Abstract
Background: There has been great interest in the use of seaweed as a functional feed ingredient for poultry in the last decade. This study aimed to assess the effects of dietary seaweed inclusion on growth performance of broiler chickens by using a systematic review and meta-analysis approach.
Methods: A systematic search of published research articles related to seaweed, broiler chickens, and growth performance was conducted using three online databases (Scopus, PubMed, and SciELO). Mean values, standard deviation, and sample size were extracted from each eligible study. The estimated effect size was then quantified using Hedges’ g with a 95% confidence interval (CI). Data were pooled using a fixed-effect model due to the absence of heterogeneity after being pre-checked using the I 2 statistic.
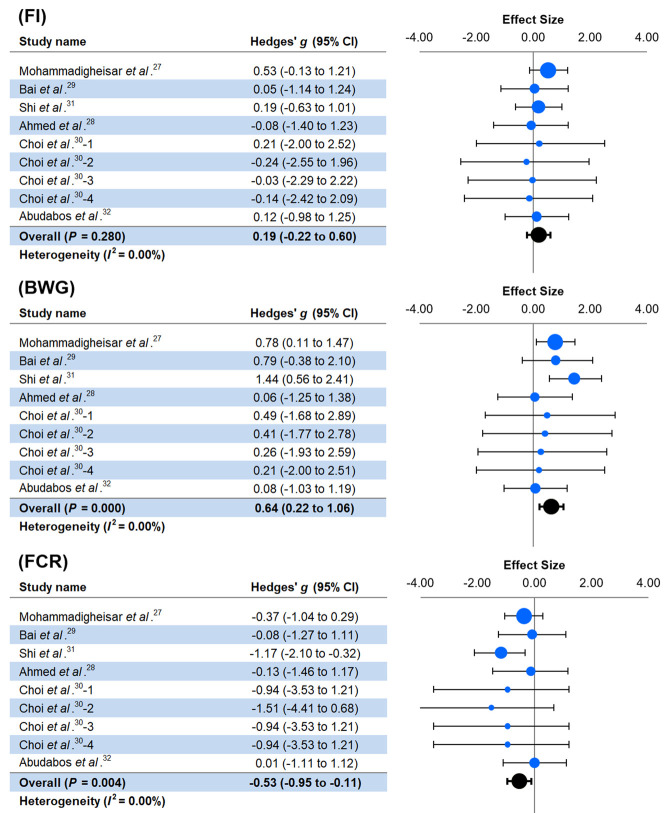
Results: A total of six studies (nine comparisons) involving 2,257 broiler chickens were accommodated in this study. The seaweed type consisted of seaweed blend, Laminaria japonica, Undaria pinnatifida, Hizikia fusiformis, and Ulva lactuca. The inclusion dose ranged from 2 to 30 g/kg, while the intervention duration ranged from 21 to 42 days. No substantial heterogeneity among studies ( I2 = 0.00%) was found for feed intake, body weight gain, and feed conversion ratio. Dietary seaweed had no significant effect on feed intake (Hedges’ g = 0.19; 95% CI = -0.22 to 0.60; P = 0.280). However, broiler chickens fed dietary seaweed had superior body weight gain (Hedges’ g = 0.64; 95% CI = 0.22 to 1.06; P = 0.000) and preferable feed conversion ratio (Hedges’ g = -0.53; 95% CI = -0.95 to -0.11; P = 0.004).
Conclusions: The current investigation highlights that dietary seaweed had growth-promoting potency for broiler chickens. However, more research on this issue is still required to build more comprehensive evidence.
Keywords: alginate, body weight gain, fucoidan, fucoxanthin, functional feed, laminarin, macroalgae, poultry
Introduction
There has been great interest in the use of seaweed as a functional feed ingredient for poultry in the last decade. The primary functional compounds in seaweed are polysaccharides, peptides, fatty acids, phlorotannins, and carotenoids 1– 3 . These compounds have antimicrobial, antioxidant, and immunomodulatory properties 4– 7 , which are essential to support production performance.
