Abstract
Purpose
To assess the effects of Serenoa repens alone or in combination with other phytotherapy compared to placebo in men with LUTS due to benign prostatic enlargement.
Materials and Methods
Following a registered protocol (CRD42021226655), we searched (December 2020) MEDLINE, CENTRAL, Embase, ClinicalTrials.gov, WHO-ICTRP trials platform and other sources with no restrictions on language, publication date or status. We included randomized controlled trials, and we critically appraised them using the Cochrane Tool for Risk of Bias Assessment (RoB 2). We conducted random-effects meta-analysis when appropriate. The primary outcomes included urinary symptoms score, quality of life, and adverse events. The certainty of the evidence was rated using GRADE.
Results
We included 27 trials with 4,853 participants. S. repens results in little to no difference in urinary symptoms, quality of life, and adverse events at short- and long-term follow-up. S. repens combined with other phytotherapy may slightly reduce urinary symptoms at short-term follow-up, but the results are uncertain. The results on quality of life and adverse events are also very uncertain.
Conclusions
S. repens alone may result in no clinical benefits for men with LUTS. There is greater uncertainty in the effects of S. repens in combination with other phytotherapy.
Keywords: Lower urinary tract symptoms, Phytotherapy, Prostatic hyperplasia, Serenoa
INTRODUCTION
Benign prostatic enlargement (BPE) commonly presents with lower urinary tract symptoms (LUTS), which can be defined as a group of urinary symptoms triggered by an obstruction, abnormality, infection, or irritation of the urethra bladder neck, urinary sphincter or prostate. These symptoms may include voiding or obstructive symptoms such as hesitancy, poor or intermittent stream, straining, prolonged micturition, feeling of incomplete bladder emptying, dribbling, etc., and storage or irritative symptoms such as frequency, urgency, urge incontinence and nocturia [1]. The prevalence of benign prostatic hyperplasia (BPH) rises with age ranging from 8% to 80% in the 4th to 9th decades of life respectively [2]. The burden of disease attributable to LUTS has been increasing in the past years. From 1990 to 2017, the years lived with disability for males of all ages rose from 1.35 million in 1990 to 2.43 million in 2017 [3]. Diagnosis usually includes patient history, including a formal assessment of symptom severity (using the International Prostate Symptom Score [IPSS] score), physical exam and targeted laboratory testing to assess secondary causes of LUTS [4]. The IPSS questionnaire assesses storage symptoms, voiding symptoms, and an additional quality of life domain [5,6]. Urodynamic assessments and ultrasound may add additional prognostic information [4]. While treatment options may include watchful waiting for those with mild symptoms, medical and surgical therapies are available to those with moderate-to-severe LUTS. Pharmacological treatments for LUTS include: alphablockers (ABs) such as tamsulosin reduce smooth-muscle tone in the prostate and bladder neck, improving symptoms measured by IPSS scores [7,8] and are the most commonly prescribed medications [9]; 5-alpha reductase inhibitors (5-ARIs) such as finasteride reduce prostatic volume by inducing epithelial atrophy, improving symptoms measured by IPSS scores [10] and they are mostly reserved for patients with larger prostates, with a latency of onset of action [4]; and combination therapy (using AB+5-ARI or in combination with antimuscarinic drugs). Patients with larger prostates or severe symptoms may be candidates for surgical therapy, including transurethral resection of the prostate or other treatment modalities: laser enucleation of the prostate, convective radiofrequency water vapor therapy (Rezum), ablation (AquaBeam), prostatic urethral lift, prostatic arterial embolization, transurethral microwave thermotherapy, among others [11].
Phytotherapeutic agents are composed of extracts derived from the roots, seeds, bark or fruits of plants. In this review, we will be focusing on the effects due to the use of the extract of the berry of the American dwarf palm (saw palmetto, Serenoa repens) in BPE, which is the most popular and widely studied phytotherapeutic agent for the treatment of LUTS. While the purported mechanism of its relief of LUTS secondary to BPE is unknown, some of those proposed are hormonal effects by inhibiting the conversion of testosterone to dihydrotestosterone [12], producing an estrogenic and antiandrogenic effect [13,14]. It may also cause a dependent inhibition of 5-ARI in the stroma and epithelium of the prostate in vitro [15], anti-inflammatory effects [14] and the promotion of apoptosis [14,16,17]. Other mechanisms include the relaxation of smooth muscles of the detrusor and the prostate via α1-adrenergic receptors and placebo effect [18].
The most commonly used extracts are hexane, ethanolic, and supercritical CO2. In this context, the hexane extract of S. repens (commercially known as Permixon) has been shown to have higher biologic activity and the lowest variability from batch to batch in free fatty acid content [19,20], possibly suggesting a higher efficacy and fewer adverse events. S. repens is usually taken in a daily dose of 320 mg. The most frequently reported adverse events are minor gastrointestinal symptoms, genitourinary problems, musculoskeletal complaints, and upper respiratory tract infections [21].
Phytotherapies are widely used in men suffering from urinary symptoms attributable to prostatic conditions, especially S. repens in BPE. Despite being widely researched, reviewed and used, these interventions are not officially recommended as a standard treatment for LUTS [4,22]. The last high-quality Cochrane review was published in 2012 [23]. Therefore, it is important to synthesize the available evidence, including a recently published trial [24] using the innovations in methodological standards for systematic review production. We, therefore, aimed to assess the effects of S. repens alone or in combination with other phytotherapy compared to placebo in men with LUTS due to BPE.
MATERIALS AND METHODS
1. Inclusion criteria
We followed a predefined protocol covering the full detail of our methods [25] and registered it in PROSPERO (CRD42021226655). Due to the nature of this study, ethical approval was not sought. We included parallel, randomized controlled trials regardless of their publication status or the language of publication as they provide a higher certainty of the effectiveness of interventions. We did not include cross-over or cluster trials. We included trials in men aged 45 years and over with LUTS/BPE with a minimum IPSS score of 8. We excluded trials of men with a known neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease and men who have been treated with surgery for BPE already. We included studies in which only a subset of participants are relevant to this review if data was available separately for the relevant subset. We included studies that compared S. repens to placebo for the main comparison. For a secondary comparison, we included phytotherapeutic agents with S. repens as a component versus placebo.
2. Outcomes
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion. Our primary outcomes included: urinary symptoms, quality of life, and adverse events. Our secondary outcomes included: peak urinary flow (Qmax), acute urinary retention, and surgical interventions for LUTS. All outcomes were assessed for short-term (<12 months) and long-term (≥12 months). We used clinically important differences to rate the overall quality of the evidence in the ‘Summary of findings’ table [26,27]. We considered an improvement of the IPSS score of three points as a minimal clinically important difference (MCID) to assess the efficacy and comparative effectiveness [28]. We used different thresholds of MCID based on the severity of IPSS with a threshold of three for men with mild LUTS, five for moderate LUTS, and eight for severe LUTS [28]. We used an MCID of one to assess the efficacy and comparative effectiveness [29].
