Abstract
T-type calcium channels (Cav3.1 to Cav3.3) regulate low-threshold calcium spikes, burst firing and rhythmic oscillations of neurons and are involved in sensory processing, sleep, and hormone and neurotransmitter release. Here, we examined four heterozygous missense variants in CACNA1I, encoding the Cav3.3 channel, in patients with variable neurodevelopmental phenotypes. The p.(Ile860Met) variant, affecting a residue in the putative channel gate at the cytoplasmic end of the IIS6 segment, was identified in three family members with variable cognitive impairment. The de novo p.(Ile860Asn) variant, changing the same amino acid residue, was detected in a patient with severe developmental delay and seizures. In two additional individuals with global developmental delay, hypotonia, and epilepsy, the variants p.(Ile1306Thr) and p.(Met1425Ile), substituting residues at the cytoplasmic ends of IIIS5 and IIIS6, respectively, were found. Because structure modelling indicated that the amino acid substitutions differentially affect the mobility of the channel gate, we analysed possible effects on Cav3.3 channel function using patch-clamp analysis in HEK293T cells. The mutations resulted in slowed kinetics of current activation, inactivation, and deactivation, and in hyperpolarizing shifts of the voltage-dependence of activation and inactivation, with Cav3.3-I860N showing the strongest and Cav3.3-I860M the weakest effect. Structure modelling suggests that by introducing stabilizing hydrogen bonds the mutations slow the kinetics of the channel gate and cause the gain-of-function effect in Cav3.3 channels. The gating defects left-shifted and increased the window currents, resulting in increased calcium influx during repetitive action potentials and even at resting membrane potentials. Thus, calcium toxicity in neurons expressing the Cav3.3 variants is one likely cause of the neurodevelopmental phenotype. Computer modelling of thalamic reticular nuclei neurons indicated that the altered gating properties of the Cav3.3 disease variants lower the threshold and increase the duration and frequency of action potential firing. Expressing the Cav3.3-I860N/M mutants in mouse chromaffin cells shifted the mode of firing from low-threshold spikes and rebound burst firing with wild-type Cav3.3 to slow oscillations with Cav3.3-I860N and an intermediate firing mode with Cav3.3-I860M, respectively. Such neuronal hyper-excitability could explain seizures in the patient with the p.(Ile860Asn) mutation. Thus, our study implicates CACNA1I gain-of-function mutations in neurodevelopmental disorders, with a phenotypic spectrum ranging from borderline intellectual functioning to a severe neurodevelopmental disorder with epilepsy.
Keywords: low-voltage-gated calcium channels, T-type calcium channels, CaV3.3, epilepsy, intellectual disability (ID)
T-type calcium channels regulate neuronal firing, are implicated in brain disease and are targets for anti-epileptic drugs. El Ghaleb et al. identify heterozygous missense variants of CACNA1I associated with variable neurodevelopmental disorders and epilepsy, and show how gain-of-function mutations cause hyper-excitability.
Introduction
Low voltage-activated, T-type calcium channels (Cav3) are expressed throughout the vertebrate nervous system and critical for normal cerebellar, thalamic, and cortical functions.1-3 Owing to their specific biophysical properties they regulate neuronal excitability and contribute to neural processing of pain, sensory, and motor functions, to neurotransmitter and hormone release, and to sleep.4 In addition, T-type calcium channels are also expressed in astrocytes.5 Mutations in CACNA1G, CACNA1H, and CACNA1I encoding Cav3 channels have been associated with a range of neurodevelopmental, neurological, and/or psychiatric disorders.6 Accordingly, T-type calcium channels are regarded as promising candidate targets in ongoing drug development ventures.7
T-type calcium channels operate at negative voltages near the resting potential of nerve cells, where they regulate excitability and the rhythmic activity of neuronal circuits.4,8,9 At rest, the great majority of T-type channels are inactivated. Following hyperpolarization, they recover and generate low threshold calcium spikes and rebound burst firing. Because of their slow deactivation kinetics considerable amounts of calcium can enter nerve cells in the wake of an action potential. Furthermore, because of the negative voltage-dependence of activation and the incomplete overlap with the voltage-dependence of inactivation, a small fraction of T-type channels remains open at rest.10 In electrophysiological analyses these currents upon repolarization and at rest are evident as transient ‘tail currents’ and as continuous ‘window currents’, respectively. When aberrantly enlarged, both these currents can become the source of an increased calcium load threatening normal development and survival of neurons.
In recent years several disease-associated Cav3 channel variants have been identified and functionally characterized in heterologous expression systems and genetic mouse models.6 The most deleterious variants detected in the CACNA1G and CACNA1H genes, encoding Cav3.1 and Cav3.2, respectively, represent de novo gain-of-function missense mutations causing congenital severe motor and cognitive impairment with cerebellar atrophy and primary aldosteronism, respectively.11–13 These missense mutations are primarily located at the cytoplasmic end of the channels’ S6 helices, which comprise the channel gate.6,14 Accordingly, the common feature of these mutations is that they affect the channel gating properties; foremost by slowing the kinetics and left-shifting the voltage-dependence of activation and inactivation. This leads to prolonged channel openings and increased window currents, which in turn result in hyper-excitability of neurons and an increased calcium load, which might be causal for neurological defects on one hand and aldosterone production and hypertension on the other.6 Involvement of both the CACNA1G and CACNA1H genes in inherited epilepsy has been proposed based on various findings in humans and mice.15–17 However, to date, none of the investigated variants have been undoubtable identified as causing seizure phenotypes and are rather classified as genetic risk factors for developing epilepsy (reviewed in Lory6).
The third member of the T-type channel family, Cav3.3 encoded by CACNA1I, was identified as genetic risk factor in schizophrenia.18–23 The schizophrenia-associated missense variant p.(Arg1346His) causes decreased membrane expression and current density when expressed in heterologous cells, while other current properties remained unaltered.24 Computer simulation suggests that such reduced current density eliminates rebound burst firing in thalamic reticular nucleus (TRN) neurons, in which Cav3.3 channels are highly expressed.25,26 Knock-in mice homozygous for the orthologous p.(Arg1346His) variant showed altered excitability of TRN neurons and deficits in sleep spindle occurrence.27 Thus, CACNA1I loss-of-function variants disrupt neuronal excitability and network activity and may contribute to the development of schizophrenia, autism and/or other complex neuropsychiatric disorders.22,28,29
Here we report three unrelated patients and one family with three affected individuals with heterozygous missense variants in the CACNA1I gene and variable neurodevelopmental phenotype. While the mother and her two children of one family showed variable cognitive impairment, the three unrelated probands had a severe phenotype with global developmental delay, hypotonia and epilepsy. Three of the four disease-associated missense variants affect an amino acid residue located at the cytoplasmic end of an S6 helix constituting the gate of the Cav3.3 calcium channel. The fourth amino acid change is located in the closely adjacent region of an S5 helix. Structure modelling suggests that the amino acid substitutions reduce the mobility of the gate by introducing new stabilizing hydrogen bond interactions. Electrophysiological analysis demonstrated slowed kinetics and left-shifted voltage-dependence of activation and inactivation of all the Cav3.3 mutant channels. These altered gating properties result in hyper-excitability, prolonged calcium currents, and a shift of firing modes when tested in the TRN neuron model and upon heterologous expression in mouse chromaffin cells. Interestingly, Cav3.3 mutant channels with p.(Ile860Asn) or p.(Ile860Met) greatly differ in the magnitude of their effects on gating properties and cellular excitability, and these differences parallel the disease severity in the affected individuals. Thus, the data presented here provide evidence for a causal link of pathogenic missense variants in the CACNA1I gene with a range of neurodevelopmental phenotypes. The gain-of-function effects caused by the amino acid substitutions offer possible functional and mechanistic explanations for the pathophysiological role of the Cav3.3 T-type calcium mutant channels in causing impaired cognitive function and epilepsy.
Materials and methods
Patients
Informed consent for genetic analyses was obtained for all patients, and genetic studies were performed clinically or as approved by the Institutional Review Boards of the relevant institutions. The patients or patients’ parents provided written informed consent for the participation in the study, clinical data and specimen collection, genetic analysis, and publication of relevant findings.
Whole-exome sequencing
Quad whole-exome sequencing (WES) was performed in Family 2 (Patients 2–4 and the father of the siblings), trio WES in Families 1 and 3 (Patients 1, 5 and their parents) and duo WES in Family 4 (Patient 6 and mother). Variant validation was performed by Sanger sequencing with DNA obtained from leucocytes of patients and parents.
