Abstract
Background
Ginkgo biloba extract (EGb) is widely used to treat impairments in memory, cognition, activities of daily living, inflammation, edema, stroke, Alzheimer's dementia, and aging.
Aim
We aimed to evaluate the safety and efficacy of EGb in treating vascular cognitive impairment (VCI).
Methods
The systematic review was performed using the latest guidelines. We searched for EGb-related trials up to March 1, 2021, in four Chinese databases, three English databases, and clinical trial registry platforms. Randomized controlled trials (RCTs) were included if the study enrolled participants with VCI. Two reviewers independently extracted the data and critically appraised the study quality. Heterogeneity was quantified with I2. Both sensitivity and subgroup analyses were used to identify the sources of heterogeneity. Publication bias was assessed with funnel plots. We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to rate the evidence quality. Outcomes included assessments using the Activities of Daily Living (ADL), Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE), Hasegawa Dementia Scale (HDS), Barthel Index (BI), Functional Activity Questionnaire (FAQ), and adverse events.
Results
In this study, a total of 2019 patients in 23 RCTs were included. EGb appeared to be more effective than control conditions as assessed by the results of cognitive function evaluation, including MMSE (MDMMSE,EGb vs.blank = 3.04, 95% CI: 0.10-5.98; MDMMSE,EGb vs.drugs for VCI = 2.70, 95% CI: 1.39-4.01; MDMMSE,EGb+drugs for VCI vs.blank = 5.90, 95% CI: 4.21-7.59; and MDMMSE,EGb+drugs for VCI vs.drugs for VCI = 3.14, 95% CI: 2.14-4.15), MoCA (MDMoCA,EGb vs.blank = 5.30, 95% CI: 2.15-8.46; MDMoCA,EGb+drugs for VCI vs.blank = 2.66, 95% CI: 1.82-3.50; and MDMoCA,EGb+drugs for VCI vs.drugs for VCI = 2.56, 95% CI: 1.85-3.27), HDS (MDHDS,EGb vs.blank = 6.50; 95% CI: 4.86-8.14; MDHDS,EGb+drugs for VCI vs.drugs for VCI = 3.60, 95% CI: 2.50-4.70), ADL (MDADL,EGb vs.blank = 7.20, 95% CI: 3.28-11.12; MDADL,EGb+drugs for VCI vs.blank = 10.00, 95% CI: 7.51-12.49; and MDADL,EGb+drugs for VCI vs.drugs for VCI = 9.20, 95% CI: 7.26-11.14), BI (MDBI,EGb+drugs for VCI vs.drugs for VCI = 5.71, 95% CI: 2.99-8.43; MDFAQ,EGb vs.drugs for VCI = −1.43, 95% CI: -2.78 to 0.08), and FAQ (MDFAQ,EGb+drugs for VCI vs.drugs for VCI = −2.17, 95% CI: -4.13 to 0.21). Evidence of certainty ranged from medium certainty to very low certainty.
Conclusion
This meta-analysis showed that EGb may be an effective and safe treatment in improving MMSE, MOCA, ADL, and BI for VCI patients within three months of diagnosis. However, given the quality of the included RCTs, more preregistered trials are needed that explicitly examine the efficacy of EGb. This systematic review has been registered on PROSPERO, with the registration number CRD42021232967.
1. Introduction
Vascular cognitive impairment (VCI) may occur as a consequence of cardiovascular disease (CVD) and covers a broad spectrum of cognitive dysfunction, ranging from subjective cognitive decline and mild cognitive impairment to dementia [1, 2]. There is little consistency in the overall incidence of VCI, possibly because of different settings and designs, as well as neuroimaging accessibility [3, 4]. VCI is a clinical syndrome that occurs as a result of many different vascular pathologies [5, 6]. As a general statement, any disease process causing cerebral ischemia or hemorrhage can cause VCI. Therefore, VCI may become the silent epidemic of the 21st century [7]. The Guidelines from the Vascular Impairment of Cognitive Classification Consensus Study (VICCCS), International Society for Vascular Behavioural and Cognitive Disorders (VASCOG), and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) have divided VCI into mild VCI and major VCI according to the severity of cognitive impairment. There are four subtypes of major VCI, including poststroke dementia (PSD), pubcortical ischemic vascular dementia (SIVaD), multi-infarct dementia (MID), and mixed dementias (MixD) [8, 9]. The clinical features of VCI are variable, depending on the type, extent, and location of the underlying cerebrovascular pathology, and include memory problems, mental slowness, and problems with executive functions. VCI is the second most common cause of dementia, accounting for 15% of dementia cases [10], with a higher prevalence of vascular dementia (VaD) in the elderly in Asia [11]. With the rise in life expectancy over the past century, the number of people affected by dementia is likely to rise. Patients with VaD have a higher level of disability and higher rates of cerebrovascular diseases, congestive heart failure, hemiplegia, paraplegia, myocardial infarction, and a higher relative risk of death compared to Alzheimer disease (AD), thus increasing both the complexity and costs of management of the disease [12, 13]. VCI represents a global problem and poses a substantial economic cost to public health systems and society in Asia now and in the near future [14]. Studies have found that VCI is common and suggest it to be an important target for treatment because it may be preventable [15]. Interventions against potentially modifiable risk factors associated with VCI [16], such as controlling diabetes and hypertension and avoiding midlife obesity, among others, have been proposed as ways of reducing dementia [17].
Extracts of the leaves of the maidenhair tree, Ginkgo biloba, have long been used for treating various disorders and are one of the most widely used plant-based products. Standardized extracts are prescribed for the treatment of various disorders, including cognitive dysfunction, headache, tinnitus, vertigo, inattention, mood disturbances, cardiovascular disease [18], coronary heart disease [19, 20], and age-related macular degeneration [21]. Ginkgo biloba is mainly used in the treatment of cerebral dysfunction. The consensus of the Asian Clinical Expert Group on Neurocognitive Disorders in 2019 recommended EGb as an important part of the clinical treatment of neurodegenerative diseases, such as AD, which has received widespread attention [22, 23]. The active components of Ginkgo biloba consist of flavonoids, terpenoids, ginkgolides, and bilobalide. Ginkgo biloba has been demonstrated to have antioxidative activity and has been shown to restore impaired mitochondrial function, thereby improving the neuronal energy supply, as well as improving compromised hippocampal neurogenesis and neuroplasticity [24], inhibiting the aggregation and toxicity of the amyloid β-peptide [25], decreasing blood viscosity, enhancing microperfusion [26], and increasing dopamine levels in the rat prefrontal cortex thus enhancing working memory and executive control [27]. Current studies have shown that EGb can influence the PI3K/Akt, CREB, and RSK1/GSK-3β signaling pathways to play a neuroprotective role [28]. Two well-defined extracts, EGb 761 and Kaveri (LI 1370), are produced from the ground leaves. In Germany, EGb 761 is one of the top five prescription medicines, while it is marketed as a food supplement and available without prescription in the UK, Canada, and the USA [28]. Ginkgo biloba has been the subject of many research reports and has been investigated in numerous clinical trials. Many systematic reviews covering different aspects of Ginkgo biloba have been published [21, 28, 29].
Despite the number of clinical trials conducted to assess its potential properties [30, 31] and the publication of several reviews documenting its efficacy in the prevention of cognitive decline and for treating cognitive impairment and dementia [32], there is still no compelling evidence on the efficacy of EGb for VCI. Therefore, we conducted a systematic review to evaluate the efficacy and safety of EGb for VCI.
2. Materials and Methods
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [33], displayed in Appendix 1.
2.1. Data Sources and Searches
Relevant studies were identified by searching seven databases and two trial registration platforms from their inception to March 1, 2021. The databases included the Chinese Biological Medical Literature Database, Chinese Wanfang data, Chinese VIP information, Chinese National Knowledge Infrastructure, PubMed, EMBASE, and the Cochrane library. The trial registration platforms were the China Clinical Trial Registration Center (ChiCTR) and ClinicalTrials.gov. We also checked the reference lists of all retrieved articles and relevant review articles to identify additional studies. We only paid attention to studies published in English and Chinese. Two individual reviewers (MZ and ZXZ) screened the titles and abstracts to select relevant studies, and duplicate studies were removed after screening each article's abstract and title. Subsequently, the eligibility criteria were used to review full-text manuscripts for available data. Any disagreements were settled via a consensus with a third researcher (XL). The detailed search strategies are shown in Appendix 2.
