally invasive surgery for EC will be truly realized in the near future.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Shanghai Changzheng Hospital (No. 2021SL035). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-441
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-441
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-441). The authors have no conflicts of interest to declare.
References
- 1.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260:259-66. 10.1097/SLA.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 3.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. 10.1056/NEJM199206113262403 [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 5.Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. 10.1007/s10388-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuschieri A.Endoscopic subtotal oesophagectomy for cancer using the right thoracoscopic approach. Surg Oncol 1993;2Suppl 1:3-11. 10.1016/0960-7404(93)90052-Z [DOI] [PubMed] [Google Scholar]
- 7.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 8.Hirano Y, Hiranuma C, Hattori M, et al. Single-incision or Single-incision Plus One-Port Laparoscopic Surgery for Colorectal Cancer. Surg Technol Int 2020;36:132-5. [PubMed] [Google Scholar]
- 9.Zhou W, Dong CZ, Zang YF, et al. Initial experience of single-incision plus one port left-side approach totally laparoscopic distal gastrectomy with uncut Roux-en-Y reconstruction. World J Gastroenterol 2020;26:4669-79. 10.3748/wjg.v26.i31.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Deng H, Mou T, et al. Short-term outcomes of single-incision plus one-port laparoscopic versus conventional laparoscopic surgery for rectosigmoid cancer: a randomized controlled trial. Surg Endosc 2019;33:840-8. 10.1007/s00464-018-6350-6 [DOI] [PubMed] [Google Scholar]
- 11.Wang YN, Peng MY, Xie WQ, et al. Short-term outcomes of single incision plus one port laparoscopic surgery for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:48-53. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Rong B, Guo M.Uniportal Thoracoscopic McKeown Esophagectomy. Indian J Surg 2020. [Epub ahead of print]. doi: . 10.1007/s12262-020-02096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Ping W, Cai Y, et al. Modified McKeown procedure with uniportal thoracoscope for upper or middle esophageal cancer: initial experience and preliminary results. J Thorac Dis 2019;11:4501-6. 10.21037/jtd.2019.11.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu W, Yuan Y, Chen L.Single-Port Thoracoscopic Minimally Invasive Esophagectomy for Esophageal Cancer. World J Surg 2019;43:567-70. 10.1007/s00268-018-4811-7 [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Chen SC, Yang SM, et al. Comparison of single- and multi-incision minimally invasive esophagectomy (MIE) for treating esophageal cancer: a propensity-matched study. Surg Endosc 2017;31:2925-31. 10.1007/s00464-016-5308-9 [DOI] [PubMed] [Google Scholar]
- 16.Zheng B, Xu JX, Wu PX, et al. Application value of closed single-port thoracoscopic and laparoscopic radical esophagectomy for esophageal cancer. Chinese Journal of Digestive Surgery 2019;18:270-3. [Google Scholar]
- 17.Wang XQ, Wang CT. Effects of closed pneumothorax single hole thoracoscopy combined with three-field lymphadenectomy in the treatment of esophageal cancer. Trauma and Critical Care Medicine 2020;8:33-35+9.
- 18.Lerut T.Uniportal video-assisted thoracoscopic surgery in esophageal diseases: an introduction. J Vis Surg 2017;3:182. 10.21037/jovs.2017.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]