Abstract
The retina is the posterior neuro-integrated layer of the eye that conducts impulses induced by light to the optic nerve for human vision. Diseases of the retina often leads to diminished vision and in some cases blindness. Diabetes mellitus (DM) is a worldwide public health issue and globally, there is an estimated 463 million people that are affected by DM and its consequences. Diabetic retinopathy (DR) is a blinding complication of chronic uncontrolled DM and is the most common cause of blindness in the United States between the ages 24–75. It is estimated that the global prevalence of DR will increase to 191.0 million by 2030, of those 56.3 million possessing vision-threatening diabetic retinopathy (VTDR). For the most part, current treatment modalities control the complications of DR without addressing the underlying pathophysiology of the disease. Therefore, there is an unmet need for new therapeutics that not only repair the damaged retinal tissue, but also reverse the course of DR. The key element in developing these treatments is expanding our basic knowledge by studying DR pathogenesis in animal models of proliferative and non-proliferative DR (PDR and NPDR). There are numerous models available for the research of both PDR and NPDR with substantial overlap. Animal models available include those with genetic backgrounds prone to hyperglycemic states, immunologic etiologies, or environmentally induced disease. In this review we aimed to comprehensively summarize the available animal models for DR while also providing insight to each model’s ocular therapeutic potential for drug discovery.
Keywords: Diabetes mellitus (DM), diabetic retinopathy (DR), animal model
Introduction
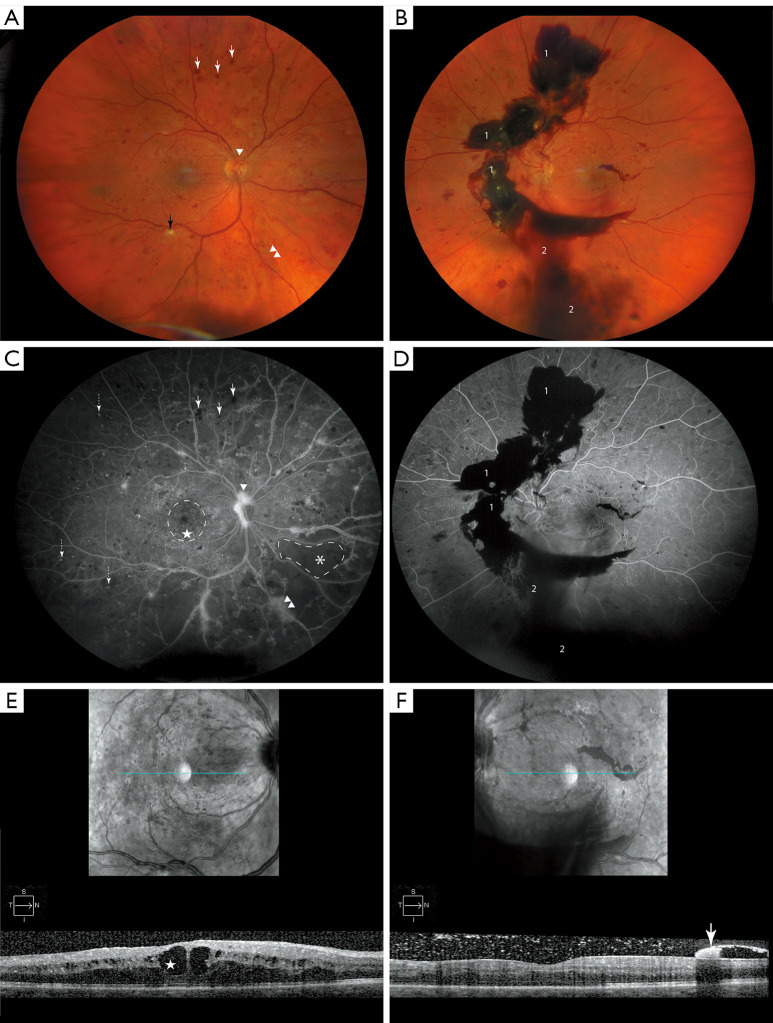
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia that increases the stress within microvasculature of many organ systems including the eye (1). Diabetic retinopathy (DR) is a major complication of DM and is the most common cause of blindness in the United States between the ages 24–75 (2,3). The development of DR is directly correlated to the duration of hyperglycemia (4,5), and the disease stages of DR follow a progression of blood vessel damage. Classification of DR can be established by the occurrence of neovascularization (NV). Pathological angiogenesis is the hallmark of proliferative DR (PDR) (6), and its absence indicates non-proliferative DR (NPDR) (Figure 1A-1F) (7,8). NPDR begins with microangiopathy that consists of pericyte loss with endothelial cell damage, increased vascular permeability of the blood retinal barrier (BRB), diabetic macular edema (DME), microaneurysms (MAs), dot blot hemorrhages (DBHs) and cotton-wool spots (CWS) (Figure 1A,1C,1E) (5,8). NPDR may progress to PDR, with increasing incidence with disease duration. Vision-threatening complications of untreated PDR are vitreous hemorrhage (VH) (Figure 1B,1D,1F) and tractional retinal detachment (TRD) that require surgical intervention (8).
Figure 1.
Features of DR. (A) Color fundus photo of the right eye of a patient with PDR, highlight pathologic changes which include ischemic damage to nerve fibers known as “cotton wool” spot (black arrow), dot-blot hemorrhages (white arrows), NVD (arrowhead) and NVE (double arrowhead). (B) The left eye of the same patient with PDR denotes advanced disease progression, highlighted are pathologic features including pre-retinal/sub-hyaloid bleeding [1] and VH [2]. (C,D) Corresponding FFA images. Pathological features shown here include MAs (dashed arrow) with corresponding features previously mentioned (A,B) and respectively denoted. Petaloid pattern of CME is encircled with dashed line. Area of capillary dropout is marked with asterix (*) and demarcated with dashed line. (E) OCT image of right eye shows cystoid macular edema CME that is more prominent in central macula (star). (F) OCT image of left eye. Hyper-reflective lesion (white arrow) representing pre-retinal/sub-hyoid hemorrhage. DR, diabetic retinopathy; PDR, proliferative diabetic retinopathy; NVD, neovascularization of the disc; NVE, neovascularization elsewhere; FFA, fundus fluorescein angiogram; MA, microaneurysm; VH, vitreous hemorrhage; CME, cystoid macular edema; OCT, optical coherence tomography.
Epidemiologic data from 2008–2018 have shown that DR is a growing public health matter, with significant adverse impacts on patients and the health system (9). The therapeutic windows for DR lie in different stages of disease progression. A major focus of ocular therapeutics is aimed at treating DME and NV. The milestone advancement was the discovery of the clinical indications of anti-vascular endothelial growth factors (VEGF) in DR (10-12). Anti-VEGF and corticosteroids have remained the mainstay treatment for many complications of DR. However, a sizeable proportion of patients (~40%) possesses DME resistant to these mono-therapies and a small percentage remain resistant (~8%) to dual therapy leading to diminished visual outcomes (13-16). Therefore, there is an unmet need for new therapeutics to treat vision-threatening sequelae of DR and reverse the course of the disease. Animal models provide means to investigate DR and develop the next generation of ocular therapeutics. In this review, we describe current DR models and outline their benefits and shortcomings in studying different stages of DR and development of novel therapeutics.
Complex diseases such as DM with multifaceted comorbidities are influenced by numerous factors that entail genetics, environmental factors, and chemical exposures (17-23). Advancement of investigational genome-wide technology has propelled the identification of genetic variants associated with DR progression (18). These variants, along with many others, can be induced or genetically engineered in animal models. Gene editing tools such as clustered regularly interspaced short palindromic repeats (CRISPR) and targeted RNA interference (RNAi) technologies allow for precise gene modifications (24,25). In addition to these major technological advancements, selectively breeding animals of desired genetic backgrounds remains as a conventional method for producing genetic animal models.
Inducible disease models can be generated with a wide variety of approaches that include diet modification, drug administration, and surgical procedures (26-29). Selection of animal species has its considerations as there is a sizeable collection to generate DR models for each stage of DR. Animals used to model DR include rats, mice, canines, feline, swine, rabbit, non-human primates, zebra fish (23).