2.2. Study Selection
This systematic review employed the PICOS strategy, an abbreviation of patient, intervention, comparison, and outcome which was used for all steps in the current systematic review. Firstly, randomized controlled trials (RCTs) that examined the efficacy of EGb for VCI were included. Secondly, we used VCI as the umbrella term encompassing vascular dementia and other cognitive syndromes with a presumed vascular basis (including mild VCI and all subdivisions of major VCI). Thirdly, we included trials with the intervention of EGb (tablets) alone or combined with a drug for VCI (hereafter referred to as DV and mainly including drugs to promote microcirculation and improve cognition, such as donepezil, nimodipine, huperzine, oxiracetam, piracetam, and butylphthalide, among others). There are three common forms of oral Ginkgo leaf products, namely, tablets, capsules, and soft capsules. It has been found in clinical practice that patients prefer tablets than the other two forms. Fourthly, the control therapy could be any kind of DV, blank, or placebo. Normally, patients would be prescribed some basic supporting treatments, including symptomatic treatment for hyperglycemia, hyperlipidemia, and hypertension. The blank or placebo would then be added to the basic supporting treatments. Fifthly, the outcomes covered measurements of cognitive function, including the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Hasegawa Dementia Scale (HDS), as well as the evaluation of daily activities, including the Activities of Daily Living (ADL), Barthel Index (BI), and Functional Activity Questionnaire (FAQ) assessments, and safety was assessed by the occurrence of adverse reactions/events. We excluded studies if they included any of the following: (1) no available full text; (2) non-RCTs (i.e., editorials, commentaries, and letters to the editor); (3) studies with faulty data; and (4) duplicate studies.
2.3. Data Extraction and Quality Assessment
All related records from databases and platforms were imported into the literature management software NoteExpress 3.2.0. The eligible studies were independently screened and selected by two reviewers (MZ and ZXZ). Then, the key information was extracted from the included studies using standardized data extraction forms, including information on the authors and study design, participant characteristics, details of the intervention and control groups, and the outcomes. The risk of bias of the included trials was evaluated by two reviewers (MZ and ZXZ) according to the Cochrane risk of bias assessment tool (Cochrane Reviewers Handbook version 6.1) [34]. Seven items were evaluated, including (1) random sequence generation, (2) allocation hiding, (3) blind setting (researchers, subjects), (4) blind evaluation of study outcomes, (5) data integrity of outcomes, (6) selective reporting of research results, and (7) Other sources of bias (such as potential bias related to special research design, baseline imbalance, and suspected fraud). Each item was evaluated as being “low risk,” “unclear,” or “high risk” and was independently completed by two evaluators (MZ and ZXZ). Disagreements during the study screening, data extraction, and quality appraising were resolved by consulting a third reviewer (XL).
2.4. Data Synthesis and Analysis
The GRADE (the Grading of Recommendations Assessment, Development, and Evaluation) system [35] was used to rate the quality of a body of evidence across outcomes. GRADE has four levels of evidence, also known as certainty in evidence or quality of evidence: very low, low, moderate, and high. We assessed the five aspects for each outcome (risk of bias, inconsistency, imprecision, indirectness, and publication bias) to evaluate the quality of the body of evidence as it related to the studies that contributed data to the meta-analyses. We created a “Summary of findings” table to summarize the effects of interventions on key outcomes, including MMSE, MoCA, HDS, ADL, BI, FAQ, and serious adverse events. We used GRADEpro GDT [36] to create the “Summary of findings.” Explanations for downgrading the quality of the evidence were listed in the footnotes.
Data analysis was performed by Review Manager 5.3 software [37] provided by the Cochrane Collaboration. We calculated the weighted mean difference (WMD) with 95% confidence interval (CI) for continuous data, and the risk ratio (RR) with 95%CI was computed for the dichotomous data. For continuous data, if the outcome was measured on different assessment scales (such as pain), we calculated the standardized mean differences (SMDs) with 95% CIs. Before we performed the meta-analysis, the clinical heterogeneity and methodological heterogeneity were assessed. If there was no clinical or methodological heterogeneity, the chi-square test with a significance level at P < 0.1 and the I2 statistic were used to quantify possible heterogeneity. If P ≥ 0.10 and I2 < 50%, there was no statistical heterogeneity between the studies in the meta-analysis and the fixed effects model was used for analysis. If P < 0.10 and I2 > 50%, this was considered to represent substantial heterogeneity between studies in the meta-analysis, and the random effects model was used to pool the data. Sensitivity or subgroup analyses were performed to determine the reasons for heterogeneity and whether the random effects model could be used for analysis or not. Descriptive analysis was used if the clinical heterogeneity was too large, or there were insufficient reports to perform a meta-analysis. All analyses were two-tailed, with alpha set at 0.05, except for heterogeneity. Publication bias was assessed using funnel plots for more than 10 studies with a particular outcome.
3. Results
3.1. Literature Search and Trial Selection
The original search of the above nine databases yielded 57,821 electronic records, including 6462 records in English and 51,359 records in Chinese. After screening the titles and abstracts, the full text of 3288 articles was reviewed. Ultimately, 23 eligible studies were selected for the present review [38–60] and were included in the qualitative and quantitative synthesis. The screening process is summarized in a flow diagram shown in Figure 1. A self-evaluation according to the PRISMA checklist is shown in Appendix 1.
Figure 1.
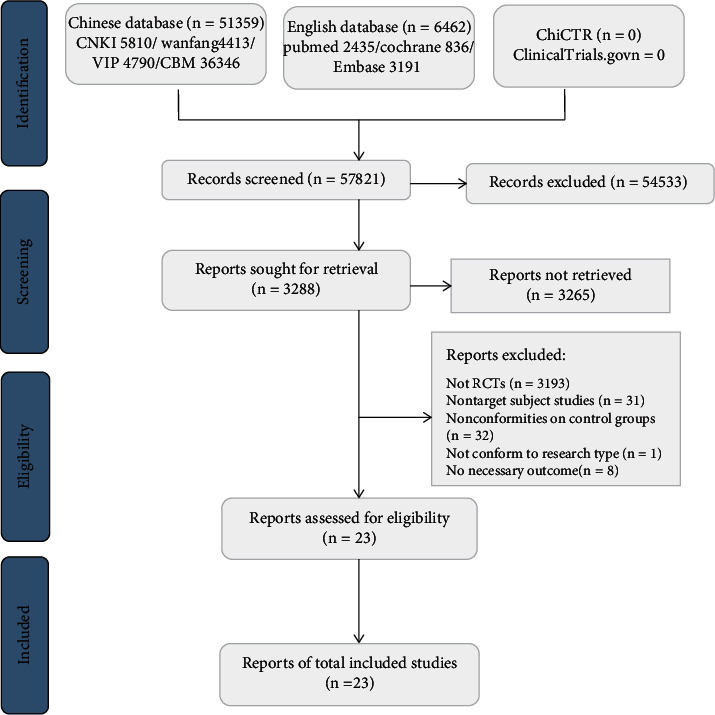
Flow chart of the study selection process.