Structure modelling
We predicted the structure of the wild-type T-type channel Cav3.3 α1-subunit and the mutants in the activated state by building a homology model based on the cryo-electron microscopy (EM) structure of the Cav3.1 α1-subunit in the inactivated state characterized by depolarized voltage-sensing domains and a closed intracellular gate (PDB accession code: 6KZO).14 The high sequence similarity of ∼85% allows a reliable structure prediction of the Cav3.3 α1-subunit. Additionally, we also generated a homology model of the Cav3.3 α1-subunit in a resting state based on the cryo-EM resting state structure of NavAb (PDB accession code: 6P6W).30
Expression plasmids and transfections
The human Cav3.3 subunit (Genebank ID AF393329)31 was transferred into an expression plasmid with an N-terminal GFP tag and the mutations were introduced by splicing by overlap extension (SOE)-PCR. HEK293T cells were transfected with the expression plasmids using FuGENE® HD reagent (Promega). Chromaffin cells from 6–8-week-old male mice were obtained as described previously32,33 and transfected by electroporation with the Mouse Neuron NucleofectorTM Kit.34
Electrophysiology and data analysis
Calcium currents in HEK293T cells were recorded with the whole-cell patch-clamp technique in voltage-clamp mode. Patch pipettes had resistances between 1.8 MΩ and 4.5 MΩ when filled with (in mM) 135 CsCl, 1 MgCl2, 10 HEPES, 4 ATP-Na2 and 10 EGTA (pH 7.4 with CsOH). The extracellular bath solution contained (in mM) 2 CaCl2, 165 choline-chloride, 10 HEPES, and 1 MgCl2 (pH 7.4 with CsOH). All five experimental groups were analysed in transiently transfected cells from four to six independent cell passages. The variants were always recorded in parallel with the wild-type Cav3.3 in cells of the same passage to obtain matched controls for statistical comparison. The means, standard error of the mean (SEM), and P-values of the experiments where the variants have separate control groups were calculated using the Student’s t-test, two-tailed, with significance criteria *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. P-values of the experiments where the variants are compared to the same control group were calculated using the ANOVA and Tukey’s or Dunnett’s post hoc test.
The whole cell current-clamp recordings of isolated mouse chromaffin cells were performed in perforated-patch mode. Patch pipettes had a resistance between 1.8 MΩ and 4 MΩ when filled with (in mM): 10 NaCl, 10 KCl, 76 K2SO4, 1 MgCl2, 5 HEPES (pH 7.35 with KOH) and supplemented with 240 µg/ml amphotericin B. The external bath solution contained (in mM): 140 NaCl, 3.6 KCl, 2 NaHCO3, 0.5 NaH2PO4, 0.5 MgSO4, 2.5 CaCl2, 5 HEPES, 5 glucose (pH 7.4 with NaOH). All three experimental groups were analysed in eGFP-positive chromaffin cells from three independent culture preparations. Wild-type Cav3.3, I860N and I860M were recorded in parallel on the same days to match controls and mutants for optimal statistical comparison. Data-points in scatter plots represent values of individual cells and means (line) ± standard error (SE). P-values were calculated using the Student’s t-test or ANOVA with Holm-Sidak post hoc test with significance criteria *P < 0.05, **P < 0.01, and ***P < 0.001.
Computer model
Modelling was performed in the NEURON simulation environment35 using the model for thalamic relay neurons36 from the model database at Yale University (https://senselab.med.yale.edu/modeldb/ accessed 21 May 2021). The electrophysiological properties of the Cav3.3 channels were modelled using Hodgkin-Huxley equations as described previously.36,37 The values of native T-type channels were substituted by the experimentally obtained values for the wild-type and the individual mutants.
Further details of the experimental procedures can be found in the Supplementary material.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary material. Consent restrictions preclude sharing of full datasets, and the consents do not cover the deposition of the next-generation sequencing data in a public database.
Results
Heterozygous missense variants in the CACNA1I gene cause a spectrum of neurological symptoms
Patient 1 is an 8-year-old female, who is the only child of healthy unrelated parents (Supplementary Table 1). Pregnancy was complicated by reduced foetal movements, breech presentation, and mild gestosis. She was born at 37 + 6 weeks of gestation by caesarean section; her birth parameters were in the normal range. Muscular hypotonia was noted at age 4 weeks, and seizures were discussed because of atypical head movements. At the age of 4 months, she received anticonvulsive therapy because of pathological EEG findings associated with possible epileptic apnoea. She showed non-epileptic hyperexcitability, and within the second year of life epileptic myoclonus was noted. Between the age of 4 and 6 years, startle seizures triggered by noise were dominating with myoclonic seizures followed by a tonic episode. Last examination at age 8 years and 1 month revealed severe global developmental delay and severe proximal muscular hypotonia and distal muscular hypertonia; she did not show any interaction. She did not achieve any motor milestones and had no speech and no cognitive development. She had myoclonic seizures daily as the predominant seizure type and rare grand mal seizures that did not respond to treatment. Cortical blindness was diagnosed. She had severe obstructive sleep apnoea syndrome that required intermittent non-invasive ventilation at the age of 6 years. She received a gastrostomy tube because of feeding difficulties. Brain MRI at age 4 months was normal. At the age of 2 years and 7 months, brain imaging revealed frontal brain atrophy, a flattened brainstem and mildly delayed myelination. We performed trio WES in Patient 1 and her parents, and did not detect any biallelic variant that could underlie her severe neurodevelopmental phenotype. However, we identified the Sanger-validated de novo variant c.2579T>A in the CACNA1I gene predicting the amino acid substitution p.(Ile860Asn) as the only potentially disease-causing variant (Supplementary Tables 1 and 2).
Through GeneMatcher,38 we identified five additional individuals with a heterozygous missense variant in CACNA1I: Family 2 with three affected members (Patients 2–4), and the two unrelated Patients 5 and 6. We included these cases in the study for clinical comparison and functional analysis. Family 2 comprised a 58-year-old female (Patient 2), her 28-year-old son (Patient 3) and 31-year-old daughter (Patient 4) who all carried the CACNA1I variant c.2580C>G/p.(Ile860Met) (Supplementary Tables 1 and 2). The mother of Patients 3 and 4 had mild cognitive impairment, and she developed seizures at the age of 58 years. Patient 3 had mild intellectual disability, and Patient 4 showed moderate intellectual disability and speech retardation. Brain CT in Patient 2 did not reveal any abnormalities (Supplementary Table 1). Interestingly, in Patient 1 and Family 2 codon 860 of CACNA1I was affected resulting in substitution of isoleucine for asparagine in Patient 1 and for methionine in Family 2 (Supplementary Table 2). WES in female Patient 5 and male Patient 6 revealed the heterozygous CACNA1I variant c.3917T>C/p.(Ile1306Thr) and c.4275G>A/p.(Met1425Ile), respectively (Supplementary Tables 1 and 2). De novo occurrence of the variant was confirmed in Patient 5, while the change was absent in the mother of Patient 6, but his father was not available for testing (Supplementary Table 1). The 21-month-old Patient 5 and the 8-year-old Patient 6 presented with severe developmental delay, hypotonia, severe speech impairment, and cortical visual impairment. Seizures began at age 2 weeks in Patient 5 and at 2 years in Patient 6 and are controlled on medication. Brain imaging at the age of 12 months was unremarkable in Patient 5; in Patient 6, several brain abnormalities were observed at the age of 5 years (Supplementary Table 1). Similar to Patient 1, Patients 5 and 6 had feeding issues with reflux that required G-tube placement in Patient 5. Patients 1 and 6 presented with postnatal growth retardation, and Patient 6 in addition had growth hormone deficiency (Supplementary Table 1).
CACNA1I is intolerant to functional genetic variation (Z-score: 5.05; observed/expected value for missense variants: 0.59),39,40 and the four different CACNA1I missense variants affect three evolutionary conserved amino acid residues that were predicted to be intolerant to variation (Supplementary Fig. 1 and Table 2).41 All variants are absent from gnomAD, and three in silico algorithms predicted the missense variants to have a damaging impact on protein function (Supplementary Table 2). In summary, based on the absence of the identified CACNA1I variants in the population databases, a predicted deleterious effect of the missense variants on protein function, and impaired cognitive function in all six affected individuals, we believed the heterozygous CACNA1I missense changes to underlie the phenotype in all of them.
Homology structure modelling predicts increased stability of activation gates in Cav3.3-I860N, -I860M and -I1306T
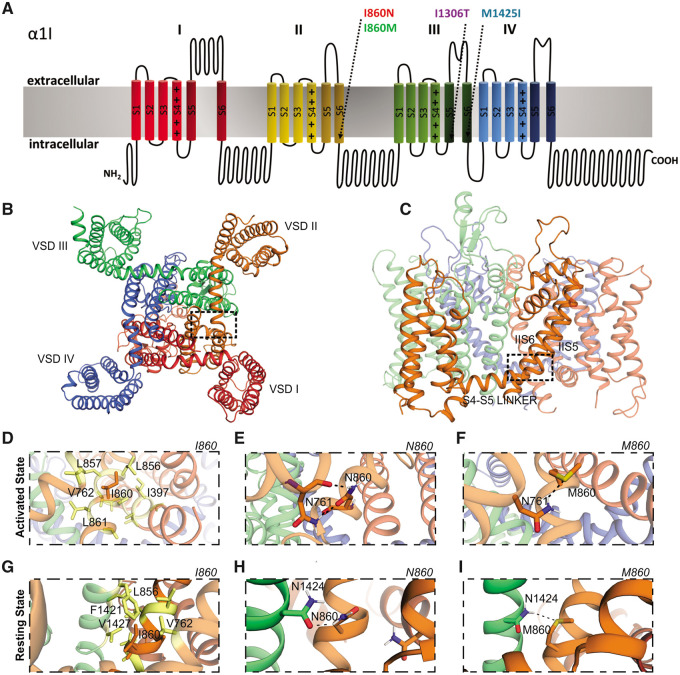
To assess the structural impact of the missense variants, we generated homology structure models of wild-type and mutant Cav3.3. Eukaryotic Cav channels are composed of four homologous repeats (I–IV), each comprising six membrane-spanning helices (S1–S6) (Fig. 1A). Helices S1–S4 of each repeat form separate voltage-sensing domains, while the four S5 and S6 helices and the connecting pore-loops form the channel pore with the selectivity filter and the activation gate (Fig. 1B and C). Because the two different substitutions of I860 (p.I860N and p.I860M) were found in individuals with greatly differing disease severity, we first focused our attention on these two variants. The I860 residue is located at the intracellular end of the IIS6 helix, a position known to be part of the channel activation gate (Fig. 1A–C).42 The structure homology model of the activated state of Cav3.3, based on the cryo-EM structure of Cav3.1 in the activated state,14 shows that I860 in wild-type Cav3.3 forms multiple hydrophobic interactions with neighbouring residues in the IIS5, IIS6 and the S4–S5 linker (Fig. 1D). These hydrophobic interactions in the activation gate area have only a weak stabilizing effect, which allows the flexibility necessary for opening and closing the channel gate. When the I860 residue is replaced by asparagine (N860), the model predicts the formation of two strong hydrogen bonds between N860 and N761 in the S4–S5 linker (Fig. 1E). This stabilizes the activation gate in the activated state and probably perturbs S6 and S4–S5 linker movement upon deactivation and inactivation. Replacing the I860 residue with methionine (M860) results in the formation of a single stabilizing sulphur hydrogen bond between M860 and N761 (Fig. 1F). This sulphur hydrogen bond is considerably weaker than either one of the classical hydrogen bonds formed by the I860N variant. Thus, M860 may also stabilize the activation gate in the activated state and potentially perturb S6 and S4–S5 linker movement, but to a lesser extent than the I860N variant (Table 1).