Several reviews have compiled studies regarding the effect of dietary seaweed inclusion on poultry performance 8– 13 . However, those reviews were based on a narrative approach, which mostly led to an inconclusive epilogue due to the contradictory results among studies. The use of systematic review and meta-analysis has become popular in animal science 14– 18 . This methodology can integrate and determine the overall effect of interventions from several studies to provide more accurate insight than the narrative review. Therefore, this study aimed to assess the effect of dietary seaweed inclusion on the growth performance of broiler chickens using a systematic review and meta-analysis approach.
Methods
This study was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 19. The PRISMA checklist is presented in Reporting guidelines 20.
Eligibility criteria
Research articles published in peer-reviewed journals between the years of 2000 to 2020 and written in English were eligible. Additionally, eligible studies also should fulfill the participants, interventions, comparisons, outcomes, and study design (PICOS) criteria given in Table 1.
Table 1. PICOS criteria.
| Items | Criteria |
|---|---|
| Participants | Broiler chickens |
| Interventions | Inclusion of dietary seaweed either as
such or fermented product |
| Comparisons | Diet without seaweed inclusion (control) |
| Outcomes | Feed intake, body weight gain, and feed
conversion ratio |
| Study design | Controlled trials |
Searching strategy
The online search was conducted using three databases, namely Scopus, PubMed, and SciELO, with the queries in Table 2. The final search was on 25 June 2020. The references from the included studies were also screened to find additional eligible studies.
Table 2. The search query in Scopus, PubMed, and SciELO databases.
| Database | Search query |
|---|---|
| Scopus | (TITLE-ABS-KEY (seaweed OR macroalgae) AND TITLE-ABS-KEY (growth OR performance) AND
TITLE-ABS-KEY (broiler OR chicken)) |
| PubMed | ((seaweed[Title/Abstract] OR macroalgae[Title/Abstract]) AND (growth[Title/Abstract] OR
performance[Title/Abstract])) AND (broiler[Title/Abstract] OR chicken[Title/Abstract]) |
| SciELO | (ab:(seaweed OR macroalgae)) AND (ab:(growth OR performance)) AND (ab:(broiler OR chicken)) |
Study selection
Firstly, the duplicate reports were removed from the database in Microsoft Excel for Microsoft 365 software. After that, the title and abstract were examined. Irrelevant studies, non-English reports, and review articles were then excluded from the list. The full text was further evaluated according to the eligibility criteria.
Data collection
Mean values, standard deviations, and sample sizes were extracted from each included study. The target variables in this study were feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR). When a study used the standard error of means as a variance measure, it was converted into standard deviation 21. In the case of more than one seaweed type used in a study, each treatment was coded individually. On the other hand, the treatment was pooled when a study used more than one dose of the same seaweed type 22. None of the authors were contacted for further clarification.
Data analysis
Data analysis was performed using Meta-Essential version 1.5 23. The estimated effect size (the difference between seaweed intervention and control) was quantified using Hedges’ g with a 95% confidence interval (CI) 24. Data were pooled using a fixed-effect model due to the absence of heterogeneity after being pre-checked using the I 2 statistic 25. A significant effect was declared when the overall estimated effect size had P < 0.05. Publication bias was not evaluated because the number of the included studies was fewer than 10 26.
