Abstract
Brassinosteroids are plant steroid hormones that are essential for plant growth. When germinated rice seeds were treated with brassinolide (BL), stems were elongated and root spiral formation was observed at 5 nM of BL. Such root spiral formation was not induced by other plant hormones such as auxin and gibberellin. Since weak non-steroidal brassinolide-like compound (NSBR1) also induced spiral formation, this root spiral induction can be used as the index in the search for BL-like compounds.
Keywords: brassinolide, brassinosteroid, rice seeds, Oryza sativa, NSBR1
Introduction
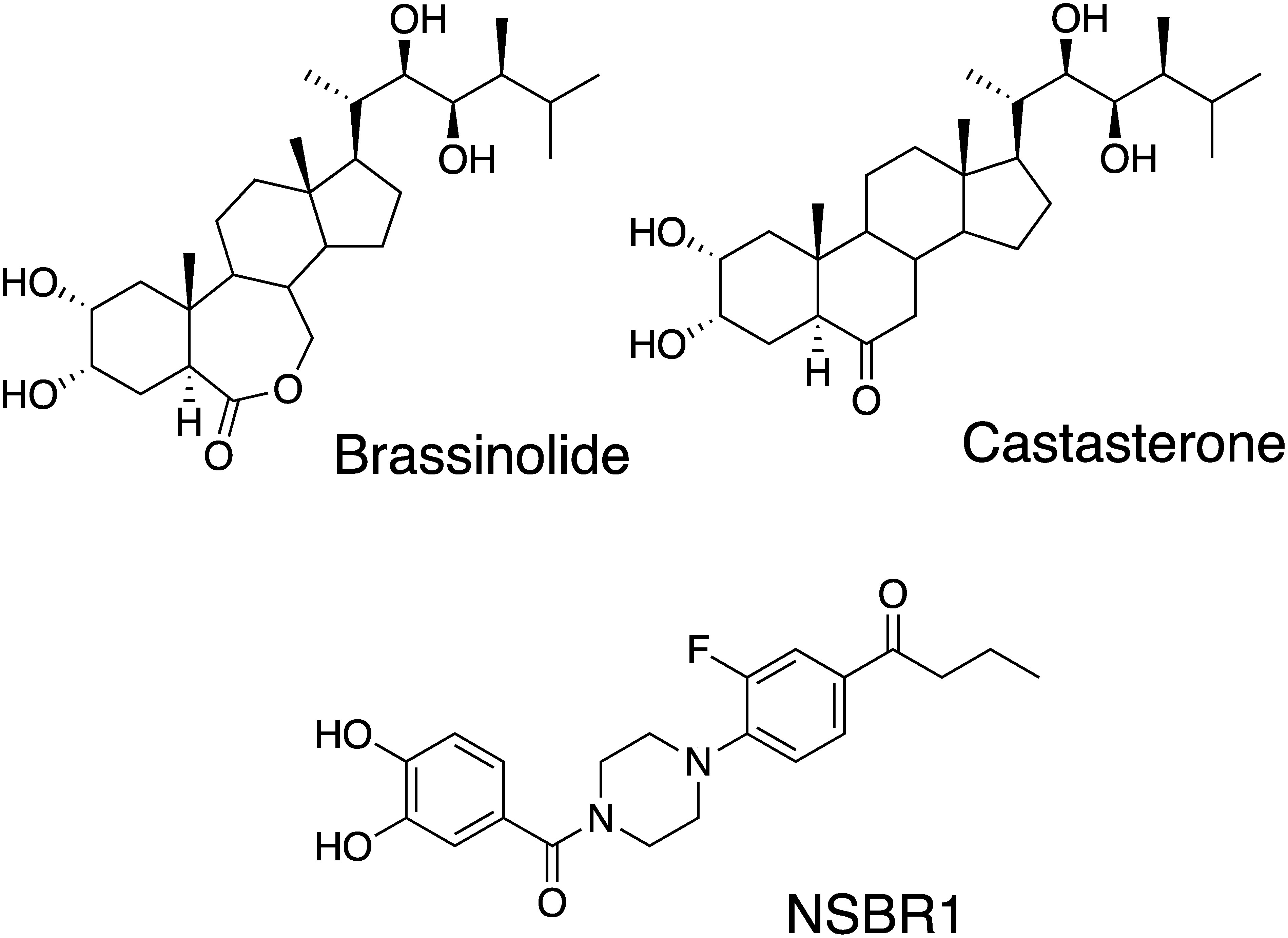
About a half century ago, Michel et al. of the USDA reported that Zea mays pollen contained ingredients expressing plant growth–promoting activity. The title of the paper is “Brassins—a new family of plant hormones from rape pollen.”1) The structure of the brassin was first characterized in 1979 by X-ray analysis and named brassinolide (BL; Fig. 1).2) A few years later, Yokota et al. found castasterone (CS; Fig. 1), from chestnut insect gall, which contains a six-membered cyclohexanone ring instead of a seven-membered lactone ring of BL.3) These compounds have sterol skeletons constructed from four fused rings (A/B/C/D) and hydroxyalkyl chains at the C17 position that were collectively called brassinosteroids (BRs).4)
Fig. 1. Structures of brassinosteroids and an agonist (NSBR1).