Figure 1.
Structure modelling of the variants I860N and I860M in Cav3.3. (A) Domain structure of Cav3.3 indicating the location of the four pathogenic missense variants at the cytoplasmic end of transmembrane helices IIS6, IIIS5, and IIIS6 (arrows) which form part of the channel’s activation gate. (B–F) Structure homology model of Cav3.3 based on the cryo-EM structure of Cav3.1 in the activated state, colour-coded as in A. (B) Bottom view and (C) side view, frames show the areas depicted in D–I. VSDI–IV = voltage-sensing domains. (D) In wild-type, I860 in IIS6 forms numerous hydrophobic interactions with neighbouring residues in the IIS5, IIS6 and the S4-S5 linker. (E) The N860 variant forms two strong, stabilizing hydrogen bonds between N860 and N761 in the S4-S5 linker. (F) The M860 variant forms one weak sulphur hydrogen bond with N761. (G–I) Interactions of the different variants in the resting state. (G) Wild-type I860 forms numerous hydrophobic interactions with neighbouring residues in IIIS6 and IIS5. (H) The N860 variant forms one strong stabilizing hydrogen bond between N860 and N1424 in IIIS6. (I) The M860 variant forms one sulphur hydrogen bond with N1424.
Table 1.
Predicted interaction partners‘ of all four CACNA1I variants (N860, M860, T1306, I1425) and their corresponding wild-type residues (I860, I1306, M1425) in the activated and resting state
| Variant | Interaction partners activated state | Interaction partners resting state | Nature of interaction | Stabilization |
|---|---|---|---|---|
| I860 | I397, V762, L856, L857, L861 | V762, L856, F1421, V1427 | Hydrophobic | Low |
| N860 | N761 | N1424 | 2 H-bonds | High |
| M860 | N761 | N1424 | 1 S-H bond | Medium |
| I1306 | V1429 | V1726 | Hydrophobic | Low |
| T1306 | E1432 | N1723 | Charged H-bond | High |
| M1425 | V1310, I1309 | I1306 | Hydrophobic | Low |
| I1425 | V1310, I1309 | I1306 | Hydrophobic | Low |
Next we analysed possible interactions of wild-type I860 and mutant N860 and M860 in a homology model of Cav3.3 in the resting state, based on the resting state structure of the prokaryotic sodium channel NavAb30 (Fig. 1G–I). Again, wild-type I860 formed multiple hydrophobic interactions (Fig. 1G). In contrast, N860 (Fig. 1H) and M860 (Fig. 1I) formed a single hydrogen bond and a sulphur hydrogen bond, respectively, with an asparagine (N1424) in the neighbouring IIIS6 helix, thus stabilizing the channel gate in the resting state and thereby potentially hampering channel opening upon activation.
A similar effect was found for the I1306T variant, which formed stabilizing hydrogen bonds in the resting and activated states (Table 1 and Supplementary Fig. 2A–D). Only the M1425I variant in IIIS6 was different, in that it did not form stabilizing hydrogen bonds, but further increased the hydrophobicity (Table 1 and Supplementary Fig. 2E–H). Interestingly, however, this substitution strengthened the van der Waals interaction with I1306 in the neighbouring IIIS5 helix, indicating reciprocal effects within this critical interaction network in the channel gate. The hydrophobic interactions in wild-type Cav3.3 are consistent with the necessary mobility of the cytoplasmic ends of the S5 and S6 helices in the gating process. On the other hand, the introduction of stabilizing interactions in the resting and activated states of the disease-associated variants is anticipated to impede both opening and closing of the gate.
CACNA1I variants alter the gating properties of Cav3.3 to different extents
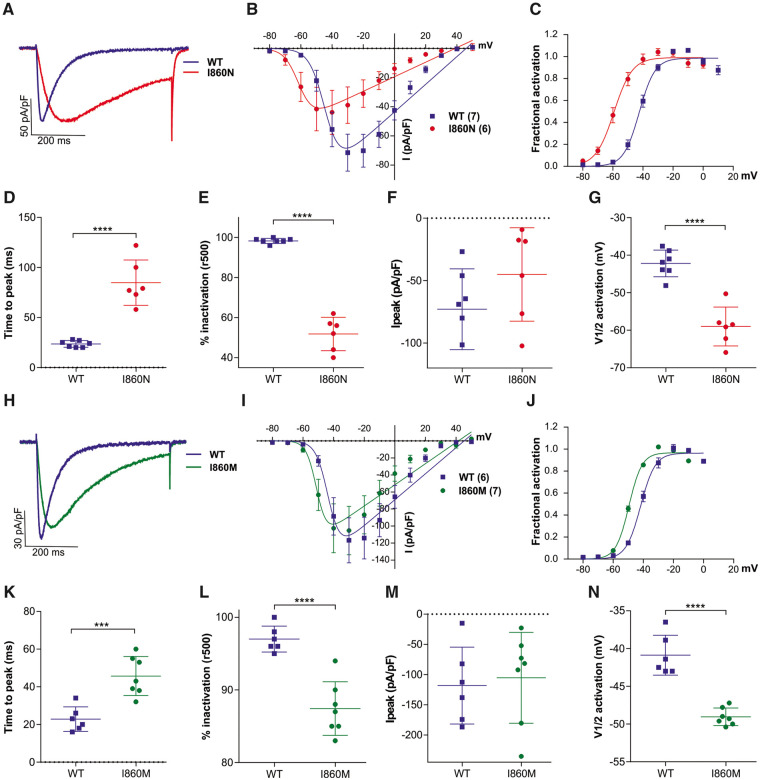
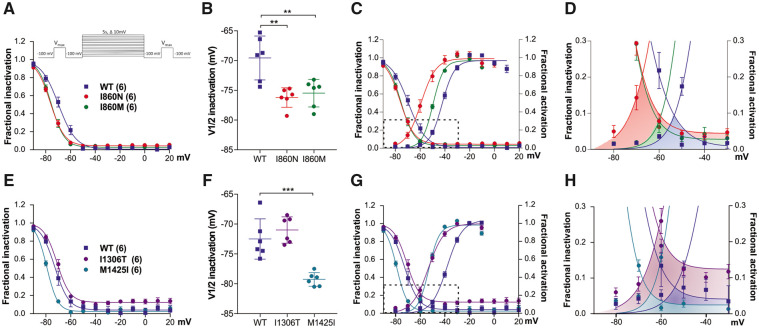
To study the effects of the four disease-associated amino acid substitutions on the biophysical gating properties of the channel we performed whole-cell patch clamp recordings of HEK293T cells transfected with wild-type and mutant Cav3.3 channels. In a first set of experiments we individually compared the current properties of the two substitutions of I860 with wild-type controls. The representative recordings and quantitative analyses presented in Fig. 2 and Supplementary Table 3 demonstrate that the I860N substitution caused a striking slowing of current kinetics (Fig. 2A–E), and a similar but milder effect was observed for the I860M mutant (Fig. 2H–L). At the voltage of maximal activation (Vmax) the time to peak of Cav3.3-I860N was increased by more than 3-fold compared to that of wild-type Cav3.3 (Fig. 2D) and ∼2-fold for Cav3.3-I860M (Fig. 2K). However, analysis of the activation time constants at all test potentials revealed that for the Cav3.3 mutants activation kinetics were significantly increased only at voltage steps to −50 mV and for Cav3.3-I860N also at −40 mV, but not at higher test potentials (Supplementary Fig. 4). Also, both Cav3.3 mutants showed a significant slowing of the inactivation kinetics with a substantially greater effect for Cav3.3-I860N compared to Cav3.3-I860M. During a 500-ms depolarization to Vmax the calcium current of wild-type Cav3.3 inactivated almost completely (95–98%), while inactivation of the Cav3.3-I860N current only reached ∼52% (Fig. 2E) and Cav3.3-I860M ∼87% (Fig. 2L). Fitting the decay phase of the current during 5-s test pulses showed significantly increased time constants for inactivation between −40 and +20 mV for Cav3.3-I860N, but for Cav3.3-I860M this increase does not reach significance (Supplementary Fig. 5). Furthermore, the I/V curve (Fig. 2B and I) and fractional activation plotted against the voltage of the depolarizing test pulse (Fig. 2C and J) show that the voltage dependence of activation was significantly shifted in the hyperpolarizing direction in both Cav3.3 mutants. Compared to wild-type Cav3.3 the Cav3.3-I860N mutant resulted in a 15.5 mV left shift of the activation curve (Fig. 2G and Supplementary Table 3). The voltage dependence of activation of Cav3.3-I860M was 8.1 mV left-shifted compared to wild-type Cav3.3 (Fig. 2N and Supplementary Table 3).