3.2. Description of the Included Trials
In this study, 23 RCTs on the use of EGb in the treatment of VCI were included. All trials were performed in mainland China and published in Chinese. A total of 2019 patients were included, including 1012 patients in the experimental group and 1007 patients in the control group. The patients' ages ranged from 49 to 83 years. There were no significant differences in sex, age, course of disease, or condition of the subjects between the study groups, with comparable baselines. Among the 23 RCTs, four assessed poststroke cognitive impairment (PSCI) [44, 50, 51, 56]; three studied vascular cognitive impairment, no dementia (VCIND) [47, 53, 59]; one did not mention sepcial subtype [42]; the remaining 15 reported on vascular dementia (VD) [38–41, 43, 45, 46, 48, 49, 52, 54, 55, 57, 58, 60]. Eleven studies [39, 42–45, 47, 48, 52, 53, 55, 56] used the diagnostic criteria of the Neurology Society of Chinese Medical Association, seven studies [38, 40, 41, 46, 49, 54, 57] used the American Psychiatric Association criteria, and the remaining five studies [50, 51, 58–60] were unclear. Sixteen RCTs [38, 40–47, 49–54, 60] used EGb in combination with DV (eight trials [42–45, 50–52, 60] with donepezil, three trials [38, 47, 53] with nimodipine, five trials [40, 41, 46, 49, 54] with huperzine, oxiracetam, piracetam, butylphthalide, ergoloid, and XueSaiTong) as the treatment group versus DV as the control group. Two RCTs [48, 57] used EGb as the monotherapy in the treatment group versus DV alone in the control group. Three RCTs [39, 55, 59] used EGb as the monotherapy in the treatment group versus a blank group. The duration of studies lasted from two to six months. As the outcome measurements, sixteen studies [38–40, 43, 44, 46, 48–52, 54–57, 60] used MMSE, nine studies [39, 42, 45–47, 50, 53, 58, 59] used MoCA, six studies [38–41, 45, 56] used ADL, three studies [44, 51, 55] used HDS, four studies [43, 46, 54, 60] used BI, and three studies [48, 49, 57] used FAQ. The total clinical efficacy rate was observed in 11 studies [41, 43–45, 48–50, 54, 55, 57, 60]. Adverse effects were reported in 15 studies [38–40, 43–45, 48–52, 54, 56, 57, 60]. The characteristics of the 23 trials are summarized in Table 1.
Table 1.
Characteristics of the included studies.
| Study ID | Types of cognitive impairment | Sample size | Interventions | Treatment course/month | Outcomes | Adverse events | ||
|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||
| [50] | PSCI | 42 | 42 | EGb 9.6 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ①② | The treatment group had 3 cases with mild dizziness. In the control group, 1 patient had mild dizziness and 1 patient had poor appetite |
| [51] | PSCI | 34 | 34 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ①④ | One patient in the treatment group had dizziness/nausea/poor appetite. The control group had 7 patients with dizziness/nausea/poor appetite |
| [44] | PSCI | 36 | 36 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ①④ | In the treatment group, 1 case had anorexia, 1 case had dizziness, 2 cases had insomnia, and 2 cases had diarrhea. In the control group, there were 2 cases with loss of appetite, 1 case with nausea and vomiting, 2 cases with dizziness, 1 case with insomnia, and 1 case with diarrhea |
| [38] | VD | 42 | 41 | EGb 40 mg tid + nimodipine 30 mg tid | Nimodipine 30 mg tid | 3 | ①③ | - |
| [40] | VD | 32 | 32 | EGb 0.5 g tid + huperzine 100 mg bid | Piracetam 1.2 g tid | 2 | ①③ | - |
| [52] | VD | 20 | 20 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ① | 3 cases with nausea/loss of appetite and 2 cases with dizziness |
| [60] | VD | 41 | 41 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ①⑤ | - |
| [39] | VD | 30 | 30 | EGb 24 mg tid | Conventional treatment | 3 | ①②③ | Nausea was observed in 2 patients in the treatment group In the control group, 2 patients had nausea and 1 patient had gingival bleeding |
| [58] | VD | 44 | 44 | EGb 19.2 mg tid + oxiracetam 800 mg bid | Conventional treatment | 3 | ② | Not reported |
| [55] | VD | 63 | 63 | EGb 19.2 mg tid | Conventional treatment | 2 | ①④ | Not reported |
| [41] | VD | 52 | 52 | EGb 1piece tid + piracetam 3 piece tid | Piracetam 1.2 g tid | 6 | ③ | Not reported |
| [54] | VD | 46 | 46 | EGb 19.2 mg tid + butylphthalide 0.2 g bid | Butylphthalide 0.2 g bid | 2 | ①⑤ | In the treatment group, there were 2 cases with dizziness, 1 case with nausea and vomiting, and 1 case with rash. The control group had 1 case with dizziness, 1 case with nausea and vomiting, and 1 case with elevated transaminase |
| [45] | VD | 55 | 55 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ②③ | - |
| [43] | VD | 43 | 43 | EGb 19.2 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 3 | ①⑤ | In the treatment group, there were 1 case with dizziness, 3 cases with nausea, and 1 case with abdominal distension. The control group had 1 case with dizziness and 1 case with nausea |
| [42] | VCI | 30 | 30 | EGb 40 mg tid + donepezil 5 mg qn | Donepezil 5 mg qn | 6 | ② | Not reported |
| [47] | VCIND | 60 | 60 | EGb 19.2 mg tid + nimodipine 30 mg tid | Nimodipine 30 mg tid | 3 | ② | Not reported |
| [53] | VCIND | 60 | 60 | EGb 19.2 mg tid + nimodipine 30 mg tid | Nimodipine 30 mg tid | 2 | ② | Not reported |
| [49] | VD | 42 | 40 | EGb 2 pieces tid + ergoloid mesylate sustained release capsules, 1 grain bid | Piracetam 0.8 g tid | 6 | ①⑥ | In the treatment group, there were 2 cases with nausea, 3 cases with dry mouth, and 1 case with dizziness |
| [46] | VD | 64 | 64 | EGb 2 pieces tid + huperzine 0.1 mg tid | Huperzine 0.1 mg tid | 6 | ①②⑤ | Not reported |
| [56] | PSCI | 30 | 31 | EGb 19.2 mg tid + XueSaiTong JiaoNang 0.2 g tid | Conventional treatment | 2 | ①③ | - |
| [48] | VD | 43 | 40 | EGb 0.4 g tid | Flunarizine hydrochloride 5 mg qd | 6 | ①⑥ | The treatment group had 2 cases with dizziness. The control group had 17 patients with headache/dizziness/drowsiness |
| [57] | VD | 43 | 43 | EGb 80 mg tid | Piracetam 0.8 g tid | 6 | ①⑥ | In the treatment group, there were 2 cases with rash and 2 cases with dizziness |
| [59] | VCIND | 60 | 60 | EGb 19.2 mg tid | Conventional treatment | 3 | ② | Not reported |
T: treatment group; C: control group; PSCI: cognitive impairment after cerebral infarction; VD: vascular dementia; VCIND: vascular cognitive impairment without dementia; ①: MMSE; ②: MoCA; ③: ADL; ④: HDS; ⑤: BI; ⑥: FAQ; -: no adverse reactions.
3.3. Risk of Bias Assessment of the Included Studies
All 23 trials claimed randomization, however, none of them described the allocation of concealment methods, and none used placebo controls or registered their protocols. The method of random sequence generation was described in eight trials as a random number table [41–44, 47, 50, 53, 54]; others did not report specific methods. Only one trial used a single-blind method for patients [42]; one trial used a double-blind method for both patients and researchers [46]. Adverse events were reporeted in 15 trials; only one trial reported dropout [38]. The selective reporting assessments of all RCTs were defined as “low” for their clear inclusion and exclusion criteria. The results of the risk of bias assessment of the included studies are shown in Figure 2.
Figure 2.
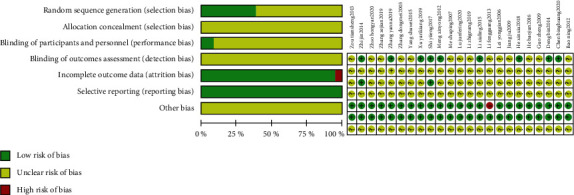
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies and each item for each included study.
3.4. Meta-Analysis
3.4.1. Analysis of MMSE
The effect of EGb compared to the blank on MMSE is summarized in Figure 3. We used random effects models for pooling the effect estimates in two studies (n = 186 patients) [39, 55]. There was a significant difference in favor of EGb for improving MMSE (MD: 3.04; 95% CI: 0.10-5.98; P = 0.04). However, the heterogeneity was substantial (I2 = 94%).
Figure 3.