Before the characterization of BL by the USDA group, Marumo’s group at Nagoya University also had been trying to isolate and characterize new auxin-like substances that are called Distylium factors. They used the rice lamina inclination assay (RLIA), which was originally designed to detect synthetic auxins,8) to measure the activity of BRs.5–7) Wada et al. also reported a simple bioassay for brassinosteroids using a wheat leaf–unrolling test.9)
With the discovery of BR-deficient mutants,10) BRs were recognized as the sixth plant hormone. Arabidopsis mutants det2 and cpd are cabbage-like dwarfs in the light, and these phenotypes were not recovered to wild type by classical hormones such as auxins and gibberellin but were only rescued by BRs. Asami et al. discovered a BR biosynthesis inhibitor, brassinazole (Brz), which induced dwarfing in Arabidopsis.11,12) To that point, more than 70 BRs had been identified in nature, chemically synthesized, and the activity evaluated using rice, Arabidopsis, cress, beans, etc. In these plants, RLIA is a good method because the activity can be determined quantitatively. Using RLIA, we measured the hormonal activity quantitatively and discussed the structure activity relationship.13–15) With further study, we discovered the novel non-steroidal brassinolide-like compound NSBR1 (Fig. 1)16) using in silico screening; in this study, however, we used the bioassays of Arabidopsis, since RLIA was too time consuming and inappropriate for assaying a number of candidate compounds.
Even BRs show interesting activity and are expected to be utilized in agriculture, as the bioassay methods that have been used to date are time consuming and not reproducible. Therefore, a simple and user-friendly bioassay method is desired. Here we developed a new and simple BL-specific bioassay method using rice seeds.
Materials and methods
Rice seeds were obtained from Oryza sativa Koshihikari cultivated in Shiga Prefecture. Seeds were soaked in running tap water for a few days. Ten germinated seeds with buds (less than 2 mm) were put on the bottoms of 50 mL beakers, and then 2 mL of water containing test compounds was added. Ten microliters of the stock solution in ethanol or DMSO of the test compound was added to 2 mL of water in a glass test tube and mixed by repetitive pipetting. DMSO can be used to prepare the stock solution, although DMSO solution is not suitable for RLIA. Seeds were grown at 25°C for 2 days in the light, and the growth of rice plants was observed. This method is shown in Fig. 2.
Fig. 2. Germination of rice seeds and observation of the root spiral formation. (A) Seeds were soaked in running tap water for a few days. (B) Ten germinated seeds with buds were transferred to a 50 mL beaker, and 2 mL of water was added. (C) Seeds, shoots, and roots were grown at 25°C for 2 days in the light.

Results and discussion
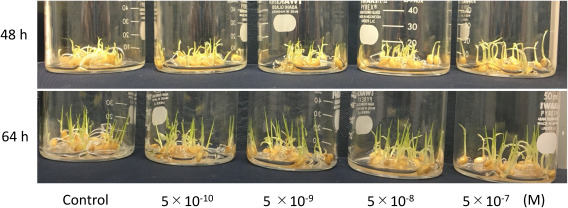
Rice plants were grown in water containing BL at various concentrations after germination. As shown in Fig. 3, the hypocotyl length became longer with BL treatment at higher concentrations 48 hr after germination. Hypocotyl elongation was stimulated in the concentration-dependent manner shown in Fig. 3. This phenomenon was observed reproducibly; however, measuring the hypocotyl length was cumbersome and not appropriate for the quantitative assay. We could not see any significant difference among treatments with different concentrations on the next day (64 hr after treatment), as shown in Fig. 3.
Fig. 3. Growth of rice plant in water containing BL at various concentrations.