Of animals used to model DR, the most widely selected are rodents as they provide low cost, short life span, and quick breeding rates; indeed, mice and rats can readily be engineered to exhibit a propensity towards DR pathogenesis (23,30-35). Canines with DR phenotypes have been reported to closely model human DR. However, ethical concerns and cost efficiency impedes widespread use (23,35-40). Felines can be induced to develop DR by altering oxygenation with models generated as early as 1950s (41,42). Recently there has been a growing interest for the use of non-human primates for drug discovery in DR, although previously non-human primates have demonstrated resistance to development of PDR (43,44). Zebrafish, surprisingly, have similar eye structure to humans and are growing in use for their efficient short life span and minimal cost (35,45-48). Although there is a wide range of animal models to study each stage of DR, there is no single model that fully encompasses the entire DR pathogenesis of the human eye.
Rodent models
Rodent models are the most frequently utilized DR animal models. There are several methodologies available to induce various stages of DR, that range from pharmacologic induction, genetic manipulation, environmental exposures, and surgical procedures (23,49-70).
Induction of pharmacologic DR is classically performed by administration of alloxan (a toxic glucose analogue) or the antibiotic streptozotocin (STZ) via destruction of the pancreas seen in type 1 DM (T1DM). Of these two, STZ is the preferred drug for inducing DR, because of the faster rate of phenotype manifestation (52). However, animals in the STZ model rarely develop PDR.
Genetic mouse models include five main genotypes: Ins2Akita (Ins2Akita), nonobese diabetic (NOD), db/db (Leprdb), Kimba, and Akimba. Rat specific models focus around six genotypes: Zucker Diabetic Fatty (ZDF), Otsuka Long-Evans Tokushima fatty (OLETF), biobreeding (BB), Wistar Bonn/Kobori (WBN/Kob), spontaneously diabetic Torii (SDT), and Goto-Kakizaki (GK). Each genetic model varies in the mode of etiology, inheritance, pathology, and disease progression (64-66,68,70-76).
Environmental induction includes diet and oxygen exposure during early stages of life. A surgical procedure able to induce DR is pancreatectomy. Each model possesses careful considerations when selecting stage of disease, time to presenting phenotype, costs, and pathology limitations.
Pharmacologically induced rodent models
Dunn and McLetchie performed the first pharmacologic induction of DM in 1942 with administration of the uric acid derivative alloxan (50). The mechanism of DM induced by alloxan is by direct targeting of insulin producing β cells of the pancreas through inhibition of the glucose-insulin pathway enzyme, glucokinase (51). The alloxan induced PDR mouse phenotype manifests between 2–9 months after induction at the age of 8–10 weeks (77). A major limitation of alloxan is the toxic effects on the liver and kidney, although weight adjusted dosing could avoid these adverse effects. Additionally, instability of alloxan at room temperature is a notable issue, and makes handling and administration challenging (50). Due to its side effects, there has been a gradual shift toward the use of STZ.
Initial reports of STZ administration causing DM in rats and dogs date back to 1963 (52). STZ is an antibiotic that was investigated for its use as a chemotherapy and later discovered to induce hyperglycemia with characteristic symptoms of DM. Induction of DM by STZ is by targeting of pancreatic islet with the destruction of β cells via DNA fragmentation facilitated cell death (52). β cells take up STZ via the low affinity glucose transporter 2 (GLUT2), as STZ chemical structure closely resembles that of glucose and N-acetyl glucosamine. As compared to alloxan, STZ shows less nephro- and hepatotoxicity, and it is favored over alloxan for its ease of handling. STZ closely mimics the pathogenesis of DM with induction of hyperglycemia within 2 weeks after induction (35).
Both rats and mice readily develop diabetes with STZ treatment. Surprisingly, rats require lower doses of STZ to exhibit pathologic hyperglycemia (23,35,69). STZ-induced DR typically manifests after long term exposure to high glucose levels greater than 150 mg/dL. Both mouse and rats with STZ DR are widely used to screen compounds for treatment of early stages of T1DM (69), and stem cell therapy (78). Mice with STZ-induced DR exhibit ganglion cell proliferation beginning 4 weeks after onset of hyperglycemia (79). At 16 weeks, the ocular phenotype resembles NPDR seen in humans, with characteristic vascular changes, and thinning of the INL and ONL (80,81). Although rare, the presence of NV has also been reported (82). In rats, STZ-induced DR begins with compromised BRB structural integrity by 2 weeks of diabetes onset, followed ONL thinning at 4 weeks, NV occurring at 8 weeks, and basement membrane thickening at 1 year (23,69,83). Pharmacologically induced DR models are reliable and straightforward for study designs. The limitations for pharmacologic DR rodent models are mainly related to the adverse effects of the drugs and constraint of disease progression.
Diet-induced rodent models
Diet induced diabetes in rodent models is classically conducted by high-galactose rich diets (27). C57BL/6J mice on high-fat diets (45% fat, 35% carbohydrate, 20% protein) have also shown propensity to develop type 2 DM (T2DM) and provide a model to investigate diet induced DR (27,84). DR phenotypes induced by high-fat diets in mice develop from a similar etiology seen in humans, where DM is a manifestation of high-caloric western diets. DR changes due to hyperglycemia are observed after 21 months with formation of MAs and capillary basement membrane thickening in 30% of mice (7,27,85,86). Rodents with diet-induced DR are commonly used; however, they do not exhibit the exact microvascular damage seen in humans and often lack the proliferative NV (27). As a result, to achieve a good size study group with microvascular manifestations, a large starting sample size as well as extended period of hyperglycemia is required.
Genetic rodent models
Genetic factors play a vital role in the development of DR (87). The majority of rodent genetic models have well characterized genetic backgrounds and are readily manipulated with genetic tools to generate knockouts, knockdowns, and transgenic hybrids. There are five main mouse (Ins2Akita, NOD, Leprdb, Kimba, and Akimba) and six main rat (ZDF, OLETF, BB, WBN/Kob, SDT, and GK) specific genetic models of DR. The limitations and advantages vary greatly among genetic models. The following will briefly explain each rodent model and describe similarities in disease pathogenesis.
T1DM models
A missense mutation in insulin 2 gene causes hyperglycemia via a T1DM-like mechanism. Accumulation of misfolded insulin in β-cells defines the Ins2Akita mouse phenotype and leads to apoptosis (64,66). The Ins2Akita model is typically used to study T1DM with destruction of β-cells present at 8 weeks. Retinal vascular disease is prominent for up to 36 weeks, with increased permeability and inflammatory damage to the BRB, marked reduction in retinal ganglion cells, and continued damage to both the inner nuclear layer (INL) and inner plexiform layer (IPL) (23,88,89). Limitations of Ins2Akita model include its lack of similarity with the vascular changes seen in human DR. These limitations must be considered when using Ins2Akita model, as conclusions drawn may require additional supporting evidence to assert correlation (90). The major advantage of the Ins2Akita model is its utility for early progression and detection of retinal glial cell damage seen in DR, which provides a useful screening tool for treatments that involve neuroprotection (7). One such treatment involves pigment epithelium-derived factor (PEDF), hypothesized to be neuroprotective via modulation of glutamate transporter expression (91,92). The Ins2Akita model is an ideal candidate for PEDF studies or similar treatment investigations.
An alternative to the Ins2Akita model is the NOD model, in which mice characteristically develop a T1DM hyperglycemia via an autoimmune response in which CD4+ and CD8+ cells target pancreatic β-cells (23,30,65,93). This model was first discovered in 1974 by Yoshihiro Tochino, who initially observed a female mouse spontaneously developed hyperglycosuria (93,94). Diabetes in NOD mice shows phenotypic resemblance to humans, exhibiting a polygenic etiology with numerous genetic loci correlated to T1DM phenotype (94-97). The NOD model develops DM at 12 weeks and retinal vasculature damage beginning with signs of apoptosis of endothelial cells and retinal glial cells. DR changes, including thickening of the retinal capillary basement membrane begin after 1 month of hyperglycemia (98). Vasoconstriction and degeneration of major blood vessels with compensatory NV manifest by 4 months of hyperglycemia (23,57,99,100). Vitreal injections of pro-inflammatory cytokines (IL-1β and TNF-α) in NOD leads to enhanced disease progression, providing a more directed model to study DR (101,102). Limitations of the NOD model includes a significant gender bias where DM develops by 30 weeks in 80% of female and only 20% of male mice (30). The gender bias in the NOD model may be influenced by gender differences in gut microbiome (103). Underlying gender bias also infers major differences in polygenic background that should be a consideration in data interpretation. Nevertheless, the pathophysiology of T1DM in the NOD model closely follows that observed in humans and provides an excellent mouse model for studies. The NOD mouse model could be used to test compounds aimed at attenuating autoimmune responses or beta islet transplants (104).