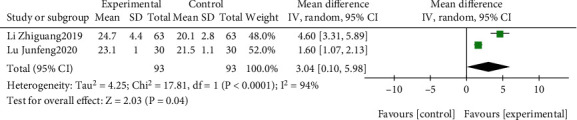
Forest plot of comparison: EGb versus blank group on MMSE levels.
The effect of EGb compared to DV on MMSE is outlined in Figure 4. We employed fixed effects models for pooling the effect estimates in two studies (n = 169 patients) [48, 57]. There was a significant difference in favor of EGb for improving MMSE (MD: 2.70; 95% CI: 1.39-4.01; P < 0.0001; I2 = 0%).
Figure 4.
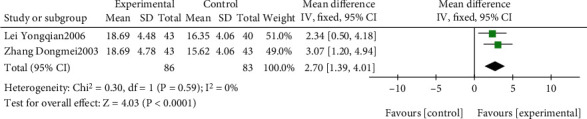
Forest plot of comparison: EGb versus drugs for VCI on MMSE levels.
The effects of EGb combined with DV compared to the blank on MMSE are summarized in Figure 5. Only one study (n = 61 patients) [56] investigated the effect of EGb in conjunction with DV. A significant difference was reported in favor of EGb in conjunction with DV to improve MMSE (MD: 5.90; 95% CI: 4.21-7.59; P < 0.00001).
Figure 5.
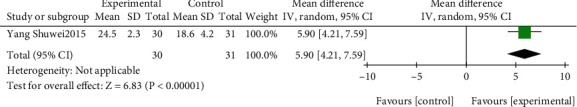
Forest plot of comparison: EGb combined with drugs for VCI versus blank group on MMSE levels.
The effect of EGb combined with DV compared to DV alone on MMSE is summarized in Figures 6 and 7. We used random effects models for pooling the effect estimates from 11 trials (n = 881 patients) [38, 40, 43, 44, 46, 49–52, 54, 60]. There was a significant difference in favor of EGb with DV for improving MMSE (MD: 3.14; 95% CI: 2.14-4.15; P < 0.00001). However, the heterogeneity was substantial (I2 = 83%). Subgroup analysis was conducted according to the different types of cognitive impairment and different courses of intervention. There was a significant difference in favor of EGb with DV for improving MMSE in three studies concerning PSCI (MD: 4.68; 95% CI: 3.25-6.12; P < 0.00001; I2 = 72%) and in eight studies concerning VD (MD: 2.43; 95% CI: 1.66-3.21; P < 0.00001; I2 = 55%). There was a significant difference in favor of EGb with DV for improving MMSE in nine studies with three-month treatment courses (MD: 3.19; 95% CI: 2.03-4.35; P < 0.00001; I2 = 86%) and in two studies with six months of treatment (MD: 2.81; 95% CI: 1.40-4.23; P < 0.0001; I2 = 0%).
Figure 6.
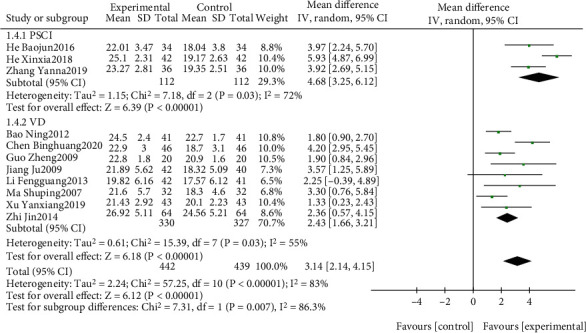
Forest plot of comparison: EGb combined with drugs for VCI versus drugs for VCI on MMSE levels (different types of cognitive impairment).
Figure 7.
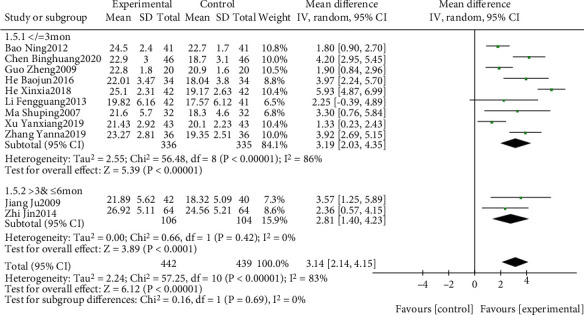
Forest plot of comparison: EGb combined with drugs for VCI versus drugs for VCI on MMSE levels (different treatment courses).
3.4.2. Analysis of MoCA
The effect of EGb compared to the blank on MoCA is summarized in Figure 8. We used random effects models for pooling the effect estimates from two trials (n = 180 patients) [39, 59]. There was a significant difference in favor of EGb for improving MoCA (MD: 5.30; 95% CI: 2.15-8.46; P = 0.001). However, the heterogeneity was substantial (I2 = 95%).
Figure 8.
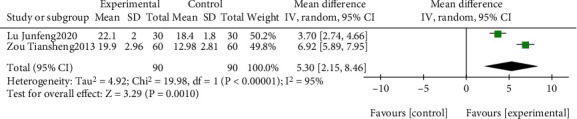
Forest plot of comparison: EGb versus blank group on MoCA levels.
The effect of EGb combined with DV compared to the blank on MoCA is summarized in Figure 9. Only one study (n = 88 patients) [58] investigated the effect of EGb in conjunction with DV. A significant difference was reported in favor of EGb in conjunction with DV to improve MoCA (MD: 2.66; 95% CI; 1.82-3.50; P < 0.00001).
Figure 9.
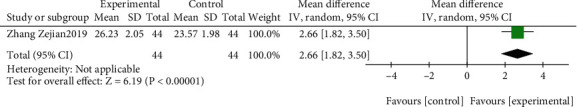
Forest plot of comparison: EGb combined with drugs for VCI versus blank group on MoCA levels.
The effect of EGb combined with DV compared to DV alone on MoCA is summarized in Figures 10 and 11. We used random effects models for pooling the effect estimates from six trials (n = 622 patients) [42, 45–47, 50, 53]. There was a significant difference in favor of EGb with DV for improving MoCA levels (MD: 2.56; 95% CI: 1.85-3.27; P = 0.006; I2 = 53%). Subgroup analysis was conducted according to the different types of cognitive impairment and different courses of intervention. There was a significant difference in favor of EGb with DV for improving MoCA in one study concerning PSCI (MD: 3.38; 95% CI: 2.66-4.10; P < 0.00001) and in two studies concerning VD (MD: 2.04; 95% CI: 1.29-2.78; P < 0.00001; I2 = 0%). There was a significant difference in favor of EGb with DV for improving MoCA in five studies with three-month treatment courses (MD: 2.59; 95% CI: 1.80-3.38; P < 0.00001; I2 = 62%) and in one study with six months of treatment (MD: 2.31; 95% CI: 0.11-4.51; P = 0.04).
Figure 10.
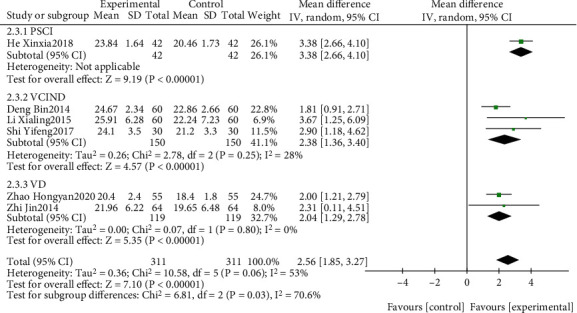
Forest plot of comparison: EGb combined with drugs for VCI versus drugs for VCI on MoCA levels (different types of cognitive impairment).
Figure 11.
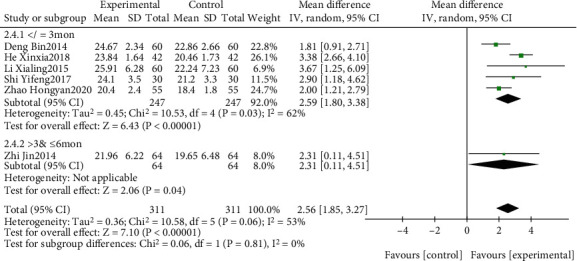
Forest plot of comparison: EGb combined with drugs for VCI versus drug treatment only for VCI on MoCA levels (different courses of treatment).
