Abstract
Both estrogen and mechanical loading regulate bone maintenance. However, mechanical overload appears less effective in enhancing bone mineral density (BMD) in estrogen-deficient women.
Objective:
To determine whether estradiol (E2) influences the early-phase bone adaptations to re-ambulation (REAMB) and/or rehabilitation exercise following hindlimb unloading (HLU) of ovariectomized (OVX) rats.
Methods:
Eighty-one 5 month old female Sprague-Dawley rats were randomized into the following groups: 1) Intact controls, 2) OVX, 3) OVX+E2, 4) OVX+ 4 weeks HLU, 5) OVX+E2+HLU, 6) OVX+HLU+ two weeks quadrupedal REAMB, 7) OVX+E2+HLU+REAMB, 8) OVX+HLU+REAMB+ supplemental climbing jumping, and balance exercises (EX), or 9) OVX+E2+HLU+REAMB+EX. Serial DXA scans were performed to track total body bone characteristics throughout the study and pQCT was utilized to determine distal femoral metaphyseal bone mineral characteristics.
Results:
Total body BMD increased 4-8% in all animals receiving supplemental E2, while BMD did not change in animals without E2. OVX reduced trabecular (t)BMD at the femoral metaphysis and HLU exacerbated this loss, while also reducing cortical (c)BMD. E2 protected against the OVX+HLU induced bone loss at the femoral metaphysis. Conversely, REAMB did not alter BMD, regardless of estrogen status. In the absence of E2, REAMB+EX resulted in severe bone loss following OVX+HLU, with tBMD and cBMD measurements that were 91% and 7% below controls (p≤0.001). However, in the presence of E2, REAMB+EX did not negatively influence bone mineral characteristics.
Conclusion:
E2 protects against bone loss resulting from combined OVX+HLU of rodents and in the absence of estrogen, exercise induces disadvantageous early-phase bone adaptations following extended disuse.
Keywords: bone, estradiol, hindlimb unloading, mechanical loading, ovariectomy, reambulation
TABLE OF CONTENTS SUMMARY
We demonstrate that in the absence of estrogen, short-duration re-ambulation in combination with moderate-intensity rehabilitation exercise was detrimental to bone mineral density (BMD) at the distal femoral metaphysis in a rodent model of severe bone loss that resulted from combined estrogen deficiency and hindlimb unloading. Conversely, in the presence of estradiol, neither re-ambulation alone nor re-ambulation plus moderate-intensity rehabilitation exercise were detrimental to BMD.
INTRODUCTION
Estrogen plays an integral role in the maintenance of bone 1. For example, the loss of estrogen that occurs at menopause 2 or with various clinical conditions in younger women 3 induces a rapid increase in bone turnover that reduces bone mineral density (BMD). Hormone therapy (HT) fully prevents the loss of BMD resulting from estrogen deficiency in humans 4; however, concerns remain regarding the safety of HT 5. Similarly, rodents experience a heightened bone resorption and increased bone turnover following ovariectomy (OVX) 6, which results in bone loss 7; while, estradiol (E2) administration prevents bone loss within this model 8.
Mechanical loading is also essential for bone maintenance 9. For example, musculoskeletal disuse resulting from space flight or neurologic insult results in a dramatic loss of BMD in humans 10. Similarly, both cast immobilization and extended bed rest also induce rapid reductions in BMD that is of clinical concern 11. Conversely, mechanical re-loading reverses this loss and when performed at a sufficient intensity, may further augment BMD 9. Several models exist to study the effects of musculoskeletal disuse on bone 12. Hindlimb unloading (HLU) of rodents represents one such model, which completely eliminates external load on the hindlimbs without eliminating voluntary contractility of the involved musculature 13. Bone loss occurs during HLU in a rapid and rather dramatic manner 14, 15 and may even persist for some time following the cessation of HLU, depending on the skeletal site 14. Re-ambulation is capable of reversing the bone loss that occurs within this model; although, full restoration of bone architecture can take a number of months to occur 14. As such, disparity exists between the time-frame in which re-ambulation fully restores BMD and with which it fully restores skeletal muscle mass, with muscle experiencing a much quicker restoration 14. This is of clinical concern, given that the duration of physical rehabilitation following extended hospitalization is typically rather short and the success of which is generally measured by the restoration of neuromuscular strength and physical function 16, not BMD.
Estrogen and mechanical loading also appear to exert synergistic effects on bone maintenance 17, 18. For example, disuse exacerbates the loss of BMD resulting from OVX 17, 19, which presents clinical concern to estrogen-deficient women experiencing extended bouts of disuse. In rodents, E2 replacement is able to protect against bone loss resulting from combined OVX and sciatic neurotomy induced disuse 17. However, in the absence of estrogen, short-duration quadrupedal re-ambulation does not appear capable of restoring BMD following HLU 19; suggesting that estrogen may influence the ability of mechanical loading to restore BMD following disuse. Clinical evidence supports this contention, as several meta-analyses indicate that postmenopausal women obtain only very limited improvements in BMD with exercise 20-23. Conversely, exercise combined with E2 replacement results in greater improvements in BMD than either treatment alone 24, 25. Despite the aforementioned evidence, few studies have directly evaluated the influence of E2 on the early-phase bone responses to re-ambulation plus rehabilitation exercise following combined OVX+HLU, a model that induces severe bone loss 15, 19 and which mimics a clinical condition in which estrogen-deficient women undergo extended disuse and subsequent physical rehabilitation.
The primary purposes of this study were to determine the independent and combined effects of E2 and short-duration moderate-intensity rehabilitation exercise on the early-phase bone adaptations in mature OVX+HLU rats. Specifically, we determined 1) whether E2 administration was capable of preserving BMD following OVX+HLU and 2) the influence of E2 on the early-phase bone adaptations to short-duration quadrupedal re-ambulation of OVX+HLU animals either with or without supplemental moderate-intensity rehabilitation exercise. We hypothesized that E2 administration would fully preserve bone mineral characteristics in OVX+HLU animals and that in the absence of estrogen, neither short-duration re-ambulation nor re-ambulation plus supplemental rehabilitation exercise would sufficiently restore bone mineral characteristics following disuse.
MATERIAL AND METHODS
Animal Care.
For this experiment, a subset of bones (n = 81) were analyzed from our companion paper which evaluated the effects of E2 replacement on the quadrupedal re-ambulation induced restoration of skeletal muscle mass following HLU of mature OVX rats 26. Briefly, five month old virgin female Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). Animals were individually housed in a temperature- and light-controlled room on a 12h light, 12h dark cycle. Rats consumed a diet of phytoestrogen-free chow (AIN 76A, Harlan Laboratories, Indianapolis, IN) and tap water ad libitum. All experimental procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia and in accordance with the Institute for Laboratory Animal Research Guide to the Care and Use of Experimental Animals.