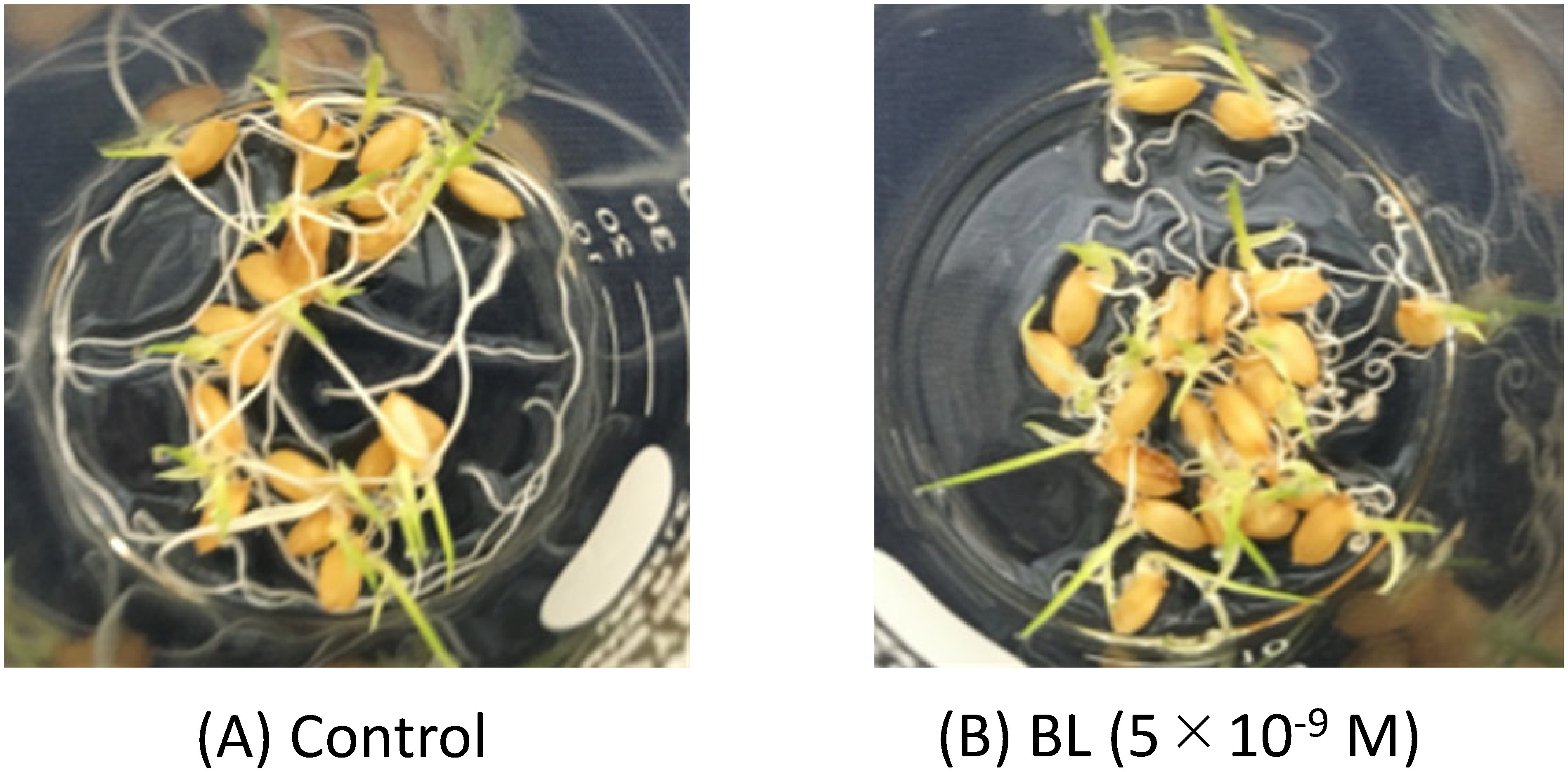
The morphology of rice plant seedlings was, however, significantly changed by BL treatment at higher concentrations. The stems became evidently robust, with stronger green color at 5×10−9 M BL. In particular, a big difference was observed in the root, in which the spiral was induced, as shown in Fig. 4.
Fig. 4. Observation of roots 64 hr after the germination of seeds treated with (A) solvent and (B) BL at 5×10−9 M.
In order to evaluate the effect on the root quantitatively, the seeds were treated with various concentrations of BL. As shown in Fig. 5, spiral induction was observed even at 5×10−9 M; as the concentration was increased, the spiral formation became more remarkable. NSBR1, the non-steroidal brassinolide-like compound16) discovered in our laboratory, also induced the spiral in two of ten seeds treated at 5×10−5 M (Fig. 5-B). We also examined the effect of an antagonist, the 3,4-difluoro-substituted analog of NSBR1, which was discovered in the assay against Arabidopsis.16) As shown in Fig. 5-C, the spiral formation induced by BL at 5×10−8 M was released by treating with an antagonist at 1.7×10−4 M. The release of spiral induction in the roots can be used to bioassay antagonists of BRs.
