Abstract
A case of bacteremia due to Ochrobactrum intermedium, with concomitant liver abscesses, in an orthotopic liver transplant recipient is presented. Identical microorganisms were isolated from fecal specimens and from an aspirate of a liver abscess that was indicative of invasion of the graft by gastrointestinal spread. 16S DNA sequence analysis of the blood isolate revealed the recovery of the recently proposed new species O. intermedium, closely related to Ochrobactrum anthropi and Brucella spp.
Until now Ochrobactrum anthropi, an unusual and infrequently encountered nonfermentative bacterium previously known as CDC group Vd (7), has been the only species in the genus Ochrobactrum. O. anthropi is widely distributed in the environment and is an opportunistic pathogen. Closely related to Brucella spp., the microorganism has been isolated from various clinical specimens and may be part of the normal flora of the large intestine (1). Identification of the microorganism by conventional determination methods is difficult, misleading, and time-consuming (6). Multiple antibiotic resistance is the rule, with most strains susceptible only to trimethoprim-sulfamethoxazole, fluoroquinolones, aminoglycosides, and imipenem (8). Nosocomial infections due to O. anthropi, particularly in patients with indwelling central venous catheters, has increased during the last decade (4). An outbreak of O. anthropi bacteremia in five organ transplant recipients (kidney, heart, and pancreas) after the administration of rabbit antithymocyte globulin during the induction phase has been reported (5).
We describe a rare manifestation of infection caused by a microorganism resembling O. anthropi in a 45-year-old female after orthotopic liver transplantation (OLT). During this procedure biliary-enteric continuity was established by a hepaticojejunostomy on a Roux-en-Y jejunal limb. The patient had a history of primary sclerosing cholangitis with Child-Pugh A liver cirrhosis complicated by portal hypertension and splenomegaly with oesophagus varices (grade 2 to 3). Less than 1 month after liver transplantation, the patient developed signs of septicemia, and a diagnosis of cholangitis was made. Two sets of blood cultures (BacT Alert; Organon Technika, Turnhout, Belgium) were collected for aerobic and anaerobic bacterial isolation at the beginning of the febrile period. Three days after collection and incubation of the blood, gram-negative rods were seen in the Gram’s smears from one anaerobic blood culture bottle and were identified as Tissierella (formerly Bacteroides) praeacuta, sensitive to metronidazole. Culture of bile obtained from a biliary drain on the fifth day of fever yielded Streptococcus intermedius and Staphylococcus epidermidis; the latter was regarded as contamination. Antibiotic therapy with intravenous piperacillin, tobramycin, and metronidazole was started. Ultrasound revealed an inhomogeneous aspect of the liver parenchyma with two cystic lesions in the left and right lobes. Magnetic resonance imaging and cholangiography of the liver revealed signs of multiple microabscesses in the liver and one large abscess in the dome of the right lobe. In the absence of hepatic arterial thrombosis proven by Doppler ultrasound, these lesions were compatible with ischemic-type biliary lesions (ITBL). After 12 days, the antibiotic treatment was changed to imipenem because of the persistence of cholangitis. Four sets of subsequent blood cultures (BacT Alert; Organon Technika) were collected at 48-h intervals for aerobic and anaerobic bacterial isolation. Three bottles yielded gram-negative rods after 24 to 48 h of aerobic incubation and were inoculated subsequently onto selective and nonselective media. The microorganism produced nonhemolytic colonies on 5% sheep blood agar medium. Pale colonies were seen on MacConkey agar after 24 h of aerobic incubation. The microorganism was identified as O. anthropi by the following