Figure 2.
The I860N and I860M substitutions slow current kinetics and left-shift the voltage-dependence of activation of Cav3.3. (A–G) Current properties of Cav3.3-I860N (red) compared to its wild-type (WT) Cav3.3 controls (blue). (H–N) Current properties of Cav3.3-I860M (green) compared to its wild-type Cav3.3 controls (blue). (A and H) Example maximal current traces at Vmax with comparable current densities show slower activation and inactivation of the I860N and I860M variants. (B and I) The current-voltage relationship and (C and J) the fractional activation curves show a 15.5 mV left-shift of activation for I860N (n = 6) and an 8.1 mV left-shift of activation for I860M (n = 7), as compared to wild-type (n = 7 and 6, respectively). (D and K) Scatter plots of the time to peak and (E and L) the fractional inactivation after 500 ms show significantly slowed activation and inactivation of the I860N and I860M variants at Vmax. (F and M) The differences in maximum current densities (Ipeak) between WT and I860N or I860M are not significant (I860N, P = 0.18; I860M, P = 0.75). (G and N) The scatter plots of the voltage at half-maximal activation (V½) show significant hyperpolarizing shifts for I860N and I860M. Mean ± SEM; P-values calculated with Student’s t-test; ***P < 0.001, ****P < 0.0001.
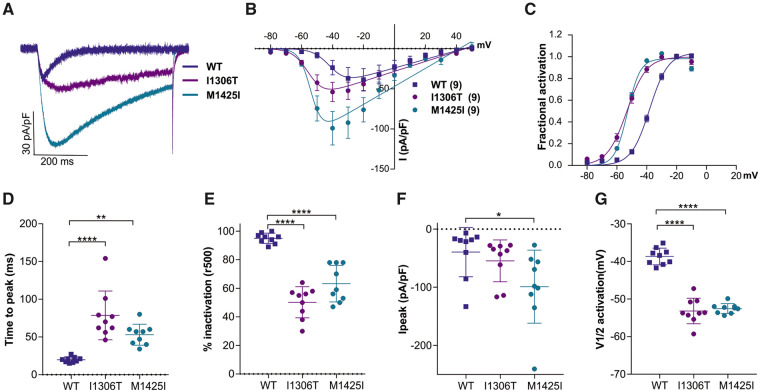
Additional electrophysiological analysis of the mutants identified in Patients 5 and 6, Cav3.3-I1306T and Cav3.3-M1425I, demonstrated similar changes on activation and inactivation kinetics as well as a left-shifted voltage-dependence of activation, 14.5 mV and 13.7 mV, respectively. M1425I also showed an almost 3-fold increase in Ipeak (Fig. 3, Supplementary Figs 4, 5 and Supplementary Table 3).
Figure 3.
The I1306T and M1425I substitutions slow current kinetics and left-shift the voltage-dependence of activation of Cav3.3. (A) Example maximal current traces of wild-type (WT) Cav3.3 (blue), Cav3.3-I1306T (purple) and Cav3.3-M1425I (cyan) show slower activation and inactivation at Vmax. (B) The current-voltage relationship and (C) the fractional activation curves show a 6.6 mV left-shift of activation for I1306T (n = 9), as compared to wild-type (n = 9), and a 5.9 mV left-shift for M1425I (n = 9). (D) Scatter plots of the time to peak and (E) the fractional inactivation after 500 ms at Vmax indicate significantly slowed activation and inactivation, respectively, of both variants. (F) The maximum current density (Ipeak) is doubled in M1425I (P = 0.03), but only insignificantly increased in I1306T (P = 0.73). (G) The scatter plot of the voltage at half-maximal activation (V½) shows a significant hyperpolarizing shift for both variants. Mean ± SEM; P-values calculated with ANOVA and Dunnett’s post hoc test; *P < 0.05, **P < 0.01, ****P < 0.0001.
Taken together, the current properties of the four Cav3.3 mutants reveal a gain-of-function effect. The left shift of voltage dependence of activation indicates that the putative disease variants activate at voltages closer to the resting membrane potential compared to wild-type Cav3.3 channels. The slowed inactivation of the mutants allows considerably more calcium to enter during depolarization. The magnitude of these effects varied between the examined Cav3.3 mutants, but consistently was highest in Cav3.3-I860N and lowest in Cav3.3-I860M.
Deactivation kinetics of Cav3.3 channels are slowed in Cav3.3-I860N, -I1306T, and -M1425I
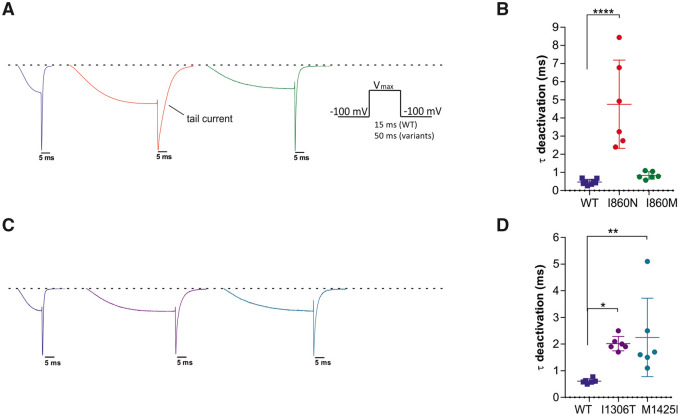
To examine whether the amino acid substitutions also affect the deactivation kinetics of the Cav3.3 channel, we performed voltage steps to Vmax for the duration necessary to achieve maximal activation, without detectable inactivation. As shown in Fig. 4A and C the tail current of the I860N, I1306T, and M1425I mutants were strikingly broader than those of wild-type and the I860M mutant. When fitting the decay of the tail current, we observed a substantial slowing of the time constant of deactivation in I860N as compared to wild-type Cav3.3, less but still significant slowing for I1306T and M1425I, but similar deactivation kinetics for I860M and wild-type (Fig. 4B and D). The up to 10-fold slower deactivation of calcium currents in the three Cav3.3 mutants suggests a considerably increased calcium influx upon repolarization as compared to both wild-type and I860M Cav3.3 channels. As the speed of deactivation is a critical determinant for the oscillatory behaviour of Cav3.3 channels,10 this channel function may be compromised in neurons expressing Cav3.3-I860N, -I1306T, or -M1425I.
Figure 4.
Current deactivation is slowed in I860N, I1306T and M1425I, but not in I860M. (A) Representative current traces, normalized to the peak of the tail currents of wild-type (blue), display a broadening of the tail current in I860N (red) and I860M (green) compared to wild-type. Inset shows the voltage-clamp protocol; voltage-steps were applied from a holding potential of −100 mV to the voltage of maximal activation for the duration necessary to achieve maximal activation without detectable inactivation [wild-type (WT) 15 ms, variants 50 ms]. (B) The scatter plot of the time constants (τ) of a mono-exponential fit of the decay of the tail currents demonstrates a significant slowing of deactivation in I860N versus wild-type (P < 0.0001), but not for I860M versus wild-type (P = 0.8). Wild-type (n = 8), I860N (n = 6), I860M (n = 6). Mean ± SEM; P-values calculated with ANOVA and Tukey’s post hoc test. ****P < 0.0001. (C) Representative normalized example current traces show that the tail currents of I1306T (purple) and M1425I (cyan) are broadened compared to wild-type. (D) The scatter plot of the time constants (τ) of a mono-exponential fit of the decay of the tail currents demonstrates a significant slowing of deactivation in I1306T versus wild-type (P = 0.02), as well as for M1425I versus wild-type (P = 0.009). n = 6 for all three experimental groups; P-values calculated with ANOVA and Dunnett’s post hoc test. *P < 0.05, **P < 0.01.
Steady state inactivation and window currents are shifted closer to the resting membrane potential
We performed a steady state inactivation protocol comparing the current size during test pulses before and after 5 s conditioning pulses at incrementally increasing potentials. Plotting the fractional inactivation against the voltage of the conditioning pulse revealed a significant left-shift of voltage-dependence of inactivation in all mutants except in I1306T (Fig. 5A, B, E and F). The overlapping area between activation and inactivation curves represents the window current (Fig. 5C and G). In all mutants the window current was left-shifted with peaks at voltages between −70 and −60 mV, as compared to window currents of wild-type Cav3.3, which peaks near −50 mV (Fig. 5D and H). In addition, I860N and I1306T experienced a substantial increase of their window currents, because in both cases the left-shift in the voltage-dependence of activation was not accompanied by a similar left-shift in the voltage-dependence of inactivation. The voltage range of Cav3.3 window current is a critical determinant of electrical activity and calcium oscillations,10 suggesting that the left-shifted and increased window current affects electrical activity of neurons expressing these Cav3.3 variants. This further implies a persistent calcium influx close to the resting membrane potential, suggesting the possibility of calcium toxicity particular for Cav3.3-I860N and Cav3.3-I1306T.