Experimental Design.
Rats were randomized according to body weight into the following groups (n = 6-12/group): 1) Intact control, 2) ovariectomy (OVX) control, 3) OVX + E2 replacement control, 4) OVX + 4 weeks hindlimb unloading (HLU), 5) OVX+E2+HLU, 6) OVX+HLU + 2 weeks re-ambulation (REAMB), 7) OVX+E2+HLU+REAMB, 8) OVX+HLU+REAMB + 2 weeks exercise (EX), and 9) OVX+E2+HLU+REAMB+EX. Mature animals that were estrogen deficient as a result of OVX were used in order to evaluate the individual and combined effects of estrogen and mechanical loading on bone, without potential old age-related changes influencing our musculoskeletal outcomes 27. All animals underwent a whole body DEXA scan using a Hologic 1000 (Bedford MA) at the initiation of the study and subsequent DEXA scans following hindlimb unloading (if applicable) and/or prior to sacrifice (Figure 1). One control group was scanned twice to determine machine error which was found to be less than 1%. Animals were sacrificed by decapitation while under deep anesthesia and femurs were removed, stripped of soft tissue, wrapped in saline soaked gauze, and stored at −20° C in order to maintain the mechanical characteristics of the bone 28.
Figure 1.
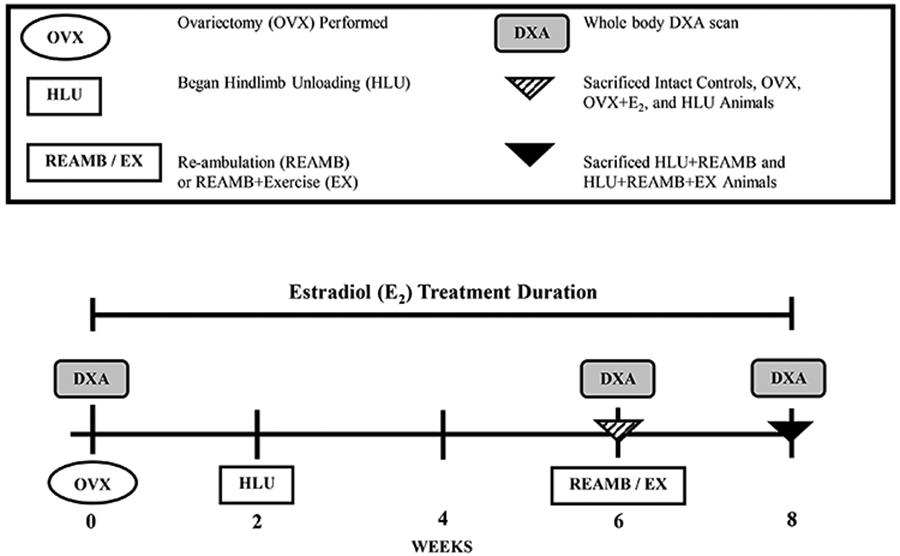
Experimental Design.
Surgery, Drug Administration, and Tail Ring Insertion.
All animals, with the exception of intact controls, received aseptic bilateral OVX under isoflurane anesthesia, according to our previously published protocol 19, 26. Following OVX, silastic catheters were implanted into the abdomen of each rat which contained at total of 12 mg E2 (Sigma, St. Louis, MO) or which remained empty. Results published in our companion paper 26 verified that at sacrifice, serum E2 concentrations were below the sensitivity of the assay (< 2 pg/ml) in OVX animals and that serum E2 was similar between intact control animals and E2 treated groups. The success of OVX and E2 replacement was further verified by uterine weights which were 577 ± 162 mg in intact controls, reduced 70-75% in all OVX groups, and restored to the level of intact controls in all E2 treated groups 26. Animals were subsequently prepped for HLU by placing two stainless steel wires (Small Parts, Inc., SWX-4029) through the tail and a 1.5 inch plaster bandage around the thorax in order to provide spinal support during HLU, as has previously been described 19, 26. After surgery, rats were allowed to freely ambulate and recover to pre-surgical weights for two weeks prior to beginning the experiment.
Hindlimb Unloading, Re-ambulation, and Exercise.
Following the two-week period of recovery from surgery, the six experimental groups underwent HLU for four weeks, according to our previously published protocol 19, while the control groups did not undergo HLU. Following HLU, two groups (OVX+HLU and OVX+E2+HLU) were sacrificed and the remaining four experimental groups were returned to unrestricted quadrupedal weight bearing for the remainder of the experiment.
In addition to quadrupedal weight bearing, two groups performed daily moderate-intensity rehabilitation exercise for a total of 13 consecutive days, including1) walking up and down a 4ft long, 20 degree ramp 3 times in each direction, 2) jumping out of a 4 inch high box a total of 10 times, 3) climbing a 15 step ladder with progressively overloaded weights (commencing with 0 g and progressing in 10 g increments until a maximum of 100 g was achieved) attached to the body, and 4) balancing on a mesh screen that was tilted up, down, and sideways twice during each exercise session for a duration of 1 minute each time 26.These exercises were chosen because they 1) mimicked the recommended duration and type of rehabilitation exercises performed following extended bed rest in hospital settings 16, 2) have previously been shown to improve muscle force in older animals 29, 3) are well tolerated by rats recovering from HLU 26, and 4) involved daily hindlimb exercises performed in a manner similar to what has been recommended to improve bone architecture in rodent models 30.
Peripheral Quantitative Computerized Tomography (pQCT)
Prior to peripheral quantitative computerized tomography (pQCT), the femurs were thawed to room temperature and were kept in saline-soaked gauze except during measurement. The left distal femoral metaphyses were scanned by pQCT with a Stratec XCT Research M Instrument (Norland Medical Systems, Fort Atkinson, WI). Scans were performed at a distance of 5 mm proximal to the distal end of the femur for measurements of cortical and cancellous bone structure. The structural variables that were measured include total, trabecular, and cortical bone area (mm2), content (mg/mm), and density (mg/cm3). Additionally, the contra-lateral femur from one group (OVX+HLU+REAMB+EX) was scanned in order to verify our findings and no bone mineral differences were observed between left and right bones (See Table, Supplemental Digital Content 1, http://links.lww.com/MENO/A34).