3.4.3. Analysis of ADL
The effect of EGb compared to the blank on ADL is summarized in Figure 12. Only one trial (n = 60 patients) [39] reported a significant difference in favor of EGb for improving ADL (MD: 7.20; 95% CI: 3.28-11.12; P = 0.0003).
Figure 12.
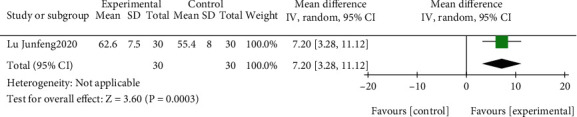
Forest plot of comparison: EGb versus blank group on ADL levels.
The effect of EGb combined with DV compared to the blank on ADL is summarized in Figure 13. Only one study (n = 61 patients) [56] investigated the effect of EGb in conjunction with DV. A significant difference was reported in favor of EGb in conjunction with DV to improve ADL (MD: 7.20; 95% CI: 3.28-11.12; P = 0.0003).
Figure 13.

Forest plot of comparison: EGb combined with drugs for VCI versus blank group on ADL levels.
The effect of EGb combined with DV compared to DV on ADL is summarized in Figure 14. We used fixed effects models for pooling the effect estimates from four trials (n = 361 patients) [38, 40, 41, 45]. There was no significant difference between the two groups (MD: 9.20; 95% CI: 7.26-11.14; P = 0.44; I2 = 0%). Subgroup analysis was conducted according to different courses of intervention. There was a significant difference in favor of EGb with DV for improving ADL in five studies with three-month treatment courses (MD: 8.00, 95% CI: 5.59-10.41; P < 0.00001; I2 = 0%) and in one study with six months of treatment (MD: 11.41; 95% CI: 8.14-14.68; P < 0.00001).
Figure 14.
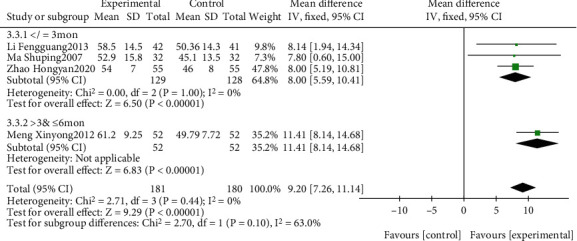
Forest plot of comparison: EGb combined with drugs for VCI versus drug treatment only for VCI on ADL levels (different treatments).
3.4.4. Analysis of HDS
The effect of EGb compared to the blank on HDS is summarized in Figure 15. Only one trial (n = 126 patients) [55] reported that a significant difference in favor of EGb for improving HDS (MD: 6.50; 95% CI: 4.86-8.14; P < 0.00001).
Figure 15.
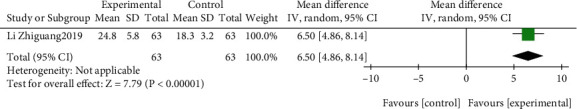
Forest plot of comparison: EGb versus blank group on HDS levels.
The effect of EGb combined with DV compared to DV on HDS is summarized in Figure 16. We used random effects models for pooling the effect estimates from two trials (n = 140 patients) [44, 51]. There was a significant difference between the two groups (MD: 3.60; 95% CI: 2.50-4.70; P < 0.00001; I2 = 69%).
Figure 16.
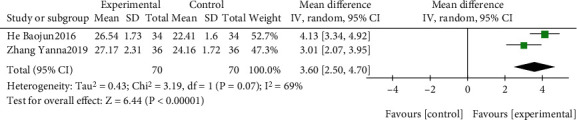
Forest plot of comparison: EGb combined with drugs for VCI versus drug treatment only for VCI on HDS levels.
3.4.5. Analysis of BI
The effect of EGb combined with DV compared to DV alone on BI is summarized in Figure 17. We used random effects models for pooling the effect estimates from four trials (n = 388 patients) [43, 46, 54, 60]. There was a significant difference between the two groups (MD: 5.71; 95% CI: 2.99-8.43; P = 0.0002; I2 = 85%). Subgroup analysis was conducted according to different intervention courses. There was a significant difference in favor of EGb with DV for improving BI in three studies with three-month treatment courses (MD: 6.78; 95% CI: 3.64-9.91; P < 0.0001; I2 = 84%) and in one study with six months of treatment (MD: 2.59; 95% CI: 0.52-4.66; P = 0.01).
Figure 17.
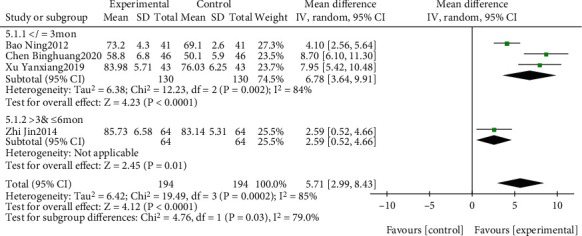
Forest plot of comparison: EGb combined with drugs for VCI versus drug treatment alone for VCI on BI levels (different treatments).
3.4.6. Analysis of FAQ
The effect of EGb compared to DV alone on FAQ is summarized in Figure 18. We used fixed effects models for pooling the effect estimates from two trials (n = 169 patients) [48, 57]. There was a significant difference in favor of EGb for improving FAQ (MD: -1.43; 95% CI: -2.78 to 0.08; P = 0.04; I2 = 0%).
Figure 18.
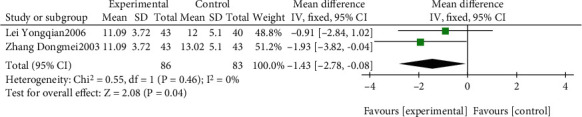
Forest plot of comparison: EGb versus drug treatment only for VCI on FAQ levels.
The effect of EGb combined with DV compared to DV alone on FAQ is summarized in Figure 19. Only one study (n = 82 patients) [49] investigated the effect of EGb in conjunction with DV. A significant difference was reported in favor of EGb in conjunction with DV to improve FAQ (MD: -2.17; 95% CI: -4.13 to 0.21; P = 0.03).
Figure 19.

Forest plot of comparison: EGb combined with drugs for VCI versus drugs for VCI on FAQ levels.
3.5. Adverse Events
Adverse effects were reported in 15 studies [38–40, 43–45, 48–52, 54, 56, 57, 60] of the 23 included studies, but were not mentioned in the remaining eight studies [41, 42, 46, 47, 53, 55, 58, 59]. Five out of the 15 studies reported that no adverse effects occurred during the trial [38, 40, 45, 56, 60]. The remaining 10 studies [39, 41–44, 46–55, 57–59] reported nine different kinds of adverse events, in which methods for judging adverse events were participant and carer reports, medical notes, or clinical observation or a combination of these. These adverse events were reported in the treatment group and in the control group respectively, except one study which did not mention which group. Six studies reported both groups experienced dizziness/nausea/vomiting/insomnia and diarrhea [43, 44, 50, 51, 54, 57]. Two studies reoported skin rashes [54, 57] and one study reported dry mouth [49] happened in the treatment group, while one study reported gingival bleeding [39] and one study [54] reported elevated transaminase happened in the control group. However, life-threatening adverse effects were not reported in any of the studies. All the details of the reported adverse effects are described in the last column in Table 1.
3.6. Publication Bias
To explore the issue of publication bias, a funnel plot was constructed in which the standard error of the mean difference was plotted against the mean difference. The funnel plots for the MMSE from 11 studies [38, 40, 43, 44, 46, 49–52, 54, 60] that compared EGb with DV versus DV alone suggested publication bias as many of the smaller studies had more positive results (Figure 20).
Figure 20.
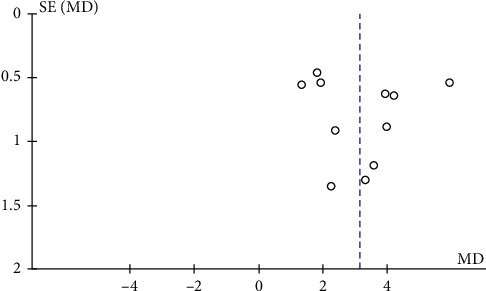
Funnel plot of comparison of EGb with drugs for VCI versus drugs alone on MMSE. The horizontal axis shows the mean difference between the estimated effects of EGb with drugs versus drugs alone on MMSE, while the vertical axis shows the standard error of the intervention effect on a reversed scale.
