Abstract
Background
Vancomycin (VCM) is an antibiotic widely used to treat a range of serious bacterial infections; however, it is associated with nephrotoxicity. Vitamin C (VC) is a classical antioxidant that can alleviate various organ injuries and inflammatory responses by reducing inflammation and oxidative stress. This study aimed to examine the effect of VC on VCM-related nephrotoxicity in mice.
Methods
Mice were randomized into four groups: control, VCM (400 mg/kg/day), VCM (400 mg/kg/day) + VC (200 mg/kg/day), and VC (200 mg/kg/day) groups. Both VCM and VC were administered via intraperitoneal injection for 7 d, after which kidney and blood samples were collected and evaluated. Creatinine (Cr), blood urea nitrogen (BUN), superoxide dismutase (SOD), malondialdehyde (MDA), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and nuclear factor-κB (NF-κB) were measured.
Results
In the VCM group, kidney index, renal injury score, cell apoptosis, serum Cr and BUN, and kidney Cr, BUN, MDA, IL-1β, IL-6, TNF-α, and NF-κB were higher compared to the control group (all P<0.05), while body weight and kidney SOD activity were lower (both P<0.05). By contrast, no differences were observed between the control and VC groups (VC and VCM + VC groups) for all these indicators.
Conclusions
The antioxidant VC reduces VCM-related renal injury by reducing oxidative stress, cell apoptosis, and inflammation.
Keywords: Vitamin C (VC), vancomycin (VCM), nephrotoxicity, oxidative stress, inflammation
Introduction
Vancomycin (VCM) is an antibiotic that has been applied since 1954 in the treatment of Gram-positive bacterial infections, especially methicillin-resistant Staphylococcus aureus (MRSA) (1,2). It acts by inhibiting polymerization of the peptidoglycans on the bacterial cell wall (2). A concentration of 15–20 mg/L VCM is usually recommended for patients with infection (3); yet, at certain doses, VCM has also been associated with a high risk of VCM-related nephrotoxicity (4,5). For example, a previous study showed that the incidence of VCM-related acute kidney injury (AKI) in patients treated with 10–15, 15–20, 20–35, and >35 mg/L VCM was 3.1%, 10.6%, 23.6%, and 81.8%, respectively (6).
Oxidative stress induced by reactive oxygen species (ROS) is considered one of the main mechanisms associated with VCM-related nephrotoxicity (7,8). Cell death and inflammatory events generated by oxidative stress might cause tubular cell damage and result in VCM-related nephrotoxicity (9,10). In addition, VCM can alter mitochondrial function and induce a dose-dependent proliferation of proximal tubular cells.
The use of antioxidants may decrease the risk of VCM-related AKI (11,12). Vitamin C (VC) is a classical antioxidant that can alleviate various organ injuries and inflammatory responses by reducing inflammation and oxidative stress (13-15). It can reduce drug-induced apoptosis by scavenging the superoxide anion produced by dysfunctional mitochondria (16).
Water-solubility is one characteristic of VC, and it can be safely administered at high amounts in vivo (100–200 mg/kg/day) (17,18). A high-dose (4 g/kg) VC pre-administration study revealed reduced VCM-associated nephrotoxicity in mice via the reduction of renal cell apoptosis (19). Yet, the study did not investigate VC’s exact mechanism of action (19). A clinical trial in China showed that high-dose VC reduces VCM-related nephrotoxicity and hospital stay (20). Conversely, some studies have suggested that an overdose of VC is associated with a high risk of acute oxalate nephropathy (21,22).
In this study, we examined the effect of high-dose VC on VCM-induced AKI in mice. The results indicated that VC was a safe, effective, and inexpensive method to prevent VCM-induced renal injury and is suitable for clinical use. We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3294).
Methods
Chemicals
Lilly (Eli Lilly Japan K.K, Seishin Laboratories, Kobe, Hyogo, Japan) provided the VCM, and Sangon Biotech (Shanghai, China) provided the VC.
Animals
We obtained 6-week-old C57BL/6J male mice, weighing 22–24 g, from the Laboratory Animal Center of Shanghai Jiao Tong University School of Medicine, Shanghai, China. All animals were housed in an environment with a temperature of 22±1 °C, relative humidity of 50%±1%, a light/dark cycle of 12/12 hr, and had free access to food and water. All animal studies (including the mice euthanasia procedure) were conducted in compliance with the regulations and guidelines of Shanghai Jiao Tong University School of Medicine institutional animal care and according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Institutional Animal Care and Use Committee (IACUC) guidelines. This study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. A protocol was prepared before the study without registration.