Another rodent model frequently used to study T1DM is the biobreeding (BB) rat model, in which an autoimmune mediated apoptosis of pancreatic β-cells causes diabetes. A frameshift mutation found in immune-associated nucleotide-binding protein gene (Ian4) is one genetic factor for triggering of autoimmune disease in this model. Additionally, this phenotype produces diabetes associated lymphopenia (105,106). Within 1-year BB rats typically present with pericyte dysfunction, microvascular degeneration, and MAs (75,107). Gut microbiome of BB rats can contribute to the spontaneous T1DM phenotype (108). Further investigation of the exact mechanism of spontaneous T1DM poses a potential limitation of the BB rat model. However, the BB rat model still provides a strong T1DM phenotype for investigations.
T2DM models
The obesity epidemic in the United States has driven the need for research animal models (109,110). T2DM is frequently associated with obesity (111). Two commonly used obesity rodent models include the ZDF rat model and the Leprdb mouse model that hold mutations in the leptin receptor gene leading to obesity and hyperglycemia (33,112,113).
ZDF rats display a reduction in leptin receptor expression that promotes obesity and serve as an excellent model for research of associated metabolic abnormalities (114). However, these rats may not be an ideal DR model, as retinal vascular damage is often absent even up to 42 weeks of chronic hyperglycemia (114).
Leprdb mice carry a mutation in the leptin receptor that results in defective leptin signaling. Homozygous animals become insensitive to leptin, leading to development of obesity and chronic hyperglycemia within the first 2 months of age (112,115). The pathophysiology of diabetes in Leprdb mouse model closely mimics that seen in T2DM, with late stages leading to atrophy of pancreatic β cells and dependence on exogenous insulin (112,115). In the retina of Leprdb mice, damage to retinal glia cells and increased central retinal membrane thickness is detected after 6 weeks of hyperglycemia (110,116). Characteristics of Leprdb mouse include thinning of the ONL seen by 8 weeks and thinning of the INL by 14 weeks of hyperglycemia (116,117). Acellular capillaries and retinal NV present by 18 weeks (118). Limitations of the Leprdb mouse model are related to the rapid continuation of severe disease with increasing blood sugar levels and worsening of cardiovascular disease that shortens lifespan to about 10 months (119,120). The primary advantage of the Leprdb mouse is it provides an exceptional model for screening of late stage DR treatments designed to alleviate the DR symptoms of reactive gliosis and severe vascular damage.
The OLETF rat model spontaneously develops T2DM via a mutation in G-protein-coupled receptor 10 (GPR10) that leads to obesity (72,73). Onset of diabetes is present by 6 months, with abrupt increases in blood glucose (73). Microvasculature changes manifest approximately 6 weeks following diabetes onset with inflammation throughout retinal microcirculation (34). Vascular leakage is noted by 3 months of hyperglycemia, due to damaged endothelial cells and pericyte loss (121,122). Limitations of this model are delayed onset diabetes and absence of a common feature of DR pathology, acellular capillaries.
Another rodent model for studying T2DM is the WBN/Kob rat model that spontaneously develops hyperglycemia via an unknown mechanism regulated by sex hormones (7,123). The causal genes have not been elucidated, but reports suggest regions of significance on chromosome 7 in WBN/Kob locus 1 (pdwk1) (124,125). Retinal disease in this model is marked by intraretinal angiopathy consisting of NV and hyalinization. Although the genotype is unknown, the phenotype elicited is this model still provides a viable research model for understanding DR progression.
Additional rat models are those that develop nonobese T2DM, the SDT and the GK models. Insulin insensitivity is the primary mechanism by which T2DM develops, with atrophy of pancreatic β-cells in late disease. Out of these two rat models, the SDT rat more closely resembles the pathophysiology seen in humans. The genes associated with the SDT model are correlated to three primary genes: Gisdt1, Gisdt2, and Gisdt3, on chromosome 1, 2 and X, respectively; all are related to glucose intolerance (76,126,127). SDT rats develop glycosuria between 20–45 weeks depending on gender (126). Diabetes manifests in male rats by 40 weeks with complete conversion at 65 weeks; however, only 33% of female rats go on to develop disease (126).
SDT rats exhibit retinal disease that features NV without underlying ischemia, as well as apoptosis in both the ganglion cell layer (GCL) and the INL of the retina. Other ocular features of SDT rat include retinal detachment and fibrous remodeling (128), and large retinal folds with extensive vascular leakage surrounds the optic disk, which closely resembles human disease (129-131). A transgenic hybrid, SDT(fa), generated by introduction of the fatty (fa) allele from the ZDF rat model into SDT rats, displays a more rapid onset of DR (132). One limitation of the SDT model is the lack of MA or NV; therefore, the SDT model is better suited for NPDR research. Similar to the Ins2Akita mouse model, SDT model possess a prominent gender bias in onset of disease that is a major consideration for study designs. The major benefit of the SDT rat model is the development of TRD in the late stages of DR.
The GK rat model develops diabetes earlier than SDT model with presentation of hyperglycemia within 4 weeks and DM by 6 weeks (133,134). There is a polygenic background contributing to the GK disease phenotype with reports of 192 potential genes (135). Disease manifestations of GK are defined by reduced retinal blood flow with absence of diameter variation of veins and arteries (7,134). The GK rat model exhibits microcirculatory changes in the retina and loss of pericytes that leads to BRB permeability increase at 3 months following disease onset. This is a good model to study circulatory changes in DR, but it does not show a clear genotype-to-phenotype linkage.
Non-diabetic/hybrid pathology models
Several genetic murine models have been developed with the aim of generating models physiologically similar to human retinal disease. An example is the non-diabetic Kimba mouse, that is genetically modified to overexpress retinal VEGF; driven by mutations at the rhodopsin promoter loci (67,136). The Kimba mouse model manifests retinal disease within the first week of life with markedly reduced ONL and INL. Abnormalities of retinal microvascular are noted by 1 month and pericyte loss by 2 months (136). An additional mouse line, Akimba, was generated by cross breeding Ins2Akita mice and Kimba mice (68). The hybrid Akimba mouse, as expected, possesses characteristics from both parental strains that includes hyperglycemia with significant edema and NV (68). A limitation of the Kimba mouse model is early disease development and rapid progression that is present at birth and is focused on the late stage of disease without hyperglycemic etiology. The research benefits of Kimba mice are that DR pathogenesis is controlled by VEGF production, providing an efficient model for treatment development and research focused on NV. Akimba mice are an excellent model for DR research with few limitations common to all animal models. The characteristics from the parental strains are additive and as a result significantly bias retinal disease progression (68).
Environmental exposure rodent models
Oxygen-induced retinopathy (OIR)
Anomalous NV in the posterior segment of the eye is the hallmark of late stage DR and the distinguishing feature of PDR from NPDR (8,23). The pathogenesis of PDR is driven by chronic hyperglycemia that ultimately leads to vascular damage, and subsequent retinal ischemia that promotes NV. Cycling of hyperoxia and hypoxia produces OIR that physiologically mimics ischemic events and induce compensatory NV. Rodent models of OIR are used to study vascular changes in retinal diseases such as DR (137-139). In OIR models, after exposure to cycling hyperoxia, rodents exhibit two phases of vascular retinal disease. Initially, vasculature regresses in hyperoxic conditions; ensuing ischemia leads to compensatory hypoxia-induced NV (59,137). This hypoxia results in release of angiogenic factors such as VEGF that stimulate angiogenesis in avascular areas to reproduce DR vascular disease (58,140).