3. Search methods for identification of studies
We searched the following sources from the inception of each database to the date of search and did not place restrictions on the language of publication.
We will search the following databases and trials registers:
- Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO; https://www.cochranelibrary.com/) from inception to searched 11 December 2020
- MEDLINE (Ovid MEDLINE ALL 1946 to Daily Update) from inception to searched 11 December 2020
- Embase (https://www.elsevier.com/) from 1974 to searched 11 December 2020
- ClinicalTrials.gov (https://www.clinicaltrials.gov/) from inception to searched 11 December 2020
- World Health Organization International Clinical Trials Registry Platform (ICTRP; https://trialsearch.who.int) from inception to searched 11 December 2020
Details of the search strategies are in the Supplementary File.
4. Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta-analyses, and health technology assessment reports. We also contacted the study authors of included trials to identify any further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished trials. We searched for unpublished studies by hand, searching the abstract proceedings of the annual meetings of the American Urological Association, European Association of Urology, and International Continence Society for the last three years (2018–2020).
5. Selection of studies and data extraction
We used Covidence to identify and remove potential duplicate records [30]. Four review authors working in pairs (GAA, LFT, NS, and CF) independently scanned the abstract, title, or both, of remaining records retrieved to determine which studies should be assessed further through Covidence. Four review authors (GAA, LFT, NS, and CF) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, following the criteria for each provided in the Cochrane Handbook [31]. We resolved all discrepancies through consensus or recourse to a fifth review author (JVAF or JHJ). We presented a PRISMA flow diagram showing the process of study selection [32].
For studies that fulfilled the inclusion criteria, four review authors (GAA, LFT, NS, and CF) independently extracted information on study design, study dates, setting, and country, participant's characteristics, details of the intervention, comparison, and outcomes. We also collected data on funding sources and conflict of interest.
6. Assessment of risk of bias in included studies
We assessed the risk of bias in each study using a recently developed revision of the Cochrane ‘Risk of bias’ tool (RoB 2: a revised tool to assess the risk of bias in randomized trials) [33]. Four review authors (GAA, LFT, NS, and CF) independently assessed five domains of bias for each outcome considering the effect of assignment to the intervention. When the four authors disagreed, we decided on the final rating by consensus, with the involvement of a fifth author (JVAF). We used the RoB 2.0 Excel tool to manage the data supporting the answers to the signaling questions and risk of bias judgments (available at https://www.riskofbias.info/). The excel file with supporting judgments is available as supplementary material in the Open Science Framework platform [25].
7. Data synthesis
We calculated the mean difference (MD) with a 95% confidence interval (CI) for continuous outcomes and the risk ratio (RR) with 95% CI for dichotomous outcomes [34]. We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity in the meta-analysis [35,36]. We interpreted the I2 statistic following the guidance of the Cochrane Handbook [37]. When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics. Publication bias: when ten or more studies investigating a particular outcome were included, we used funnel plots to assess small study effects. We summarized data using a random-effects model. We used Review Manager 5 software to perform the analysis [38]. We intended to perform subgroup analysis by age, the severity of symptoms and type of preparation, but the heterogeneity across interventions was too low. We performed sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes: restricting the analysis by considering the risk of bias, by excluding studies at an overall ‘high risk’ of bias. We intended to explore heterogeneity considering the baseline severity of symptoms, age, and type of S. repens preparation, but the overall heterogeneity was low. Only for the primary analysis heterogeneity could be explained by a single study (see RESULTS).
8. Summary of findings table
We rated the overall quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, which takes into account criteria related only to internal validity: study limitations (overall risk of bias), inconsistency, imprecision, and publication bias; but also to external validity; indirectness of results [39]. For each comparison, each of the authors independently rated the quality of evidence for each outcome as high, moderate, low, or very low. We constructed “Summary of findings” tables and resolved every discrepancy that appeared by consensus or, when needed, via arbitration by other review authors using GRADEpro [40]. These tables provided key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and studies addressing each important outcome, and the rating of overall confidence in effect estimate for each outcome [41,42].
RESULTS
See Fig. 1 for the PRISMA flow diagram. We identified 6,684 records from databases and, after removing duplicates, we screened 5,520, of which we sought to retrieve 104. We could not retrieve 28 full-text articles, mostly older studies from the 80s and 90s, so 76 were assessed for eligibility. We excluded 49 studies for several reasons (see Supplementary File for a complete list of studies that were excluded, ongoing, or awaiting classification). We included 27 randomized controlled trials performed in an outpatient setting [24,43,44,45,46,47,48,49,50,51,52,53,54,56,57,58,59,60,61,62,63,64,65,66,67,68]. Most studies included men in their 60s with moderate LUTS and moderate-sizes prostate (see Table 1 for the characteristics of included studies). These studies included several types of formulation of S. repens which were divided into two comparisons: S. repens extract vs. placebo (main comparison, 19 studies, 3,630 randomized participants) and S. repens extract as a component of combined phytotherapy regimes vs. placebo (secondary comparison, 8 studies, 1,223 randomized participants). One of the studies included two arms of S. repens: in standard and high concentration [58]. We only included the standard dose to preserve comparability across studies. Two studies were funded by government agencies [44,46], ten studies were funded by the manufacturers of the product [24,50,51,54,58,59,61,63,65,66], and the other studies did not specify their funding sources.
Fig. 1. PRISMA flow diagram.
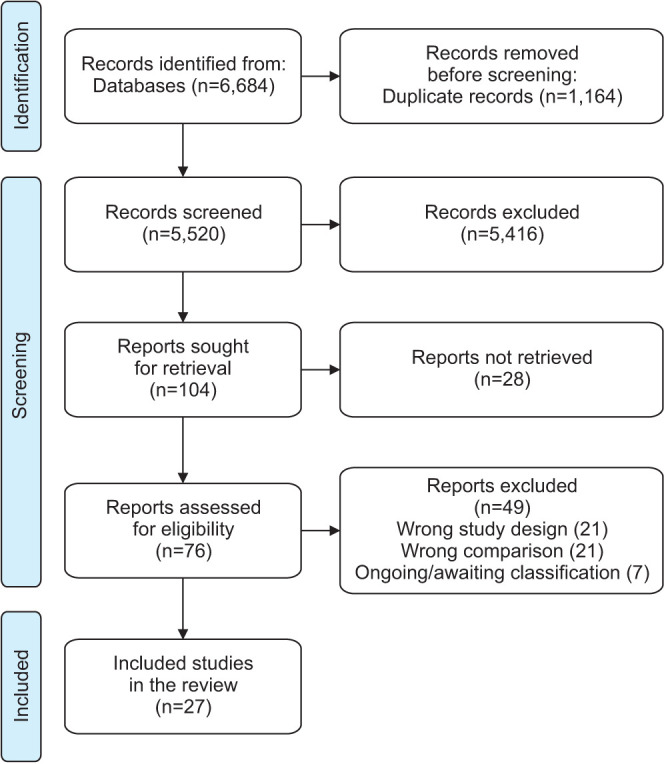
Table 1. Characteristics of the included studies.