characteristics. It was motile and positive for oxidase, catalase, urease, esculin hydrolysis, nitrate and nitrite reduction, utilization of glucose, arabinose, rhamnose, adonitol, and mannitol. The organism was negative for arginine dihydrolase, growth at 42°C, indole production, lysine decarboxylase, ornithine decarboxylase, and gelatin, and for utilization of lactose, maltose, cellobiose, and salicin (1). The microorganism was also identified by its biochemical reactions by using the API 20 NE system (bio-Merieux). An identical microorganism was recovered from two fecal specimens obtained just before and during the episodes of bacteremia by growth on MacConkey agar after 24 to 48 h of aerobic incubation. All five Ochrobactrum isolates were susceptible to imipenem, ciprofloxacin, and trimethoprim-sulfamethoxazole and resistant to amoxicillin, piperacillin, cefuroxime, cefotaxime, and ceftazidime (Table 1). MICs were determined by E tests (AB Biodisk, Solna, Sweden) on Isosensitest agar after 18 h of incubation in an ambient atmosphere. E-test MICs were interpreted by using the National Committee for Clinical Laboratory Standards (NCCLS) breakpoints for Pseudomonas spp. and Acinetobacter spp. published in the guidelines for performance of antimicrobial susceptibility testing by dilution methods (11). Because the blood isolates were resistant to aminoglycosides, ciprofloxacin was added to the treatment. However, the fever persisted, and metronidazole was reinstituted as part of the antibiotic regimen. Because of the irreversible bile duct destruction due to ITBL, the patient underwent a re-OLT 6 weeks after the primary transplantation. During retransplantation, the large abscess in the dome of the right lobe perforated as a result of manipulation of the infected graft. This abscess contained more than 1 liter of pus, causing bacterial contamination of the abdominal cavity. After removal of the primary graft, the abdominal cavity was rinsed several times with liters of sterile saline, and debridement of the abscess cavity in the right upper quadrant of the abdomen was performed. Subsequent culturing of aspirated pus obtained from the large abscess in the liver yielded pure growth of a similar microorganism identified by biochemical testing as O. anthropi. The antibiotic susceptibility pattern of the isolate from the liver was similar to those previous isolates from blood and fecal specimens (Table 1). In addition, environmental investigation was performed by culturing tapwater samples obtained from the patient’s hospital room. Three subsequent cultures of 1-liter water samples did not reveal the recovery of O. anthropi with nonselective media, including blood agar medium, or with selective media supplemented with vancomycin (20 mg/liter), piperacillin combined with tazobactam (3 mg/liter), and amphotericin B (20 mg/liter) after aerobic incubation at 35°C. The postoperative period was complicated only by a cytomegalovirus infection 4 weeks after retransplantation. The patient was treated with ganciclovir and slowly improved. She was discharged from the hospital 2 months after retransplantation.
TABLE 1.
E-test MICs of nine antimicrobial agents for three Ochrobactrum isolates from blood, liver, and fecal specimens
| Agent | MIC (μg/ml)
|
MIC breakpointa (μg/ml)
|
|||
|---|---|---|---|---|---|
| Blood | Liver | Feces | Sensitive | Resistant | |
| Ampicillin | >256 | >256 | >256 | ≤8 | ≥32 |
| Piperacillin | >256 | >256 | >256 | ≤16 | ≥128 |
| Cefuroxime | >256 | >256 | >256 | ≤8 | ≥32 |
| Cefotaxime | >256 | >256 | >256 | ≤8 | ≥64 |
| Ceftazidime | >256 | >256 | >256 | ≤8 | ≥32 |
| Tobramycin | 16 | 16 | 16 | ≤4 | ≥16 |
| Ciprofloxacin | 0.19 | 0.19 | 0.19 | ≤1 | ≥4 |
| Imipenem | 1.5 | 1.0 | 1.0 | ≤4 | ≥16 |
| Trimethoprim-sulfamethoxazole | 0.094 | 0.125 | 0.125 | ≤2/38b | ≥4/76b |
Delineated by the National Committee for Clinical Laboratory Standards (11).
Value for trimethoprim/value for sulfamethoxazole.