Results
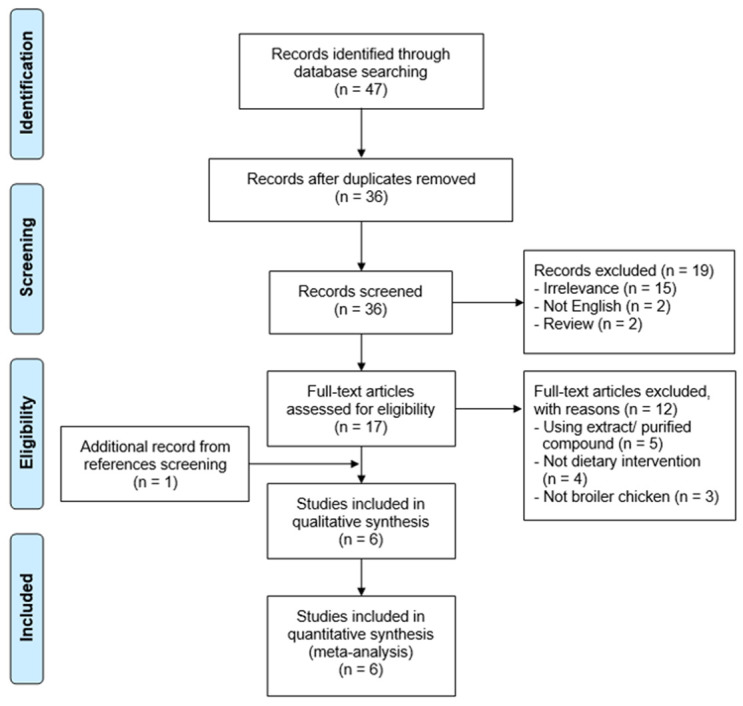
The PRISMA flow diagram is shown in Figure 1. The search using three online databases identified 47 records. Of these, five studies met the eligibility criteria. Additionally, one study from reference screening also found to be eligible. Therefore, a total of six studies, with nine comparisons were included in the synthesis.
Figure 1. PRISMA flow diagram.
The details of the included studies are shown in Table 3. A total of 2,257 broiler chickens were involved in this study. The seaweed type used included seaweed blend 27, Laminaria japonica 28, 30, Undaria pinnatifida 29, 31, Hizikia fusiformis 31, and Ulva lactuca 32. The inclusion dose ranged from 2 to 30 g/kg, while the intervention duration ranged from 21 to 42 days. The extracted data of target variables is presented as Extended data 33.
Table 3. Details of the included studies.
| Study name | N | Strain | Sex | Diet type | Seaweed type | Dose (g/kg) | Period (d) |
|---|---|---|---|---|---|---|---|
| Mohammadigheisar et al. 27 | 864 | Ross | Male | Corn-SBM | Blend of brown, green, and red seaweed | 5, 10, and 20 | 1-42 |
| Bai et al. 28 | 144 | Arbor Acres | Mixed | Corn-SBM | L. japonica | 10 | 1-42 |
| Shi et al. 29 | 384 | Ross | Mixed | Corn-SBM | Fermented U. pinnatifida | 2 | 1-35 |
| Ahmed et al. 30 | 70 | Ross | Mixed | Corn-SBM | Fermented L. japonica | 5 | 1-35 |
| Choi et al. 31 | 750 | Ross | Male | Corn-SBM |
U. pinnatifida (as such and fermented)
and H. fusiformis (as such and fermented) |
5 | 1-35 |
| Abudabos et al. 32 | 45 | Ross | Male | Corn-SBM | U. lactuca | 10 and 30 | 12-33 |
n: number of broiler chickens, SBM: soybean meal.
As shown in Figure 2, no substantial heterogeneity was found for any variables ( I 2 = 0.00%). Dietary seaweed had no significant effect ( P > 0.05) on FI. However, this intervention significantly improves ( P < 0.05) the BWG and FCR of broiler chickens. The overall estimated effect size values for BWG and FCR were 0.64 and -0.53, respectively, which were equivalent to the raw mean difference of 77.24 g and -0.07, respectively.
Figure 2. Forest plot showing the effect of dietary seaweed inclusion on growth performance of broiler chicken.
FI: feed intake, BWG: body weight gain, FCR: feed conversion ratio, CI: confidence interval.
