Abstract
Introduction
Drug-resistant tuberculosis (TB) remains a global health threat, with little over 50% of patients successfully treated. Novel regimens like the ones being studied in the TB-PRACTECAL trial are urgently needed. Understanding anti-TB drug exposures could explain the success or failure of these trial regimens. We aim to study the relationship between the patients’ exposure to anti-TB drugs in TB-PRACTECAL investigational regimens and their treatment outcomes.
Methods and analysis
Adults with multidrug-resistant TB randomised to investigational regimens in TB-PRACTECAL will be recruited to a nested pharmacokinetic-pharmacodynamic (PKPD) study. Venous blood samples will be collected at 0, 2 and 23 hours postdose on day 1 and 0, 6.5 and 23 hours postdose during week 8 to quantify drug concentrations in plasma. Trough samples will be collected during week 12, 16, 20 and 24 visits. Opportunistic samples will be collected during weeks 32 and 72. Drug concentrations will be quantified using liquid chromatography-tandem mass spectrometry. Sputum samples will be collected at baseline, monthly to week 24 and then every 2 months to week 108 for MICs and bacillary load quantification. Full blood count, urea and electrolytes, liver function tests, lipase, ECGs and ophthalmology examinations will be conducted at least monthly during treatment.
PK and PKPD models will be developed for each drug with nonlinear mixed effects methods. Optimal dosing will be investigated using Monte-Carlo simulations.
Ethics and dissemination
The study has been approved by the Médecins sans Frontières (MSF) Ethics Review Board, the LSHTM Ethics Committee, the Belarus RSPCPT ethics committee and PharmaEthics and the University of Witwatersrand Human Research ethics committee in South Africa. Written informed consent will be obtained from all participants. The study results will be shared with public health authorities, presented at scientific conferences and published in a peer-reviewed journal.
Trial registration number
Keywords: tuberculosis, clinical trials, pharmacology
Strengths and limitations of this study.
This is the first study that prospectively evaluates the pharmacokinetic (PK) and PK-pharmacodynamic properties of three novel exclusively oral, short course multidrug-resistant-tuberculosis treatments; bedaquiline, pretomanid, linezolid in absence and presence of either moxifloxacin or clofazimine.
The study is including participants from key populations (HIV positive and on antiretrovirals) as well as in ethnically diverse populations (South Africa and Belarus).
Being a nested study, the sample size is mainly determined by the parent study.
Introduction
Tuberculosis (TB) remains the deadliest infectious disease in the world, killing an estimated 1.4 million people of the 10 million people who developed the disease in 2019. TB that is resistant to the most powerful anti-TB drug, rifampicin resistant (RR)-TB, caused disease in half a million people representing 5% of all TB and yet is estimated to have caused death in 15% (182 000).1 WHO currently recommends use of either a shorter treatment regimen (9–12 months) or a longer regimen lasting up to 20 months for the treatment of multidrug-resistant (MDR)/RR-TB depending on prior exposure or proven resistance to second line anti-TB drugs. A 6–9 months regimen consisting of bedaquiline (B), pretomanid (Pa) and linezolid (Lzd) which was used in the NiX-TB study2 has been recommended for use in operational research.3
PRAgmatic Clinical Trial for a more Effective, Concise And Less toxic MDR-TB regimens (TB-PRACTECAL) is a multicentre, open label, phase 2–3 randomised controlled trial evaluating 6 months duration, exclusively oral regimens containing bedaquiline, pretomanid, linezolid±moxifloxacin (Mfx) or clofazimine (Cfz) for the treatment of microbiologically confirmed pulmonary RR-TB. In the TB-PRACTECAL trial, B is dosed at 400 mg daily for 2 weeks and then 200 mg three times a week for 22 weeks. Pretomanid is dosed at 200 mg daily for 24 weeks. Linezolid is dosed at 600 mg daily for 16 weeks and then reduced to 300 mg daily for 8 weeks. Mfx is given at 400 mg daily for 24 weeks and Cfz is dosed at 100 mg daily for body weight above 33 kg and 50 mg daily below 33 kg for 24 weeks.