The mice were acclimated to their new environment for 7 d before the experiments. They were then randomly divided into four groups with 7 mice in each: control, VC (200 mg/kg/day), VCM (400 mg/kg/day), and VCM (400 mg/kg/day) + VC (200 mg/kg/day). Both VCM and VC were injected intraperitoneally for 7 d, and the control group received the same volume of saline.
The mice were weighed and sacrificed under anesthesia with sodium pentobarbital on day 8. Blood was harvested through the eyeballs and centrifuged at 3,000 rpm at 4 °C for 10 min. The serum was stored at –80 °C until analysis. The kidneys were collected, weighed, and stored at –80 °C for further analysis.
Histological detection of tubular injury and apoptosis
The kidney tissue samples were fixed in 10% formalin buffer, embedded in paraffin, and cut into 4-µm sections. Hematoxylin and eosin (HE, Servicebio Technology, Wuhan, Hubei, China) staining was used for the histological detection of tubular necrosis. We scored the tubular damage by calculating the percentage of tubules that displayed tubular epithelial cell necrosis, vacuolization, brush border loss, tubular dilatation, atrophy, casts, and interstitial inflammatory cell infiltration. At least 10 fields for each section were reviewed and scored. All histopathological parameters were scored as follows: 0, none; 1, injury ≤25%; 2, injury 26–50%; 3, injury 51–75%; and 4, injury ≥76%. Study personnel who participated in injection, scoring, and data analysis were blind to group allocation.
Tubular cell apoptosis detection
Tubular cell apoptosis was detected with 4’,6-diamidino-2-phenylindole (DAPI) stain (Servicebio Technology) and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) stain using an In Situ Cell Death Detection Kit (Roche Molecular Systems, Mannheim, Germany).
Measurement of creatinine (Cr), blood urea nitrogen (BUN), malondialdehyde (MDA) levels, and superoxide dismutase (SOD) activity
Sections of kidney tissue were homogenized in nine volumes of cold saline, and 10% (w/v) tissue homogenate was prepared. The supernatants of kidney sections were obtained from 3,000 rpm at 4 °C for 10 min centrifugation and were used to measure Cr and BUN levels (Changchun Huili Biotech, Changchun, Jilin, China), SOD activity, and MDA levels (Nanjing Jiancheng Bioengineering, Nanjing, China) using commercial assay kits, according to the manufacturer’s instructions. The serum was also used to measure Cr and BUN levels.
Measurement of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and nuclear factor (NF)-κB levels
The supernatants of kidney tissues were used to determine IL-1β, IL-6, TNF-α (Thermo Fisher Scientific, Vienna, Austria), and NF-κB levels (Cloud-Clone Corp., Wuhan, Hubei, China) using commercial enzyme-linked immunosorbent assay (ELISA) kits, according to manufacturers’ instructions.
Statistical analysis
Numerical variables were summarized as mean ± standard deviation (SD). Data were analyzed using the software SPSS version 25.0 (IBM Corp., Armonk, NY, USA). The groups were compared with analysis of variance (ANOVA) followed by the post-hoc Student’s t-test or the Mann-Whitney U-test. A P value <0.05 was considered statistically significant.
Results
VC reduces VCM-induced nephrotoxicity
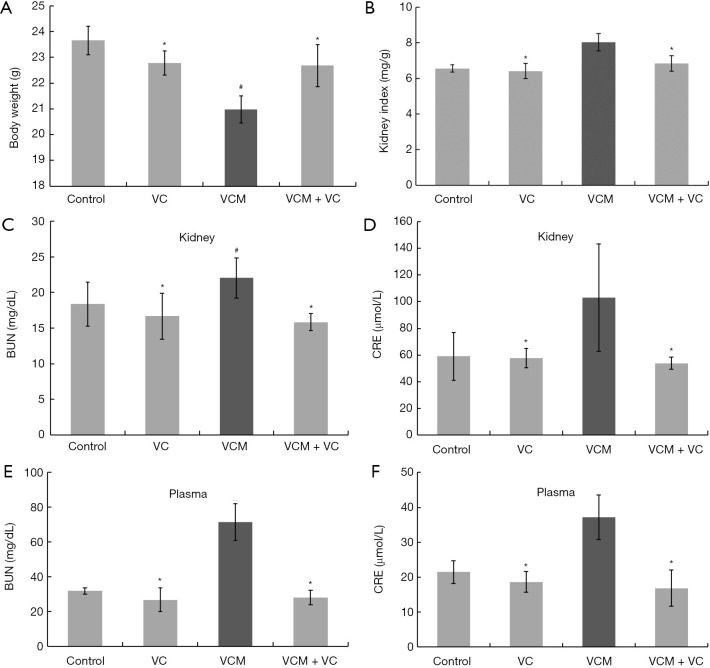
To determine the effects of VC on renal injury induced by VCM, we first observed the kidney’s histological status and the serum and kidney biochemical indicators in different animal groups. The mean body weight of the VCM group was significantly lower (Figure 1A), while the mean kidney index was significantly higher compared to other groups (Figure 1B, all P<0.05). In addition, the BUN and Cr levels in the kidney tissue or in the plasma of VCM-treated mice were significantly higher compared with those in the control, VC, and VCM + VC groups (Figure 1C-1F, P<0.05). In contrast, no differences in body weight, kidney index, and biochemical indicators were found between the VC group, VCM + VC groups, and the control group (all P>0.05).