Discussion
In this study, the use of dietary seaweed had a beneficial impact on BWG and FCR of broiler chickens. According to Cohen 34, the overall estimated effect size of BWG and FCR in the present study was categorized into the medium (0.5) to large (0.8) standardized effect size. In agreement with this finding, other studies also showed that the use of seaweed could improve production performance in laying hens 35– 37 and geese 38. Seaweed contained numerous unique bioactive substances such as alginate, ulvan, laminarin, fucoidan, and fucoxanthin. Those compounds could inhibit the colonization of pathogenic bacteria ( Escherichia coli and Salmonella Enteritidis), promote the growth of beneficial gut microbes (lactic acid bacteria), improve small intestinal architecture, antioxidant status, and immune response 39– 43 . Together, those mechanisms could ultimately improve the growth performance of broiler chickens.
Nevertheless, this finding is accompanied by the limited number of included studies. It is possible that not all relevant studies were captured by the searching strategies. For those reasons, the current results should be elucidated with caution. Moreover, due to the enormous diversity of seaweed in nature (around twenty thousand species) 44, future studies regarding seaweed intervention in broiler chickens are still open and strongly encouraged to provide a robust body of knowledge.
Conclusions
The current systematic review and meta-analysis highlight that dietary seaweed had no adverse effect on FI. Instead, they could improve BWG and FCR of broiler chickens. However, more research on this issue is still required to build more comprehensive evidence.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: Extended data for ‘The effects of dietary seaweed inclusion on growth performance of broiler chickens: a systematic review and meta-analysis’. https://doi.org/10.6084/m9.figshare.12721454.v1 33.
This project contains the following extended data in DOC format:
-
-
Extended data 1 – extracted data of feed intake
-
-
Extended data 2 – extracted data of body weight gain
-
-
Extended data 3 – extracted data of feed conversion ratio
-
-
Extended data 4 – list of included studies
Reporting guidelines
Figshare: PRISMA checklist for 'The effect of dietary seaweed inclusion on growth performance of broiler chickens: a systematic review and meta-analysis'. https://doi.org/10.6084/m9.figshare.12721118.v1 20.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Funding Statement
This work was supported by the Indonesian Endowment Fund for Education, Ministry of Finance, Republic of Indonesia.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved
References
- 1.Holdt SL, Kraan S: Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23(3):543–597. 10.1007/s10811-010-9632-5 [DOI] [Google Scholar]
- 2.Cardoso SM, Pereira OR, Seca AM, et al. : Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar Drugs. 2015;13(11):6838–6865. 10.3390/md13116838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rengasamy KR, Mahomoodally MF, Aumeeruddy MZ, et al. : Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem Toxicol. 2020;135:111013. 10.1016/j.fct.2019.111013 [DOI] [PubMed] [Google Scholar]
- 4.Shannon E, Abu-Ghannam N: Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar Drugs. 2016;14(4):81. 10.3390/md14040081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poveda-Castillo GD, Rodrigo D, Martínez A, et al. : Bioactivity of fucoidan as an antimicrobial agent in a new functional beverage. Beverages. 2018;4(3):64. 10.3390/beverages4030064 [DOI] [Google Scholar]
- 6.Corino C, Modina SC, Di Giancamillo A, et al. : Seaweeds in pig nutrition. Animals (Basel). 2019;9(12):1126. 10.3390/ani9121126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Zavaglia A, Prieto Lage MA, Jimenez-Lopez C, et al. : The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants (Basel). 2019;8(9):406. 10.3390/antiox8090406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans FD, Critchley AT: Seaweeds for animal production use. J Appl Phycol. 2014;26(2):891–899. 10.1007/s10811-013-0162-9 [DOI] [Google Scholar]