Figure 5.
Steady state inactivation and window currents are left-shifted in all four missense variants of Cav3.3. (A–D) Steady-state inactivation and window currents of Cav3.3-I860N (red) and -I860M (green) compared to wild-type (WT) controls (blue); (E–H) the same for Cav3.3-I1306T (purple) and Cav3.3-M1425I (cyan). (A, B, E and F) Fractional inactivation curves and scatter plot of V½ of inactivation show that, compared to wild-type, the voltage-dependence of inactivation is left-shifted in I860N (6.6 mV, P = 0.0018), I860M (5.9 mV, P = 0.0045; n = 6), and M1425I (6.8 mV, P < 0.001), but not in I1306T (P = 0.47). The inset in A shows the steady state inactivation protocol used for these experiments. (C and G) The simultaneous display of the fractional activation and inactivation curves shows that activation of Cav3.3-I860N and Cav3.3-I1306T is left-shifted to greater extent than inactivation, resulting in greatly increased window currents. (D and H) Enlarged area indicated by the frames in (C and G) show the size and voltage-range of the window currents for wild-type (shaded in blue), I860N (red), I860M (green), I1306T (purple), and M1425I (cyan). Window currents of Cav3.3-I860M and -M1425I are left-shifted but not enlarged. n = 6 for all experimental groups. Mean ± SEM; P-values calculated with ANOVA and Dunnett’s post hoc test. **P < 0.01, ***P < 0.001.
The mutant Cav3.3 channels increase persistent and action potential-induced calcium currents
The observed alterations in activation, deactivation, and inactivation properties of the disease-associated Cav3.3 mutants are expected to affect neuronal excitability and the magnitude of calcium influx during repetitive action potential firing. Therefore, we next examined the calcium currents in action potential clamp experiments. Action potential-like depolarizations, modelled on the shape of thalamic neurons,43 were repeated 99 times at 20 Hz, and currents were recorded from HEK293T cells heterologously expressing wild-type and mutant Cav3.3 channels (Supplementary Fig. 3A and B). In all conditions, T-type calcium currents were facilitated during the first few action potentials and then experienced a continuous attenuation during the rest or the protocol, due to accumulating inactivation of Cav3.3. Importantly, with Cav3.3-I860N, -I860M, -I1306T, and -M1425I calcium currents before (persistent pace-making current), during, and after the action potential were increased by several-fold (Supplementary Fig. 3C). The increase was greatest in the early sweeps, where the persistent current of the mutants was an order of magnitude larger than that of wild-type and about twice the size for I860N and I1306T compared to I860M and M1425I (Supplementary Fig. 3D). Also, the calcium influx during the action potential was several-fold larger than in wild-type, with I860N, -I1306T and -M1425I, but not for -I860M (Supplementary Fig. 3E and F). Together, the action potential clamp recordings demonstrate that during repetitive action potential firing calcium influx is increased substantially and to different degrees in cells expressing the examined Cav3.3 mutants. However, action potential clamp experiments do not account for the dynamic feedback regulation of the continually changing T-type currents onto the shape of the neuronal firing patterns.
The mutant Cav3.3 channels increase neuronal firing in a model of thalamic neurons
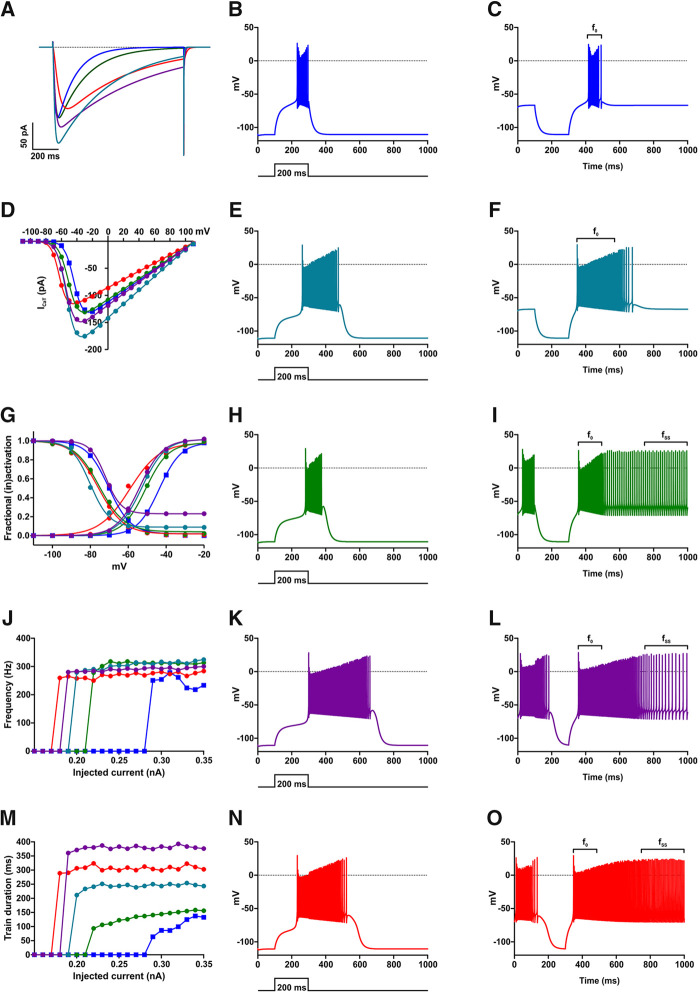
Since Cav3.3 is highly expressed in thalamic neurons and these neurons are implicated in the pathophysiology of epilepsy,17,37,44 we simulated the effects of altered gating properties of the four Cav3.3 disease-associated variants in a computer model of TRN neurons.11,45 In the model the parameters of the T-type calcium conductance were adjusted to closely resemble the current properties determined in the heterologous cell system (Fig. 6A, D and G; cf. Figs 2, 3 and 5). Simulation of neuronal excitability with the wild-type Cav3.3 showed that depolarizing current injection triggers burst firing with a starting frequency of ∼250 Hz that is steadily decreasing until the burst ends abruptly at the end of the current injection (Fig. 6B and J). In simulations for the Cav3.3 mutants eliciting burst firing required substantially less current injection (Fig. 6J and M) indicating that the gating defects in the Cav3.3 mutants lower the neuron’s rheobase. The firing frequency was somewhat elevated (up to 300 Hz), but strikingly the burst duration was prolonged, exceeding the duration of the depolarizing current injection (Fig. 6 B, E, H, K, M and N). These effects on rheobase and burst duration were strongest for I860N and weakest for I860M (Fig. 6J and M).
Figure 6.
Computer modelling of a TRN neuron shows that all amino acid substitutions increase electrical activity of Cav3.3, with I860N showing a stronger effect. The experimentally determined values for τactivation, τinactivation, V1/2activation, kact, V1/2inactivation, and kinact were entered in the computer model to generate ICaT currents with identical kinetics (A) and voltage dependence of activation and inactivation (D and G) as experimentally measured (Figs 2–5) for wild-type (WT) Cav3.3 (blue), M1425I (cyan), I860M (green), I1306T (purple) and I860N (red) channels. To test the effects of mutations on neuronal excitability, the resting membrane potential was set to −110 mV and 200 ms long depolarizing current pulses of increasing amplitudes were applied. (B, E, H, K and N) show the electrical activity at the minimum current injection (rheobase) necessary to stimulate an action potential. (J) The wild-type model needs a depolarizing pulse of 0.29 nA to initiate a train of action potentials, while M1425I fires at 0.20 nA current injection, I860M at 0.22 nA, I1306T at 0.19 nA, and I860N at 0.18 nA. (M) While at maximum current injection of 0.35 nA the 200-ms long pulses initiate in wild-type and I860M models an action potential train with a comparable duration (wild-type t = 132.7 ms and I860M t = 156.3 ms) the other Cav3.3 mutations substantially increased the train duration (M1425I t = 243.5 ms, I1306T t = 376.3 ms, and I860N t = 302.9 ms) even at lower current injection levels. Running the models for 10 s (here shown 1 s) with the resting membrane potential unclamped showed that the wild-type model fires short action potential trains only after a brief hyperpolarizing pulse to −110 mV (C) with a frequency f0=255.1 Hz, while I860M (I), I1306T (L) and I860N (O) models display a continuous electrical activity, with I860M showing an initial frequency of f0 = 355.5 Hz and final frequency of fSS = 91.5 Hz, I1306T of f0 = 320.9 Hz and fSS = 44.9 Hz, and I860N of f0 = 320.3 Hz and fSS = 129.2 Hz. (F) The M1425I model does not show a continuous electrical activity but a train with a frequency f0 = 338.2 Hz.
When the simulations were run from a membrane potential of −60 mV, corresponding to the up-state of thalamic neurons, in the wild-type model hyperpolarizing steps to −110 mV were followed by transient burst lasting ∼100 ms (Fig. 6C). In contrast, in simulations of the mutants the bursts were lengthened, stopping spontaneously only with Cav3.3-M1425I. For Cav3.3-I860M, -I860N, and -I1306T bursting started at high frequency for ∼100 ms and then switched to a steady firing pattern at reduced frequency continuing until the end of the 10-s recording (Fig. 6F, I, L and O). Together, the data from the computer simulations of TRN neurons indicate that the altered gating properties of the Cav3.3 mutants cause a neuronal gain-of-function effect with severe hyper-excitability.