Figure 1.
VC attenuated VCM-induced nephrotoxicity in mice. (A) Body weight. (B) Kidney index. (C) Kidney BUN. (D) Kidney Cr. (E) Serum BUN. (F) Serum Cr. The results are presented as group means ± SD (n=7 mice/group). *, P>0.05 vs. the control group; #, P<0.05 vs. the control group. VC, vitamin C; VCM, vancomycin; BUN, blood urea nitrogen; Cr, creatinine; SD, standard deviation.
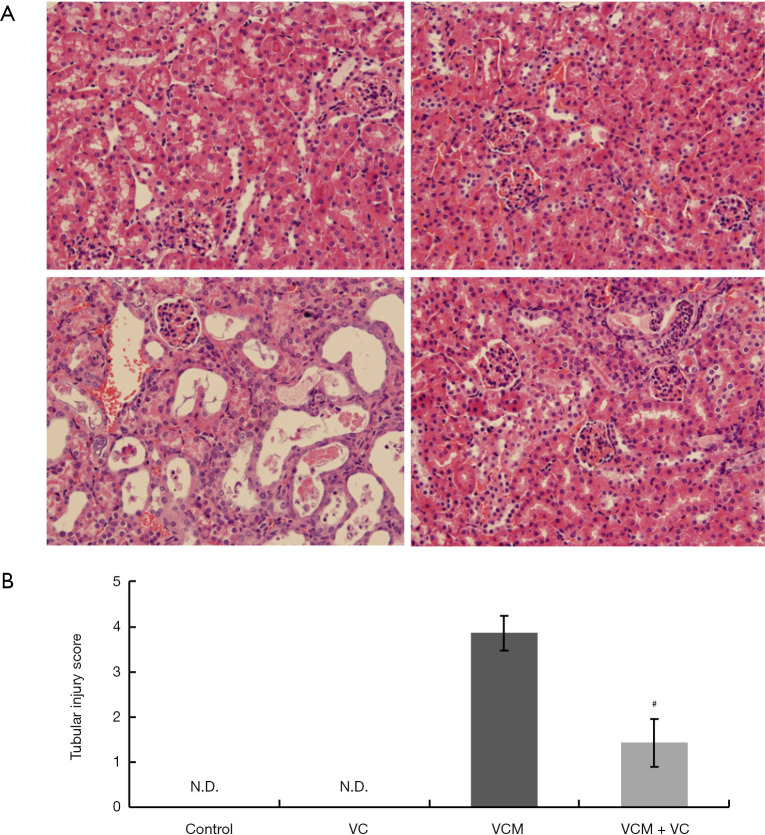
The HE staining of kidney sections from VCM-treated mice revealed obvious renal tubular damage, including epithelial cell degeneration (necrosis, vacuolization, and brush border loss), tubular degeneration (dilatation, atrophy, and casts), and interstitial inflammatory cell infiltration (Figure 2A, left bottom panel). Moreover, compared to the VCM group, tubular damage was significantly relieved in the tissue sections of the VCM + VC group (Figure 2A, right bottom panel), as supported by the tubular injury score (Figure 2B).
Figure 2.