Fig. 5. Spiral induction in rice seeds. (A) BL at various concentrations. (B) NSBR1 at 5×10−5 M and 2.5×10−4 M. (C) BL antagonist at 1.7×10−4 M.
We also examined the effect of gibberellin on the growth of roots. No spiral was induced in the root at 5×10−6 M of GA3. At a higher concentration (5×10−5 M) of GA3, root growth was significantly inhibited. Likewise, auxins did not induce the spiral, even at the higher concentration (5×10−5 M). Since this spiral induction is specific to BRs and the potency can be determined quantitatively using minimum effective concentrations, a study of the structure–activity relationship for spiral induction by various BRs13–15) is in progress.
Acknowledgments
We thank Mr. Sadao Hirai for kindly supplying the rice seeds.
References
- 1).J. W. Mitchel, N. Mandava, J. F. Worley, J. R. Plimmer and M. V. Smith: Brassins—a new family of plant hormones from rape pollen. Nature 225, 1065–1066 (1970). [DOI] [PubMed] [Google Scholar]
- 2).M. D. Grove, G. F. Spencer, W. K. Rohwedder, N. Mandava, J. F. Worley, J. D. Warthen Jr., G. L. Steffens, J. L. Flippen-anderson and J. C. Cook Jr.: Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281, 216–217 (1979). [Google Scholar]
- 3).T. Yokota, M. Arima and N. Takahashi: Castasterone, a newphytosterol with plant-hormone potency, from chestnut insect gall. Tetrahedron Lett. 23, 1275–1278 (1982). [Google Scholar]
- 4).J. Liu, D. Zhang, X. Sun, T. Ding, B. Lei and C. Zhang: Structure–activity relationship of brassinosteroids and their agricultural practical usages. Steroids 124, 1–17 (2017). [DOI] [PubMed] [Google Scholar]
- 5).K. Wada and S. Marumo: Synthesis and plant growth–promoting activity of brassinolide analogues. Agric. Biol. Chem. 45, 2579–2585 (1981). [Google Scholar]
- 6).K. Wada, S. Marumo, N. Ikekawa, M. Morisaki and K. Mori: Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant Cell Physiol. 22, 323–325 (1981). [Google Scholar]
- 7).S. Fujioka, T. Noguchi, S. Takatsuto and S. Yoshida: Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry 49, 1841–1848 (1998). [Google Scholar]
- 8).E. Maeda: Rate of lamina inclination in excised rice leaves. Physiol. Plant. 18, 813–827 (1965). [Google Scholar]
- 9).K. Wada, H. Kondo and S. Marumo: A simple bioassay for brassinosteroids: A wheat leaf–unrolling test. Agric. Biol. Chem. 49, 2249–2251 (1985). [Google Scholar]
- 10).S. D. Clouse: Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 10, 1–8 (1996). [DOI] [PubMed] [Google Scholar]
- 11).T. Asami and S. Yoshida: Brassinosteroid biosynthesis inhibitors. Trends Plant Sci. 4, 348–353 (1999). [DOI] [PubMed] [Google Scholar]
- 12).W. Rozhon, S. Akter, A. Fernandez and B. Poppenberger: Inhibitors of brassinosteroid biosynthesis and signal transduction. Molecules 24, 4372 (2019). https://doi.org/10.3390/molecules24234372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).S. Uesusuki, B. Watanabe, S. Yamamoto, J. Otsuki, Y. Nakagawa and H. Miyagawa: Synthesis of brassinosteroids of varying acyl side chains and evaluation of their brassinolide-like activity. Biosci. Biotechnol. Biochem. 68, 1097–1105 (2004). [DOI] [PubMed] [Google Scholar]
- 14).S. Yamamoto, B. Watanabe, J. Otsuki, Y. Nakagawa, M. Akamatsu and H. Miyagawa: Synthesis of 26,27-biscastasterone analogs and analysis of conformation–activity relationship for brassinolide-like activity. Bioorg. Med. Chem. 14, 1761–1770 (2006). [DOI] [PubMed] [Google Scholar]
- 15).B. Watanabe, S. Yamamoto, T. Yokoi, A. Sugiura, S. Horoiwa, T. Aoki, H. Miyagawa and Y. Nakagawa: Brassinolide-like activity of castasterone analogs with varied side chains against rice lamina inclination. Bioorg. Med. Chem. 25, 4566–4578 (2017). [DOI] [PubMed] [Google Scholar]
- 16).A. Sugiura, S. Horoiwa, T. Aoki, S. Takimoto, Y. Yamagami, T. Nakano, Y. Nakagawa and H. Miyagawa: Discovery of a nonsteroidal brassinolide-like compound, NSBR1. J. Pestic. Sci. 42, 105–111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]