Mouse OIR model
The mouse OIR model was first reported by Smith et al. in 1994 (58). Commonly used to model retinopathy of prematurity (ROP) but pathologically includes similar features of DR. In it, OIR is generated by exposing neonatal mice to an environment of 75% oxygen at 7 days of age for a duration of at least 5 days, followed by a return to room air for 5 days. The retinopathy begins under hyperoxia with regression of retinal blood vessels from the central zone resulting in vaso-obliteration (138). Exposure to normoxic conditions leads to ischemia and release of hypoxic-inducible factors, such as erythropoietin and VEGF that trigger angiogenesis (58,60,140). Gradual vascularization of the vaso-obliterated areas mimics human proliferative retinopathies such as DR and ROP (58,141). Disease features of the OIR mouse model include reduction in INL and IPL thickness and degeneration of BRB with increased vascular leakage. New blood vessels are formed distinctly and termed “endothelial tufts” that may protrude into the vitreous (58,141). The OIR mouse model provides an excellent example to study PDR, because the pathologic angiogenesis resembles human disease. Studies using the OIR mouse model demonstrate that the inhibition of VEGF signaling via soluble VEGF receptor 1 antibody reduces pathologic retinal NV (63). Major limitation of this model are the absence of chronic hyperglycemia, thus limiting the studies to microvasculature changes seen in PDR and retinal NV which is transient and spontaneously regress, unlike PDR.
Canine DR models
Surgical procedures were the primary method used to induce diabetes in early animal models. Historically insulin was first isolated from pancreas of canines for treatment of “Juvenile Diabetes” (49), an immune mediated T1DM. Canines develop diabetes shortly after pancreatectomy and provided one of the earliest animal models to study diabetes (7,29). In addition to pancreatectomy, other methodologies used to generate canine DR models include high-galactose diet induction, alloxan/STZ administration, and growth hormone regimens (7). Canines on long-term high galactose diets develop DR, with severe stages of disease manifesting by 5 years (37,142,143). Morphology of DR in canines closely resembles disease seen in humans (144), and includes many of the classical features such as MAs, pericyte loss, thickened capillary basement membrane, and dot and blot hemorrhages (145). Pathologic vascularization is often absent, limiting this model to NPDR studies. The high cost of canine care and the ethical unpopularity of using canines in research have impeded the use of canine models for DR research studies.
Feline models
Felines can develop DM and display the same pathological characteristics as human T1DM and T2DM (144,146). Pancreatectomy is often used to induce glucose intolerance. However, the time to feline DR phenotype ranges from 5 to 9 years of chronic hyperglycemia (146,147). Felines can display retinal changes including CWS, vascular leakage, and NV (147,148). MAs in felines have been reported in some studies and absent in others (148). Alloxan and STZ are also effective modalities in developing DR in felines and often used as complements to partial pancreatectomy (149). The feline OIR model was first described in the 1950s, as one of the earliest models exploring the effects of oxygen concentration on retinal disease (41). Similar to the rodent OIR model, kittens were exposed to hyperoxic conditions (80% oxygen) for 4–5 days, which induced retinal vessel regression and vaso-obliteration. When kittens are returned to room air (21% oxygen), hypoxia-induced angiogenesis develops (41,42). The feline OIR model exhibits similar disease features to mouse OIR model. However, felines do not display retinal detachment. There are multiple limitations with using feline models, including long incubations required for DR development and unavailability of research reagents. These limitations make this model difficult to use. However, felines exhibit a moderate resistance to DR and may offer a novel platform to investigate severe stages of disease in future studies.
Swine models
Swines have found utility in many disciplines of disease research. The human eye and the swine eye share similarity in size, retinal structure, and vasculature; these are appealing characteristics for modeling DR (150). The majority of swine eye research is not performed with in vivo models, but rather using in vitro experiments with swine retinal cells, specifically Müller cells (151,152). Swine in vivo DR models can be pharmacologically induced by administration of alloxan or STZ (151), and biologically induced with intravitreal injections of retinal pigment epithelial (RPE) cells (153). Alloxan-induced DR in swine exhibit retinal basement membrane thickening, pericyte destruction, and vascular edema within 4–6 months of hyperglycemia (154). Transgenic swine models also demonstrate signs of DR. Swine carrying a mutated human HNF-1α (P291fsinsC) developed chronic hyperglycemia (>200 mg/dL) by 1 month of age. NPDR features such as retinal hemorrhage and CWS were detected by 4 months of hyperglycemia, yet PDR was not observed in this model. This limited study requires further investigation (155). Development of swine DR models may provide an excellent means for therapeutic development that may overshadow the cost limitations and scarcity of research reagents.
Rabbit models
Rabbit models of DR have been generated by pharmacologic agents, altered diet, or direct implantations of VEGF (inherently non-diabetic) into the retina. Each has its own drawbacks. Pharmacologic induction is the most common method to induce DR in rabbits. Typical agent use is STZ (100 mg/kg), which abruptly increases blood glucose levels (>200 mg/dL) (156). DR lesions present with variable distribution within 5 months of STZ administration. Approximately 50% of rabbits develop PDR. Interestingly, 40% only show MAs, DBH, and thrombosis. The remaining 10% exhibit only hard exudates and moderate microangiopathy (35,156).
Diet-induced DR in rabbits is achieved with high-caloric-hyperlipidemic diet (157). However, minimal DR development and long incubation time make this approach less attractive.
Direct implantation of VEGF is a viable method to induce DR changes in rabbits (158-160). Rabbits develop PDR with NV within 3 weeks of VEGF polymeric implant (160). However, regression of NV occurs 5 weeks after implantation leaving a short window for study. The use of human recombinant basic fibroblast growth factor (bFGF) in a polymeric implant yields more efficient DR development within 1–2 weeks of implantations and DR features are hemorrhage, NV, and TRD (158).
The rabbit model provides an excellent means to screen therapeutic compounds, as the DR phenotype can be achieved in a short time, and the resultant retinal pathology is similar to human disease. The limitations of this model are high cost and variation of DR among rabbit species (159).
Non-human primate models
DM may be elicited in non-human primates by chemical or surgical induction (44,161,162). Despite the presence of long-term hyperglycemia in these models, non-human primates are particularly resistant to PDR (44). After administration of Alloxan or STZ, a long duration of hyperglycemia is required before early sings of DR are detected (44,161). This resilience suggests that non-human primates possess means to minimize the effects of widely uncontrolled chronic hyperglycemia.
Cynomolgus macaque or obese rhesus monkeys display spontaneous T2DM, with retinal ischemia, MAs, CWS, intra-retinal hemorrhages and hard exudates in the macula without NV (43,161). One of the major limitations of non-human primates for drug discovery is the long housing duration before DR develops (43). Due to lack of NV, non-human primates are limited to NPDR studies (7). The major benefit that non-human primate models provide is the presence of a macula that is not found in other animal models. Future directions for developing DR models of non-human primate require a better understanding of biological mechanisms that play a role in NV during chronic hyperglycemia.
Zebrafish models
A unique DR animal model that surprisingly shares similar eye structure to the human eye is zebrafish. DR can be generated in adult zebrafish with hypoxia induction (163,164). Transgenic fluorescent zebrafish Tg(fli1:EGFP)y that are exposed to hypoxic conditions with ~10% air for 12 days develop new blood vessels that are readily identifiable with GFP imaging (165). Genetic mutation of the von Hippel-Lindau tumor suppressor gene (vhl1) also results in a DR-like angiogenesis (166). Zebrafish carrying this vhl1 mutation have pronounced retinal vascular formation and upregulation of VEGF. DR features in this model include increased number of hyaloid vasculatures with simultaneous vascular leakage, retinal edema, severe NV and ensuing retinal detachment (166). A major advantage of using zebrafish is its ease of genetic manipulation, in addition to its low cost and quick breeding rate. Zebrafish serve as a viable DR model that requires further characterization and can expedite empiric drug screenings exponentially (45).