A cumulating body of evidence has shown that anti-TB drug exposure especially in HIV positive patients varies significantly.4Moreover, low-drug concentrations are linked to poor outcomes,5 particularly microbiological failure.6 Identifying the optimal dose and duration of drugs such as linezolid in treating RR-TB remains a global research priority.3
If the TB-PRACTECAL trial identifies successful regimens, the PRACTECAL-pharmacokinetic-pharmacodynamic (PKPD) substudy will provide explanatory evidence to why the tested regimens at the chosen doses and administration scenario are efficacious. And if the regimens have not been shown to be non-inferior, allow the understanding of whether variability of particular drug exposures could have played a part in the efficacy or safety outcomes and make appropriate recommendations for further research.7
We; therefore, aim to study the relationship between the patients’ exposure to anti-TB drugs in the TB-PRACTECAL trial investigational regimens and their respective treatment outcomes.
Method and analysis
Study design development
The Fisher Information Matrix (FIM)8 was used to optimise a venous blood sampling design that supports PK model development in that expected PK model parameter estimate precision will be ≤20%.
Identification of prior information
A structured literature search was done in Medline, Embase and PubMed databases and relevant conferences in August 2017, with the following search terms (population pharmacokinetics AND drug_name) to identify published population PK models. The search yielded 5, 0, 3, 1 and 5 relevant papers for linezolid,9–13 pretomanid, bedaquiline,14 Cfz15 and Mfx,16–20 respectively. Authors were contacted when full text articles were not accessible and drug developers were contacted with the request to share unpublished models.21 Where more than one suitable PK model was available, the following hierarchically listed criteria were used to select a suitable model to be used for design optimisation: study population (MDR-TB, TB, non-TB patients or healthy subjects), original PK study sample size (larger sample sizes preferred), and a critical appraisal of the publications including whether the authors reported enough parameters to allow for parameterisation of the model.
Identification and definition of constraints
The FIM was maximised given a series of design constrains. First, samples could only be collected on scheduled visits with planned laboratory sample collection as per main study protocol. Second, venous plasma samples could only be scheduled during day nurse working and laboratory opening hours in order to warrant access to staff, centrifuges and freezers. Lastly, the sampling intervals could not be shorter than 15 min in order to warrant that the protocol is executable by clinical and laboratory staff.
Sampling schedule optimisation
ED design optimisation with uncertainty on clearance estimates, using PopED; an R-package22 (V.0.3.2), was used to simultaneously optimise a venous blood sampling schedule for pretomanid21 and linezolid13 in first instance. Subsequently, bedaquiline,23 Cfz15 and Mfx19 expected elimination clearance estimates were evaluated given the optimal venous plasma sampling designs for pretomanid and linezolid. The two-step approach was chosen due to the distinctly different PK profiles of bedaquiline, Cfz and Mfx, with an elimination phase outside the 24-hour dosing schedule, when compared with pretomanid and linezolid.
Design evaluation
The optimal venous blood sampling schedule was subsequently evaluated for each study drug using the stochastic simulation and estimation (SSE) function of PsN24 with NONMEM (V.7.3). The optimal evaluated design was for 240 patients sampled at day 0 (0, 2, 23 hours), week 8 (0, 6.5, 23 hours), trough at weeks 12, 16, 20 and 24 and opportunistic at week 32 and 72. The expected relative SEs (RSEs) for clearance were below 20% for each drug (table 1).
Table 1.
Expected clearance RSEs per drug, using the chosen sampling schedule
| Drug | Simulated clearance (mL/min) |
Mean re-estimated (mL/min) |
RSE re-estimated (%) |
| Linezolid | 1.86 | 1.96 | 16.6 |
| Clofazimine | 10 | 11.2 | 6.32 |
| Pretomanid | 7.71 | 4.16 | 2.02 |
| Bedaquiline | 2.78 | 2.84 | 6.08 |
| Moxifloxacin | 10.6 | 12.6 | 1.47* |
*The moxifloxacin PK model used an informative prior from literature in the SSE.
PK, pharmacokinetic; RSE, relative SE; SSE, stochastic simulation and estimation.
Study design
Main study question
What is the relationship between the patients’ exposure to anti-TB drugs in the TB-PRACTECAL trial investigational regimens and their respective treatment outcomes?
Primary objective
Measure the plasma concentrations of pretomanid, linezolid, bedaquiline, Cfz and Mfx in a subset of patients in the TB-PRACTECAL trial and using population PK models, estimate the population exposure metrics (Cmin, Cmean, Cmax, area under the curve (AUC)) for the individual drugs in the TB-PRACTECAL trial.
Secondary objectives
Develop PK models for each of the study drugs.
Develop a PKPD model to characterise the relationship between drug exposure, baseline clinical covariates, baseline minimum inhibitory concentrations and early bactericidal effect.