| Study | Trial period | Country | n | Follow-up | Brand (when available) | Age (y) | IPSS | Prostate volume | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | ||||||
| Studies comparing Serenoa repens with placebo | |||||||||||
| Argirović et al. 2013 [43] | 2008–2010 | Serbia | 199 | 6 months | Prostamol Uno | 59.2±7.8 | 56.8±7.7 | 18.0±4.9 | 16.2±4.7 | 35.2±10.3 | 38.6±11.6 |
| Barry et al. 2011 [44] | 2008–2010 | USA | 369 | 72 weeks | Prosta-Urgenin Uno | 61.25±8.72 | 60.7±8.08 | 14.42±4.29 | 14.69±4.75 | N/A | N/A |
| Bauer et al. 1999 [45] | N/A | Germany | 101 | 6 months | Talso uno | N/A | N/A | N/A | N/A | N/A | N/A |
| Bent et al. 2006 [46] | 2001–2004 | USA | 225 | 14 months | Carbon dioxide extract | 62.9±8.0 | 63.0±7.4 | 15.7±5.7 | 15.0±5.3 | 34.7±13.9 | 33.9±15.2 |
| Boccafoschi and Annoscia 1983 [47] | N/A | Italy | 22 | 60 days | Permixon | 68 (55–80) | 68 (54–78) | N/A | N/A | N/A | N/A |
| Champault et al. 1984 [48] | N/A | France | 110 | 30 days | Permixon | N/A | N/A | N/A | N/A | N/A | N/A |
| Descotes et al. 1995 [49] | 1995 | France | 176 | 30 days | Permixon | 65.6±8.4 | 67±7.6 | N/A | N/A | N/A | N/A |
| BASTA 2006 [50] | N/A | International | 1,011 | 12 months | Permixon | N/A | N/A | N/A | N/A | N/A | N/A |
| Gerber et al. 2001 [51] | 1999–2000 | USA | 85 | 6 month | Serenoa repens | 64.6±9.9 | 65.3±9.7 | 16.7±4.9 | 15.8±4.8 | N/A | N/A |
| Glemain et al. 2002 [52] | N/A | France | 329 | 52 weeks | Permixon | 65.2±7.9 | 64.4±7.7 | 16.2±5.2 | 16.3±5.6 | 40.8±16.5 | 38.6±15 |
| Hizli and Uygur 2007 [53] | 2005 | Turkey | 60 | 6 months | Permixon | 60.2±6.3 | 58.9±5.7 | 15.6±3.2 | 16.2±4.7 | 31.2±4.2 | 38.6±11.6 |
| Hong et al. 2009 [68] | N/A | Korea | 62 | 12 months | Serenoa repens | 52.0 | 53.1 | 18.3 | 15.4 | 26.1 | 23.2 |
| Marks et al. 2000 [54] | 1997–1998 | USA | 44 | 6 months | Lipoidal extract | 65.1±8.1 | 62.9±9.3 | 18.1±7.2 | 16.6±5.3 | 58.5±29.8 | 55.6±26.7 |
| Reece Smith et al. 1986 [55] | N/A | UK | 70 | 12 weeks | Permixon | 66.15±5.86 | 67.03±6.03 | N/A | N/A | N/A | N/A |
| Ryu et al. 2015 [56] | 2012–2013 | Korea | 120 | 12 months | Permixon | 62.5±1.21 | 63.4±1.44 | 19.6±0.73 | 20±0.85 | 30.1±0.93 | 30.2±0.67 |
| Shi et al. 2008 [57] | N/A | China | 94 | 3 months | Prostataplex | 65.91 | 64.04 | 16.85 | 14.46 | 47.72 | 48.38 |
| Sudeep et al. 2020 [58] | N/A | India | 99 | 12 weeks | SPO | 57.76±7.25 | 55.18±8.56 | 20.00±4.41 | 20.00±3.74 | N/A | N/A |
| Willetts et al. 2003 [59] | 1999–2000 | Australia | 100 | 12 weeks | Carbon dioxide extract | 62.1±1.2 | 63.9±1.3 | N/A | N/A | N/A | N/A |
| Ye et al. 2019 [24] | 2014–2016 | China | 354 | 24 weeks | Serenoa repens | 61.47±5.20 | 60.32±5.96 | 14.42±3.88 | 14.34±4.08 | 37.0±19.7 | 37.3±25.4 |
| Studies comparing phytotherapy containing S. repens with placebo | |||||||||||
| Carbin et al. 1990 [60] | 1990 | Sweden | 55 | 3 month | Curbicin | 62.0±6.7 | 61.2±5.8 | N/A | N/A | N/A | N/A |
| Coulson et al. 2013 [61] | N/A | Australia | 60 | 3 months | ProstateEZE Max | 63±10.1 | 64.9±9.6 | 19.5 | 18 | N/A | N/A |
| Iacono et al. 2015 [62] | N/A | Italy | 185 | 6 months | +Tradamixina | N/A | N/A | 20.6±5.4 | N/A | N/A | N/A |
| Lopatkin et al. 2005 [63] | 1997–2000 | Russia | 257 | 24 weeks | PRO 160/120 | 67±7 | 68±6 | 17.4±3.3 | 17.8±3.3 | 43.5±17.6 | 44.8±17.6 |
| Metzker et al. 1996 [64] | N/A | Germany | 40 | 12 months | Prostagutt forte | 66.0 | 65.1 | 18.6 | 19.0 | N/A | N/A |
| Morgia et al. 2014 [65] | 2011–2012 | Italy | 225 | 12 months | Profluss | 65 | 66 | 20 | 19 | 45 | 45 |
| Preuss et al. 2001 [66] | N/A | USA | 144 | 3 months | Cernitin AF | N/A | N/A | 18.9 | 17.1 | N/A | N/A |
| Schulz 2006 [67] | N/A | Germany | 257 | 24 weeks | Verum | N/A | N/A | 18 | 18 | N/A | N/A |
Values are presented as mean±standard deviation, median (interquartile range), or mean only.
IPSS, International Prostate Symptom Score; I, intervention; C, control; N/A, not available.
1. Risk of bias
We here summarize the risk of bias of studies included in our analysis and summary of findings table. Only four studies were found to be at an overall low risk of bias [44,46,57,58]. Three studies were considered at an overall high risk of bias considering that they were open-label and outcome assessors were not blinded or due to problems with missing outcome data [53,56,68]. We rated the rest of the studies with ‘some concerns’ due to lack of detail of the randomization process and a lack of a protocol or analysis plan, which precluded an assessment of selective outcome reporting. See the Supplementary File to summarize the risk of bias assessment in each analysis and the supplementary file (excel spreadsheet) at the Open Science Framework [25].