Conclusions
The prevalence of diabetes and its complications continue to remain a major public health concern in the United States. The utilization of animal models serves a vital role by furthering the understanding of DR pathophysiology, progression, and etiology. Future advancement of DR animal models is required as DR is a multifaceted disease including vascular and neurologic components that are influenced by both genetics and environmental exposures. While no single animal model encompassing the entire DR pathogenesis exists, we find promise in furthering comprehension of genetic models and development of new high-order animal models. To this end, development of ocular therapeutics for DR treatments holds a favorable future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by an unrestricted Grant from Research to Prevent Blindness (RPB) to the Department of Ophthalmology & Visual Sciences; startup funding from the Department of Ophthalmology & Visual Sciences and the College of Medicine.EY026556 (AY) and NIGMS MSTP Training Grant T32GM007288 (JQ).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Susanna S. Park) for the series “Novel Tools and Therapies for Ocular Regeneration” published in Annals of Translational Medicine. The article has undergone external peer review.
Peer Review File: Available at https://dx.doi.org/10.21037/atm-20-6737
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-20-6737). The series “Novel Tools and Therapies for Ocular Regeneration” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Guthrie RA, Guthrie DW. Pathophysiology of diabetes mellitus. Crit Care Nurs Q 2004;27:113-25. 10.1097/00002727-200404000-00003 [DOI] [PubMed] [Google Scholar]
- 2.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007;14:179-83. 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes 2015;6:489-99. 10.4239/wjd.v6.i3.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha-Vaz J. Characterization and relevance of different diabetic retinopathy phenotypes. Dev Ophthalmol 2007;39:13-30. 10.1159/000098497 [DOI] [PubMed] [Google Scholar]
- 6.Liu CH, Wang Z, Sun Y, et al. Animal models of ocular angiogenesis: from development to pathologies. Faseb J 2017;31:4665-81. 10.1096/fj.201700336R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson R, Barathi VA, Chaurasia SS, et al. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech 2012;5:444-56. 10.1242/dmm.009597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124-36. 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 9.Cheloni R, Gandolfi SA, Signorelli C, et al. Global prevalence of diabetic retinopathy: protocol for a systematic review and meta-analysis. BMJ Open 2019;9:e022188. 10.1136/bmjopen-2018-022188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006;26:699-700. 10.1097/01.iae.0000225351.87205.69 [DOI] [PubMed] [Google Scholar]
- 11.Oshima Y, Sakaguchi H, Gomi F, et al. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2006;142:155-8. 10.1016/j.ajo.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Chen HX, Gore-Langton RE, Cheson BD. Clinical trials referral resource: Current clinical trials of the anti-VEGF monoclonal antibody bevacizumab. Oncology (Williston Park) 2001;15:1017, 1020, 1023-6. [PubMed] [Google Scholar]
- 13.Lois N, McCarter RV, O’Neill C, et al. Endothelial progenitor cells in diabetic retinopathy. Front Endocrinol (Lausanne) 2014;5:44. 10.3389/fendo.2014.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simó R, Hernández C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 2015;48:160-80. 10.1016/j.preteyeres.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Choi MY, Jee D, Kwon JW. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS One 2019:14:e0222364. 10.1371/journal.pone.0222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol 2016;9:1427-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simó-Servat O, Hernández C, Simó R. Diabetic retinopathy in the context of patients with diabetes. Ophthalmic Res 2019;62:211-7. 10.1159/000499541 [DOI] [PubMed] [Google Scholar]
- 18.Cabrera AP, Mankad RN, Marek L, et al. Genotypes and phenotypes: a search for influential genes in diabetic retinopathy. Int J Mol Sci 2020;21:2712. 10.3390/ijms21082712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang H, Luo S, Huang G, et al. Advances in knowledge of candidate genes acting at the beta-cell level in the pathogenesis of T1DM. Front Endocrinol (Lausanne) 2020;11:119. 10.3389/fendo.2020.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz M, Romppel M, Grande G. Built environment and health: a systematic review of studies in Germany. J Public Health (Oxf) 2018;40:8-15. [DOI] [PubMed] [Google Scholar]
- 21.Feng J, Glass TA, Curriero FC, et al. The built environment and obesity: a systematic review of the epidemiologic evidence. Health Place 2010;16:175-90. 10.1016/j.healthplace.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 22.Gary-Webb TL, Suglia SF, Tehranifar P. Social epidemiology of diabetes and associated conditions. Curr Diab Rep 2013;13:850-9. 10.1007/s11892-013-0427-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares AM, Althoff K, Chen GF, et al. Animal models of diabetic retinopathy. Curr Diab Rep 2017;17:93. 10.1007/s11892-017-0913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014;157:1262-78. 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sledz CA, Williams BR. RNA interference in biology and disease. Blood 2005;106:787-94. 10.1182/blood-2004-12-4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel DD, Lipinski DM. Validating a low-cost laser speckle contrast imaging system as a quantitative tool for assessing retinal vascular function. Sci Rep 2020;10:7177. 10.1038/s41598-020-64204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preguiça I, Alves A, Nunes S, et al. Diet-induced rodent models of diabetic peripheral neuropathy, retinopathy and nephropathy. Nutrients 2020;12:250. 10.3390/nu12010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagami Y, Hatano E, Chayama Y, et al. An anti-PLVAP antibody suppresses laser-induced choroidal neovascularization in monkeys. Eur J Pharmacol 2019;854:240-6. 10.1016/j.ejphar.2019.04.035 [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Singh R, Vasudeva N, et al. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc Diabetol 2012;11:9. 10.1186/1475-2840-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makino S, Kunimoto K, Muraoka Y, et al. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 1980;29:1-13. 10.1538/expanim1978.29.1_1 [DOI] [PubMed] [Google Scholar]
- 31.Thayer TC, Wilson SB, Mathews CE. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:541-61. 10.1016/j.ecl.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun 2016;66:76-88. 10.1016/j.jaut.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 1966;153:1127-8. 10.1126/science.153.3740.1127 [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto K, Hiroshiba N, Tsujikawa A, et al. In vivo demonstration of increased leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci 1998;39:2190-4. [PubMed] [Google Scholar]
- 35.Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res 2013;2013:106594. 10.1155/2013/106594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engerman RL, Bloodworth JM, Jr. Experimental diabetic retinopathy in dogs. Arch Ophthalmol 1965;73:205-10. 10.1001/archopht.1965.00970030207013 [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Kubo E, Takahashi Y, et al. Retinal vessel changes in galactose-fed dogs. Arch Ophthalmol 1998;116:785-9. 10.1001/archopht.116.6.785 [DOI] [PubMed] [Google Scholar]
- 38.Kador PF, Takahashi Y, Wyman M, et al. Diabeteslike proliferative retinal changes in galactose-fed dogs. Arch Ophthalmol 1995;113:352-4. 10.1001/archopht.1995.01100030108031 [DOI] [PubMed] [Google Scholar]
- 39.Miller EJ, Brines CM. Canine diabetes mellitus associated ocular disease. Top Companion Anim Med 2018;33:29-34. 10.1053/j.tcam.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 40.McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 1996;37:300-11. [PubMed] [Google Scholar]