Statistical Analysis
Results are reported as means ± SEM, and p ≤ 0.05 was defined as the threshold of significance. One-way ANOVAs (for normally distributed data) were used to separately analyze dependent variables and the Tukey’s posthoc test was performed for multiple comparisons among groups when appropriate. The Kruskal-Wallis test was performed when data were not normally distributed and when indicated pair wise comparisons were further evaluated with the Mann-Whitney U test. Change scores representing the change from baseline to post-HLU and from baseline to sacrifice were computed for all DXA bone characteristics via non-parametric tests in order to determine whether treatments altered the magnitude of change across time points. Data were analyzed with the SPSS v15.0.0 statistical software package.
RESULTS
Body Mass
At baseline, mean body mass in intact control animals was 270 ± 15 g. As reported in our companion paper 26, body mass was similar across all groups at baseline and at sacrifice; however, several groups experienced a significant within groups increase in body mass from baseline to sacrifice (See Table, Supplemental Digital Content 2, http://links.lww.com/MENO/A35).
Dual X-Ray Absorptiometry (DXA)
Total body bone mineral characteristics at baseline, following HLU, and at sacrifice are presented in Tables 1-3. Baseline total body bone area was similar among all groups, while total body BMC and total body BMD started approximately 7-12% lower in OVX+E2 animals (p ≤ 0.05). No other differences in baseline bone characteristics were present among groups.
Table 1.
Total body bone mineral content (BMC) as measured by DXA.
| Group | BMC (g) At Baseline |
BMC (g) Following HLU |
BMC (% Change) Following HLU |
BMC (g) At Sacrifice |
BMC (% Change) At Sacrifice |
|
|---|---|---|---|---|---|---|
| Intact Control | (a) | 9.5 ± 0.2 | N/A | N/A | 10.5 ± 0.2 | 11%e |
| OVX | (b) | 9.6 ± 0.2c* | N/A | N/A | 10.4 ± 0.2 | 8% |
| OVX+E2 | (c) | 8.3 ± 0.3a*,b*,d*,h | N/A | N/A | 8.8 ± 0.2 | 6% |
| OVX+HLU | (d) | 9.5 ± 0.2c* | ----See Values for Sacrifice---- | 10.0 ± 0.2 | 5% | |
| OVX+E2+HLU | (e) | 9.3 ± 0.3 | ----See Values for Sacrifice---- | 9.6 ± 0.3 | 3%a | |
| OVX+HLU+REAMB | (f) | 9.4 ± 0.2 | 9.8 ± 0.2 | 4% | 10.3 ± 0.2 | 10% |
| OVX+E2+HLU+REAMB | (g) | 9.2 ± 0.1 | 9.5 ± 0.2 | 3% | 9.7 ± 0.1 | 5% |
| OVX+HLU+REAMB+EX | (h) | 9.5 ± 0.2c* | 9.7 ± 0.2 | 2% | 10.2 ± 0.2 | 7% |
| OVX+E2+HLU+REAMB+EX | (i) | 9.2 ± 0.2 | 9.4 ± 0.4 | 2% | 9.8 ± 0.2 | 7% |
Values are Means ± SEM of 4-12/group. Letters a-i indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs. OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2+HLU, f = vs. OVX+HLU+REAMB, g = vs. OVX+E2+HLU+REAMB, h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX).
Table 3.
Total body bone mineral density (BMD) as measured by DXA.
| Group | BMD (g) At Baseline |
BMD (g) Following HLU |
BMD (% Change) Following HLU |
BMD (g) Sacrifice |
BMD (% Change) At Sacrifice |
|
|---|---|---|---|---|---|---|
| Intact Control | (a) | 0.178 ± 0.002c* | N/A | N/A | 0.180 ± 0.001 | 2%c |
| OVX | (b) | 0.176 ± 0.002c* | N/A | N/A | 0.175 ± 0.002 | 0%c*,i |
| OVX+E2 | (c) | 0.157 ± 0.003a*,b*,d*,e*,f*,h | N/A | N/A | 0.169 ± 0.003 | 8%a,b*,d*,f*,h |
| OVX+HLU | (d) | 0.175 ± 0.002c* | ----See Values for Sacrifice---- | 0.170 ± 0.002 | −3%c*,e,i* | |
| OVX+E2+HLU | (e) | 0.173 ± 0.004c* | ----See Values for Sacrifice---- | 0.179 ± 0.004 | 4%d | |
| OVX+HLU+REAMB | (f) | 0.174 ± 0.001c* | 0.169 ± 0.002 | −3% | 0.173 ± 0.002 | 0%c* |
| OVX+E2+HLU+REAMB | (g) | 0.172 ± 0.001 | 0.175 ± 0.004 | 2% | 0.178 ± 0.004 | 4% |
| OVX+HLU+REAMB+EX | (h) | 0.168 ± 0.002c | 0.173 ± 0.002 | 3% | 0.170 ± 0.002 | 1%c |
| OVX+E2+HLU+REAMB+EX | (i) | 0.169 ±0.004 | 0.174 ± 0.004 | 3% | 0.178 ± 0.002 | 6%b,d* |
Values are Means ± SEM of 4-12/group. Letters a-i indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs.OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2+HLU, f = vs. OVX+HLU+REAMB, g = vs. OVX+E2+HLU+REAMB, h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX).
All groups experienced a similar 2-5% increase in total body BMC following HLU (Table 1). At sacrifice, total body BMC had increased 11% in intact control animals and all other groups experienced a similar 5-10% increase, with the exception that OVX+E2+HLU animals experienced only a 3% increase in total body BMC that was less than controls (p ≤ 0.05).
Following HLU, the change in total body bone area was similar across groups (Table 2). At sacrifice, total body bone area had increased 8% in intact control animals and all groups undergoing OVX (without supplemental E2) experienced a similar elevation in total body bone area. Conversely, this increase was suppressed in all groups receiving E2 administration, resulting in elevations in total body bone area that were less than was observed in controls and in several OVX groups (p ≤ 0.05).
Table 2.