Histopathological changes in the kidney tissues of mice treated with VCM and/or VC. (A) HE-stained mouse kidney sections from the control group (top left), VC group (top right), VCM group (bottom left), and VCM + VC group (bottom right), 40× magnification. (B) The kidney tubular injury score in each group. Tubular injury was scored by calculating the percentage of tubules that displayed tubular epithelial cell degeneration (necrosis, vacuolization, and brush border loss), tubular alterations (dilatation, atrophy, and casts), and interstitial inflammatory cell infiltration as 0, none; 1, injury percentage ≤25%; 2, injury percentage 26–50%; 3, injury percentage 51–75%; 4, injury percentage ≥76%. The results are presented as group means ± SD (n=7 mice/group). #, P<0.05 compared to the VCM group. VCM, vancomycin; VC, vitamin C; HE, hematoxylin and eosin; SD, standard deviation.
These findings suggest a significant protective effect of VC co-administration against renal injury induced by VCM.
VC reverses VCM-induced oxidative stress in kidney tissue
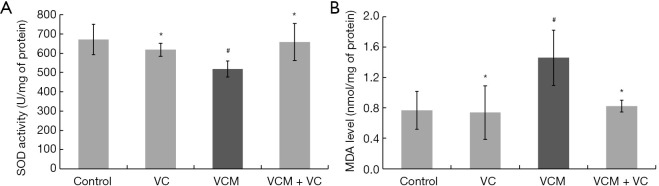
Significant induction of oxidative stress, with markedly decreased SOD activity and increased MDA levels, was observed in the kidney tissues of the VCM group compared to other groups (Figure 3, all P<0.05). Meanwhile, no differences in oxidative stress indicators were found among the control, VC, and VCM + VC group.
Figure 3.
VC reversed VCM-induced oxidative stress in the kidney tissues of mice. (A) SOD activity. (B) MDA levels. The results are presented as group means ± SD (n=7 mice/group). *, P>0.05 vs. the control group; #, P<0.05 vs. the control group. VC, vitamin C; VCM, vancomycin; SOD, superoxide dismutase; MDA, malondialdehyde; SD, standard deviation.
VC attenuates inflammatory cytokine production in kidneys activated by VCM
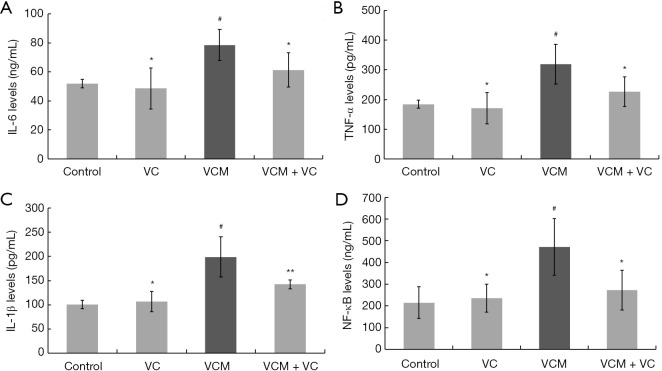
To investigate how VC protects renal injury from VCM, we examined the inflammatory mediators and NF-κB levels in each group (Figure 4). Compared to the control group, significant increases in IL-1β, IL-6, TNF-α, and NF-κB were observed in the VCM group (all P<0.05 vs. the control group). These markers were all lower in the VCM + VC group compared to the VCM group. Hence, these data suggest that VC significantly alleviates VCM-induced inflammatory response in the kidney in vivo. This effect of VC might be related to the inhibition of NF-κB signaling.
Figure 4.
VC reversed VCM-induced inflammatory cytokine production in the kidney tissues of mice. (A) IL-6. (B) TNF-α. (C) IL-1β. (D) NF-κB. The results were presented as group means ± SD (n=7 mice/group). *, P>0.05 vs. the control group; **, P<0.05 vs. the VCM group; #, P<0.05 vs. the control group. VC, vitamin C; VCM, vancomycin; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; NF-κB, nuclear factor-κB; SD, standard deviation.