2. Effect of interventions
1) Main comparison: Serenoa repens versus placebo
We included 19 studies with 3,630 participants in this comparison; however, not all studies reported the outcomes relevant to this review. The majority of studies did not specify the proprietary name of the intervention; however, some were identified: Permixon (8 studies), Prostamol Uno (1 study), Prosta-Urgenin Uno (1 study), Talso uno (1 study), Prostataplex (1 study), and SPO (1 study). See Table 2 for a summary of the main results and the Supplementary File for the supporting analysis.
Table 2. Summary of findings for the main comparison: Serenoa repens compared to placebo/no treatment for lower urinary symptoms due to benign prostatic hyperplasia.
| Outcomes | No. of participants (studies) Follow-up | Certainty of the evidence (GRADEa) | Relative effect (95% CI)b | Anticipated absolute effects | ||
|---|---|---|---|---|---|---|
| Risk with placebo/no treatment | Risk difference with S. repens | |||||
| Urologic symptom score | 1,725 (10 RCTs) | ⨁⨁⨁⨁ HIGH |
- | The mean urologic symptom score was 14.23 | MD 0.84 lower (1.65 lower to 0.03 lower) | |
| Measured by IPSS scores (range 0–35) | ||||||
| Higher scores indicate worse symptoms | ||||||
| Follow-up: 2 to 6 months | ||||||
| Quality of life | 1,001 (5 RCTs) | ⨁⨁⨁⨁ HIGH |
- | The mean quality of life was 3.10 | MD 0.15 lower (0.30 lower to 0.01 lower) | |
| Measured by IPSS-QoL score (range 0–6) | ||||||
| Follow-up: 2 to 6 months | ||||||
| Adverse events | 2,443 (13 RCTs) | ⨁⨁⨁⨁ HIGH |
RR 1.04 (0.80 to 1.34) | 179 per 1000 | 7 more per 1000 (36 fewer to 61 more) | |
| Cumulative incidence | ||||||
| Follow-up: 2 to 17 months | ||||||
Patient or population: lower urinary symptoms due to benign prostatic hyperplasia. Setting: Australia, China, France, Germany, India, Italy, Korea, Serbia, Turkey, United Kingdom, and United States of America. Intervention: S. repens. Comparison: placebo/no treatment.
GRADE, Grading of Recommendations Assessment, Development, and Evaluation; CI, confidence interval; IPSS, International Prostate Symptom Score; -, not available; MD, mean difference; QoL, quality of life; RR, risk ratio.
a:GRADE Working Group grades of evidence: (1) High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. (2) Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. (3) Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. (4) Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
b:The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
We did not downgrade the certainty of the evidence due to the risk of bias because these results are based on the sensitivity analysis excluding studies with high risk of bias. Moreover, we found no other concerns to further downgrade the certainty of the evidence.
(1) Urinary symptoms
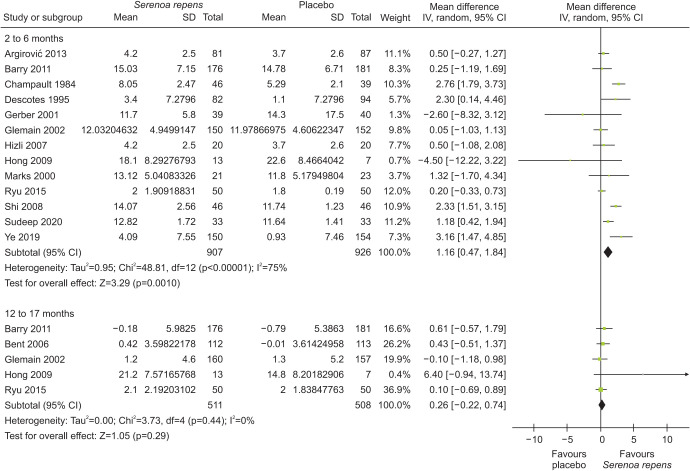
S. repens results in little to no difference in urinary symptoms at short-term follow-up (2 to 6 months, MD −0.84, 95% CI −1.65 to −0.03; 1,725 participants; 10 studies; I2=63%, high certainty of the evidence). All of the heterogeneity is explained by a single study of 304 participants that compared S. repens to placebo and showed a difference in IPSS scores of −2.77 (95% CI −3.71 to −1.83) [24], which is statistically significant but clinically unimportant compared to the minimal important difference of three points [6]; therefore we did not downgrade due to inconsistency considering a minimally contextualized approach [69]. We also did not downgrade for risk of bias since our main analysis is based on the sensitivity analysis, excluding studies at high risk of bias [53,56,68]. Some studies could not be included in the meta-analysis since they only reported p-values for each comparison. One study with 101 participants found that S. repens results in a reduction of urinary symptoms (p<0.01) [45]. Another study with 1,011 participants found a decrease in urinary symptoms with S. repens compared to placebo at 12 months follow-up (p=0.04) [50]. S. repens results in little to no difference in urinary symptoms at a long-term follow-up (12 to 17 months, MD 0.01, 95% CI −0.58 to 0.59; 1,018 participants; 5 studies; I2=0%).
We found no difference based on the type of extract (Hexanic versus non-hexanic) (p-value=0.27, see Analysis 1.1.4. in the Supplementary File).
(2) Quality of life
S. repens results in little to no difference in the quality of life at short-term follow-up (2 to 6 months, MD −0.15, 95% CI −0.30 to −0.01; 1,001 participants; 5 studies; I2=0%, high certainty of the evidence). Moreover, S. repens results in little to no difference in quality of life at long-term follow-up (12 to 17 months, MD −0.12, 95% CI −0.37 to 0.13; 1,002 participants; 5 studies; I2=39%). We did not downgrade for risk of bias since our main analysis is based on the sensitivity analysis, excluding studies at high risk of bias [53,56,68].
(3) Adverse events
S. repens does not increase the risk of adverse events (2 to 17 months, RR 1.04, 95% CI 0.80 to 1.34; 2,443 participants; 13 studies; I2=16%, high certainty of the evidence). We did not downgrade for risk of bias since our main analysis is based on the sensitivity analysis, randomized excluding studies at high risk of bias [53,56]. Three studies were not included in the meta-analysis since they reported no adverse events [45,57,58]. The most commonly reported adverse events were: headache, gastrointestinal disorders (e.g., diarrhea, nausea and vomiting, stomach upset), upper respiratory (e.g., rhinitis), ejaculation disorders, musculoskeletal (e.g., arthralgia in the knees and muscular arm pain), and dizziness.
Two studies classified adverse events as severe and non-severe [50,52]. In one study, the severe adverse events described were dizziness in the placebo group and hypotension in the intervention group [52], whereas in the other study those described in the intervention group included colon cancer, gastrointestinal hemorrhage, urinary retention, and myocardial ischemia [50].
(4) Peak urinary flow (Qmax)
S. repens may result in an increase in Qmax compared to placebo at 2 to 6 months follow-up (MD 1.16, 95% CI 0.47 to 1.84; 1,833 participants; 13 studies; I2=75%), however this effect dissolve at 12 to 17 month follow-up (MD 0.26, 95% CI −0.22 to 0.74; n=1,019; 5 studies; I2=0%). See Fig. 2 for further details.