Total body bone area as measured by DXA.
| Group | Area (cm2) At Baseline |
Area (cm2) Following HLU |
Area (% Change) Following HLU |
Area (cm2) Sacrifice |
Area (% Change) At Sacrifice |
|
|---|---|---|---|---|---|---|
| Intact Control | (a) | 54.0 ± 1.2 | N/A | N/A | 58.4 ± 0.8 | 8%c*,e |
| OVX | (b) | 54.6 ± 1.0 | N/A | N/A | 59.4 ± 1.5 | 9%c*,e*,i |
| OVX+E2 | (c) | 53.2 ± 1.0 | N/A | N/A | 51.8 ± 0.8 | −2%a*,b*,d*,f*,h |
| OVX+HLU | (d) | 54.3 ± 1.0 | ----See Values for Sacrifice---- | 58.8 ± 1.0 | 8%c*,e | |
| OVX+E2+HLU | (e) | 53.8 ± 0.8 | ----See Values for Sacrifice---- | 53.7 ± 1.4 | 0%a,b*,d,f* | |
| OVX+HLU+REAMB | (f) | 53.8 ± 0.8 | 58.0 ± 1.3 | 8% | 59.1 ± 1.2 | 10%c*,e*,i |
| OVX+E2+HLU+REAMB | (g) | 53.5 ± 0.4 | 54.4 ± 1.3 | 2% | 54.6 ± 1.0 | 0% |
| OVX+HLU+REAMB+EX | (h) | 55.8 ± 0.9 | 56.4 ± 0.7 | 1% | 59.5 ± 1.3 | 6%c |
| OVX+E2+HLU+REAMB+EX | (i) | 54.5 ± 0.9 | 53.8 ± 1.4 | −1% | 55.2 ± 1.2 | 1%b,f |
Values are Means ± SEM of 4-12/group. Letters a-i indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs. OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2+HLU, f = vs. OVX+HLU+REAMB, g = vs. OVX+E2+HLU+REAMB, h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX).
Following HLU, the change in total body BMD was not different among groups (Table 3). Throughout the study, total body BMD increased approximately 2% in intact control animals. Groups undergoing OVX (without supplemental E2) did not experience an increase in total body BMD throughout the intervention; although values were not different than controls. Conversely, total body BMD increased 4-8% throughout the study in all groups receiving supplemental E2. The largest magnitude change occurred in the OVX+E2 group that experienced an approximate 8% increase in total body BMD, a result that was greater than controls and all groups receiving OVX without supplemental E2 (p ≤ 0.05).
Distal Femoral Metaphysis Total Bone Mineral Characteristics
The total bone mineral characteristics at the distal femoral metaphysis are presented in Table 4. In OVX animals, total BMC (p ≤ 0.01) and total BMD (p ≤ 0.001) were 14-18% below that of intact controls, with no difference present between these groups for total bone area. Estradiol (E2) treatment did not prevent the OVX-induced reduction in total BMC, but did induce a 10-13% reduction in total bone area compared with controls and OVX animals (p ≤ 0.05). The OVX-induced reduction in total BMC and E2-induced reduction in total bone area combined to produce a total BMD measurement that was 15% higher than OVX animals (p ≤ 0.001) and not different from intact controls.
Table 4.
Total bone mineral characteristics at the distal femoral metaphysis as measured by pQCT.
| Group | Total BMC (mg/mm) |
Total Bone Area (mm2) |
Total BMD (mg/cm3) |
|
|---|---|---|---|---|
| Intact Control | (a) | 16.5 ± 0.4b*,c*d*,e,f*,h*,i | 20.7 ± 0.4c*,e,h | 796 ± 14b*,d*,f*,h*,i* |
| OVX | (b) | 14.1 ± 0.6a*,d*,f,h* | 21.6 ± 1.0c | 652 ± 11a*,c*,d*,e*,f*,h* |
| OVX+E2 | (c) | 14.0 ± 0.7a*,d*,f,h* | 18.6 ± 0.5a*,b,d,g*,h* | 752 ± 26b*,d*,f*,h* |
| OVX+HLU | (d) | 9.6 ± 0.3a*,b*,c*,e*,g*,i* | 20.8 ± 0.4c,e | 464 ± 13a*,b*,c*,e*,f,g*,i* |
| OVX+E2+HLU | (e) | 14.5 ± 0.8a,f*,h* | 18.8 ± 0.7a,d,g,h* | 773 ± 40b*,d*,f*,h* |
| OVX+HLU+REAMB | (f) | 11.0 ± 0.6a*,b,c,e*,g,i | 20.5 ± 0.7h | 531 ± 16a*,b*,c*,d,e*,g,h* |
| OVX+E2+HLU+REAMB | (g) | 15.7 ± 1.1d*,f,i | 22.2 ± 1.0c*,e | 709 ± 50d*,f,g* |
| OVX+HLU+REAMB+EX | (h) | 9.9 ± 0.5a*,b*,c*,e*,g*,i* | 23.2 ± 0.8a,c*,e*,f | 427 ± 17a*,b*c*,e*f*,g*,i* |
| OVX+E2+HLU+REAMB+EX | (i) | 13.7 ± 0.8a,d*,f,h* | 21.0 ± 1.0 | 658 ± 40a*,d*,h* |
Values are Means ± SEM of n = 6-12/group. Letters a-f indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs. OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2 + HLU, f = vs. OVX+HLU+REAMB, g = vs. OVX+E2+HLU+REAMB, h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX). BMC = Bone mineral content, BMD = bone mineral density.
Hindlimb unloading (HLU) of OVX animals exacerbated the OVX-induced total bone loss at the distal femoral metaphysis, resulting in total BMC and total BMD measurements that were 29-32% less than OVX (p ≤ 0.001) and 42% less than controls (p ≤ 0.001), but did not alter total bone area. Estradiol treatment partially prevented the loss of BMC induced by OVX+HLU resulting in a total BMC measurement that was 51% higher than OVX+HLU animals (p ≤ 0.001) and not different than OVX+E2 controls. Additionally, total bone area was 9-10% lower in OVX+E2+HLU animals compared to OVX+HLU and controls (p ≤ 0.05). The E2-associated increase in total BMC and reduction in total bone area combined to produce a total BMD measurement that was 66% greater than OVX+HLU treatment (p ≤ 0.001) and not different from intact controls.
In OVX+HLU+REAMB animals, total BMC (non-significant) and total BMD (p ≤ 0.01) were 14% higher than in OVX+HLU animals, with no difference in total bone area between groups; however, both total BMC and total BMD remained 33% below intact controls (p ≤ 0.001). Conversely, E2-treatment of OVX+HLU+REAMB animals resulted in total BMC and total BMD measurements that were 53-63% higher than OVX+HLU animals (p ≤ 0.001) and were ultimately maintained at the level of intact controls.
In OVX+HLU+REAMB+EX animals, total BMC and total BMD remained 40-46% below intact controls (p ≤ 0.001) and were not different than OVX+HLU animals. In addition, total bone area was 11% higher in OVX+HLU+REAMB+EX animals than in intact controls (p ≤ 0.05). Conversely, E2-treatment of OVX+HLU+REAMB+EX animals resulted in a total BMC and total BMD that were 40-46% higher than OVX+HLU and OVX+HLU+REAMB+EX animals (p ≤ 0.001); although, total BMC and total BMD remained 17% below intact controls (p ≤ 0.05).