Expression of Cav3.3-I860N and -I860M variants in mouse chromaffin cells shifts their firing mode
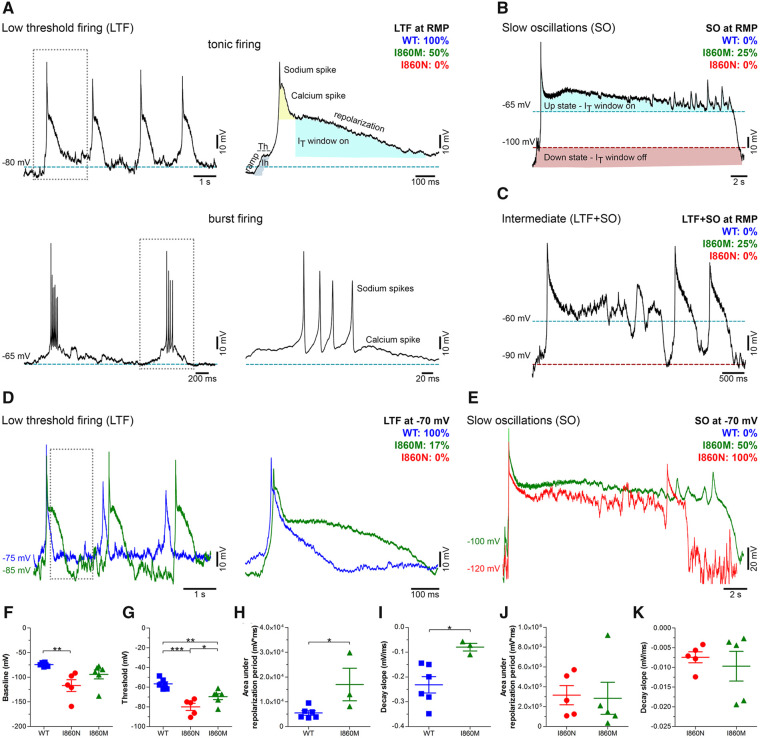
Finally, to examine the effects of the altered gating properties of the putative Cav3.3 disease variants on the firing patterns in native excitable cells, we overexpressed wild-type Cav3.3 and two representative mutants in freshly prepared mouse chromaffin cells, an established model for studying neuron-like action potential firing.46,47 We chose Cav3.3-I860N and -I860M, because structurally they are substitutions of the same residue and functionally represent the two extremes of the observed effects on gating properties and excitability. Consistent with the critical role of Cav3 channels in regulating neuronal excitability,4,44 and as predicted by our neuronal simulation, expression of wild-type Cav3.3 in mouse chromaffin cells induced low-threshold firing (either tonic or repetitive burst-firing) (Fig. 7A). Presumably, activation of the T-type calcium current depolarizes the membrane potential to above the threshold of the sodium spikes before its gradual inactivation terminates the burst and allows the repolarization of the membrane potential.4 As long as inactivation of Cav3.3 is incomplete the cell remains in the up-state with the membrane potential oscillating in the range of Cav3.3’s window current near −50 mV. Only after the membrane potential had returned to below the lower threshold of the window current the cell would fire spikes and bursts again. This firing behaviour observed in mouse chromaffin cells expressing wild-type Cav3.3 is reminiscent of the firing pattern of thalamic neurons,4,37 therefore, chromaffin cells represent a useful model system for studying the effects of altered T-type calcium currents on thalamic neuron-like burst firing.
Figure 7.
Wild-type, I860M and I860N Cav3.3 channels differently shape firing patterns of mouse chromaffin cells. (A–C) Representative current-clamp recordings of isolated mouse chromaffin cells (MCCs) at resting membrane potential (RMP, 0 pA current injection). MCCs expressing wild-type (WT) Cav3.3, Cav3.3-I860M or Cav3.3-I860N exhibit distinct firing modes depending on the contribution of T-type window currents (IT window) and hyperpolarization-activated sodium conductances (Ih)4: low threshold tonic or burst firing (LTF, A), slow oscillations (SO, B) resulting from membrane potential bistability, and intermediate (LTF+SO, C). The percentages of MCCs displaying the different firing modes for wild-type (blue), I860M (green) and I860N (red) are indicated in each panel. Note that all firing cells expressing wild-type channels show LTF, while I860M shifts firing more towards SO (25% SO and 25% LTF+SO; wild-type n = 4, I860M n = 4). MCCs expressing the I860N variant display no spontaneous activity (n = 11). Hatched lines indicate the membrane potential at which IT window current is on (blue) or off (red). (D and E) Representative current-clamp recordings after clamping the resting membrane potential to −70 mV. All firing cells expressing wild-type Cav3.3 show LTF (D). I860M shifts firing more towards SO [17% LTF (D), 50% SO and 33% LTF+SO (E)]. MCCs expressing I860N display only SO (E) (P = 0.001) (wild-type n = 6, I860N n = 5, I860M n = 6). (F) Expression of I860N channel in MCCs hyperpolarizes the membrane potential to −116.9 ± 11.9 mV (P = 0.01) compared to −74.3 ± 1.5 mV for wild-type and −94.2 ± 9.1 mV for I860M. (G) While the depolarization threshold of MCCs expressing wild-type Cav3.3 channels is −56.7 ± 2.1 mV, expression of Cav3.3-I860N shifts the depolarization threshold to −79.9 ± 3.6 mV (P < 0.001) and Cav3.3-I860M to −69.6 ± 2.9 mV (P = 0.01). (H) The area under the action potential plateau phase is significantly higher (P < 0.05) for MCCs expressing I860M (16 940 ± 6562 mV × ms) compared to wild-type (5471 ± 926.8 mV × ms) when the cells show LTF activity, while it is similar for MCCs expressing I860M or I860N in the SO firing mode (J). The action potential decay slope (between 90% and 10%) was significantly increased in MCCs expressing I860M (−0.08 ± 0.01 mV/ms, P = 0.02) compared to wild-type (−0.23 ± 0.03 mV/ms) (I), but was similar for cells expressing I860M or I860N (K). Chi-square test, t-test or one-way ANOVA with Holm-Sidak post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001.
When the Cav3.3-I860M variant was expressed in chromaffin cells the decay of low-threshold spikes was significantly delayed, resulting in a prolonged calcium spike (Fig. 7D) or in persistent low-frequency oscillations in the up-state (Fig. 7B and E). In variance to what was predicted by the neuronal computer model, this did not result in prolonged spike firing but in occasional spikes in some of the recorded chromaffin cells (Fig. 7B, C and E). Either the oscillations mostly remained below the threshold of the sodium spikes or the incomplete recovery from inactivation of the sodium channels prevented the generation of repetitive sodium spikes. Eventually the membrane potential returned to the down-state and the cells were ready for firing again. The delayed repolarization and the resulting prolonged slow oscillation are consistent with the reduced rate of inactivation kinetics and the left-shifted window current of the Cav3.3-I860M channel observed in the patch-clamp analysis. When the Cav3.3-I860N variant was expressed in chromaffin cells, spike-firing ceased completely, and all recorded cells remained in the slow oscillation mode (Fig. 7E). Apparently, because of the substantially slowed T-type current inactivation and severely left-shifted and enlarged window current, cells expressing this disease-associated Cav3.3 variant mostly persist in the up-state showing low-frequency oscillations without sodium spikes.
The shift in firing modes from low-threshold firing to slow oscillations in the two Cav3.3 mutants was accompanied by a hyperpolarization of the baseline membrane potential (Fig. 7F) and a significant reduction of the firing threshold of the calcium spike (Fig. 7G). This is consistent with the notion that the left-shifted voltage dependence of activation in Cav3.3-I860M and -I860N results in hyper-excitability of the cell. Moreover, the delayed hyperpolarization and persistent slow oscillations cause a substantially increased and potentially harmful calcium influx in cells expressing Cav3.3-I860M and -I860N (Fig. 7H–K). This notion was examined by monitoring the seal stability during the recording. While initial seal quality of chromaffin cells expressing I860M or I860N was good and comparable to that of wild-type cells, after action potential firing ∼80% of I860N and 9% of I860M cells displayed leaky and unstable seal properties (Supplementary Fig. 6). This is indicative of increased cellular stress experienced, probably due to the increased calcium influx through the mutant channels.
Discussion
In this study we report heterozygous gain-of-function mutations in CACNA1I associated with a congenital neurodevelopmental disorder of variable severity in three unrelated patients and one family. Patients 2–4 belonging to one family share the heterozygous CACNA1I missense variant p.(Ile860Met) and have borderline intellectual functioning or mild or moderate intellectual disability as main clinical feature. Patient 2 developed late-onset seizures. The other three patients (Patients 1, 5 and 6) show a severe neurodevelopmental disorder and share severe global developmental delay, absence of speech, gross motor delay, muscular hypotonia, early-onset seizures, cortical visual impairment, and feeding difficulties. Variable clinical features include various brain malformations, startle response or seizures, postnatal growth retardation, gastroesophageal reflux, and gastrostomy. The CACNA1I variants p.(Ile860Asn) and p.(Ile1306Thr) were de novo in Patients 1 and 5, respectively, while Patient 6 carried the heterozygous p.(Met1425Ile) variant absent in his mother.