Fig. 2. Effects of Serenoa repens on peak urinary flow (Qmax). SD, standard deviation; CI, confidence interval.
(5) Acute urinary retention
Two studies found little to no difference in the incidence of acute urinary retention between S. repens and placebo; however, the CIs included substantial benefits and harms (2 to 17 months, RR 3.30, 95% CI 0.52 to 21.05; 409 participants; 2 studies; I2=0%, see analysis in Supplementary File). Another study with 1,011 participants reported no difference in the incidence of acute urinary retention between participants who received S. repens and placebo (p-value not available) [50]. Moreover, two studies reported no cases of acute urinary retention [55,56].
None of the included studies reported the effects of S. repens on surgical interventions for LUTS.
2) Secondary comparison: phytotherapeutic agents with various agents including Serenoa repens versus placebo
We included eight studies with 1,223 participants in this comparison. These studies compared the effects of the following agents containing S. repens as a component: Curbicin, ProstateEZE Max, Serenoa repens plus Tradamixine, PRO 160/120, Prostagutt forte, Profluss, Cernitin AF, and Verum. See Table 3 for a summary of the main results and the Supplementary File for the supporting analysis.
Table 3. Summary of findings for the secondary comparison: phytotherapy with Serenoa repens versus placebo.
| Outcomes | No. of participants (studies) Follow-up | Certainty of the evidence (GRADEa) | Relative effect (95% CI)b | Anticipated absolute effects | ||
|---|---|---|---|---|---|---|
| Risk with placebo/no treatment | Risk difference with phytotherapy with S. repens | |||||
| Urologic symptom score | 416 (3 RCTs) | ⨁◯◯◯ VERY LOWc,d,e |
- | The mean urologic symptom score was 11.1 | MD 2.94 lower (5.55 lower to 0.32 lower) | |
| Measured by IPSS scores (range 0–35) | ||||||
| Higher scores indicate worse symptoms | ||||||
| Follow-up: 12 to 48 weeks | ||||||
| Quality of life | 265 (2 RCTs) | ⨁◯◯◯ VERY LOWc,f,g |
One study reported improvements (p<0.05) while the other did not. | |||
| Measured by IPSS-QoL score (range 0–6) | ||||||
| Follow-up: 6 to 12 months | ||||||
| Adverse events | 437 (3 RCTs) | ⨁⨁◯◯ LOWc,e |
RR 0.87 (0.56–1.36) | 158 per 1,000 | 21 fewer per 1,000 (70 fewer to 57 more) | |
| Cumulative incidence | ||||||
| Follow-up: 12 to 48 weeks | ||||||
Patient or population: lower urinary symptoms due to benign prostatic hyperplasia. Setting: Sweden, Australia, Italy, Russia, Germany, USA. Intervention: S. repens with other phytotherapy. Comparison: placebo/no treatment.
GRADE, Grading of Recommendations Assessment, Development, and Evaluation; CI, confidence interval; IPSS, International Prostate Symptom Score; -, not available; MD, mean difference; QoL, quality of life; RR, risk ratio.
a:GRADE Working Group grades of evidence: (1) High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. (2) Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. (3) Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. (4) Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
b:The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
c:Downgraded one level due to concerns about bias: all studies did not have a pre-specified analysis plan or protocol (some concerns of selective outcome reporting).
d:Downgraded one level due to concerns about inconsistency: high statistical inconsistency (I2=77%).
e:Downgraded one level due to imprecision: wide confidence interval.
f:Downgraded one levels due to imprecision: the included studies reported p-values and we are uncertain about effect sizes.
g:Downgraded one level due to inconsistency: the included studies reported different effects.
(1) Urinary symptoms
Phytotherapeutic agents with various agents, including S. repens, may reduce urinary symptoms compared to placebo at short-term follow-up but the evidence is very uncertain (12 to 48 weeks, MD −2.94, 95% CI −5.55 to −0.32; 416 participant; 3 studies; I2=77%, very low certainty of the evidence). Three studies could not be included in the meta-analysis since they only reported p-values for each comparison: one study with 60 participants found a 36% reduction in the total IPSS median score in the active group (S. repens, lycopene, Prunus Africana, Epilobium parviflorum, and Cucurbita pepo) compared to 8% in the placebo group at three months followup (p<0.05) [61]. Another study with 225 participants found a greater decrease in IPSS scores for combination therapy (S. repens, lycopene, and selenium) compared to control at 12-month follow-up (median change 2.0, range −3 to −1, p<0.01) [65]. Finally, a study with 257 participants found a mean decrease in the intervention group of 6 points in the IPSS score in comparison with 4 points in the placebo group at 24 weeks follow-up (p<0.01) [67]. One study reported as an abstract did not provide comparative data (only a decrease in IPSS in the intervention group) [62].
(2) Quality of life
We are very uncertain on the effects of these agents on quality of life (very low certainty of the evidence). One study with 40 participants found that 84.2% of the participants in the intervention group had improvements in their quality of life after six months of treatment in comparison with 11.1% of improvement in the placebo group (p<0.001) [64]. Another study with 225 participants found little to no difference in the quality of life scores (median change 0, range −0.1 to 1) [65].
(3) Adverse events
Phytotherapeutic agents with various agents, including S. repens, may result in little to no difference in the occurrence of adverse events; however, the CIs included substantial benefits and harms (12 to 48 weeks, RR 0.87, 95% CI 0.56 to 1.36; 437 participants; 3 studies; I2=0%, low certainty of evidence). Two studies reported that there were no adverse events [60,61]. Another study with 225 participants reported no significant differences in treatment-related adverse events (p=0.67) [65]. The most commonly reported adverse events were: headache, gastrointestinal disorders (e.g. diarrhoea, nausea and vomiting, stomach upset), upper respiratory (e.g. rhinitis), ejaculation disorders, musculoskeletal (e.g. arthralgia in the knees and pain), and dizziness.
(4) Peak urinary flow (Qmax)
The effects of these phytotherapeutics agents on this parameter were inconsistent (MD 1.46, 95% CI −0.53 to 3.45; 220 participants; 3 studies; I2=76%). Another study with 152 participants found little to no difference in the change of Qmax in the intervention group compared with placebo (median change 0.8, range 0.1 to 1.7) [65].
None of the included studies in this comparison reported the effects of these treatments on acute urinary retention and surgical interventions.
DISCUSSION
We conducted a systematic review including 27 randomized controlled trials assessing the effects of S. repens alone or in combination with other phytotherapy. For S. repens alone, high certainty evidence indicates that there is little to no clinical benefits for patients with LUTS. For S. repens in combination with phytotherapy, we found similar results but with greater uncertainty.