- 41.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol 1954;38:397-432. 10.1136/bjo.38.7.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps DL, Rosenbaum AL. Effects of marginal hypoxemia on recovery from oxygen-induced retinopathy in the kitten model. Pediatrics 1984;73:1-6. [PubMed] [Google Scholar]
- 43.Shimazawa M, Hara H. Establishment of retinal disease models using non-human primates and its strategy for drug discovery. Nihon Yakurigaku Zasshi 2018;152:139-46. 10.1254/fpj.152.139 [DOI] [PubMed] [Google Scholar]
- 44.Tso MO, Kurosawa A, Benhamou E, et al. Microangiopathic retinopathy in experimental diabetic monkeys. Trans Am Ophthalmol Soc 1988;86:389-421. [PMC free article] [PubMed] [Google Scholar]
- 45.Jo DH, Cho CS, Kim JH, et al. Animal models of diabetic retinopathy: doors to investigate pathogenesis and potential therapeutics. J Biomed Sci 2013;20:38. 10.1186/1423-0127-20-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldsmith JR, Jobin C. Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol 2012;2012:817341. 10.1155/2012/817341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coomer CE, Morris AC. Capn5 expression in the healthy and regenerating zebrafish retina. Invest Ophthalmol Vis Sci 2018;59:3643-54. 10.1167/iovs.18-24278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali Z, Zang J, Lagali N, et al. Photoreceptor degeneration accompanies vascular changes in a zebrafish model of diabetic retinopathy. Invest Ophthalmol Vis Sci 2020:61:43. 10.1167/iovs.61.2.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banting FG, Best CH. The internal secretion of the pancreas. 1922. Indian J Med Res 2007;125:251-66. [PubMed] [Google Scholar]
- 50.McLetchie NG. Alloxan diabetes: a discovery, albeit a minor one. J R Coll Physicians Edinb 2002;32:134-42. [PubMed] [Google Scholar]
- 51.Dixon KC, King AJ, Malinin T. Protein in dying beta-cells of the pancreatic islets. Q J Exp Physiol Cogn Med Sci 1960;45:202-12. 10.1113/expphysiol.1960.sp001458 [DOI] [PubMed] [Google Scholar]
- 52.Rakieten N, Rakieten ML, Nadkarni MV. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep 1963;29:91-8. [PubMed] [Google Scholar]
- 53.Eleazu CO, Eleazu KC, Chukwuma S, et al. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord 2013:12:60. 10.1186/2251-6581-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engerman RL, Kern TS. Experimental galactosemia produces diabetic-like retinopathy. Diabetes 1984;33:97-100. 10.2337/diab.33.1.97 [DOI] [PubMed] [Google Scholar]
- 55.Joussen AM, Doehmen S, Le ML, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis 2009;15:1418-28. [PMC free article] [PubMed] [Google Scholar]
- 56.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995;15:4738-47. 10.1523/JNEUROSCI.15-07-04738.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res 2010;29:500-19. 10.1016/j.preteyeres.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101-11. [PubMed] [Google Scholar]
- 59.Lange C, Ehlken C, Stahl A, et al. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefes Arch Clin Exp Ophthalmol 2009;247:1205-11. 10.1007/s00417-009-1116-4 [DOI] [PubMed] [Google Scholar]
- 60.Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 1995;92:905-9. 10.1073/pnas.92.3.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Connor KM, Aderman CM, et al. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci 2009;50:1329-35. 10.1167/iovs.08-2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 2009;4:1565-73. 10.1038/nprot.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A 1995;92:10457-61. 10.1073/pnas.92.23.10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izumi T, Yokota-Hashimoto H, Zhao S, et al. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 2003;52:409-16. 10.2337/diabetes.52.2.409 [DOI] [PubMed] [Google Scholar]
- 65.Serreze DV, Chapman HD, Varnum DS, et al. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol 1997;158:3978-86. [PubMed] [Google Scholar]
- 66.Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999;103:27-37. 10.1172/JCI4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997;151:281-91. [PMC free article] [PubMed] [Google Scholar]
- 68.Rakoczy EP, Ali Rahman IS, Binz N, et al. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am J Pathol 2010;177:2659-70. 10.2353/ajpath.2010.090883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70:5.47.1-5.47.20. [DOI] [PubMed]
- 70.Schmidt RE, Dorsey DA, Beaudet LN, et al. Analysis of the Zucker Diabetic Fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol 2003;163:21-8. 10.1016/S0002-9440(10)63626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokoi N, Hoshino M, Hidaka S, et al. A novel rat model of type 2 diabetes: the Zucker fatty diabetes mellitus ZFDM rat. J Diabetes Res 2013;2013:103731. 10.1155/2013/103731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe TK, Suzuki M, Yamasaki Y, et al. Mutated G-protein-coupled receptor GPR10 is responsible for the hyperphagia/dyslipidaemia/obesity locus of Dmo1 in the OLETF rat. Clin Exp Pharmacol Physiol 2005;32:355-66. 10.1111/j.1440-1681.2005.04196.x [DOI] [PubMed] [Google Scholar]
- 73.Lu ZY, Bhutto IA, Amemiya T. Retinal changes in Otsuka long-evans Tokushima Fatty rats (spontaneously diabetic rat)--possibility of a new experimental model for diabetic retinopathy. Jpn J Ophthalmol 2003;47:28-35. 10.1016/S0021-5155(02)00631-7 [DOI] [PubMed] [Google Scholar]
- 74.Miyamura N, Amemiya T. Lens and retinal changes in the WBN/Kob rat (spontaneously diabetic strain). Electron-microscopic study. Ophthalmic Res 1998;30:221-32. 10.1159/000055479 [DOI] [PubMed] [Google Scholar]
- 75.Sima AA, Chakrabarti S, Garcia-Salinas R, et al. The BB-rat--an authentic model of human diabetic retinopathy. Curr Eye Res 1985;4:1087-92. 10.3109/02713688509003353 [DOI] [PubMed] [Google Scholar]
- 76.Yamada H, Yamada E, Higuchi A, et al. Retinal neovascularisation without ischaemia in the spontaneously diabetic Torii rat. Diabetologia 2005;48:1663-68. 10.1007/s00125-005-1809-0 [DOI] [PubMed] [Google Scholar]
- 77.Schröder S, Palinski W, Schmid-Schönbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 1991;139:81-100. [PMC free article] [PubMed] [Google Scholar]
- 78.Yazdanyar A, Zhang P, Dolf C, et al. Effects of intravitreal injection of human CD34+ bone marrow stem cells in a murine model of diabetic retinopathy. Exp Eye Res 2020;190:107865. 10.1016/j.exer.2019.107865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar S, Zhuo L. Longitudinal in vivo imaging of retinal gliosis in a diabetic mouse model. Exp Eye Res 2010;91:530-6. 10.1016/j.exer.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 80.Martin PM, Roon P, van Ells TK, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 2004;45:3330-6. 10.1167/iovs.04-0247 [DOI] [PubMed] [Google Scholar]
- 81.Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci 2005;46:4281-7. 10.1167/iovs.04-1361 [DOI] [PubMed] [Google Scholar]
- 82.Su L, Ji J, Bian J, et al. Tacrolimus (FK506) prevents early retinal neovascularization in streptozotocin-induced diabetic mice. Int Immunopharmacol 2012;14:606-12. 10.1016/j.intimp.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Wu Y, Jin Y, et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 2008;49:732-42. 10.1167/iovs.07-0721 [DOI] [PubMed] [Google Scholar]
- 84.Surwit RS, Kuhn CM, Cochrane C, et al. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988;37:1163-7. 10.2337/diab.37.9.1163 [DOI] [PubMed] [Google Scholar]
- 85.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J 2004;18:1450-52. 10.1096/fj.03-1476fje [DOI] [PubMed] [Google Scholar]
- 86.Robison WG, Jr, Jacot JL, Glover JP, et al. Diabetic-like retinopathy: early and late intervention therapies in galactose-fed rats. Invest Ophthalmol Vis Sci 1998;39:1933-41. [PubMed] [Google Scholar]
- 87.Simó-Servat O, Hernández C, Simó R. Genetics in diabetic retinopathy: current concepts and new insights. Curr Genomics 2013;14:289-99. 10.2174/13892029113149990008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 2005:46:2210-8. 10.1167/iovs.04-1340 [DOI] [PubMed] [Google Scholar]