Distal Femoral Metaphysis Trabecular Bone Mineral Characteristics
The trabecular bone mineral characteristics at the distal femoral metaphysis are presented in Table 5. In OVX and OVX+E2 animals, trabecular (t)BMC was 32-40% lower and tBMD was 35% lower than intact controls (p ≤ 0.001). No differences in trabecular bone area were present between OVX and intact controls; however, trabecular bone area was 10-14% lower in OVX+E2 animals compared with both intact controls (p ≤ 0.01) and OVX animals (p ≤ 0.05).
Table 5.
Trabecular bone mineral characteristics at the distal femoral metaphysis as measured by pQCT.
| Group | Trabecular BMC (mg/mm) |
Trabecular Bone Area (mm2) |
Trabecular BMD (mg/cm3) |
|
|---|---|---|---|---|
| Intact Control | (a) | 3.2 ± 0.2b*,c*,d*,e,f*,h*,i* | 6.2 ± 0.1c*,e,h | 516 ± 22b*c*,d*,f*,h*,i* |
| OVX | (b) | 2.2 ± 0.1a*,d*,f*,h* | 6.5 ± 0.3c | 333 ± 15a*,d*,f*,h* |
| OVX+E2 | (c) | 1.9 ± 0.3a*,d*,h* | 5.6 ± 0.2a*,b,d,g*,h* | 333 ± 45a*,d*,f*,h* |
| OVX+HLU | (d) | 1.0 ± 0.1a*,b*,c*,e*,g*,h*,i | 6.2 ± 0.1c,e | 160 ± 9a*,b*,c*,e*,g*,h* |
| OVX+E2+HLU | (e) | 2.4 ± 0.3a,d*,f,h* | 5.6 ± 0.2a,d,g,h* | 428 ± 53b,d*,f,h* |
| OVX+HLU+REAMB | (f) | 1.2 ± 0.1a*,b*,e,g*,h* | 6.2 ± 0.2h | 185 ± 17a*,b*,c*,e,g*,h* |
| OVX+E2+HLU+REAMB | (g) | 2.7 ± 0.3d*,f*,h* | 6.7 ± 0.3c*,e | 407 ± 55d*,f*,h* |
| OVX+HLU+REAMB+EX | (h) | 0.3 ± 0.1a*,b*,c*,d*,e*,f*g*,i* | 6.9 ± 0.2a,c*,e*,f | 44 ± 10a*,b*,c*,d*,e*,f*,g*,i* |
| OVX+E2+HLU+REAMB+EX | (i) | 1.9 ± 0.3a*,d,h* | 6.3 ± 0.3 | 313 ± 48a*,h* |
Values are Means ± SEM of n = 6-12/group. Letters a-f indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs.OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2+HLU, f = OVX+HLU+REAMB, g = OVX+E2+HLU+REAMB), h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX. BMC = Bone mineral content, BMD = bone mineral density.
Hindlimb unloading exacerbated the OVX-induced loss of trabecular bone, resulting in tBMC and tBMD measurements that were approximately 52-54% less than OVX controls (p ≤ 0.001), while trabecular bone area remained unaltered. Conversely, in OVX+E2+HLU animals tBMC and tBMD were increased 140-168% above OVX+HLU animals (p ≤ 0.01) and trabecular bone area was 10% lower than both intact controls and OVX+HLU animals (p ≤ 0.05). Ultimately, both tBMC and tBMD in OVX+E2+HLU animals were maintained at the level of OVX+E2 animals, but remained 25% (p ≤ 0.05) and 17% (p ≤ 0.001) below intact controls, respectively.
In OVX+HLU+REAMB animals, trabecular bone mineral characteristics were similar to those of OVX+HLU animals, with tBMC and tBMD remaining 62-64% below intact controls (p ≤ 0.001). Conversely, E2-treatment of OVX+HLU+REAMB animals resulted in tBMC and tBMD measurements that were 120-125% higher than OVX+HLU+REAMB animals (p ≤ 0.01), and ultimately maintained trabecular bone mineral characteristics at the level of intact controls.
In OVX+HLU+REAMB+EX animals, tBMC and tBMD were 70-76% below OVX+HLU animals (p ≤ 0.001) and approximately 90% below intact controls (p ≤ 0.001). In addition, trabecular bone area was elevated 11% in OVX+HLU+REAMB+EX animals, compared with intact controls (p ≤ 0.05). Conversely, E2-treatment of OVX+HLU+REAMB+EX animals resulted in tBMC and tBMD measurements that were 6-7 fold higher than OVX+HLU+REAMB+EX animals (p ≤ 0.001), 90-95% higher than OVX+HLU animals (p ≤ 0.05), and not different than OVX+E2 controls; although, tBMC and tBMD remained lower than intact controls (p ≤ 0.01).
Distal Femoral Metaphysis Cortical Bone Mineral Characteristics
The cortical bone mineral characteristics at the distal femoral metaphysis are presented in Table 6. In OVX animals, cortical (c)BMC and cortical bone area were 32-34% lower than intact controls (p ≤ 0.001), with no difference in cBMD present between these groups. Estradiol treatment of OVX animals prevented the loss of cortical bone at this skeletal site resulting in a cBMC measurement that was 30% higher than OVX controls (p ≤ 0.05) and not different than intact controls; while, cortical bone area remained 18% below controls (p ≤ 0.05). The preservation of cBMC and concomitant reduction in cortical bone area in OVX+E2 animals resulted in a cBMD measurement that was 7-11% higher than both OVX and intact controls (p ≤ 0.001).
Table 6.
Cortical bone mineral characteristics at the distal femoral metaphysis as measured by pQCT.