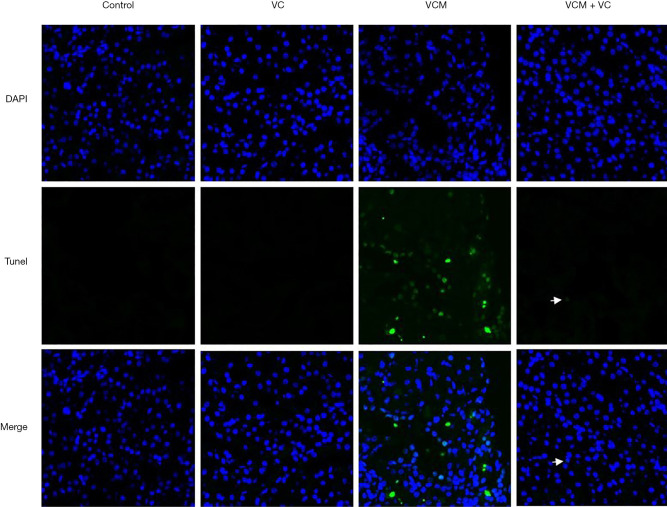
VC reduces VCM-induced cell apoptosis in kidneys
The results of TUNEL analysis are shown in Figure 5. We used DAPI staining to localize the kidney cells' nucleus in each group. Briefly, a high number of apoptotic nuclei were found in the kidneys of the VCM-treated mice. Co-treatment with VC significantly reduced the number of positive cells caused by VCM in the kidneys. Moreover, only very few TUNEL-positive cells (white arrow) were found in the VCM + VC group. In summary, VC treatment significantly ameliorated VCM-induced renal cell apoptosis.
Figure 5.
VC alleviated VCM-induced apoptosis in kidney sections of mice. TUNEL-positive (green) cells were observed in the kidney sections of mice treated with VCM. Nuclei were counterstained with DAPI (blue). The white arrows indicate the location of the positive cells; 90× magnification. VC, vitamin C; VCM, vancomycin; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling; DAPI, 4’,6-diamidino-2-phenylindole.
Discussion
Preclinical studies have demonstrated that drug accumulation in proximal tubule cells, resulting in cellular oxidative stress and apoptosis, is a major cause of VCM-related nephrotoxicity. The nephrotoxicity of VCM can be reduced by extending the infusion time, reducing the maximum concentration, using antioxidants, and using drugs that reduce cell aggregation (23). Rahmani reviewed clinical and preclinical evidences regarding new strategies for prevention of VCM-induced nephrotoxicity. Evidence from 2014 to end of 2019, including twelve animal studies and one clinical trial, were evaluated. The conclusion shows that, although incidence of VCM-induced nephrotoxicity was not reduced significantly in the clinical trial, antioxidants reduced incidence of VCM-induced nephrotoxicity in preclinical studies. In preclinical studies, antioxidants including VC, vitamin E, cilastatin, melatonin, zingerone, rutin, naringenin, saffron, silymarin and dexmedetomidine were nephroprotective against VCM-induced nephrotoxicity (24).
VC as a water-soluble antioxidant plays an integral part in the cellular oxidative metabolism, as it efficiently scavenges ROS and reactive nitrogen species produced under various stress conditions. At present, the pharmacological effects and clinical application of VC have been studied deeply. Research shows that VC has an anti-sepsis effect by reducing inflammatory response and oxidative stress as well as suppressing immunological dysfunction (25). Besides, VC in pharmacological concentration can contribute to the suspended formation of hydroxyl radical via the Fenton reaction, and induce the death of cancer cells, thus serving as an important element of the oxidative stress therapy against cancer cells (26). Recently, VC has been used for the treatment and prevention of COVID-19 complications, due to its multiple pharmacological characteristics, including antiviral, antioxidant, anti-inflammatory, and immunoregulatory effects (27,28). High doses of intravenous VC can reduce the risk of COVID-19 infection with advanced cytokine storm (29).
The antibiotic VCM can induce nephrotoxicity (4,5), which may be inhibited by VC (19,22). Pre-clinical evidence showed that VC suppresses excessive cytokine release leading to sepsis-induced organ dysfunction (30). Our data further suggested that VC reduces VCM-related renal injury by reducing oxidative stress, cell apoptosis, and inflammation.
The clinical use of VCM is limited by drug-induced nephrotoxicity. Oxidative stress reduction in the kidney is critical for suppressing VCM-related nephrotoxicity (31,32). A recent study showed that high-dose VC might reduce the nephrotoxicity of VCM in severe cases (22). Published study revealed high-dose VC pre-administration reduced VCM-associated nephrotoxicity in mice via the reduction of renal cell apoptosis. In this report, high-dose VC pre-administration decreased the plasma Cr and BUN levels increased by VCM, and reduced the characteristics of VCM-associated nephrotoxicity in histological evidence (19). However, it did not fully clarify the exact mechanism of VC in reducing VCM-associated nephrotoxicity. In present study, based on the observation of renal histological status and serum and kidney biochemical indicators in different animal groups, we further explored the role of VC in alleviating VCM-related renal injury by reducing oxidative stress, apoptosis and inflammation. In the VCM group, body weight decreased, and the kidney index increased after 7 d of VCM injection compared with the control group, and renal damage was considered to have caused these changes (19); tubular injury was observed in the kidney tissue sections, and BUN and Cr levels were increased in both serum and kidney tissue. Histologically, tubular casts, epithelial cell vacuolization, and brush border loss were evident in the VCM group. In contrast, VC treatment reversed VCM-induced kidney injury, which inferred that high-dose VC might prevent VCM-induced renal injury.