- 89.Han Z, Guo J, Conley SM, et al. Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci 2013;54:574-84. 10.1167/iovs.12-10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLenachan S, Chen X, McMenamin PG, et al. Absence of clinical correlates of diabetic retinopathy in the Ins2Akita retina. Clin Exp Ophthalmol 2013;41:582-92. 10.1111/ceo.12084 [DOI] [PubMed] [Google Scholar]
- 91.Shen X, Zhong Y, Xie B, et al. Pigment epithelium derived factor as an anti-inflammatory factor against decrease of glutamine synthetase expression in retinal Müller cells under high glucose conditions. Graefes Arch Clin Exp Ophthalmol 2010;248:1127-36. 10.1007/s00417-010-1362-5 [DOI] [PubMed] [Google Scholar]
- 92.Xie B, Jiao Q, Cheng Y, et al. Effect of pigment epithelium-derived factor on glutamate uptake in retinal Muller cells under high-glucose conditions. Invest Ophthalmol Vis Sci 2012;53:1023-32. 10.1167/iovs.11-8695 [DOI] [PubMed] [Google Scholar]
- 93.Johansen K. The spectrum of plasma insulin responses in middle-aged and old non-obese diabetics with various degrees of metabolic derangement. Acta Med Scand 1973;194:157-64. 10.1111/j.0954-6820.1973.tb19424.x [DOI] [PubMed] [Google Scholar]
- 94.Hanafusa T, Miyagawa J, Nakajima H, et al. The NOD mouse. Diabetes Res Clin Pract 1994;24 Suppl:S307-11. 10.1016/0168-8227(94)90267-4 [DOI] [PubMed] [Google Scholar]
- 95.Baxter AG, Cooke A. The genetics of the NOD mouse. Diabetes Metab Rev 1995;11:315-35. 10.1002/dmr.5610110403 [DOI] [PubMed] [Google Scholar]
- 96.Leiter EH, Prochazka M, Coleman DL. The non-obese diabetic (NOD) mouse. Am J Pathol 1987;128:380-3. [PMC free article] [PubMed] [Google Scholar]
- 97.Simpson PB, Mistry MS, Maki RA, et al. Cuttine edge: diabetes-associated quantitative trait locus, Idd4, is responsible for the IL-12p40 overexpression defect in nonobese diabetic (NOD) mice. J Immunol 2003;171:3333-7. 10.4049/jimmunol.171.7.3333 [DOI] [PubMed] [Google Scholar]
- 98.Li CR, Sun SG. VEGF expression and cell apoptosis in NOD mouse retina. Int J Ophthalmol 2010;3:224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shaw SG, Boden JP, Biecker E, et al. Endothelin antagonism prevents diabetic retinopathy in NOD mice: a potential role of the angiogenic factor adrenomedullin. Exp Biol Med (Maywood) 2006;231:1101-5. [PubMed] [Google Scholar]
- 100.Lee S, Harris NR. Losartan and ozagrel reverse retinal arteriolar constriction in non-obese diabetic mice. Microcirculation 2008;15:379-87. 10.1080/10739680701829802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mugisho OO, Rupenthal ID, Squirrell DM, et al. Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: modelling signs of diabetic retinopathy. PLoS One 2018;13:e0202156. 10.1371/journal.pone.0202156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mugisho OO, Green CR, Squirrell DM, et al. Connexin43 hemichannel block protects against the development of diabetic retinopathy signs in a mouse model of the disease. J Mol Med (Berl) 2019;97:215-29. 10.1007/s00109-018-1727-5 [DOI] [PubMed] [Google Scholar]
- 103.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400-12. 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niclauss N, Meier R, Bédat B, et al. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev 2016;31:146-62. 10.1159/000439412 [DOI] [PubMed] [Google Scholar]
- 105.MacMurray AJ, Moralejo DH, Kwitek AE, et al. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res 2002;12:1029-39. 10.1101/gr.412702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rutledge EA, Fuller JM, van Yserloo B, et al. Sequence variation and expression of the Gimap gene family in the BB rat. Exp Diabetes Res 2009;2009:835650. 10.1155/2009/835650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallis RH, Wang K, Marandi L, et al. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes 2009;58:1007-17. 10.2337/db08-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malaisse WJ, Courtois P, Scott FW. Insulin-dependent diabetes and gut dysfunction: the BB rat model. Horm Metab Res 2004;36:585-94. 10.1055/s-2004-825920 [DOI] [PubMed] [Google Scholar]
- 109.Zhu W, Wu Y, Meng YF, et al. Association of obesity and risk of diabetic retinopathy in diabetes patients: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2018;97:e11807. 10.1097/MD.0000000000011807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tschöp M, Heiman ML. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 2001;109:307-19. 10.1055/s-2001-17297 [DOI] [PubMed] [Google Scholar]
- 111.Kinlen D, Cody D, O’Shea D. Complications of obesity. QJM 2018;111:437-43. 10.1093/qjmed/hcx152 [DOI] [PubMed] [Google Scholar]
- 112.Chua SC, Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 1996;271:994-6. 10.1126/science.271.5251.994 [DOI] [PubMed] [Google Scholar]
- 113.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 1978;14:141-8. 10.1007/BF00429772 [DOI] [PubMed] [Google Scholar]
- 114.Caolo V, Roblain Q, Lecomte J, et al. Resistance to retinopathy development in obese, diabetic and hypertensive ZSF1 rats: an exciting model to identify protective genes. Sci Rep 2018;8:11922. 10.1038/s41598-018-29812-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84:491-5. 10.1016/S0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 116.Tang L, Zhang Y, Jiang Y, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051-63. 10.1258/ebm.2011.010400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bogdanov P, Corraliza L, Villena JA, et al. The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One 2014;9:e97302. 10.1371/journal.pone.0097302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang MK, Park SH, Kim YH, et al. Dietary compound chrysin inhibits retinal neovascularization with abnormal capillaries in db/db mice. Nutrients 2016;8:782. 10.3390/nu8120782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Belke DD, Larsen TS, Gibbs EM, et al. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 2000;279:E1104-13. 10.1152/ajpendo.2000.279.5.E1104 [DOI] [PubMed] [Google Scholar]
- 120.Aasum E, Hafstad AD, Severson DL, et al. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 2003;52:434-41. 10.2337/diabetes.52.2.434 [DOI] [PubMed] [Google Scholar]
- 121.Miyamura N, Bhutto IA, Amemiya T. Retinal capillary changes in Otsuka Long-Evans Tokushima fatty rats (spontaneously diabetic strain). Electron-microscopic study. Ophthalmic Res 1999;31:358-66. 10.1159/000055559 [DOI] [PubMed] [Google Scholar]
- 122.Bhutto IA, Lu ZY, Takami Y, et al. Retinal and choroidal vasculature in rats with spontaneous diabetes type 2 treated with the angiotensin-converting enzyme inhibitor cilazapril: corrosion cast and electron-microscopic study. Ophthalmic Res 2002;34:220-31. 10.1159/000063877 [DOI] [PubMed] [Google Scholar]
- 123.Ohashi K, Kim JH, Hara H, et al. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol 1990;6:231-47. [PubMed] [Google Scholar]
- 124.Mori M, Fu X, Chen L, et al. Hereditary pancreatitis model WBN/Kob rat strain has a unique haplotype in the Pdwk1 region on chromosome 7. Exp Anim 2009;58:409-13. 10.1538/expanim.58.409 [DOI] [PubMed] [Google Scholar]
- 125.Tsuji A, Nishikawa T, Mori M, et al. Quantitative trait locus analysis for chronic pancreatitis and diabetes mellitus in the WBN/Kob rat. Genomics 2001;74:365-9. 10.1006/geno.2001.6557 [DOI] [PubMed] [Google Scholar]
- 126.Shinohara M, Masuyama T, Shoda T, et al. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int J Exp Diabetes Res 2000;1:89-100. 10.1155/EDR.2000.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Masuyama T, Fuse M, Yokoi N, et al. Genetic analysis for diabetes in a new rat model of nonobese type 2 diabetes, Spontaneously Diabetic Torii rat. Biochem Biophys Res Commun 2003;304:196-206. 10.1016/S0006-291X(03)00548-5 [DOI] [PubMed] [Google Scholar]