3.7. Grade Evaluation of Outcomes
GRADEpro software, version 3.6.1, was used to evaluate the quality of evidence of the outcomes. Due to various biases, inconsistencies, inaccuracies, and publication bias, the evidence of certainty ranges from medium certainty to very low certainty, as shown in Table 2.
Table 2.
Summary of findings.
| EGb alone or combined with drugs for VCI versus drugs for VCI alone or blank group “Drugs for VCI” and “blank” are abbreviated as “DV” and “B,” respectively. | |||||
|
| |||||
| Patients or population: the patients diagnosed with VCI were not limited in terms of age, sex, and race Intervention: use of EGb alone or combined with drugs for VCI Comparison: drugs for VCI or blank control | |||||
|
| |||||
| Outcomes | Anticipated absolute effects∗ (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with control group | Risk with experimental group | ||||
| MMSE | |||||
| EGb vs. B | MMSE mean range 20.1-21.5 | MD 3.04 (0.10 lower to 5.98 higher) | — | 186 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3,4 |
| EGb vs. DV | The average levels in the control group were 15.62-16.35 | MD 2.7 (1.39 lower to 4.01 higher) | — | 169 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,4 |
| EGb + DV vs. B | There was a significant difference in MMSE between EGb + DV and B (P < 0.00001) in one RCT [56]. | — | 61 (1 RCT) | ⊕⊝⊝⊝ Low1,2 |
|
| EGb + DV vs. DV | MMSE range 17.57-24.56 | MD 3.14 (0 lower to 4.15 higher) | — | 881 (11 RCTs) | ⊕⊝⊝⊝ Very low1,3,5 |
| MoCA | |||||
| EGb vs. B | MoCA mean range 12.98-18.4 | MD 5.3 (2.15 lower to 8.46 higher) | — | 180 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3,4 |
| EGb + DV vs. B | There was a significant difference in MMSE between EGb + DV and B (P < 0.00001) in one RCT [58] | — | 88 (1 RCT) | ⊕⊝⊝⊝ Low1,2 |
|
| EGb + DV vs. DV | MoCA mean range 18.4-22.86 | MD 2.56 (2.66 lower to 3.27 higher) | — | 622 (6 RCTs) | ⊕⊝⊝⊝ Very low1,3,5 |
| ADL | |||||
| EGb vs. B | There was a significant difference in ADL between EGb and B (P = 0.0003) in one RCT [39] | — | 60 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,4 |
|
| EGb + DV vs. B | There was a significant difference in ADL between EGb + DV and B (P < 0.00001) in one RCT [56] | — | 61 (1 RCTs) | ⊕⊝⊝⊝ Very low1,2,4 |
|
| EGb + DV vs. DV | ADL mean range 45.1-50.36 | MD 9.2 (5.59 lower to 11.41 higher) | — | 361 (4 RCTs) | ⊕⊝⊝⊝ Medium1 |
| HDS | |||||
| EGb vs. B | There was a significant difference in HDS between EGb and B (P < 0.00001) in one RCT [55] | — | 126 (1 RCT) | ⊕⊝⊝⊝ Very low1,2,4 |
|
| EGb + DV vs. DV | HDSL mean range 22.41-24.16 | MD 3.6 (2.5 lower to 4.7 higher) | — | 140 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 |
| BI | |||||
| EGb + DV vs. DV | BI mean range 50.1-83.14 | MD 5.71 (3.64 lower to 8.43 higher) | — | 388 (4 RCTs) | ⊕⊝⊝⊝ Very low1,3,4,5 |
| FAQ | |||||
| EGb vs. DV | FAQ mean range 12-13.02 | MD1.43 (2.78 higher to 0.08 lower) | — | 169 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 |
| EGb + DV vs. DV | There was a significant difference in FAQ between EGb + DV and DV (P = 0.03) in one RCT [49] | — | 82 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 |
|
∗The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; CI: confidence interval; RCT: randomized controlled trials. 1Bias in the integrity of hidden allocation, blind method, and result data. 2Funnel plot asymmetry. 3High heterogeneity, I2 ≥ 50%. 4Wide confidence interval. 5Differences in the types of cognitive impairment of the subjects, control medication, or course of treatment. Outcomes: EGb vs. B: EGb vs. blank control; EGb vs. DV: EGb vs. drug for VCI; EGb + DV vs. B: EGb + drug for VCI vs. blank control; EGb + DV vs. DV: EGb + drug for VCI vs. drug for VCI.
4. Discussion
There is currently no early intervention strategy for cognitive impairment and preventing cognitive decline [61]. The interests of the public and the medical profession in the use of EGb for cognitive impairment and dementia have grown considerably in recent years [8, 61, 62]. There is evidence to support the efficacy of EGb treatment for dementia, and the effect has been found to be dose- and age-dependent [32]. Our analysis supports the efficacy of EGb (in tablet form) for VCI, and it appeared to be well tolerated. This study is a systematic review of the English and Chinese literature to determine the efficacy and safety of EGb for VCI. Twenty-three RCTs including a total of 2019 patients with VCI met the inclusion criteria. The main finding of this review was that EGb treatment appears to be more effective than controls as assessed by various measures of cognitive function, including MMSE, MoCA, HDS, ADL, BI, and FAQ. The evidence of certainty ranges from medium certainty to very low certainty. Although the findings appear positive, the poor methodological quality and clinical heterogeneity of the included studies limit the evidence to support the use of EGb for VCI. In addition, the safety of EGb treatment could not be confirmed because only 65% (15/23) of the studies mentioned safety issues or investigated adverse effects. Due to the limited number of included studies that analyzed safety, we failed to draw a definitive conclusion, which is one of the major issues needing further confirmation. More attention should be paid to both the monitoring and reporting of adverse effects of EGb.
There are some limitations in this study: (1) Only Chinese and English literature was included. All the participants were Chinese, and the administration of EGb was limited to tablets. This could affect the generalization of the results. (2) The data analysis was performed using published trials with positive results, suggesting that trials with negative results may have been missed, which would make the true effect substantially different from the estimate of the effect. (3) The quality of the included trials was generally low, and the certainty of evidence ranged from medium certainty to very low certainty, reducing our confidence in the estimates of the effects. Neither intention analysis nor allocation concealment strategy was mentioned in any of the studies. (4) We put the different kinds of VCI together for each outcome evaluation, although we performed subgroup analysis on the different VCI type as a supplementary analysis. This may not be in line with clinical practice. (5) The treatment courses in most of the included studies were relatively short, and the long-term consequences of EGb treatment for VCI remain unexplored. (6) Clinicians differ in their experience and use of the measurement scales, such as MMSE, MoCA, and ADL. Inconsistent treatment methods in the control group and differences in drug dosage or course may also have an impact on the evaluation of efficacy and safety. The in-homogeneity of the basic supporting treatment may have a confounding effect. Consequently, the results generated from the current review should be interpreted with caution.
Conducting clinical trials in VCI has many obstacles. Clinical outcomes in VCI patients are multifaceted, as they may experience further cognitive decline and may also experience progressive vascular morbidity, mortality, and general deterioration of function [15, 63, 64]. Outcome measures in future trials should include brain structure and function imaging and disease progression determined by macro network diagrams of the brain along with patient-reported outcomes, such as PET (positron emission tomography) and SPECT (single-photon emission computed tomography), measured using a validated rCBF (regional cerebral blood flow) scale [65–67]. In addition, the 23 included studies contained various types of VCI and different kinds of DV. VCI encompasses a heterogeneous population in terms of cognitive profile and severity of deficits, vascular brain injury, and concurrent neurodegenerative pathology [9]. Thus, the patients should be divided into specific subgroups according to different ages and EGb dosages. It is reasonable to take the most common kinds of VCI with high incidence as future target types to explore the precise benefits obtained from EGb. A long follow-up with long-term outcomes is important to determine the effectiveness and safety of EGb[68]. The safety of EGb is a major concern in clinical practice. Thus, safety monitoring of EGb in pharmacovigilance systems is needed.