The transcriptional mediator NF-κB is active in a series of cellular processes, such as inflammation, immunity, cell proliferation, and apoptosis (33). The NF-κB pathway can be activated by ROS via upregulation of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, thereby further increasing intracellular ROS production and gene expression of pro-inflammatory cytokines in endothelial cells (34-36). The activation of NF-κB is associated with increased levels of proapoptotic protein Bcl and cell death effector caspase-3 (37,38), which has a pivotal role in damage to tubular epithelial cells. A previous study found that VCM may increase NF-κB expression and produce the pro-inflammatory cytokines IL-1β and TNF-α, which leads to renal damage in rats (39). In a hemorrhagic shock rat model, VC (100–200 mg/kg) reduced renal injury by regulating Sirtuin1 (SIRT1) and acetyl (Ace)-NF-κB, thus reducing epithelial cell inflammation and apoptosis associated with oxidative stress (40).
In the present study, VCM treatment alleviated SOD expression and increased lipid peroxidation product MDA. These results confirmed the relationship between VCM-induced nephrotoxicity and oxidative stress produced by ROS. The levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and the transcriptional mediator NF-κB were markedly increased, and TUNEL-positive cells were evident in the kidneys of VCM-treated mice. Those results indicated that renal cell damage induced by oxidative stress was associated with inflammatory cell infiltration and cell apoptosis, which may be related to the NF-κB pathway. Supplementation of high-dose VC significantly decreased the expression of IL-1β, IL-6, TNF-α, and NF-κB, increased SOD activity, and decreased the MDA levels. Moreover, TUNEL-positive cells were significantly decreased in the VCM + VC group. These results indicated that ROS and NF-κB were upregulated after VCM treatment, causing renal damage by oxidative stress, cell inflammation, and apoptosis. High-dose VC provided effective protection against these reactions.
According to the signaling pathway studies on NF-κB, some other factors might be involved in the ROS/NF-κB pathway. The roles of ROS include modulation of the transcriptional activity of NF-κB in response to Toll-like receptor 4 (TLR4)-dependent signaling and activation of the production of pro-inflammatory cytokines and p38 (41). Mitogen-activated protein kinase (MAPK) (42) and Jun N-terminal kinase (JNK) (43), which are involved in inflammation, cell death, and cell survival, might be activated by oxidative stress and regulated by NF-κB. Heme oxygenase (HO)-1 and SIRT1 are upregulated by ROS and inhibit NF-κB expression from preventing multi-organ injuries in hemorrhagic shock rats (40,44). Nevertheless, few of these pathways have been investigated in VCM-induced nephrotoxicity. Some scholars used the stable isotope labeling by amino acids in cell culture (SILAC) method to detect molecular interactions and analyze related signaling pathways in VCM-induced nephrotoxicity of human proximal tubule epithelial HK-2 cells. The results showed that more than 492 proteins in HK-2 cells interact with VCM, and 290 signaling pathways and cellular functions may be regulated by VCM. These proteins and pathways may regulate cell cycle, apoptosis, autophagy, EMT, and ROS production (45). These signaling pathways may be involved in the nephrotoxicity of VCM. The present preliminary study revealed that the renal protective activity of high-dose VC might be related to the ROS/NF-κB pathway. However, further studies are needed to explore the exact mechanisms of the preventive effect of VC in VCM-induced nephropathy.
Conclusions
High-dose VC reduces VCM-related renal injury by inhibiting oxidative stress, inflammation, and cell apoptosis. Therefore, high-dose VC treatment might be an effective and safe therapeutic approach to prevent VCM-induced nephrotoxicity. Further studies are needed to more thoroughly examine the underlying molecular mechanism.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the help of the staff from the Laboratory Animal Center of Shanghai Jiao Tong University School of Medicine.