| Group | Cortical BMC (mg/mm) |
Cortical Bone Area (mm2) |
Cortical BMD (mg/cm3) |
|
|---|---|---|---|---|
| Intact Control | (a) | 12.8 ± 0.6b*,d*,f*,h*,i* | 13.8 ± 0.7b*,c,d*,f*,h*,i* | 928 ± 6c*,d*,e*,f*,h* |
| OVX | (b) | 8.4 ± 0.3a*,c,d*,f*,h* | 9.3 ± 0.4a*,d*,f*,h* | 898 ± 12c*,d*,e* |
| OVX+E2 | (c) | 10.9 ± 0.8b,d*,f*,h* | 11.0 ± 0.9a,d*,f*,h* | 993 ± 8a*,b*,d*,f*,g*,h*,i |
| OVX+HLU | (d) | 4.1 ± 0.3a*,b*,c*,e*,f,g*,h*,i* | 5.0 ± 0.2a*,b*,c*,e*,f,g*,h*,i* | 823 ± 19a*,b*,c*,e*,g*,i* |
| OVX+E2+HLU | (e) | 10.8 ± 1.2d*,f*,h* | 11.0 ± 1.1d*,f*,h* | 975 ± 9a*,b*,d*,f*,g,h* |
| OVX+HLU+REAMB | (f) | 5.7 ± 0.4a*,b*,c*,d,e*,g,i | 6.6 ± 0.5a*,b*,c*,d,e*,g,i | 870 ± 8a*,c*,e* |
| OVX+E2+HLU+REAMB | (g) | 10.5 ± 1.5d*,f,h | 11.3 ± 1.5d*,f,h | 917 ± 20c*,d*,e |
| OVX+HLU+REAMB+EX | (h) | 5.4 ± 0.3a*,b*,c*,d*,e*,g,i* | 6.2 ± 0.3a*,b*,c*,d*,e*,g,i* | 870 ± 10a*,c*,e* |
| OVX+E2+HLU+REAMB+EX | (i) | 8.8 ± 0.8a*,d*,f,h* | 9.5 ± 0.8a*,d*,f,h* | 917 ± 20c,d* |
Values are Means ± SEM of n = 6-12/group. Letters a-f indicate differences from respectively labeled groups at p ≤ 0.05 or *p ≤ 0.01 (a = vs. Intact controls, b = vs. OVX, c = vs. OVX+E2, d = vs. OVX+HLU, e = vs. OVX+E2 HLU, f = vs. OVX+HLU+REAMB, g = vs. OVX+E2+HLU+REAMB), h = vs. OVX+HLU+REAMB+EX, i = vs. OVX+E2+HLU+REAMB+EX. BMC = Bone mineral content, BMD = bone mineral density.
Hindlimb unloading exacerbated the loss of cortical bone induced by OVX, resulting in cBMC, cortical bone area, and cBMD were 51%, 46%, and 8% lower than OVX controls, respectively (p ≤ 0.01). Conversely, in OVX+E2+HLU animals, cortical bone area and cBMC were not different than intact controls, while cBMD was 18% greater than OVX+HLU animals (p ≤ 0.001) and elevated 5% above controls (p ≤ 0.001).
In OVX+HLU+REAMB animals, cBMC and cortical bone area were 39% and 32% greater than OVX+HLU animals, respectively (p ≤ 0.05), with no difference in cBMD between groups; however, cBMC and cortical bone area remained 52-54% below intact controls (p ≤ 0.001) and cBMD remained 6% below controls (p ≤ 0.001). Conversely, E2-treatment of OVX+HLU+REAMB animals resulted in cBMC, cortical bone area, and cBMD that were each higher than both OVX+HLU (p ≤ 0.01) and OVX+HLU+REAMB animals (p ≤ 0.05), and which were ultimately maintained at the level of intact controls.
In OVX+HLU+REAMB+EX animals, cortical cBMC was 32% higher and cortical bone area was 24% higher than in OVX+HLU animals, respectively (p ≤ 0.01); however, cBMD was not different between groups. Despite these improvements, cBMC and cortical bone area remained 55-58% below intact controls, and cBMD was 6% below intact controls (p ≤ 0.001), with no differences in cortical bone mineral characteristics present between OVX+HLU+REAMB and OVX+HLU+REAMB+EX animals. Conversely, E2-treatment of OVX+HLU+REAMB+EX animals resulted in cBMC and cortical bone area measurements that were higher than OVX+HLU (p ≤ 0.001) and OVX+HLU+REAMB+EX animals (p ≤ 0.01), and ultimately maintained cBMD at the level of intact controls.
DISCUSSION
Both estrogen 1 and mechanical loading 9 influence the maintenance of BMD. However, few studies have evaluated the individual and combined influences of E2 and exercise on the early-phase skeletal adaptations following OVX combined with HLU, a model which mimics the clinically relevant bone loss that occurs in estrogen-deficient women after extended disuse and the subsequent effects of musculoskeletal rehabilitation following severe bone loss. Our primary finding is that in the absence of estrogen, two weeks of moderate-intensity rehabilitation exercise produced severe trabecular and cortical bone loss at the distal femoral metaphysis when performed following a period of extended HLU. Specifically, we observed that 1) at the distal femoral metaphysis, HLU worsened the OVX induced bone loss, 2) E2 treatment prevented most of the bone mineral deficit induced by OVX and HLU, 3) in the absence of estrogen, two weeks of quadrupedal re-ambulation induced neither positive nor negative effects on bone mineral characteristics following HLU, and 4) in the presence of E2, two weeks of quadrupedal re-ambulation (either with or without adjunct moderate-intensity rehabilitation exercise) provided no benefit nor detriment to bone following OVX+HLU.
Estradiol is known to influence bone development and maintenance 1, 2. For example, OVX induces in a rapid increase in bone resorption and concomitant reflex increase in bone formation 6, which results in the net loss of BMD 6, 31, similar to the effects we observed in OVX animals. Conversely, E2 replacement reduces bone turnover and the associated loss of BMD resulting from estrogen deficiency in both humans 4 and animal models 8. In the present study, E2 replacement induced a marked elevation in BMD, as evidenced by the 4-8% net improvement in total body BMD experienced in all groups receiving E2 replacement; while a net loss or only a small change in total body BMD occurred in the OVX groups without E2 replacement. Thus, it is somewhat surprising that the trabecular bone mineral characteristics at the distal femoral metaphysis were not different between OVX and OVX+E2 groups, considering the well-established ability of E2 administration to prevent the OVX induced reductions in tBMD at this 32 and other skeletal sites 7, 8. The most likely explanation of these results stems from our DXA data which demonstrates that the total body BMD of OVX+E2 animals was significantly lower than several other groups at baseline; suggesting that any E2-induced improvement in tBMD may have been obscured by the differences in baseline values. In fact, in all E2 treated groups (including OVX+E2 animals) serum E2 concentrations were similar to that of intact controls, uterine weights were restored to the level of intact controls, and a robust improvement in total body BMD was observed, demonstrating the effectiveness of this treatment. It is also possible that the weight gain experienced following ad libitum feeding of OVX animals limited the loss of BMD within estrogen-deficient animals 33. Regardless, our results demonstrate that E2 administration induced a net improvement in total body BMD and that E2 successfully prevented the deleterious alterations in total and cortical BMD that occur at the distal femoral metaphysis, regardless of treatment.