- 128.Sasase T, Morinaga H, Abe T, et al. Protein kinase C beta inhibitor prevents diabetic peripheral neuropathy, but not histopathological abnormalities of retina in Spontaneously Diabetic Torii rat. Diabetes Obes Metab 2009;11:1084-7. 10.1111/j.1463-1326.2009.01082.x [DOI] [PubMed] [Google Scholar]
- 129.Fukuda M, Nakanishi Y, Fuse M, et al. Altered expression of aquaporins 1 and 4 coincides with neurodegenerative events in retinas of spontaneously diabetic Torii rats. Exp Eye Res 2010;90:17-25. 10.1016/j.exer.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 130.Kakehashi A, Saito Y, Mori K, et al. Characteristics of diabetic retinopathy in SDT rats. Diabetes Metab Res Rev 2006;22:455-61. 10.1002/dmrr.638 [DOI] [PubMed] [Google Scholar]
- 131.Matsuoka M, Ogata N, Minamino K, et al. Leukostasis and pigment epithelium-derived factor in rat models of diabetic retinopathy. Mol Vis 2007;13:1058-65. [PMC free article] [PubMed] [Google Scholar]
- 132.Masuyama T, Katsuda Y, Shinohara M. A novel model of obesity-related diabetes: introgression of the Lepr(fa) allele of the Zucker fatty rat into nonobese Spontaneously Diabetic Torii (SDT) rats. Exp Anim 2005;54:13-20. 10.1538/expanim.54.13 [DOI] [PubMed] [Google Scholar]
- 133.Goto Y, Suzuki K, Ono T, et al. Development of diabetes in the non-obese NIDDM rat (GK rat). Adv Exp Med Biol 1988;246:29-31. 10.1007/978-1-4684-5616-5_4 [DOI] [PubMed] [Google Scholar]
- 134.Miyamoto K, Ogura Y, Nishiwaki H, et al. Evaluation of retinal microcirculatory alterations in the Goto-Kakizaki rat. A spontaneous model of non-insulin-dependent diabetes. Invest Ophthalmol Vis Sci 1996;37:898-905. [PubMed] [Google Scholar]
- 135.Liu T, Li H, Ding G, et al. Comparative genome of GK and Wister rats reveals genetic basis of type 2 diabetes. PLoS One 2015;10:e0141859. 10.1371/journal.pone.0141859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Eeden PE, Tee LB, Lukehurst S, et al. Early vascular and neuronal changes in a VEGF transgenic mouse model of retinal neovascularization. Invest Ophthalmol Vis Sci 2006;47:4638-45. 10.1167/iovs.06-0251 [DOI] [PubMed] [Google Scholar]
- 137.Dollery CT, Bulpitt CJ, Kohner EM. Oxygen supply to the retina from the retinal and choroidal circulations at normal and increased arterial oxygen tensions. Invest Ophthalmol 1969;8:588-94. [PubMed] [Google Scholar]
- 138.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res 1994;36:724-31. 10.1203/00006450-199412000-00007 [DOI] [PubMed] [Google Scholar]
- 139.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci 1993;34:576-85. [PubMed] [Google Scholar]
- 140.Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 2010;51:2813-26. 10.1167/iovs.10-5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Downie LE, Pianta MJ, Vingrys AJ, et al. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol 2007;504:404-17. 10.1002/cne.21449 [DOI] [PubMed] [Google Scholar]
- 142.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007;2007:95103. 10.1155/2007/95103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takahashi Y, Augustin W, Wyman M, et al. Quantitative analysis of retinal vessel changes in galactose-fed dogs. J Ocul Pharmacol 1993;9:257-69. 10.1089/jop.1993.9.257 [DOI] [PubMed] [Google Scholar]
- 144.Niaz K, Maqbool F, Khan F, et al. Comparative occurrence of diabetes in canine, feline, and few wild animals and their association with pancreatic diseases and ketoacidosis with therapeutic approach. Vet World 2018;11:410-22. 10.14202/vetworld.2018.410-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gardiner TA, Stitt AW, Anderson HR, et al. Selective loss of vascular smooth muscle cells in the retinal microcirculation of diabetic dogs. Br J Ophthalmol 1994;78:54-60. 10.1136/bjo.78.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hatchell DL, Toth CA, Barden CA, et al. Diabetic retinopathy in a cat. Exp Eye Res 1995;60:591-3. 10.1016/S0014-4835(05)80074-0 [DOI] [PubMed] [Google Scholar]
- 147.Mansour SZ, Hatchell DL, Chandler D, et al. Reduction of basement membrane thickening in diabetic cat retina by sulindac. Invest Ophthalmol Vis Sci 1990;31:457-63. [PubMed] [Google Scholar]
- 148.Linsenmeier RA, Braun RD, McRipley MA, et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 1998;39:1647-57. [PubMed] [Google Scholar]
- 149.Reiser HJ, Whitworth UG, Jr, Hatchell DL, Sutherland FS, et al. Experimental diabetes in cats induced by partial pancreatectomy alone or combined with local injection of alloxan. Lab Anim Sci 1987;37:449-52. [PubMed] [Google Scholar]
- 150.Sanchez I, Martin R, Ussa F, et al. The parameters of the porcine eyeball. Graefes Arch Clin Exp Ophthalmol 2011;249:475-82. 10.1007/s00417-011-1617-9 [DOI] [PubMed] [Google Scholar]
- 151.King JL, Mason JO, 3rd, Cartner SC, et al. The influence of alloxan-induced diabetes on Müller cell contraction-promoting activities in vitreous. Invest Ophthalmol Vis Sci 2011;52:7485-91. 10.1167/iovs.11-7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vision Res 2017;139:93-100. 10.1016/j.visres.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Umazume K, Barak Y, McDonald K, et al. Proliferative vitreoretinopathy in the Swine-a new model. Invest Ophthalmol Vis Sci 2012;53:4910-6. 10.1167/iovs.12-9768 [DOI] [PubMed] [Google Scholar]
- 154.Yang Y, Hayden MR, Sowers S, et al. Retinal redox stress and remodeling in cardiometabolic syndrome and diabetes. Oxid Med Cell Longev 2010;3:392-403. 10.4161/oxim.3.6.14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Umeyama K, Nakajima M, Yokoo T, et al. Diabetic phenotype of transgenic pigs introduced by dominant-negative mutant hepatocyte nuclear factor 1α. J Diabetes Complications 2017;31:796-803. 10.1016/j.jdiacomp.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 156.Drago F, La Manna C, Emmi I, et al. Effects of sulfinpyrazone on retinal damage induced by experimental diabetes mellitus in rabbits. Pharmacol Res 1998;38:97-100. 10.1006/phrs.1998.0339 [DOI] [PubMed] [Google Scholar]
- 157.Helfenstein T, Fonseca FA, Ihara SS, et al. Impaired glucose tolerance plus hyperlipidaemia induced by diet promotes retina microaneurysms in New Zealand rabbits. Int J Exp Pathol 2011;92:40-9. 10.1111/j.1365-2613.2010.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wong CG, Rich KA, Liaw LH, et al. Intravitreal VEGF and bFGF produce florid retinal neovascularization and hemorrhage in the rabbit. Curr Eye Res 2001;22:140-7. 10.1076/ceyr.22.2.140.5528 [DOI] [PubMed] [Google Scholar]
- 159.Erb MH, Sioulis CE, Kuppermann BD, et al. Differential retinal angiogenic response to sustained intravitreal release of VEGF and bFGF in different pigmented rabbit breeds. Curr Eye Res 2002;24:245-52. 10.1076/ceyr.24.4.245.8412 [DOI] [PubMed] [Google Scholar]
- 160.Ozaki H, Hayashi H, Vinores SA, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res 1997;64:505-17. 10.1006/exer.1996.0239 [DOI] [PubMed] [Google Scholar]
- 161.Kim SY, Johnson MA, McLeod DS, et al. Retinopathy in monkeys with spontaneous type 2 diabetes. Invest Ophthalmol Vis Sci 2004;45:4543-53. 10.1167/iovs.04-0519 [DOI] [PubMed] [Google Scholar]
- 162.Jonasson O, Jones CW, Bauman A, et al. The pathophysiology of experimental insulin-deficient diabetes in the monkey. Implications for pancreatic transplantation. Ann Surg 1985;201:27-39. [PMC free article] [PubMed] [Google Scholar]
- 163.Cao Z, Jensen LD, Rouhi P, et al. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc 2010;5:1903-10. 10.1038/nprot.2010.149 [DOI] [PubMed] [Google Scholar]
- 164.Cao R, Jensen LD, Söll I, et al. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS One 2008;3:e2748. 10.1371/journal.pone.0002748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Delov V, Muth-Köhne E, Schäfers C, et al. Transgenic fluorescent zebrafish Tg(fli1:EGFP)y1 for the identification of vasotoxicity within the zFET. Aquat Toxicol 2014;150:189-200. 10.1016/j.aquatox.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 166.van Rooijen E, Voest EE, Logister I, et al. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech 2010;3:343-53. 10.1242/dmm.004036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as