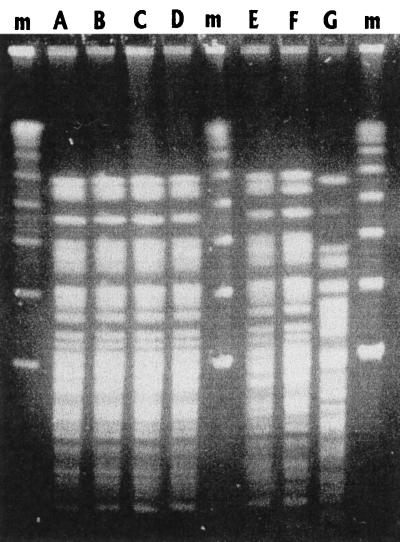
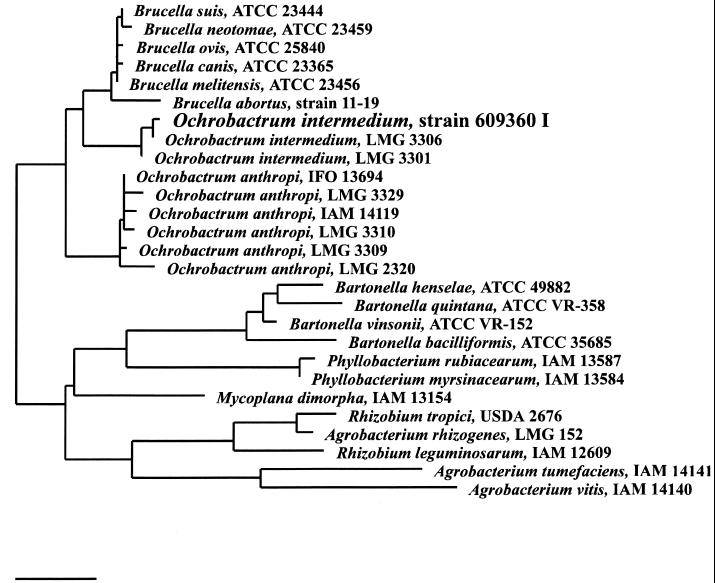
Genotypic analysis of all Ochrobactrum isolates revealed identical DNA patterns, as determined by pulsed-field gel electrophoresis (Fig. 1) (14) and randomly amplified polymorphic DNA with primers for the enterobacterial repetitive intergenic consensus (ERIC) sequences ERIC1R and ERIC2 (13). Identification of the blood isolate in our laboratory by PCR with 16S rDNA primers followed by DNA sequence analysis of the PCR products revealed the recovery of a recently reported new species with the proposed name Ochrobactrum intermedium (occupying a position intermediate between those of O. anthropi and Brucella spp.) showing a close relationship to Brucella spp. and O. anthropi (12). The 16S rRNA sequence of O. intermedium was aligned against all available sequences from public databases (10) and complemented with new sequences from GenBank with the automatic alignment tool from the ARB software program package (11a). Evolutionary distances were calculated for 27 sequences, including 1,353 sequence positions, starting from position 100 (Escherichia coli numbering). A phylogenetic tree was constructed using the neighbor joining algorithm implemented in the ARB program (Fig. 2). Since no biochemical test is currently available to discriminate between O. anthropi and O. intermedium, resistance to colistin (polymyxin E) and polymyxin B is likely to indicate a structural difference between both species of Ochrobactrum (12) as well as similarity of O. intermedium to the brucellae (12). Our isolate was resistant to polymyxin B (150 μg of Neo-sensitabs; Rosco, Taastrup, Denmark) as reported for O. intermedium. In contrast, O. anthropi is sensitive to colistin and polymyxin B. Therefore, we recommend that resistance to colistin or polymyxin B be tested for whenever an isolate is identified as O. anthropi.
FIG. 1.
Pulsed-field gel electrophoresis of XbaI restriction digests of chromosomal DNA of Ochrobactrum isolates (letters A to F) from blood, fecal specimens, and liver. Lane G designates the major restriction pattern of O. anthropi ATCC 49188 (7); lanes m, molecular size markers.
FIG. 2.
Phylogenetic tree showing the relationship between the blood isolate O. intermedium designated strain 609360 I with O. intermedium strains, O. anthropi, Brucella spp., and other members of the beta-proteobacteria. Bar, 1% estimated sequence divergence.
Reports of O. anthropi isolated from sources other than blood of immunocompromised patients are rare (2, 3), and this is the first report of a serious infection caused by a new species, named O. intermedium, in a liver transplant recipient. It is possible that certain infections thought to be caused by O. anthropi were actually caused by O. intermedium, because these microorganisms cannot be easily differentiated. While O. anthropi’s most common clinical manifestation appears to be catheter-associated bacteremia (4), this microorganism is capable of producing pyogenic infections as well, especially when they involve foreign bodies (2, 3). No evidence was found for catheter-related sepsis. Bacteremia is very common after liver transplantation, developing in approximately one-fourth of all patients (15). The source of bacteremia is most often the abdomen. Recovery of O. intermedium from fecal specimens just before and during O. intermedium bacteremia in the patient indicates that the route of infection is most likely the gastrointestinal tract. We hypothesize that Roux-en-Y biliary anastomoses in OLT patients allow colonization of the hepatic allograft with bowel flora, which reflux into the biliary tree, leading to infection (9). Under normal circumstances, such colonization is without consequence, due to the adequate clearance of gastrointestinal reflux from the biliary tree. However, in this case the clearance of the biliary tree was compromised due to multiple intrahepatic stenoses and dilatations resulting from ITBL. Despite the in vitro susceptibility of O. intermedium to imipenem, treating the patient with this antibiotic failed to eradicate the microorganism, as reported earlier (8). The pyogenic infection caused by O. intermedium was successfully managed by explantation of the infected primary graft and intravenous treatment with imipenem and tobramycin for 3 weeks. The patient underwent bowel decontamination of the gastrointestinal tract with an oral regimen including polymyxin and tobramycin which could have led to selection for O. intermedium.