A recent systematic review and network meta-analysis on the same topic included 22 randomized clinical trials with multiple comparisons of hexanic and non-hexanic extract of S. repens (HESr and nHESr) with alpha-adrenergic agonists and placebo [70]. The authors concluded that there were clinically insignificant improvements in IPSS for HESr and nHESr at 12 weeks; however, their CIs included little to no difference (placebo vs. HESr: MD −0.47, 95% IC −2.69 to 1.74; nHESr vs. placebo: MD −1.69, 95% CI −4.36 to 0.98). Moreover, the authors reported improvements in IPSS using HESr compared to nHESr; however, their reported CI includes little to no difference (nHESr vs. HESr: MD −2.16, 95% CI −5.64 to 1.30), similar to our findings in our subgroup analysis. Regarding Qmax, their results were similar to ours, with an increase of peak urinary flow of 1 to 2 points compared with placebo (nHESr +2.4 and HESr +1.04). Finally, the review was limited due to fewer studies comparing S. repens with placebo (7 in that review compared to 15 in ours) with a substantial imprecision in their results. Another systematic review included seven randomized clinical trials comparing HESr (restricted to Permixon) with placebo for the outcomes of nocturia, Qmax and adverse events but did not assess IPSS [71]. The peak urinary flow analysis reported an increase of Qmax (MD 3.37 points) compared to the increase we found (MD 1.16 points). They also found a decrease in the episodes of nocturia that may be clinically insignificant (MD −0.31, range −0.59 to −0.03); however, the findings on adverse events were similar to ours. Finally, a systematic review including 15 randomized clinical trials and 12 observational studies comparing Permixon with placebo assessed nocturia, Qmax and adverse events but did not assess IPSS [72]. This review also found a small reduction in nocturia that may be clinically insignificant (MD −0.64, range −0.98 to −0.31) and similar results regarding adverse events. This systematic review showed a peak urinary flow improvement of 2.75 mL/s which is slightly higher than our findings. While the overall clinical important effects of S. repens remain unproven, higher concentrations may result in small but positive improvements in LUTS symptoms as described in a single study [58].
The 2020 Guideline of the American Urological Association focuses on the treatment of LUTS attributed to BPH using common surgical techniques and minimally invasive surgical therapies; thus, the information on the different types of medical interventions is not deepened, much less the use of S. repens [11]. Despite this, we found a previous version of this guideline from 2010, where it is mentioned that the available data do not suggest that S. repens has a clinically significant effect on LUTS secondary to BPH [73]. Furthermore, it adds that no dietary supplement, combined herbal medicine or other unconventional therapy is recommended to manage LUTS secondary to BPH due to the paucity of high-quality published trials [73]. The European Association of Urology guidelines on the management of non-neurogenic male LUTS makes recommendations on therapeutic and surgical interventions in patients with BPH [4]. In addition, a comprehensive and exhaustive bibliographic search was carried out on herbal medicine, especially on S. repens. This guide recommends offering the hexane extract of S. repens to men with LUTS who want to avoid possible adverse events, especially those related to sexual function (weak recommendation), informing the patient that the magnitude of efficacy may be modest (strong recommendation) [4]. The guidelines by the Korean Urological Association for the evidence-based diagnosis and treatment of BPH and basic information on diagnostic testing, drug therapy, and surgical treatment [74]. This guide recommends drug therapy as the primary treatment in patients with moderate or severe symptoms, reserving surgical interventions to those with moderate to severe LUTS and for patients who develop acute urinary retention or other complications related to BPH [74]. However, this guideline does not mention phytotherapy or S. repens in managing urological symptoms in BPH.
The overall certainty of the evidence was high for the main comparison considering minor concerns due to inconsistency and focusing on our main sensitivity analysis excluding studies at high risk of bias. For the secondary comparison, however, we could not perform sensitivity analysis since all studies were found to have limitations in their report. This highlights the importance of researchers and journal editors adhering to CONSORT [75]. Moreover, the CIs were wide, and we found substantial heterogeneity, which could be partially explained by the differences in components across combined agents. Not all studies the full details of critical outcomes such as urinary symptoms, quality of life, and adverse events, which would be desirable considering patient's values and preferences [76].
Our study has several limitations. First, we initially aimed to assess the effects of S. repens compared to placebo or other treatments; however, based on the emerging evidence from other reviews, we considered that if S. repens was not more effective than placebo, comparisons to other treatments may be misleading; therefore we focused on its effects alone or in combination with other phytotherapy. Second, we could not retrieve some full-text articles; however, based on our inspection of other systematic reviews, we estimate that many of these studies might be observational or might not provide sufficient data relevant to our main outcomes [23]. This is because older studies focus on nocturia and not IPSS scores, which is currently the mainstay outcome for this condition. Third, we could not incorporate several studies in meta-analysis due to missing data (missing standard deviation or standard error), but we reported these results separately. Finally, we could not perform many predefined funnel plots, subgroup, and sensitivity analysis due to the scarcity of data, low heterogeneity across comparisons, and few trials included in each comparison. Nevertheless, our systematic review has several strengths. It is the most up-to-date comprehensive review on this topic, including the greatest number of studies per comparison due to a thorough bibliographic search and incorporating new review methods (such as Risk of Bias 2). Moreover, it is the most up-to-date review using GRADE, which allows for the seamless incorporation of evidence into recommendations, and it is the preferred approach for guideline development [77,78].
CONCLUSIONS
S. repens alone results in no clinical benefits for men with LUTS. There is greater uncertainty in the effects of S. repens in combination with other phytotherapy. While others reviews on this subject found small effect sizes for some formulations, their CI indicated no important effects. Our review incorporating GRADE includes a comprehensive interpretation of these effect estimates. Future studies need to fully report their methods (including randomization and allocation concealment together with their prospectively registered protocols) and funding sources.
ACKNOWLEDGMENTS
This protocol predominantly used a template structure (background and methods) from a previous protocol by one of the authors on a different topic [8] and the latest Cochrane review [23].
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Juan Víctor Ariel Franco and Jae Hung Jung.
- Data acquisition: Camila Micaela Escobar Liquitay, Leonel Fabrizio Trivisonno, Cecilia Fieiras, Nadia Sgarbossa, and Gustavo Ariel Alvez.
- Statistical analysis: Juan Víctor Ariel Franco and Jae Hung Jung.
- Data analysis and interpretation: All authors.
- Drafting of the manuscript: All authors.
- Critical revision of the manuscript: All authors.
- Administrative, technical, or material support: Juan Víctor Ariel Franco.
- Supervision: Juan Víctor Ariel Franco.
- Approval of the final manuscript: All authors.
SUPPLEMENTARY MATERIAL
Supplementary materials can be found via https://doi.org/10.4111/icu.20210254.
Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement: a systematic review and meta-analysis
References
- 1.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Launer BM, McVary KT, Ricke WA, Lloyd GL. The rising worldwide impact of benign prostatic hyperplasia. BJU Int. 2021;127:722–728. doi: 10.1111/bju.15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, et al. EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO) [Internet] Arnhem: EAU Guidelines Office; 2021. [cited 2021 Apr 30]. Available from: https://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ [Google Scholar]
- 5.AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170(2 Pt 1):530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 6.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald R, Wilt TJ. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: a systematic review of efficacy and adverse effects. Urology. 2005;66:780–788. doi: 10.1016/j.urology.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Jung JH, Kim J, MacDonald R, Reddy B, Kim MH, Dahm P. Silodosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2017;11:CD012615. doi: 10.1002/14651858.CD012615.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. Eur Urol. 2010;58:450–456. doi: 10.1016/j.eururo.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Tacklind J, Fink HA, Macdonald R, Rutks I, Wilt TJ. Finasteride for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2010;(10):CD006015. doi: 10.1002/14651858.CD006015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline amendment 2020. J Urol. 2020;204:799–804. doi: 10.1097/JU.0000000000001298. [DOI] [PubMed] [Google Scholar]
- 12.Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2008;179:2119–2125. doi: 10.1016/j.juro.2008.01.094. [DOI] [PubMed] [Google Scholar]
- 13.Marwick C. Growing use of medicinal botanicals forces assessment by drug regulators. JAMA. 1995;273:607–609. [PubMed] [Google Scholar]
- 14.Buck AC. Is there a scientific basis for the therapeutic effects of serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol. 2004;172(5 Pt 1):1792–1799. doi: 10.1097/01.ju.0000140503.11467.8e. [DOI] [PubMed] [Google Scholar]
- 15.Weisser H, Behnke B, Helpap B, Bach D, Krieg M. Enzyme activities in tissue of human benign prostatic hyperplasia after three months' treatment with the Sabal serrulata extract IDS 89 (Strogen) or placebo. Eur Urol. 1997;31:97–101. doi: 10.1159/000474426. [DOI] [PubMed] [Google Scholar]
- 16.Vacherot F, Azzouz M, Gil-Diez-De-Medina S, Colombel M, De La Taille A, Lefrère Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon in benign prostatic hyperplasia. Prostate. 2000;45:259–266. doi: 10.1002/1097-0045(20001101)45:3<259::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Vela-Navarrete R, Escribano-Burgos M, Farré AL, García-Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. J Urol. 2005;173:507–510. doi: 10.1097/01.ju.0000150533.94952.25. [DOI] [PubMed] [Google Scholar]
- 18.Partin AW, Peters CA, Kavoussi LR, Dmochowski RR, Wein AJ. Campbell Walsh Wein Urology. 12th ed. Philadelphia: Elsevier; 2020. p. 4096. [Google Scholar]
- 19.Habib FK, Wyllie MG. Not all brands are created equal: a comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004;7:195–200. doi: 10.1038/sj.pcan.4500746. [DOI] [PubMed] [Google Scholar]
- 20.Scaglione F, Lucini V, Pannacci M, Caronno A, Leone C. Comparison of the potency of different brands of Serenoa repens extract on 5alpha-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology. 2008;82:270–275. doi: 10.1159/000161128. [DOI] [PubMed] [Google Scholar]
- 21.Avins AL, Lee JY, Meyers CM, Barry MJ CAMUS Study Group. Safety and toxicity of saw palmetto in the CAMUS trial. J Urol. 2013;189:1415–1420. doi: 10.1016/j.juro.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 23.Tacklind J, Macdonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2012;12:CD001423. doi: 10.1002/14651858.CD001423.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Z, Huang J, Zhou L, Chen S, Wang Z, Ma L, et al. Efficacy and safety of Serenoa repens extract among patients with benign prostatic hyperplasia in China: a multicenter, randomized, double-blind, placebo-controlled trial. Urology. 2019;129:172–179. doi: 10.1016/j.urology.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Franco JVA. Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement: a systematic review and meta-analysis. [cited 2021 Apr 30];OSF. 2021 doi: 10.4111/icu.20210254. [Preprint]. Available from: https://osf.io/7x9e8/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston BC, Thorlund K, Schünemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes. 2010;8:116. doi: 10.1186/1477-7525-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salas Apaza JA, Franco JVA, Meza N, Madrid E, Loézar C, Garegnani L. Minimal clinically important difference: the basics. Medwave. 2021;21:e8149. doi: 10.5867/medwave.2021.03.8149. [DOI] [PubMed] [Google Scholar]
- 28.Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 29.Brasure M, MacDonald R, Dahm P, Olson CM, Nelson VA, Fink HA, et al. Newer medications for lower urinary tract symptoms attributed to benign prostaic hyperplasia: a review. Rockville: Agency for Healthcare Research and Quality (US);; 2016. [PubMed] [Google Scholar]
- 30.Veritas Health Innovation. Covidence systematic review software [Internet] Melbourne: Veritas Health Innovation; 2020. [cited 2021 Apr 30]. Available from: https://www.covidence.org/ [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. London: Cochrane; 2021. [Google Scholar]
- 32.Page MJ, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C d., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv. [Preprint] 2020. [cited 2021 Apr 30]. Available from: [DOI] [PubMed]
- 33.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Taylor KS, Mahtani KR, Aronson JK. Dealing with categorical risk data when extracting data for meta-analysis. BMJ Evid Based Med. 2021;26:43–45. doi: 10.1136/bmjebm-2020-111649. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeks JJ, Higgins JPT, Altman DG. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. London: Cochrane; 2020. Analysing data and undertaking meta-analyses. [Google Scholar]
- 38.The Cochrane Collaboration. Review Manager (RevMan) [Computer program] 2020. [Google Scholar]
- 39.Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave. 2021;21:e8109. doi: 10.5867/medwave.2021.02.8109. [DOI] [PubMed] [Google Scholar]
- 40.McMaster University; Evidence Prime, Inc. GRADEpro GDT: GRADEpro Guideline Development Tool [Internet]. Hamilton: McMaster University; 2020. [cited 2021 Apr 30]. Available from: https://gradepro.org/ [Google Scholar]
- 41.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. London: Cochrane; 2020. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. [Google Scholar]
- 43.Argirović A, Argirović D. Does the addition of Serenoa repens to tamsulosin improve its therapeutical efficacy in benign prostatic hyperplasia? Vojnosanit Pregl. 2013;70:1091–1096. doi: 10.2298/vsp110620029a. [DOI] [PubMed] [Google Scholar]
- 44.Barry MJ, Meleth S, Lee JY, Kreder KJ, Avins AL, Nickel JC, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer HW, Casarosa C, Cosci M, Fratta M, Blessmann G. [Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo-controlled double-blind study] MMW Fortschr Med. 1999;141:62. [PubMed] [Google Scholar]