The four different CACNA1I missense variants reported here affect amino acid residues in a known hot-spot region for gain-of-function calcium channel mutations in or next to the channel activation gate (Supplementary Fig. 1).11–13 Three of the four variants affect an amino acid residue located at the cytoplasmic end of an S6 helix; thus classifying the related disease as S6 calcium channelopathy.6 Methionine 1425 in Cav3.3 corresponds to methionine 1531 in Cav3.1 and to methionine 1549 in Cav3.2. The de novo CACNA1G variant p.(Met1531Val) has recently been reported in a patient with severe epileptic encephalopathy and global cerebellar atrophy,11 and the heterozygous CACNA1H variants p.(Met1549Val) and p.(Met1549Ile) have been associated with early-onset hypertension and hyperaldosteronism with incomplete penetrance.12,13 Electrophysiological studies of the respective Cav3 mutant channels provided evidence for a gain-of-function effect of all three mutations changing the methionine in the highly conserved MFV sequence in the Cav3 IIIS6 segment.11–13 The recurrently mutated alanine 961 in Cav3.1 [p.(Ala961Thr)], underlying severe developmental delay, cerebellar atrophy with or without epilepsy,11 is located next to the isoleucine corresponding to Ile860 in Cav3.3 which is mutated in Patient 1 and Family 2 reported here (Supplementary Fig. 1). The Cav3.1 A961T mutant showed slowed activation and inactivation kinetics and a negative shift in the steady-state activations, similar to the M1531V mutant, confirming both as gain-of-function mutations.11 For isoleucine 1306 substituted by threonine in Cav3.3, no equivalent pathogenic germline mutation could be identified in one of the other Cav channels (Supplementary Fig. 1). However, somatic mutations resulting in the corresponding substitutions I1430T in Cav3.2 (CACNA1H) and I1015S/V in Cav1.3 (CACNA1D) were identified in aldosterone-producing adenomas.48,49 Moreover, substitutions of two neighbouring amino acid residues, such as serine 1373 in Cav2.1 (CACNA1A) and arginine 998 in Cav1.4 (CACNA1F), are disease-associated (Supplementary Fig. 1), but likely cause loss- rather than gain-of-function.50–52
Electrophysiological analysis of the Cav3.3 variants revealed a gain-of-function effect on channel gating with a substantially increased calcium influx at rest and during action potential firing. These altered channel properties resulted in hyper-excitability in a model of TRN neurons. When ectopically expressed in mouse chromaffin cells, the two representative Cav3.3 variants I860M and I860N which are associated with a relatively mild and severe neurological phenotype, respectively, caused a shift of firing modes from burst firing to slow oscillation. Importantly, the magnitude of all functional changes determined experimentally was consistently lower for the Cav3.3-I860M variant than for Cav3.3-I860N and -I1306T corresponding well to the relatively mild phenotype in patients with the I860M variant. Together, our data add CACNA1I to the list of disease genes associated with motor and cognitive impairment with or without epilepsy.
All four disease-associated Cav3.3 variants show substantial gain-of-function effects on the gating properties. The up to 16 mV shift in the voltage-dependence of activation to hyperpolarizing potentials is the likely cause of the observed increased excitability of neurons expressing the Cav3.3 channel variants. This notion is supported by the reduced threshold and increased frequency and duration of firing in the model of TRN neurons. Moreover, in the case of Cav3.3-I860M and -I860N, it is evident in the lower threshold for calcium spikes recorded in mouse chromaffin cells. As TRN neurons are implicated in the generation of abnormal rhythms associated with absence epilepsy,37 hyper-excitability of the Cav3.3 mutant channel expressed in thalamic neurons represents a plausible cause of the epileptic phenotype in the three patients with the p.(Ile860Asn), p.(Ile1306Thr) and p.(Met1425Ile) variants. Coherent with this interpretation, increased T-type calcium currents have previously been observed in TRN neurons of a genetic rat model of generalized epilepsy.9,17
The Cav3.3 mutants showed reduced activation, deactivation, and inactivation kinetics. Slowed activation would represent a loss-of-function, but this effect was only significant at the most negative test potentials. However, the significantly slowed deactivation and inactivation kinetics will result in prolonged channel openings and increased calcium influx in active neurons, and thus represents a gain of calcium channel function. Action potential clamp experiments indicated that during physiological depolarization patterns the effects on voltage-dependence of inactivation dominate and cause substantially increased calcium influx before, during, and after the neuronal action potential. Slowed inactivation, in part also combined with altered voltage-dependence of activation and inactivation, have previously been described for disease-causing mutations in CACNA1G and CACNA1H encoding the T-type calcium channels Cav3.1 and Cav3.2, respectively,11,12 as well as in L-, PQ-, and R-type calcium channels.53–57 In all these genes, mutations affect highly conserved amino acid residues at the cytoplasmic end of the S6 helices, which line the channel gate. The associated phenotypes comprise neurodevelopmental anomalies and/or epileptic encephalopathy in the majority of cases. Thus, there is substantial evidence indicating that increased calcium influx because of a delayed inactivation causes a congenital neurodevelopmental disorder. Our present study adds another member of the voltage-gated calcium channel family to the S6 channelopathies, all of which probably share common pathophysiological mechanisms.
Because of their specific biophysical properties, with activation at low membrane potentials, T-type calcium channels support calcium influx at rest. The partial overlap of the activation and inactivation curves indicates the voltage range where a fraction of channels becomes activated but not yet inactivated, called the window current. In cells expressing T-type channels this causes a bi-stable resting potential and cells in the up-state generate slow, calcium-dependent oscillations of the membrane potential at about −50 mV.10 Particularly in the Cav3.3-I860N and -I1306T variants the greater left-shift of the voltage-dependence of activation compared to that of inactivation causes a substantial left-shift and enlargement of the window current. This most probably causes the complete switch from the low-threshold firing mode to the slow oscillation mode observed in mouse chromaffin cells expressing Cav3.3-I860N. As this mode is accompanied by a constant influx of calcium it will increase the likelihood of calcium toxicity and consequently may lead to aberrant development and death of neurons.58 We propose that these neuronal cell abnormalities represent a major cause of the severe neurodevelopmental defects observed in Patients 1, 5 and 6.
The modest and profound electrophysiological alterations observed in the Cav3.3-I860M and -I860N channel variants, respectively, strikingly demonstrate that the properties of the substituted amino acid rather than the position or nature of the changed amino acid residue determine the severity of the gating defects and the clinical phenotype. At the molecular level our structure models of wild-type and mutant Cav3.3 provide a possible mechanistic explanation for the left-shifted voltage-dependence of activation and slowed inactivation, as well as for the different severity of the effects in the Cav3.3-I860M and -I860N channel variants. In the wild-type situation Cav3.3 I860 interacts with its environment through multiple weak hydrophobic interactions. This endows the cytoplasmic end of the IIS6 helix with the necessary flexibility for its radial motion upon opening and closing of the channel gate. In the Cav3.3-I860N and -I860M disease variants the substituted amino acids form hydrogen bonds with neighbouring residues, both in the activated and resting state. Stabilization of both states by addition of hydrogen bonds can easily be envisaged to slow the kinetics and left-shift activation, by slowing the state transitions and stabilizing the activated state, respectively. The addition of stabilizing interactions by the substituted amino acids, rather than the removal of a critical property of the original amino acid, is consistent with the observed dominant gain-of-function effects and also explains how two distinct substitutions at the same position can result in different disease severity. By forming two classical hydrogen bonds the stability of Cav3.3-I860N substantially exceeds that of Cav3.3-I860M, which only forms a single sulphur hydrogen bond. This difference in the strength of the immobilizing interactions corresponds well with the differences in the experimentally determined effects on the voltage-dependence of activation and the kinetics of deactivation and inactivation. Consistent with this notion, also the Cav3.3-I1306T variant forms a hydrogen bond in an otherwise hydrophobic position. However, Cav3.3-M1425I does not form hydrogen bonds; instead the substitution increases the hydrophobicity and probable van der Waals interactions with neighbouring I1306, thus further highlighting the importance of the molecular interactions of these amino acids at the cytoplasmic ends of IIIS5 and IIIS6 for proper channel gating.
Together, our functional analysis of the CACNA1I variants linked to neurodevelopmental disorders provide evidence for two parallel pathomechanisms contributing to the aetiology of the epileptic phenotype. First, from early development on calcium toxicity in neurons expressing the Cav3.3 variants with a more severe gain-of-function effect may lead to aberrant development and loss of neurons, structural brain changes, global developmental delay, and seizures.58 Second, because of the specific function of Cav3.3 in regulating neuronal firing patterns in the thalamus,1,4 defects in the channel gating properties observed in the Cav3.3 variants are expected to result in hyper-excitability and firing mode shift in thalamic neurons and thus may directly contribute to epileptic seizures. At present it remains uncertain to what extent deficiencies in early neuronal development and acutely altered excitability contribute to hyper-excitability on the network level and consequently to seizures. An involvement of glia cells, which also express Cav3.3, cannot be excluded.5,58 Nevertheless, the identification of CACNA1I gain-of-function mutations to be causative for a range of neurodevelopmental phenotypes, including difficult-to-treat epilepsy, makes Cav3.3 an attractive target for pharmacological treatment with licensed T-type channel blockers, as well as for the development of new Cav3.3-specific inhibitors.7 As a case in point, targeted treatment of Patient 1 with the non-selective T-type channel blocker ethosuximide resulted in improved control of seizures, in particular in a reduction of myoclonic seizures. Further studies are required to establish a target therapy of T-type calcium channel blockers in patients with this new type of S6 calcium channelopathy.