- 9.Rajauria G: Seaweeds: a sustainable feed source for livestock and aquaculture.In: Brijesh KT, Declan JT, eds. Seaweed Sustainability.San Diego, CA, USA: Academic Press.2015;389–420. 10.1016/B978-0-12-418697-2.00015-5 [DOI] [Google Scholar]
- 10.Angell AR, Angell SF, de Nys R, et al. : Seaweed as a protein source for mono-gastric livestock. Trends Food Sci Technol. 2016;54:74–84. 10.1016/j.tifs.2016.05.014 [DOI] [Google Scholar]
- 11.Makkar HP, Tran G, Heuzé V, et al. : Seaweeds for livestock diets: A review. Anim Feed Sci Technol. 2016;212:1–17. 10.1016/j.anifeedsci.2015.09.018 [DOI] [Google Scholar]
- 12.Haberecht S, Wilkinson S, Roberts J, et al. : Unlocking the potential health and growth benefits of macroscopic algae for poultry. Worlds Poult Sci J. 2018;74(1):5–20. 10.1017/S0043933917001052 [DOI] [Google Scholar]
- 13.Øverland M, Mydland LT, Skrede A: Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J Sci Food Agric. 2019;99(1):13–24. 10.1002/jsfa.9143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belluco S, Barco L, Roccato A, et al. : Escherichia coli and Enterobacteriaceae counts on poultry carcasses along the slaughterline: A systematic review and meta-analysis. Food Control. 2016;60:269–280. 10.1016/j.foodcont.2015.07.033 [DOI] [Google Scholar]
- 15.Vieira BS, Silva FG, Oliveira CF, et al. : Does citric acid improve performance and bone mineralization of broilers when combined with phytase? A systematic review and meta-analysis. Anim Feed Sci Technol. 2017;232:21–30. 10.1016/j.anifeedsci.2017.07.016 [DOI] [Google Scholar]
- 16.Ozdemir M, Kopuzlu S, Topal M, et al. : Relationships between milk protein polymorphisms and production traits in cattle: a systematic review and meta-analysis. Arch Anim Breed. 2018;61(2):197–206. 10.5194/aab-61-197-2018 [DOI] [Google Scholar]
- 17.Toledo TD, Pich CS, Roll AA, et al. : The effect of litter materials on broiler performance: a systematic review and meta-analysis. Br Poult Sci. 2019;60(6):605–616. 10.1080/00071668.2019.1639143 [DOI] [PubMed] [Google Scholar]
- 18.McCarthy KM, McAloon CG, Lynch MB, et al. : Herb species inclusion in grazing swards for dairy cows—A systematic review and meta-analysis. J Dairy Sci. 2020;103(2):1416–1430. 10.3168/jds.2019-17078 [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andri F, Dono ND, Sasongko H, et al. : PRISMA checklist for 'The effect of dietary seaweed inclusion on growth performance of broiler chickens: a systematic review and meta-analysis'.2020. 10.6084/m9.figshare.12721118.v1 [DOI] [PMC free article] [PubMed]
- 21.Greig JD, Waddell L, Wilhelm B, et al. : The efficacy of interventions applied during primary processing on contamination of beef carcasses with Escherichia coli: A systematic review-meta-analysis of the published research. Food Control. 2012;27(2):385–397. 10.1016/j.foodcont.2012.03.019 [DOI] [Google Scholar]
- 22.Higgins JPT, Li T, Deeks JJ: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition.Chichester, UK: John Wiley & Sons.2019;143–176. 10.1002/9781119536604.ch6 [DOI] [Google Scholar]
- 23.Suurmond R, van Rhee H, Hak T: Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537–553. 10.1002/jrsm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedges LV, Olkin I: Statistical Methods for Meta-Analysis. San Diego, CA USA: Academic Press.1985. Reference Source [Google Scholar]
- 25.Higgins JPT, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Sutton AJ, Ioannidis JP, et al. : Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 27.Mohammadigheisar M, Shouldice VL, Sands JS, et al. : Growth performance, breast yield, gastrointestinal ecology and plasma biochemical profile in broiler chickens fed multiple doses of a blend of red, brown and green seaweeds. Br Poult Sci. 2020;1–9. 10.1080/00071668.2020.1774512 [DOI] [PubMed] [Google Scholar]