Study correlations between baseline clinical covariates, baseline minimum inhibitory concentrations, drug exposure and longitudinal PKPKD markers and long-term treatment outcome defined as success at end of treatment and remaining relapse free for 1 year after successful treatment.
Develop PKPD models investigating associations between PK parameters and treatment emergent toxicity.
Use results from the aforementioned algorithms to develop a hypothesis on the optimal dosing of linezolid and Cfz using Monte-Carlo simulations.
Patient and public involvement
Patients were not directly involved in the design of this study. However, the parent clinical trial engaged patients in the setup and implementation.25
Study setting
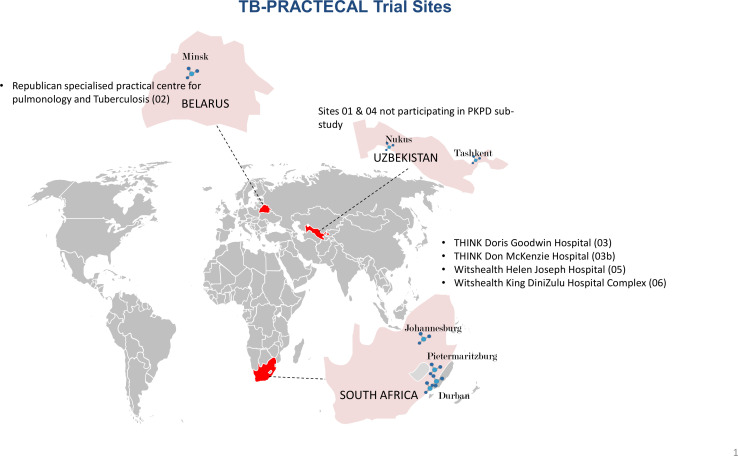
The study is recruiting and being implemented in five hospitals (figure 1) in Belarus and South Africa. The drug quantification bioanalysis will be conducted at the University of Liverpool, Liverpool, UK and the mycobacteriology is done at Republican Specialised Practical Centre for Pulmonology and Tuberculosis National Reference Laboratory in Minsk for Belarus samples and Cytespace Africa laboratories in Pretoria, South Africa.
Figure 1.
Trial sites participating in the PRACTECAL-PKPD sub-study. PKPD, pharmacokinetic-pharmacodynamic; TB, tuberculosis.
Study population
We are seeking to describe the population drug exposures and their variability in the target population, so a traditional power calculation was not done. The total number of patients recruited into the study is driven by the timing of starting the substudy and proportion of patients consenting. Based on the optimal design parameters, we aimed to recruit a maximum of 240 patients resulting in just under 3000 drug concentration observations. We recruited 97 patients with an expected 1164 samples to be available for bioanalysis and at least 3492 drug concentration measurements for the four study drugs.
Inclusion/exclusion criteria
All adult patients recruited into the investigational arms of the parent TB-PRACTECAL trial in the approved sites are eligible to join the study (https://clinicaltrials.gov/ct2/show/NCT02589782) with the following eligibility criteria:
Inclusion criteria:
Patients eligible for inclusion in the trial must fulfil all of the following criteria:
Male or female subjects aged 15 years of age or above, regardless of HIV status.
Microbiological test (molecular or phenotypic) confirming presence of Mycobacterium tuberculosis.
Resistant to at least rifampicin by either molecular or phenotypic drug susceptibility test.
Completed informed consent form (ICF).
Exclusion criteria:
Known allergies, hypersensitivity or intolerance to any of the study drugs.
Pregnant or breast feeding; or unwilling to use appropriate contraceptive measures.
Liver enzymes >3 times the upper limit of normal.
Any condition (social or medical) which, in the opinion of the investigator, would make study participation unsafe.
Taking any medications contraindicated with the medicines in the trial; QTcF >450 ms.
One or more risk factors for QT prolongation (excluding age and gender) or other uncorrected risk factors for Torsades de Pointes.
History of cardiac disease, syncopal episodes, symptomatic or asymptomatic arrhythmias (with the exception of sinus arrhythmia).
Any baseline biochemical laboratory value consistent with grade 4 toxicity.
Moribund.
Known resistance to bedaquiline, pretomanid, delamanid or linezolid.
Prior use of bedaquiline and/or pretomanid and/or linezolid and/or delamanid for one or more months.
-
Patients not eligible to start a new course of MDR-TB or extensively drug resistant (XDR)-TB treatment according to local protocol, including but not limited to:
Currently on MDR-TB treatment for more than 2 weeks (and not failing).