Funding: This work was supported by the Science and Technology Fund Project of Shanghai Jiao Tong University School of Medicine (JDYX2016QN001) and the Bethune Medical Science Research Foundation (N111ES).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal studies (including the mice euthanasia procedure) were conducted in compliance with the regulations and guidelines of Shanghai Jiao Tong University School of Medicine institutional animal care and according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Institutional Animal Care and Use Committee (IACUC) guidelines. This study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3294
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3294
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3294). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Zamoner W, Prado IRS, Balbi AL, et al. Vancomycin dosing, monitoring and toxicity: critical review of the clinical practice. Clin Exp Pharmacol Physiol 2019;46:292-301. 10.1111/1440-1681.13066 [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Preuss CV, Bernice F. Vancomycin. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing, 2020. [Google Scholar]
- 3.Ye ZK, Li C, Zhai SD. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS One 2014;9:e99044. 10.1371/journal.pone.0099044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horey A, Mergenhagen KA, Mattappallil A. The relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran's population: a retrospective analysis. Ann Pharmacother 2012;46:1477-83. 10.1345/aph.1R158 [DOI] [PubMed] [Google Scholar]
- 5.Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017;102:459-69. 10.1002/cpt.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008;52:1330-6. 10.1128/AAC.01602-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oktem F, Arslan MK, Ozguner F, et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 2005;215:227-33. 10.1016/j.tox.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto Y, Yano T, Hanada Y, et al. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur J Pharmacol 2017;800:48-56. 10.1016/j.ejphar.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 9.Kandemir FM, Yildirim S, Kucukler S, et al. Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed Pharmacother 2018;105:981-91. 10.1016/j.biopha.2018.06.048 [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Zou J, Zhang T, et al. Protective effects of DHA-PC against vancomycin-induced nephrotoxicity through the inhibition of oxidative stress and apoptosis in BALB/c mice. J Agric Food Chem 2018;66:475-84. 10.1021/acs.jafc.7b04565 [DOI] [PubMed] [Google Scholar]
- 11.Nishino Y, Takemura S, Minamiyama Y, et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 2003;37:373-9. 10.1080/1071576031000061002 [DOI] [PubMed] [Google Scholar]
- 12.Elyasi S, Khalili H, Hatamkhani S, et al. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur J Clin Pharmacol 2013;69:747-54. 10.1007/s00228-012-1406-3 [DOI] [PubMed] [Google Scholar]
- 13.Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 2014;18:460. 10.1186/s13054-014-0460-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, Fei J, Chen Y, et al. Pharmacological preconditioning with vitamin C attenuates intestinal injury via the induction of heme oxygenase-1 after hemorrhagic shock in rats. PLoS One 2014;9:e99134. 10.1371/journal.pone.0099134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu PR, Hu YC, Huang TC, et al. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways. Int J Mol Sci 2016;18:38. 10.3390/ijms18010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel U, Nickel A, Kuntz S, et al. Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis 2004;25:703-12. 10.1093/carcin/bgh079 [DOI] [PubMed] [Google Scholar]
- 17.Long CL, Maull KI, Krishnan RS, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 2003;109:144-8. 10.1016/S0022-4804(02)00083-5 [DOI] [PubMed] [Google Scholar]
- 18.Kahn SA, Beers RJ, Lentz CW. Resuscitation after severe burn injury using high-dose ascorbic acid: a retrospective review. J Burn Care Res 2011;32:110-7. 10.1097/BCR.0b013e318204b336 [DOI] [PubMed] [Google Scholar]
- 19.Takigawa M, Yatsu T, Takino Y, et al. High-dose vitamin C preadministration reduces vancomycin-associated nephrotoxicity in mice. J Nutr Sci Vitaminol (Tokyo) 2019;65:399-404. 10.3177/jnsv.65.399 [DOI] [PubMed] [Google Scholar]
- 20.He J, Mao E, Xu W, et al. High dose vitamin C significantly reduces the nephrotoxicity of vancomycin in critically ill patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:468-72. [DOI] [PubMed] [Google Scholar]