Our results are based on published studies, the number of included studies was small, and the quality was poor, which may lead to low credibility of the conclusions. Future research on EGb in VCI should implement higher quality research methodology to limit the potential for bias. More large-scale, multicenter randomized controlled clinical trials on related mechanisms should be implemented in a scientifically designed manner, clinically important outcomes should be selected, and longer treatments and follow-up periods should be used. We recommend that the SPIRIT 2013 and the CONSORT 2010 statement [69–72] should be used as a guideline when designing and reporting RCTs for EGb in the future.
5. Conclusion
In summary, in patients with VCI, Ginkgo biloba extract tablets can be taken separately or in addition to other medication. Although the available evidence from the present review supported the efficacy of Gingko biloba extract, recommendations for its routine use for the treatment of VCI are limited by the poor methodological quality and clinical heterogeneity of the included studies. Nevertheless, we have identified an area that is worthy of further study. Further well-designed rigorous RCTs of Ginkgo biloba extract for VCI are needed.
Acknowledgments
This study was supported by CACMS Innovation Fund (No. CI2021A00701-3); NATCM TCM Inheritance and Innovation “Hundred-Thousand-Ten Thousand” Talents Project (QiHuang Scholar)-National TCM Leading Personnel Support Program (NATCM Personnel and Education Department [2018] No. 12); the project from the China Center for Evidence Based Traditional Chinese Medicine (No. 2020YJSZX-2); the project of Evidence-Based Medicine of Traditional Chinese Medicine (No. ZZ13-024-3); the Fundamental Research Funds of the Central Public Welfare Research Institutes Grant (No. ZZ13-YQ-075); the National Natural Science Foundation Project of China (No. 81873168); and the National Key Research and Development Program of China (No. 2018YFC1704303).
Contributor Information
Yunling Zhang, Email: yunlingzhang2004@163.com.
Xing Liao, Email: okfrom2008@hotmail.com.
Data Availability
All relevant data are within the article and its supporting information files. The data supporting this systematic review and meta-analysis are from published literature and accessible datasets, which have been cited. The original included articles used in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors' Contributions
Zhan Min and Xing Liao designed the study, conducted the analysis, and drafted the manuscript. Zeng Zixiu and Shen Wei performed the literature selection. Sun Linjuan and Liu Jianxun did the quality assessment. Wang Ying and Han Fuhua performed the data extraction. Shi Jingzi, Zeng Xinyun, and Lu Xiyue did part of the statistical work. Zhang Yunling and Liao Xing critically revised the manuscript.
Supplementary Materials
Appendix 1: checklist of items for the meta-analysis according to PRISMA statement.
Appendix 2: supplementary retrieval information: RCTs of Ginkgo biloba Extract in the treatment of VCI.
References
- 1.Levine D. A., Langa K. M. Vascular cognitive impairment: disease mechanisms and therapeutic implications. Journal of the American Society for Experimental NeuroTherapeutics. 2011;8(3):361–373. doi: 10.1007/s13311-011-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter A., Pillai J. A. Treatment of vascular cognitive impairment. Current Treatment Options in Neurology. 2015;17(8):p. 367. doi: 10.1007/s11940-015-0367-0. [DOI] [PubMed] [Google Scholar]
- 3.Kalaria R. N., Maestre G. E., Arizaga R., et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurology. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters F. J., Ikram M. A. Epidemiology of vascular Dementia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(8):1542–1549. doi: 10.1161/ATVBAHA.119.311908. [DOI] [PubMed] [Google Scholar]
- 5.Dichgans M., Leys D. Vascular cognitive impairment. Circulation Research. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 6.van der Flier W. M., Flier W., Skoog I., Schneider J. Vascular cognitive impairment nature reviews. Disease primers. 2018;4 doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 7.Román G. C. Vascular dementia may be the most common form of dementia in the elderly. Journal of the Neurological Sciences. 2002;203:7–10. doi: 10.1016/s0022-510x(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev P., Kalaria R., O'Brien J., et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Disease and Associated Disorders. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelick P. B., Scuteri A., Black S. E., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien T. J., Thomas A. Vascular dementia. The Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 11.Davis G., Baboolal N., Mc Rae A., Stewart R. Dementia prevalence in a population at high vascular risk: the Trinidad national survey of ageing and cognition. BMJ Open. 2018;8(2, article e018288) doi: 10.1136/bmjopen-2017-018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillit H., Hill J. The costs of vascular dementia: a comparison with Alzheimer's disease. Journal of the Neurological Sciences. 2002;203-204:35–39. doi: 10.1016/S0022-510X(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 13.Knopman D. S., Rocca W. A., Cha R. H., Edland S. D., Kokmen E. Survival study of vascular dementia in Rochester, Minnesota. Archives of Neurology. 2003;60(1):85–90. doi: 10.1001/archneur.60.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Sun M.-K. Potential therapeutics for vascular cognitive impairment and dementia. Current Neuropharmacology. 2018;16(7):1036–1044. doi: 10.2174/1570159X15666171016164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorhouse P., Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. The Lancet Neurology. 2008;7(3):246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 16.Pendlebury S. T., Rothwell P. M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. The Lancet Neurology. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 17.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Defeudis F., Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions basic studies and clinical applications. Current Drug Targets. 2000;1(1):25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- 19.Shaito A., Thuan D. T. B., Phu H. T. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Frontiers in pharmacology. 2020;11 doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y. Z., Li S. Q., Zu X. G., du J., Wang F. F. Ginkgo biloba extract improves coronary artery circulation in patients with coronary artery disease: contribution of plasma nitric oxide and endothelin-1. Phytotherapy Research. 2008;22(6):734–739. doi: 10.1002/ptr.2335. [DOI] [PubMed] [Google Scholar]
- 21.Evans J. R. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database of Systematic Reviews. 2013;(1, article CD001775) doi: 10.1002/14651858.cd001775.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G., Wang Y., Sun J., Zhang K., Liu J. Ginkgo biloba for mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Current Topics in Medicinal Chemistry. 2016;16(5):520–528. doi: 10.2174/1568026615666150813143520. [DOI] [PubMed] [Google Scholar]
- 23.Kandiah N., Ong P. A., Yuda T., et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: expert consensus on the use ofGinkgo bilobaextract, EGb 761®. CNS Neuroscience & Therapeutics. 2019;25(2):288–298. doi: 10.1111/cns.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchantchou F., Xu Y., Wu Y., Christen Y., Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer's disease. The FASEB Journal. 2007;21(10):2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y., Wu Z., Butko P., et al. Amyloid- -Induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. Journal of Neuroscience. 2006;26(50):13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Költringer P., Langsteger W., Eber O. Dose-dependent hemorheological effects and microcirculatory modifications following intravenous administration of Ginkgo biloba special extract EGb 761. Clinical hemorheology. 1995;4(15):649–656. [Google Scholar]