Unstable address.
Lost to follow-up in previous treatment with no change in circumstance and motivation.
Tuberculous meningoencephalitis, brain abscesses, osteomyelitis or arthritis.
The additional criteria for inclusion into the PRACTECAL-PKPD study is for the patient to be aged 18 years or older, to sign the sub-study ICF after agreeing to the additional blood draws.
Study outline
Study period
The first batch of samples’ bioanalysis will start in the second quarter of 2021 and continue at regular intervals on batched samples. The data cleaning and analysis will be continuous until end of study.
Study procedures
Patients undergoing recruitment into the TB-PRACTECAL trial will be systematically requested to join the PRACTECAL-PKPD study as well if eligible. After screening and randomisation, only patients who have been randomised to the investigational regimens will be available to be recruited into the PRACTECAL-PKPD.
At least 4 mL (vacutainer tube, lithium heparin) of blood will be collected from the hand, forearm or antecubital vein at each sampling occasion and moment for the PK. The sampling occasions are on Day 1, Weeks 8, 12, 16, 20, 24, 32 and 72 (figure 2). On day 1, blood will be collected just before drugs intake, then 2 and 23 hours after drugs intake. On week 8, blood will be collected just before drugs intake, then 6.5 and 23 hours postdose. These multiple blood sample occasions may require the patient to stay in hospital overnight. At weeks 12, 16, 20 and 24 the blood will be collected just before taking the dose. Both the planned and actual blood collection times should be documented at the earliest opportunity. Samples from week 32 and 72 will be collected whenever feasible after the patients have completed their treatment so blood collection is not relative to drug intake on that occasion. These have been included to capture the elimination phases of the drugs which have long terminal half-lives.
Figure 2.
The PRACTECAL–PKPD study investigational schedule. Cfz, clofazimine; Mfx, moxifloxacin; PKPD, pharmacokinetic-pharmacodynamic.
Collected PK blood samples will be centrifuged at 1000 G for 5 min within 30 min of blood drawing, if this is not possible the sample will be refrigerated for a maximum of 60 min. The supernatant plasma will be aspirated and pipetted into two equal aliquots approximately 1.5 mL each and stored in temperature of max −20°C within 60 min of collection, it should then be transferred to dry ice if transport is required and stored in a −80°C freezer. At defined intervals, these frozen samples will be shipped on dry ice to the University of Liverpool laboratory.
Bioanalytical plan
Individual drugs concentrations will be quantified in a Good Clinical Laboratory Practice compliant bioanalytical facility using validated liquid chromatography-tandem mass spectrometry assay methods. Assay performance, sample chromatograms, standard curves and the validity of methods will also be reported.
Data collection
Demographic data will include age, sex and site. Data for safety outcomes will be collected as part of the main TB-PRACTECAL trial. These include triplicate ECGs at baseline, predose and at 4–6 hours postdose on day 7 and then weekly up to week 8. After week 8, triplicate ECGs predose only every 4 weeks up to week 24, every 8 weeks up to week 48 and then week 72. Full blood count, urea and electrolytes, liver function tests and lipase will be performed on day 0, weekly up to week 8 and monthly up to week 24, at weeks 32, 72 and 108. Audiometry and ophthalmological assessments are also conducted as per investigational schedule. These will be reported as serious adverse events (AE), AE of special interest and other AEs with their respective severity grading using the MSF Severity Grading Scale.26
Data for the assessment of efficacy outcomes will be collected as part of the main TB-PRACTECAL trial and include: sputum for smear, culture (time to positivity in MGIT) and MIC at baseline and monthly up to week 24 then every 2 months to week 108. Weight and height at baseline then weight at every visit until completion. Chest X-ray at baseline and week 24.
Other relevant covariate data collected include history of TB treatment and baseline blood glucose levels.
PK-specific data collection
Study-specific electronic clinical report forms will include scheduled sample collection time, actual time sample taken, time separation completed, time stored at −20°C or lower, time last dose taken, prior exposure to drugs of interest, covariates such as time last meal taken, concomitant medications especially ARVs and the time of the last dose.