- 46.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 47.Boccafoschi C, Annoscia S. [Serenoa repens extract and placebo in prostatic benign hyperplasia: clinical results] Urologia. 1983;50:1257–1268. Italian. [Google Scholar]
- 48.Champault G, Patel JC, Bonnard AM. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br J Clin Pharmacol. 1984;18:461–462. doi: 10.1111/j.1365-2125.1984.tb02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Descotes JL, Rambeaud JJ, Deschaseaux P, Faure G. Placebo-controlled evaluation of the efficacy and tolerability of Permixon® in benign prostatic hyperplasia after exclusion of placebo responders. Clin Drug Investig. 1995;9:291–297. [Google Scholar]
- 50.BASTA. Efficacy of two formulations of Sabal serrulata; a double-blind; randomized; placebo-controlled phase III study [Internet] Amsterdam: European Medicines Agency; 2006. Dec 29, [cited 2021 Jun 14]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2006-003532-30. [Google Scholar]
- 51.Gerber GS, Kuznetsov D, Johnson BC, Burstein JD. Randomized, double-blind, placebo-controlled trial of saw palmetto in men with lower urinary tract symptoms. Urology. 2001;58:960–964. doi: 10.1016/s0090-4295(01)01442-x. discussion 964-5. [DOI] [PubMed] [Google Scholar]
- 52.Glemain P, Coulange C, Billebaud T, Gattegno B, Muszynski R, Loeb G Groupe de l'essai OCOS. [Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: the OCOS trial] Prog Urol. 2002;12:395–403. discussion 404. French. [PubMed] [Google Scholar]
- 53.Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int Urol Nephrol. 2007;39:879–886. doi: 10.1007/s11255-006-9106-5. [DOI] [PubMed] [Google Scholar]
- 54.Marks LS, Partin AW, Epstein JI, Tyler VE, Simon I, Macairan ML, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000;163:1451–1456. [PubMed] [Google Scholar]
- 55.Reece Smith H, Memon A, Smart CJ, Dewbury K. The value of permixon in benign prostatic hypertrophy. Br J Urol. 1986;58:36–40. doi: 10.1111/j.1464-410x.1986.tb05424.x. [DOI] [PubMed] [Google Scholar]
- 56.Ryu YW, Lim SW, Kim JH, Ahn SH, Choi JD. Comparison of tamsulosin plus serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Urol Int. 2015;94:187–193. doi: 10.1159/000366521. [DOI] [PubMed] [Google Scholar]
- 57.Shi R, Xie Q, Gang X, Lun J, Cheng L, Pantuck A, Rao J. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. J Urol. 2008;179:610–615. doi: 10.1016/j.juro.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 58.Sudeep HV, Thomas JV, Shyamprasad K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020;20:86. doi: 10.1186/s12894-020-00648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willetts KE, Clements MS, Champion S, Ehsman S, Eden JA. Serenoa repens extract for benign prostate hyperplasia: a randomized controlled trial. BJU Int. 2003;92:267–270. doi: 10.1046/j.1464-410x.2003.04316.x. [DOI] [PubMed] [Google Scholar]
- 60.Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. Br J Urol. 1990;66:639–641. doi: 10.1111/j.1464-410x.1990.tb07199.x. [DOI] [PubMed] [Google Scholar]
- 61.Coulson S, Rao A, Beck SL, Steels E, Gramotnev H, Vitetta L. A phase II randomised double-blind placebo-controlled clinical trial investigating the efficacy and safety of ProstateEZE Max: a herbal medicine preparation for the management of symptoms of benign prostatic hypertrophy. Complement Ther Med. 2013;21:172–179. doi: 10.1016/j.ctim.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Iacono F, Ruffo A, Prezioso D, Romeo G, Illiano E, Romis L, et al. Combined treatment with Tradamixina® and Serenoa repens decreases PSA levels and prostate inflammation, improving the lower urinary tract symptoms (LUTS). A randomized, double-blind, placebo-controlled study on 185 patients: PS-04-009. J Sex Med. 2015;12:201 [Google Scholar]
- 63.Lopatkin N, Sivkov A, Walther C, Schläfke S, Medvedev A, Avdeichuk J, et al. Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms--a placebo-controlled, double-blind, multicenter trial. World J Urol. 2005;23:139–146. doi: 10.1007/s00345-005-0501-9. [DOI] [PubMed] [Google Scholar]
- 64.Metzker H, Kieser M, Hölscher U. [Efficacy of a combined Sabal-Urtica preparation in the treatment of benign prostatic hyperplasia (BPH)] Urologe B. 1996;36:292–300. German. [Google Scholar]
- 65.Morgia G, Russo GI, Voce S, Palmieri F, Gentile M, Giannantoni A, et al. Serenoa repens, lycopene and selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicenter double-blinded randomized study between single or combination therapy (PROCOMB trial) Prostate. 2014;74:1471–1480. doi: 10.1002/pros.22866. [DOI] [PubMed] [Google Scholar]
- 66.Preuss HG, Marcusen C, Regan J, Klimberg IW, Welebir TA, Jones WA. Randomized trial of a combination of natural products (cernitin, saw palmetto, B-sitosterol, vitamin E) on symptoms of benign prostatic hyperplasia (BPH) Int Urol Nephrol. 2001;33:217–225. doi: 10.1023/a:1015227604041. [DOI] [PubMed] [Google Scholar]
- 67.Schulz V. [Phytotherapie der benignen Prostatahyperplasie -Wirksamkeit eines Sabal-Urtica-Präparates durch weitere Doppelblindstudie bestätigt] Z Phytother. 2006;27:22–23. [Google Scholar]
- 68.Hong H, Kim CS, Maeng S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr Res Pract. 2009;3:323–327. doi: 10.4162/nrp.2009.3.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. doi: 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russo GI, Scandura C, Di Mauro M, Cacciamani G, Albersen M, Hatzichristodoulou G, et al. Clinical efficacy of Serenoa repens versus placebo versus alpha-blockers for the treatment of lower urinary tract symptoms/benign prostatic enlargement: a systematic review and network meta-analysis of randomized placebo-controlled clinical trials. Eur Urol Focus. 2021;7:420–431. doi: 10.1016/j.euf.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Novara G, Giannarini G, Alcaraz A, Cózar-Olmo JM, Descazeaud A, Montorsi F, et al. Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. Eur Urol Focus. 2016;2:553–561. doi: 10.1016/j.euf.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Vela-Navarrete R, Alcaraz A, Rodríguez-Antolín A, Miñana López B, Fernández-Gómez JM, Angulo JC, et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018;122:1049–1065. doi: 10.1111/bju.14362. [DOI] [PubMed] [Google Scholar]
- 73.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 74.Yeo JK, Choi H, Bae JH, Kim JH, Yang SO, Oh CY, et al. Korean clinical practice guideline for benign prostatic hyperplasia. Investig Clin Urol. 2016;57:30–44. doi: 10.4111/icu.2016.57.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dahm P, Franco J. Re: a systematic review of patients' values, preferences, and expectations for the diagnosis and treatment of male lower urinary tract symptoms. Eur Urol. 2021;80:254–255. doi: 10.1016/j.eururo.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Jung JH, Franco JV, Dahm P. Moving towards evidence-based clinical practice guidelines. Urogenit Tract Infect. 2018;13:45–50. [Google Scholar]
- 78.Quilodrán C, Kirmayr M, Valente B, Pérez-Bracchiglione J, Garegnani L, Franco JVA. The GRADE approach, Part 2: evidence to decision frameworksoutlining decision-making in health. Medwave. 2021;21:e8182. doi: 10.5867/medwave.2021.04.8182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement: a systematic review and meta-analysis