Supplementary Material
Acknowledgements
We thank the patient families for the participation in this study. We further thank I. Mahlknecht, S. Demezt, and N. Kranebitter at the Division of Physiology, Medical University Innsbruck for excellent technical support. We thank Emilio Carbone, Claudio Franchino and Chiara Calorio from the Department of Drug Science and Technology, University of Turin, Italy, for providing helpful advice on chromaffin cell cultures. We thank Maja Hempel for genetic counselling of Family 1 and Inka Jantke for skilful technical assistance, both at the Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg.
Funding
This study was supported by grants from the Austrian Science Fund (FWF) P30402 and DOC30 to B.E.F., P31434 to P.T., T855 to M.C., and grants from the Deutsche Forschungsgemeinschaft (KU 1240/6-2 and KU 1240/10-1 to K.K.) and the Federal Ministry of Education and Research (01DQ17003 to K.K.).
Competing interests
R.E.P. is an employee of GeneDx, Inc. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- TRN
thalamic reticular nucleus
References
- 1.Huguenard JR.Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Adv Neurol. 1999;79:991–999. [PubMed] [Google Scholar]
- 2.Monteil A, Chemin J, Leuranguer V, et al. Specific properties of T-type calcium channels generated by the human alpha 1I subunit. J Biol Chem. 2000;275:16530–16535. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Reyes E.Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. [DOI] [PubMed] [Google Scholar]
- 4.Cain SM, Snutch TP.. Contributions of T-type calcium channel isoforms to neuronal firing. Channels. 2010;4:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA.. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41:347–353. [DOI] [PubMed] [Google Scholar]
- 6.Lory P.Neuronal Cav3 channelopathies: Recent progress and perspectives. Pflugers Arch. 2020;472(7):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss N, Zamponi GW.. T-type channel druggability at a crossroads. ACS Chem Neurosci. 2019;10:1124–1126. [DOI] [PubMed] [Google Scholar]
- 8.Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P.. Specific contribution of human T-type calcium channel isotypes (α1G, α1H and α1I) to neuronal excitability. J Physiol. 2002;540:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broicher T, Kanyshkova T, Meuth P, Pape H-C, Budde T.. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–399. [DOI] [PubMed] [Google Scholar]
- 10.Chevalier M, Lory P, Mironneau C, Macrez N, Quignard J-F.. T-type Cav3.3 calcium channels produce spontaneous low-threshold action potentials and intracellular calcium oscillations. Eur J Neurosci. 2006;23:2321–2329. [DOI] [PubMed] [Google Scholar]
- 11.Chemin J, Siquier-Pernet K, Nicouleau M, et al. De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain. 2018;141:1998–2013. [DOI] [PubMed] [Google Scholar]
- 12.Daniil G, Fernandes-Rosa FL, Chemin J, et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMed. 2016;13:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholl UI, Stölting G, Nelson-Williams C, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Huang G, Wu Q, et al. Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature. 2019;576:492–491. [DOI] [PubMed] [Google Scholar]
- 15.Eckle V-S, Shcheglovitov A, Vitko I, et al. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol. 2014;592:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Monteil A, Bidaud I, et al. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Hum Mutat. 2007;28:524–525. [DOI] [PubMed] [Google Scholar]
- 17.Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC.. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly P, Barroso I, Blackwell J, et al. ; ISGC and the WTCCC 2. Genome-wide association study implicates HLA-C01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry. 2012;72:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulsuner S, Walsh T, Watts AC, et al. ; PAARTNERS Study Group. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manoach DS, Pan JQ, Purcell SM, Stickgold R.. Reduced sleep spindles in schizophrenia: A treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry. 2016;80:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripke S, Neale B, Corvin A, et al. SWG of the PGC. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Huang D, Wei L, Luo X-J.. Further evidence for the genetic association between CACNA1I and schizophrenia. Hereditas. 2018;155:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Liu Y, Chen J, et al. Genetic risk between the CACNA1I gene and schizophrenia in Chinese Uygur population. Hereditas. 2018;155:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ.. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Sci Rep. 2016;6:34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astori S, Wimmer RD, Prosser HM, et al. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X-B, Murray KD, Jones EG.. Low-threshold calcium channel subunit Ca(v) 3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol. 2011;519:1181–1195. [DOI] [PubMed] [Google Scholar]
- 27.Ghoshal A, Uygun DS, Yang L, et al. Effects of a patient-derived de novo coding alteration of CACNA1I in mice connect a schizophrenia risk gene with sleep spindle deficits. Transl Psychiatry. 2020;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu AT-H, Dai X, Martinez-Agosto JA, Cantor RM.. Support for calcium channel gene defects in autism spectrum disorders. Mol Autism. 2012;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Roige S, Fontanillas P, Elson SL, et al. Genome-wide association studies of impulsive personality traits (BIS-11 and UPPS-P) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J Neurosci. 2019;39:2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisedchaisri G, Tonggu L, McCord E, et al. Resting-state structure and gating mechanism of a voltage-gated sodium channel. Cell. 2019;178:993–1003.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomora JC, Murbartian J, Arias JM, Lee JH, Perez-Reyes E.. Cloning and expression of the human {T-type channel Ca(v)3.3}: insights into prepulse facilitation. Biophys J. 2002;83:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calorio C, Gavello D, Guarina L, et al. Impaired chromaffin cell excitability and exocytosis in autistic Timothy syndrome TS2-neo mouse rescued by L-type calcium channel blockers. J Physiol. 2019;597:1705–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcantoni A, Vandael DHF, Mahapatra S, et al. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci. 2010;30:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courel M, Vasquez MS, Hook VY, Mahata SK, Taupenot L.. Sorting of the neuroendocrine secretory protein Secretogranin II into the regulated secretory pathway: Role of N- and C-terminal alpha-helical domains. J Biol Chem. 2008;283:11807–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hines ML, Carnevale NT.. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. [DOI] [PubMed] [Google Scholar]
- 36.Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR.. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996;16:169–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huguenard JR, Prince DA.. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobreira N, Schiettecatte F, Valle D, Hamosh A.. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karczewski KJ, Francioli LC, Tiao G, et al. ; Genome Aggregation Database Consortium. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C.. MetaDome: Pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat. 2019;40:1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catterall WA, Lenaeus MJ, El-Din TMG.. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol. 2020;60:133–154. [DOI] [PubMed] [Google Scholar]
- 43.Cazade M, Bidaud I, Lory P, Chemin J.. Activity-dependent regulation of T-type calcium channels by submembrane calcium ions. Elife. 2017;6:e22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamponi GW, Lory P, Perez-Reyes E.. Role of voltage-gated calcium channels in epilepsy. J Physiol. 2010;460:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chemin J, Nargeot J, Lory P.. Neuronal T-type alpha 1H calcium channels induce neuritogenesis and expression of high-voltage-activated calcium channels in the NG108-15 cell line. J Neurosci. 2002;22:6856–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahapatra S, Calorio C, Vandael DHF, Marcantoni A, Carabelli V, Carbone E.. Calcium channel types contributing to chromaffin cell excitability, exocytosis and endocytosis. Cell Calcium. 2012;51:321–330. [DOI] [PubMed] [Google Scholar]
- 47.Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E.. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium. 2007;42:397–408. [DOI] [PubMed] [Google Scholar]
- 48.NabaK, , BlinderAR, , Hattangady NG, . et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75(3):645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.OrtnerNJ, , KasererT, , CopelandJN, , Striessnig J.. De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflugers Arch. 2020;472(7):755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byers HM, Beatty CW, Hahn SH, Gospe SM.. Dramatic response after lamotrigine in a patient with epileptic encephalopathy and a De NovoCACNA1A variant. Pediatr Neurol. 2016;60:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damaj L, Lupien-Meilleur A, Lortie A, et al. CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet. 2015;23:1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeitz C, Michiels C, Neuillé M, et al. Where are the missing gene defects in inherited retinal disorders? Intronic and synonymous variants contribute at least to 4% of CACNA1F-mediated inherited retinal disorders. Hum Mutat. 2019;40:765–787. [DOI] [PubMed] [Google Scholar]
- 53.Helbig KL, Lauerer RJ, Bahr JC, et al. De novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and dyskinesias. Am J Hum Genet. 2018;103(5):666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofer NT, Tuluc P, Ortner NJ, et al. Biophysical classification of a CACNA1D de novo mutation as a high-risk mutation for a severe neurodevelopmental disorder. Mol Ecular Autism. 2020;11: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang X, Raju PK, D'Avanzo N, et al. Both gain‐of‐function and loss‐of‐function de novo CACNA 1A mutations cause severe developmental epileptic encephalopathies in the spectrum of Lennox‐Gastaut syndrome. Epilepsia. 2019;60:1881–1894. [DOI] [PubMed] [Google Scholar]
- 56.Pinggera A, Lieb A, Benedetti B, et al. CACNA1D de novo mutations in autism spectrum disorders activate cav1.3 l-type calcium channels. Biol Psychiatry. 2015;77:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Splawski I, Timothy KW, Sharpe LM, et al. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. [DOI] [PubMed] [Google Scholar]
- 58.Steinlein OK.Calcium signaling and epilepsy. Cell Tissue Res. 2014;357:385–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary material. Consent restrictions preclude sharing of full datasets, and the consents do not cover the deposition of the next-generation sequencing data in a public database.