- 27.Yoshitake T., Yoshitake S., Kehr J. The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. British Journal of Pharmacology. 2010;159(3):659–668. doi: 10.1111/j.1476-5381.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birks J., Grimley Evans J., Cochrane Dementia and Cognitive Improvement Group Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD003120.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Gauthier S., Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clinical Interventions in Aging. 2014;9, article 2065 doi: 10.2147/cia.s72728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amieva H., Meillon C., Helmer C., Barberger-Gateau P., Dartigues J. F. Ginkgo biloba extract and long-term cognitive decline: a 20-year follow-up population-based study. PLoS One. 2013;8(1, article e52755) doi: 10.1371/journal.pone.0052755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeKosky S., Williamson J. D., Fitzpatrick A. L., et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H.-F. An overview of systematic reviews of Ginkgo biloba extracts for mild cognitive impairment and dementia. Frontiers in Aging Neuroscience. 2016;8 doi: 10.3389/fnagi.2016.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page M. J., McKenzie J. E., Bossuyt P. M., et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. Journal of Clinical Epidemiology. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Cumpston M., Li T., Page M. J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:p. ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balshem H., Helfand M., Schünemann H. J., et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Gradepro G. Computer program. McMaster University (developed by Evidence Prime) GRADEpro GDT Hamilton (ON) McMaster University (developed by Evidence Prime); 2015. [Google Scholar]
- 37.Collaboraion T. C. Collaboraion T C. Review Manager (RevMan)[Computer program]. Version [5, 3]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboraion. 2014;5(3) [Google Scholar]
- 38.Li F., Xu Y., Li C., Nie H. Effect of nimodipine combined with gko leaf on vascular dementia [J] Nerve injury and functional reconstruction. 2013;1:30–32. [Google Scholar]
- 39.Lu Junfeng F. F., Jinxia Z. The effect of gingko leaf on vascular endothelial function in patients with vascular dementia [J] Chinese modern doctors. 2020;17(58):16–19+24. [Google Scholar]
- 40.Ma Shuping Z. X. Effect of huperzine A tablet combined with Ginkgo biloba leaf on cognitive ability and hemorheology of patients with vascular dementia. Chinese Community Physicians (General Edition) 2007;6(9):p. 13. [Google Scholar]
- 41.Meng Xinyong Z. Z. Clinical study of Ginkgo biloba combined with piracetam tablet in the treatment of vascular dementia. Chinese Journal of Physician Training. 2012;16:46–47. [Google Scholar]
- 42.Yifeng S. Clinical observation of Ginkgo biloba combined with donepezil in the treatment of vascular cognitive impairment. Nanjing University of Chinese Medicine; 2017. [Google Scholar]
- 43.Yanxiang X. Observation on the curative effect of Ginkgo biloba combined with donepezil in the treatment of vascular dementia patients. Medical Innovation in China. 2019;18:121–124. [Google Scholar]
- 44.Zhang Yanna H. S., Zhouna W. Clinical effect of donepezil combined with Ginkgo biloba leaf in the treatment of cognitive impairment after cerebral infarction. Chinese Journal of Traditional Chinese Medicine. 2019;7:1785–1788. [Google Scholar]
- 45.Zhao H., Xu C., Zheng Y., Su J. Clinical effect observation of ggo biloba leaf combined with donepezil in treatment of vascular dementia [J] Journal of Practical Medical Technology. 2020;77:837–839. [Google Scholar]
- 46.Zhi Jin D. B., Jiwen P., et al. Observation on the curative effect of Ginkgo biloba combined with huperzine A tablet in the treatment of vascular dementia. Shanxi Traditional Chinese Medicine. 2014;11:1468–1469. [Google Scholar]
- 47.Xialing L. Effect of Ginkgo biloba leaf combined with nimodipine on 60 cases of vascular cognitive dysfunction without dementia. Chinese Medicine Guide. 2015;17:187–188. [Google Scholar]
- 48.Yongqian L. Observation on the curative effect of Ginkgo biloba leaf preparation on 43 cases of vascular dementia. Journal of Xiangnan University. 2006;3:26–27. [Google Scholar]
- 49.Ju J. Ginkgo biloba combined with peilei can treat vascular dementia. Modern Hospital. 2009;8:56–57. [Google Scholar]
- 50.Xinxia H. Efficacy and safety of donepezil combined with Ginkgo biloba in the treatment of cognitive dysfunction after cerebral infarction. International Medical and Health Review. 2018;24(14):2105–2106. [Google Scholar]
- 51.Baojun H. Efficacy and safety analysis of donepezil combined with Ginkgo biloba in the treatment of cognitive dysfunction after cerebral infarction. Modern drug application in China. 2016;13:215–216. [Google Scholar]
- 52.Guo Zheng M. B. Clinical observation on 20 cases of vascular dementia treated with donepezil hydrochloride combined with Ginkgo biloba leaves. Chinese Community Physician (Medical Specialty Bimonthly) 2009;11(12):p. 56. [Google Scholar]
- 53.Bin D. Clinical study of Ginkgo biloba leaf combined with nimodipine in the treatment of VCIND. Chinese Tropical Medicine. 2014;2:210–212. [Google Scholar]
- 54.Chen B., Su Q., Shi Q., Lin P., Znhag Y. Treatment of 46 cases of vascular dementia with ginkgo biloba leaves combined with butylphthalide [J] Fujian traditional Chinese medicine. 2020;51(1):83–84. [Google Scholar]
- 55.Li Zhiguang X. G., Haifeng W. Clinical study of Ginkgo biloba leaf combined with sodium cytidine in the treatment of vascular dementia. Journal of New Chinese Medicine. 2019;51(5):126–128. [Google Scholar]
- 56.Shuwei Y. Clinical observation of Ginkgo biloba combined with XueSaitong in the treatment of cognitive impairment after cerebral infarction. Jilin Medical Journal. 2015;36(17):3842–3843. [Google Scholar]
- 57.Zhang Dongmei L. D. Observation of therapeutic effect of Ginkgo biloba leaf preparation on vascular dementia. Practical gerontology. 2003;17(4):197–199. [Google Scholar]
- 58.Zhang Zejian F. F. H. Clinical observation of Ginkgo biloba leaf combined with olacetam capsule in the treatment of mild cognitive impairment after stroke. Grassroots Medical Forum. 2019;23(20):2815–2817. [Google Scholar]
- 59.Zou Tiansheng W. J., Shanshan H. Combination of traditional Chinese and western medicine in the treatment of non - dementia vascular cognitive dysfunction. JiLin journal of traditional chinese medicine. 2013;33(5):487–489. [Google Scholar]
- 60.Bao Ning H. Y. Clinical effect of donepezil hydrochloride combined with Ginkgo biloba leaf in the treatment of vascular dementia. Chinese Medicine. 2012;7(8):965–966. [Google Scholar]
- 61.Skrobot O. A., Black S. E., Chen C., et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the vascular impairment of cognition classification consensus study. Alzheimer's & Dementia. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Writing Group of Dementia and Cognitive Impair Group N S, Chinese Medical Association. Guidelines for the management of vascular cognitive disorders. Chinese Journal of Neurology. 2011;2(44) [Google Scholar]
- 63.Van Der Flier W. M., Skoog I., Schneider J. A., et al. Vascular cognitive impairment. Nature Reviews Disease Primers. 2018;4(1):1–16. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 64.O'Brien T. J., Erkinjuntti T., Reisberg B., et al. Vascular cognitive impairment. The Lancet Neurology. 2003;2(2):89–98. doi: 10.1016/S1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 65.Zuo X.-N., Xing X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience & Biobehavioral Reviews. 2014;45:100–118. doi: 10.1016/j.neubiorev.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Papo D., Buldú J. M., Boccaletti S., Bullmore E. T. Complex network theory and the brain. The Royal Society; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7, article e68910) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H., Zhou C., Yu M., et al. The effect of Ginkgo biloba dropping pills on hemorheology and blood lipid: a systematic review of randomized trials. Evidence-based Complementary and Alternative Medicine. 2019;2019:12. doi: 10.1155/2019/2609625.2609625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Faes L., Calvert M. J., Denniston A. K. Extension of the CONSORT and SPIRIT statements. The Lancet. 2019;394(10205):p. 1225. doi: 10.1016/S0140-6736(19)31819-7. [DOI] [PubMed] [Google Scholar]
- 70.Eldridge S. M., Chan C. L., Campbell M. J., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:p. i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulz K. F., Altman D. G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of internal medicine. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 72.Dutton S. J. The SPIRIT 2013 statement. Maturitas. 2014;78(1):1–2. doi: 10.1016/j.maturitas.2014.02.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: checklist of items for the meta-analysis according to PRISMA statement.
Appendix 2: supplementary retrieval information: RCTs of Ginkgo biloba Extract in the treatment of VCI.
Data Availability Statement
All relevant data are within the article and its supporting information files. The data supporting this systematic review and meta-analysis are from published literature and accessible datasets, which have been cited. The original included articles used in this study are available from the corresponding author upon request.