- 21.Buehner M, Pamplin J, Studer L, et al. Oxalate nephropathy after continuous infusion of high-dose vitamin C as an adjunct to burn resuscitation. J Burn Care Res 2016;37:e374-9. 10.1097/BCR.0000000000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurm H, Sheta MA, Nivera N, et al. Vitamin C-induced oxalate nephropathy: a case report. J Community Hosp Intern Med Perspect 2012. doi: . 10.3402/jchimp.v2i2.17718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pais GM, Liu J, Zepcan S, et al. Vancomycin-induced kidney injury: animal models of toxicodynamics, mechanisms of injury, human translation, and potential strategies for prevention. Pharmacotherapy 2020;40:438-54. 10.1002/phar.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahmani H, Khalili H. Prevention of vancomycin-induced nephrotoxicity; an update review of clinical and preclinical studies. Infect Disord Drug Targets 2021. [Epub ahead of print]. doi: . 10.2174/1871526521666210331164552 [DOI] [PubMed] [Google Scholar]
- 25.Li R, Guo C, Li Y, et al. Therapeutic targets and signaling mechanisms of vitamin C activity against sepsis: a bioinformatics study. Brief Bioinform 2021;22:bbaa079. [DOI] [PMC free article] [PubMed]
- 26.Szarka A, Kapuy O, Lőrincz T, et al. Vitamin C and cell death. Antioxid Redox Signal 2021;34:831-44. 10.1089/ars.2019.7897 [DOI] [PubMed] [Google Scholar]
- 27.Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther 2020;18:99-101. 10.1080/14787210.2020.1706483 [DOI] [PubMed] [Google Scholar]
- 28.Abobaker A, Alzwi A, Alraied AHA. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep 2020;72:1517-28. 10.1007/s43440-020-00176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020;12:100190. 10.1016/j.phanu.2020.100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brant EB, Angus DC. Is high-dose vitamin C beneficial for patients with sepsis? JAMA 2019;322:1257-8. 10.1001/jama.2019.11643 [DOI] [PubMed] [Google Scholar]
- 31.Im DS, Shin HJ, Yang KJ, et al. Cilastatin attenuates vancomycin-induced nephrotoxicity via P-glycoprotein. Toxicol Lett 2017;277:9-17. 10.1016/j.toxlet.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 32.Cetin H, Olgar S, Oktem F, et al. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin Exp Pharmacol Physiol 2007;34:1181-5. 10.1111/j.1440-1681.2007.04695.x [DOI] [PubMed] [Google Scholar]
- 33.Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys 2013;42:443-68. 10.1146/annurev-biophys-083012-130338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siomek A. NF-κB signaling pathway and free radical impact. Acta Biochim Pol 2012;59:323-31. 10.18388/abp.2012_2116 [DOI] [PubMed] [Google Scholar]
- 35.Zhang GG, Bai YP, Chen MF, et al. Asymmetric dimethylarginine induces TNF-alpha production via ROS/NF-kappaB dependent pathway in human monocytic cells and the inhibitory effect of reinioside C. Vascul Pharmacol 2008;48:115-21. 10.1016/j.vph.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 36.Qi S, Xin Y, Guo Y, et al. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int Immunopharmacol 2012;12:278-87. 10.1016/j.intimp.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 37.Tao Y, Yan D, Yang Q, et al. Low K+ promotes NF-kappaB/DNA binding in neuronal apoptosis induced by K+ loss. Mol Cell Biol 2006;26:1038-50. 10.1128/MCB.26.3.1038-1050.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta J, Fan Y, Gupta N, et al. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 2006;25:6800-16. 10.1038/sj.onc.1209938 [DOI] [PubMed] [Google Scholar]
- 39.Qu S, Dai C, Lang F, et al. Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob Agents Chemother 2018;63:e01545-18. 10.1128/AAC.01545-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi MZ, Yao Y, Xie RL, et al. Intravenous vitamin C attenuates hemorrhagic shock-related renal injury through the induction of SIRT1 in rats. Biochem Biophys Res Commun 2018;501:358-64. 10.1016/j.bbrc.2018.04.111 [DOI] [PubMed] [Google Scholar]
- 41.Asehnoune K, Strassheim D, Mitra S, et al. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 2004;172:2522-9. 10.4049/jimmunol.172.4.2522 [DOI] [PubMed] [Google Scholar]
- 42.Sakon S, Xue X, Takekawa M, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 2003;22:3898-909. 10.1093/emboj/cdg379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 2009;47:891-905. 10.1016/j.freeradbiomed.2009.06.033 [DOI] [PubMed] [Google Scholar]
- 44.Zhao B, Fei J, Chen Y, et al. Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complement Altern Med 2014;14:442. 10.1186/1472-6882-14-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li ZL, Zhou SF. A SILAC-based approach elicits the proteomic responses to vancomycin-associated nephrotoxicity in human proximal tubule epithelial HK-2 cells. Molecules 2016;21:148. 10.3390/molecules21020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as