Data analysis
PK and PKPD models will be developed for each drug based on the plasma concentrations in the study. Nlme modelling software packages (eg, NONMEM or nlmixR) using first-order conditional estimation method with interaction (focei) will be used. Several combinations of absorption models (first order, first order with lag-time and transit absorption), distribution models (one-compartment, two-compartment and three-compartment distribution), variability models (between-subject variability and between occasion variability), and error models (additive, proportional and combined additive and proportional error models) will be assessed. Relative oral bioavailability will be evaluated as a fixed parameter (100% for the population), to allow estimation of the between-subject and between-occasion variability of the relative bioavailability. Competing models will be evaluated during the model building process by the objective function value (OFV—computed as minus twice the log likelihood of the data), physiological plausibility, and goodness-of-fit diagnostics. A significant (p=0.05) improvement will be concluded if the OFV dropped with 3.84 points or more (after the introduction of one new parameter, that is, one degree of freedom). Effects of covariates on PK model parameter estimates will be assessed using a stepwise covariate modelling approach. During the forward inclusions a p=0.05 will be considered a significant improvement of the model fit while during the backward eliminations a p=0.01 will be considered significant improvement of the model fit. Model evaluation will include residual plots and visual predictive checks.27 Prior information in a Bayesian framework will be applied where the venous plasma sampling design fails to support sufficient precision on PK parameter estimates.
Direct linear, EMAX and sigmoid EMAX models will be studied in order to characterise the concentration–effect relationship with a PKPD model. Effects of covariates on PKPD model parameters will be assessed, including the effects of relevant concomitant medications, using identical statistical criteria as described for the PK models in combination with physiological and pharmacological plausibility.
For adverse events both direct and delayed, that is, using an effect compartment, linear, EMAX and sigmoid EMAX models will be investigated to the concentration–effect relationships.
Model development will start as soon as we have the samples from at least 60 patients analysed, that is, interim analysis. Structural models from the interim analysis will be re-evaluated and full covariate analyses will be done once the full dataset becomes available.
For dose optimisations, first, a virtual patient population will be simulated on the basis of observed baseline characteristics among patients enrolled in the study. Then clinically feasible dosing scenarios will be formulated. Lastly, Monte-Carlo simulations done in order to study PK, PKPD and toxicity endpoints following the various dosing scenarios.
Regulatory and ethical considerations
The study has been approved by the MSF Ethics Review Board (reference no. 1541) and the LSHTM Ethics Committee (reference no. 16249) from the two leading institutions. The Belarus RSPCPT ethics committee and the regulator-Centre of Excellence for the Minsk site. PharmaEthics for the Don Mckenzie and Dorris Goodwin hospitals sites, University of Witwatersrand Human Research ethics committee for the Helen Joseph and King DiniZulu Hospitals sites and the South Africa Health Products Regulatory Authority.
The informed consent process will be in line with International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH) Good Clinical Practice guidance. The information given and informed consent process will be in the patient’s preferred language and documented on a written consent form signed by both the patient and investigator. Where the patient is illiterate, a thumb print of the participant as well as the signature of a witness independent of the study will be documented.
Dissemination
The results of the study will be presented at scientific conferences and published in a peer-reviewed journal. If the TB-PRACTECAL trial successfully identifies effective and safe regimens, the results of this study may be used to inform a WHO guidelines process by potentially answering specific questions on recommended dosages of the B, Pa, Lzd-based regimens as well as potentially informing study countries decisions.
Supplementary Material
Acknowledgments
This study is part of the TB-PRACTECAL trial project. The authors would like to thank the TB-PRACTECAL Project Management Team, Steering Committee, Scientific Advisory Committee and investigator teams at the trial sites in Belarus and South Africa in setting up and implementing this study.
Footnotes
Twitter: @docwak77
Contributors: B-TN, GRD, FK, IM, CB, AB and DM designed the study. FK and B-TN conducted the optimal design analysis. B-TN wrote the first draft of the manuscript and all authors reviewed and approved the final version of the manuscript.