- 28.Bai J, Wang R, Yan L, et al. : Co-supplementation of dietary seaweed powder and antibacterial peptides improves broiler growth performance and immune function. Braz J Poult Sci. 2019;21(2):eRBCA-2018–0826. 10.1590/1806-9061-2018-0826 [DOI] [Google Scholar]
- 29.Shi H, Kim SH, Kim IH: Effect of dietary inclusion of fermented sea mustard by-product on growth performance, blood profiles, and meat quality in broilers. J Sci Food Agric. 2019;99(9):4304–4308. 10.1002/jsfa.9663 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed ST, Mun HS, Islam MM, et al. : Effects of fermented corni fructus and fermented kelp on growth performance, meat quality, and emission of ammonia and hydrogen sulphide from broiler chicken droppings. Br Poult Sci. 2014;55(6):745–751. 10.1080/00071668.2014.960804 [DOI] [PubMed] [Google Scholar]
- 31.Choi YJ, Lee SR, Oh JW: Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australas J Anim Sci. 2014;27(6):862–870. 10.5713/ajas.2014.14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abudabos AM, Okab AB, Aljumaah RS, et al. : Nutritional value of green seaweed ( Ulva lactuca) for broiler chickens. Ital J Anim Sci. 2013;12(2):e28. 10.4081/ijas.2013.e28 [DOI] [Google Scholar]
- 33.Andri F, Dono ND, Sasongko H, et al. : Extended data for ‘The effects of dietary seaweed inclusion on growth performance of broiler chickens: a systematic review and meta-analysis’.2020. 10.6084/m9.figshare.12721454.v1 [DOI] [PMC free article] [PubMed]
- 34.Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, USA: Lawrence Erlbaum Associates.1988. Reference Source [Google Scholar]
- 35.Kulshreshtha G, Rathgeber B, Stratton G, et al. : Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult Sci. 2014;93(12):2991–3001. 10.3382/ps.2014-04200 [DOI] [PubMed] [Google Scholar]
- 36.Choi Y, Lee EC, Na Y, et al. : Effects of dietary supplementation with fermented and non-fermented brown algae by-products on laying performance, egg quality, and blood profile in laying hens. Asian-Australas J Anim Sci. 2018;31(10):1654–1659. 10.5713/ajas.17.0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal AB, Biswas A, Mir NA, et al. : Effects of dietary supplementation of Kappaphycus alvarezii on productive performance and egg quality traits of laying hens. J Appl Phycol. 2019;31(3):2065–2072. 10.1007/s10811-018-1707-8 [DOI] [Google Scholar]
- 38.Ma WQ, Cheng HZ, Zhao DH, et al. : Effects of dietary Enteromorpha powder supplementation on productive performance, egg quality, and antioxidant performance during the late laying period in Zi geese. Poult Sci. 2020;99(2):1062–1068. 10.1016/j.psj.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan GL, Guo YM, Yuan JM, et al. : Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poult Sci. 2011;90(7):1441–1448. 10.3382/ps.2011-01364 [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Li D, Wang J, et al. : Effects of polymannuronate on performance, antioxidant capacity, immune status, cecal microflora, and volatile fatty acids in broiler chickens. Poult Sci. 2015;94(3):345–352. 10.3382/ps/pev006 [DOI] [PubMed] [Google Scholar]
- 41.Sweeney T, Meredith H, Vigors S, et al. : Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species Laminaria digitata improved growth rate and intestinal structure in young chicks, but does not influence Campylobacter jejuni colonisation. Anim Feed Sci Technol. 2017;232:71–79. 10.1016/J.ANIFEEDSCI.2017.08.001 [DOI] [Google Scholar]
- 42.Gumus RE, Gelen SU, Koseoglu S, et al. : The effects of fucoxanthin dietary inclusion on the growth performance, antioxidant metabolism and meat quality of broilers. Braz J Poult Sci. 2018;20(3):487–496. 10.1590/1806-9061-2017-0666 [DOI] [Google Scholar]
- 43.Li Q, Luo J, Wang C, et al. : Ulvan extracted from green seaweeds as new natural additives in diets for laying hens. J Appl Phycol. 2018;30(3):2017–2027. 10.1007/s10811-017-1365-2 [DOI] [Google Scholar]
- 44.Tanna B, Choudhary B, Mishra A: Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018;36:96–105. 10.1016/j.algal.2018.10.019 [DOI] [Google Scholar]