Mechanical load also influences the maintenance of BMD 9. As evidence, the reduced musculoskeletal loads following disuse result in a rapid and dramatic reduction in BMD 10, 11. Conversely, physical activity reduces osteoporotic fracture risk in older adults 34 and load bearing exercise improves BMD in young individuals 9 and older women receiving HT 24, 25, 35. However, several meta-analyses have reported that both pre- 36, 37 and postmenopausal 20-23 women experience very little, if any, elevation in BMD following exercise interventions; suggesting that estrogen status influences mechanical loading induced bone proliferation, as has been demonstrated in vitro 38 and in vivo in various animal models 17, 39, 40. In the present study, HLU worsened the OVX-induced loss of BMD at the femoral metaphysis, similar to what others have reported 15, 19. We expand upon these findings by demonstrating that E2 replacement fully prevents the reduction in BMD resulting from OVX+HLU, ultimately maintaining bone mineral characteristics at the level of intact controls. Conversely, we report that two weeks of re-ambulation neither enhanced nor produced negative effects on BMD in the early phase of rehabilitation, irrespective of estrogen status; which supports the findings of others who have reported that short-duration quadrupedal re-ambulation is an insufficient stimulus to restore BMD following disuse 14, 19 and that the mechanical loading induced restoration of BMD following disuse takes significantly longer than the restoration of skeletal muscle mass 14.
The loss of BMD following disuse is profound and occurs rather rapidly. Conversely, in rodents, the mechanical loading induced restoration of bone architecture following extended disuse can take upwards of two to three times the duration of disuse 14. However, a clinical discord is present, as the duration of in-patient physical rehabilitation following extended hospitalization is typically only several weeks 16. In our study, we utilized a re-ambulation plus rehabilitation exercise protocol that involved daily functional loading cycles comprised of progressively overloaded climbing, jumping-type, and balance exercises which primarily involved use of the hindlimbs, similar to what others have recommended to improve BMD in rodents 30. In addition, this protocol mimicked the typical frequency, duration, and type of rehabilitation exercises that are recommended clinically for the restoration of neuromuscular and physical function following extended disuse 16 and this protocol has been previously demonstrated to improve skeletal muscle mass in older female rats 29. Thus, it is with great surprise that we report the potentially worrisome observation that in the absence of estrogen, re-ambulation in combination with moderate-intensity rehabilitation exercise produced early-phase bone loss at the femoral metaphysis following extended disuse. In order to verify our findings we performed pQCT analysis on the contralateral limbs of all OVX+HLU+REAMB+EX animals and report that the femoral metaphysis tBMC and tBMD measurements of both limbs were the lowest of all groups and less than 1/10th that of intact control animals at sacrifice. The apparently disadvantageous effect of this rehabilitation exercise protocol within our estrogen deficient OVX+HLU rodent model is further highlighted because quadrupedal re-ambulation (either with or without adjunct rehabilitation exercise) did not worsen BMD when animals were administered E2. Similar to these observations, our recently published companion paper 26 reported that in the absence of estrogen, short-duration re-ambulation plus exercise does not improve hindlimb muscle mass in estrogen-deficient OVX+HLU animals; whereas full restoration of muscle mass occurred in animals receiving E2 replacement while undergoing re-ambulation plus exercise.
Importantly, previous results from our laboratory 19 and the results of our current study both demonstrate that two weeks of quadrupedal re-ambulation (without adjunct exercise) does not induce bone loss in estrogen-deficient female rats following extended disuse. However, others have reported that the proximal tibia of intact rats undergoes an approximate 10% loss of BMD over a period of 28 days following the cessation of HLU-alone, even when animals are allowed unrestricted quadrupedal re-ambulation 14. The severe bone loss that we observed in OVX+HLU animals following quadrupedal re-ambulation combined with exercise differs from these previous studies in three ways, 1) our animals were completely estrogen-deficient, as a result of OVX prior to HLU, 2) our animals performed moderate-intensity exercise as an adjuvant to unrestricted quadrupedal re-ambulation versus re-ambulation alone in previous studies 14, 19, and 3) the loss of BMD occurred to a much greater degree in our study (i.e., a 76-91% reduction in trabecular BMD and 20-46% reduction in total BMD compared with all other groups) versus previous studies (i.e., an approximate 10% reduction compared with age-matched controls) 14. Given the magnitude of bone loss that we observed in OVX+HLU rats undergoing re-ambulation plus exercise, we find it unlikely that the bone loss that we observed was simply an artifact of that which accompanies exercise-induced bone remodeling; although, this remains a possibility because we only evaluated BMD at a single time-point. We believe it is more likely that we have found a unique paradigm and time-frame in which moderate-intensity rehabilitation exercise appears to produce disadvantageous changes in bone architecture, especially given the combination of our current results and those of previous studies. Regardless, it remains unknown whether the loss of BMD that accompanied the moderate-intensity rehabilitation exercise regimen we employed would ultimately be reversed if exercise were continued for an extended period of time, as others have demonstrated 14. Additionally, it should be acknowledged that the non-parametric Mann-Whitney U statistical test utilized in this study for post hoc pair wise comparisons carries an inherent risk for committing Type 2 errors when evaluating a large number of groups, as was the case in this study. In order to satisfy the stringent requirements for use of the non-parametric Mann-Whitney U test we first utilized the Kruskal-Wallis test and only when significance was observed were further post hoc pair wise comparisons conducted using the Mann-Whitney U. Regardless, a large number of pair wise comparisons were performed. As such, confirmation of our findings with future studies is warranted. In addition, future research examining the longitudinal adaptations to bone architecture and bone strength within the unique model of OVX+HLU followed by subsequent quadrupedal re-ambulation and exercise would assist in determining whether the disadvantageous early-phase adaptations that we observed persist or whether exercise is capable of facilitating bone remodeling within our animal model.
The severe rehabilitation exercise-induced bone loss that we observed in OVX+HLU rats also draws attention to a potential clinical concern for estrogen-deficient women experiencing musculoskeletal disuse, given that a similar duration of clinically-prescribed rehabilitation exercises are recommended to combat the rapid loss of neuromuscular and physical function which occurs following extended bed rest 16. In fact, we are unaware of any study that has evaluated the skeletal responses to rehabilitation exercise in estrogen-deficient women following extended disuse. As such, our results suggest that a significant gap may exist within the clinical literature regarding this unique paradigm. Future studies comparing the time-course changes in muscle and bone in estrogen-deficient women who undergo extended disuse and subsequent rehabilitation exercise would assist in the further development of safe and effective clinical guidelines for the restoration of musculoskeletal tissue within a subset of the population that is at high-risk for both osteoporosis and sarcopenia.