Funding: This work was supported by Médecins sans Frontières. Award/Grant number is not applicable.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.World Health Organization . Global tuberculosis report 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020;382:893–902. 10.1056/NEJMoa1901814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020. [PubMed] [Google Scholar]
- 4.van Heeswijk RPG, Dannemann B, Hoetelmans RMW. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother 2014;69:2310–8. 10.1093/jac/dku171 [DOI] [PubMed] [Google Scholar]
- 5.Pasipanodya JG, McIlleron H, Burger A, et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013;208): :1464–73. 10.1093/infdis/jit352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloprogge F, Mwandumba HC, Banda G, et al. Longitudinal pharmacokinetic-pharmacodynamic biomarkers correlate with treatment outcome in drug-sensitive pulmonary tuberculosis: a population pharmacokinetic-pharmacodynamic analysis. Open Forum Infect Dis 2020;7:ofaa218. 10.1093/ofid/ofaa218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dooley KE, Hanna D, Mave V, et al. Advancing the development of new tuberculosis treatment regimens: the essential role of translational and clinical pharmacology and microbiology. PLoS Med 2019;16:e1002842. 10.1371/journal.pmed.1002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarons L, Ogungbenro K. Optimal design of pharmacokinetic studies. Basic Clin Pharmacol Toxicol 2010;106:250–5. 10.1111/j.1742-7843.2009.00533.x [DOI] [PubMed] [Google Scholar]
- 9.Luque S, Grau S, Alvarez-Lerma F, et al. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int J Antimicrob Agents 2014;44:409–15. 10.1016/j.ijantimicag.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 10.McGee B, Dietze R, Hadad DJ, et al. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2009;53:3981–4. 10.1128/AAC.01378-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meagher AK, Forrest A, Rayner CR, et al. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob Agents Chemother 2003;47:548–53. 10.1128/AAC.47.2.548-553.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plock N, Buerger C, Joukhadar C, et al. Does linezolid inhibit its own metabolism? Population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab Dispos 2007;35:1816–23. 10.1124/dmd.106.013755 [DOI] [PubMed] [Google Scholar]
- 13.Tsuji Y, Holford NHG, Kasai H, et al. Population pharmacokinetics and pharmacodynamics of linezolid-induced thrombocytopenia in hospitalized patients. Br J Clin Pharmacol 2017;83:1758–72. 10.1111/bcp.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson EM, Murray S, Karlsson MO. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J Antimicrob Chemother 2015;70:1106–14. 10.1093/jac/dku504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganesan SSG, Hughes D. Identification of optimal dose and dosing regimen of clofazimine for the treatment of multidrug-resistant tuberculosis (MDR-TB) based on pharmacokinetic modelling. 46th Conference on Lung Health of the UNION, Cape Town, South AFrica, 2015. [Google Scholar]
- 16.Chang MJ, Jin B, Chae J-W, et al. Population pharmacokinetics of moxifloxacin, cycloserine, p-aminosalicylic acid and kanamycin for the treatment of multi-drug-resistant tuberculosis. Int J Antimicrob Agents 2017;49:677–87. 10.1016/j.ijantimicag.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 17.Peloquin CA, Hadad DJ, Molino LPD, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2008;52:852–7. 10.1128/AAC.01036-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pranger AD, Kosterink JGW, van Altena R, et al. Limited-sampling strategies for therapeutic drug monitoring of moxifloxacin in patients with tuberculosis. Ther Drug Monit 2011;33:350–4. 10.1097/FTD.0b013e31821b793c [DOI] [PubMed] [Google Scholar]
- 19.Zvada SP, Denti P, Geldenhuys H, et al. Moxifloxacin population pharmacokinetics in patients with pulmonary tuberculosis and the effect of intermittent high-dose rifapentine. Antimicrob Agents Chemother 2012;56:4471–3. 10.1128/AAC.00404-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zvada SP, Denti P, Sirgel FA, et al. Moxifloxacin population pharmacokinetics and model-based comparison of efficacy between moxifloxacin and ofloxacin in African patients. Antimicrob Agents Chemother 2014;58:503–10. 10.1128/AAC.01478-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salinger DH, Subramoney V, Everitt D, et al. Population pharmacokinetics of the antituberculosis agent Pretomanid. Antimicrob Agents Chemother 2019;63:e00907–19. 10.1128/AAC.00907-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyberg J, Ueckert S, Strömberg EA, et al. PopED: an extended, parallelized, nonlinear mixed effects models optimal design tool. Comput Methods Programs Biomed 2012;108:789–805. 10.1016/j.cmpb.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 23.McLeay SC, Vis P, van Heeswijk RPG, et al. Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob Agents Chemother 2014;58:5315–24. 10.1128/AAC.01418-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindbom L, Pihlgren P, Jonsson EN, et al. PsN-Toolkit-a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 2005;79:241–57. 10.1016/j.cmpb.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 25.Douch E. Engaging communities in tuberculosis research: the experience of the TB-PRACTECAL trial. the BMJ opinion 2018. [Google Scholar]
- 26.Medecins SANS Frontieres PV-TB-D12 MSF severity grading scale 2016.
- 27.Nguyen THT, Mouksassi M-S, Holford N, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 2017;6:87–109. 10.1002/psp4.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.