In conclusion, we have demonstrated that O. intermedium is capable of producing pyogenic infection of the liver after gastrointestinal spread in liver transplant recipients, thereby expanding the known pathogenic potential of Ochrobactrum spp. in immunocompromised patients.
Acknowledgments
We thank M. van Opstal, W. Postma, K. van Slochteren, and B. C. Meijer from the Department of Medical Microbiology and Laboratory for Public Health Groningen, University Hospital Groningen, for technical assistance.
REFERENCES
- 1.Alnor D, Frimodt-Moller N, Espersen F, Frederiksen W. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin Infect Dis. 1994;18:914–920. doi: 10.1093/clinids/18.6.914. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C, Campbell D B. Pancreatic abscess associated with Achromobacter group Vd, biovar 1. J Clin Microbiol. 1980;12:282–283. doi: 10.1128/jcm.12.2.282-283.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cieslak T J, Drabick C J, Robb M L. Pyogenic infections due to Ochrobactrum anthropi. Clin Infect Dis. 1996;22:845–847. doi: 10.1093/clinids/22.5.845. [DOI] [PubMed] [Google Scholar]
- 4.Cieslak T J, Robb M L, Drabick C J, Fischer G W. Catheter-associated sepsis caused by Ochrobactrum anthropi: report of a case and review of related nonfermentative bacteria. Clin Infect Dis. 1992;14:902–907. doi: 10.1093/clinids/14.4.902. [DOI] [PubMed] [Google Scholar]
- 5.Ezzedine H, Mourad M, van Ossel C, Logghe C, Squifflet J P, Renault F, Wauters G, Gigi J, Wilmotte L, Haxhe J J. An outbreak of Ochrobactrum anthropi bacteremia in five organ transplant patients. J Hosp Infect. 1994;27:35–42. doi: 10.1016/0195-6701(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 6.Haditsch M, Binder L, Tschurtschenthaler G, Watschinger R, Zauner G, Mittermayer H. Bacteremia caused by Ochrobactrum anthropi in an immunocompromised child. Infection. 1994;22:291–292. doi: 10.1007/BF01739922. [DOI] [PubMed] [Google Scholar]
- 7.Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1988;38:406–416. [Google Scholar]
- 8.Kern W V, Oethinger M, Kaufhold A, Rozdzinski E, Marre R. Ochrobactrum anthropi bacteremia: report of four cases and short review. Infection. 1993;21:306–310. doi: 10.1007/BF01712451. [DOI] [PubMed] [Google Scholar]
- 9.Korvick J A, Marsh J W, Starlz T E, Yu V L. Pseudomonas aeruginosa bacteremia in patients undergoing liver transplantation: an emerging problem. Surgery. 1991;109:62–68. [PMC free article] [PubMed] [Google Scholar]
- 10.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–110. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Sixth informational supplement. NCCLS document M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 11a.Strunk O, Ludwig W. ARB software package. Munich, Germany: Technische Universtät München; 1996. [Google Scholar]
- 12.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol. 1998;48:759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 13.Van Belkum A, Meis J. Polymerase chain reaction-mediated genotyping in microbial epidemiology. Clin Infect Dis. 1994;18:1017–1018. doi: 10.1093/clinids/18.6.1017. [DOI] [PubMed] [Google Scholar]
- 14.Van Dijck P, Delmée M, Ezzedine H, Deplano A, Struelens M J. Evaluation of pulsed-field gel electrophoresis and rep-PCR for epidemiological analysis of Ochrobactrum anthropi strains. Eur J Clin Microbiol Infect Dis. 1995;14:1099–1102. doi: 10.1007/BF01590948. [DOI] [PubMed] [Google Scholar]
- 15.Winston D J, Emmanouilides C, Busuttil R W. Infections in liver transplant recipients. Clin Infect Dis. 1995;21:1077–1091. doi: 10.1093/clinids/21.5.1077. [DOI] [PubMed] [Google Scholar]