The mechanism(s) underlying the pathophysiological bone loss that occurred following moderate-intensity rehabilitation exercise within our estrogen-deficient OVX+HLU rodent model are not intrinsically clear, especially considering well-established ability of exercise to restore BMD following disuse 12 and to protect against the bone loss induced solely by estrogen-deficiency 41-43. As such, the combined effects of estrogen deficiency and extended disuse would appear to either 1) induce early-phase maladaptive responses to mechanical loading within bone and/or 2) suppress BMD to such a degree that exercise produces adverse effects on bone mineralization, as others have suggested could occur 14. One mechanism through which OVX+HLU may exert deleterious influence on bone is through reduced estrogen receptor (ER) expression within bone; although, we did not measure this in our study. Functional estrogen receptors (ERs) are required for muscle 44 and bone maintenance 18, 45 and, in particular, ERα regulates mechanical loading induced bone mineral accretion 18, 45. OVX is known to reduce ERα expression within bone 46 and E2 treatment upregulates mRNA expression of ERα in osteocytes and osteoblast-like cells in vitro 47. Additionally, both estrogen48 and mechanical loading 49, 50 are known to influence sclerostin expression. Sclerostin is the protein encoded by the SOST gene and is a potent negative regulator of bone mass 51. SOST−/− knockout mice express much higher bone mass than wild-type littermates 52, while transgenically elevated SOST/sclerostin severely reduces trabecular BMD 50. Specifically, estrogen deprivation increases circulating sclerostin, a result that is positively associated with changes in bone resorption but not formation 48. Conversely, E2 replacement reduces circulating sclerostin and normalizes bone turnover 48. Similarly, HLU increases sclerostin protein expression in the hindlimbs, a result that ablates bone formation; while enhanced loading reduces sclerostin and restores bone formation in a strain dependent manner 49. We are unaware of any studies that have examined the combined influence of OVX and HLU on sclerostin expression; however, it is possible that in the absence of estrogen exercise is unable to reduce sclerostin expression, given that ERα activation is required for osteoblasts to stimulate the normal bone proliferation response to mechanical strain both in vitro 38 and in vivo 18. Regardless of the underlying mechanism(s), our results suggest that in the absence of estrogen, moderate-intensity rehabilitation exercise induced severe early-phase bone loss at the distal femoral metaphysis following combined OVX+HLU. These findings appear to be in contrast to the well-established ability of exercise to prevent bone loss following OVX alone 41-43 or to restore bone mineral density following HLU alone 12.
CONCLUSIONS
In summary, our primary finding is that in the absence of E2, short-duration re-ambulation plus moderate-intensity rehabilitation exercise worsened BMD at the distal femoral metaphysis in a rodent model of combined estrogen deficiency and disuse atrophy (OVX+HLU) that results in severe bone loss. These findings may have potential clinical relevance given that our rodent model mimics the clinical condition in which estrogen deficient women undergo disuse and subsequent neuromuscular rehabilitation. The apparently negative findings of exercise within this unique rodent paradigm are underscored by our findings that 1) E2 replacement induced a significant prevention of the loss of bone resulting from OVX+HLU, 2) regardless of estrogen status, short-duration re-ambulation alone induced neither positive nor negative effects on bone, and 3) in the presence of E2, short-duration re-ambulation plus exercise did not induce negative bone mineral changes. However, the long-term skeletal adaptations to exercise within this animal model remain to be determined. In addition, future research examining the skeletal adaptations in estrogen deficient women undergoing physical rehabilitation following extended disuse would assist in determining if our findings translate clinically and whether short-duration E2 administration is an appropriate and safe adjuvant therapy to rehabilitation exercise regimens intended to restore musculoskeletal tissue in estrogen deficient women following extended disuse; especially considering that the safety of long-term HT continues to remain controversial following the findings of the Women’s Health Initiative 5.
Supplementary Material
Supplemental Table 1. Comparison of bone mineral characteristics at the distal femoral metaphysis from the contralateral limbs of animals undergoing ovariectomy (OVX) + hindlimb unloading (HLU) + reambulation (REAMB) + exercise (EX).
Supplementary Table 2. Body Mass At Baseline and Sacrifice.
ACKNOWLEDGEMENTS
The authors disclose no conflicts of interest with the presentation of this material. This manuscript was supported in part by a VA CDA-2 (JFY), a VA Merit Award (SEB), and a NASA NA65-12300 and AG15796 (MB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Compston JE. Sex steroids and bone. Physiol Rev 2001;81:419–447. [DOI] [PubMed] [Google Scholar]
- 2.Clarke BL, Khosla S. Female reproductive system and bone. Archives of biochemistry and biophysics 2010;503:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meczekalski B, Podfigurna-Stopa A, Genazzani AR. Hypoestrogenism in young women and its influence on bone mass density. Gynecol Endocrinol 2010;26:652–657. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. Jama 2003;290:1729–1738. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 6.Yarrow JF, Conover CF, Purandare AV, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. American journal of physiology 2008;295:E1213–1222. [DOI] [PubMed] [Google Scholar]
- 7.Wronski TJ, Dann LM, Scott KS, Cintron M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcified tissue international 1989;45:360–366. [DOI] [PubMed] [Google Scholar]
- 8.Wronski TJ, Cintron M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology 1988;123:681–686. [DOI] [PubMed] [Google Scholar]
- 9.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Medicine and science in sports and exercise 2004;36:1985–1996. [DOI] [PubMed] [Google Scholar]
- 10.Amin S Mechanical factors and bone health: effects of weightlessness and neurologic injury. Current rheumatology reports 2010;12:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield SA. Disuse osteopenia. Curr Osteoporos Rep 2010;8:91–97. [DOI] [PubMed] [Google Scholar]
- 12.Giangregorio L, Blimkie CJ. Skeletal adaptations to alterations in weight-bearing activity: a comparison of models of disuse osteoporosis. Sports medicine (Auckland, NZ 2002;32:459–476. [DOI] [PubMed] [Google Scholar]
- 13.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 2005;10:7–40. [DOI] [PubMed] [Google Scholar]
- 14.Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. Journal of musculoskeletal & neuronal interactions 2006;6:217–225. [PubMed] [Google Scholar]
- 15.Bagi CM, Miller SC. Comparison of osteopenic changes in cancellous bone induced by ovariectomy and/or immobilization in adult rats. The Anatomical record 1994;239:243–254. [DOI] [PubMed] [Google Scholar]
- 16.Perme C, Chandrashekar R. Early mobility and walking program for patients in intensive care units: creating a standard of care. Am J Crit Care 2009;18:212–221. [DOI] [PubMed] [Google Scholar]
- 17.Westerlind KC, Wronski TJ, Ritman EL, et al. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proceedings of the National Academy of Sciences of the United States of America 1997;94:4199–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev 2004;32:64–68. [DOI] [PubMed] [Google Scholar]
- 19.Tou JC, Foley A, Yuan YV, Arnaud S, Wade CE, Brown M. The effect of ovariectomy combined with hindlimb unloading and reloading on the long bones of mature Sprague-Dawley rats. Menopause (New York, NY 2008;15:494–502. [DOI] [PubMed] [Google Scholar]
- 20.Kelley GA, Kelley KS. Exercise and bone mineral density at the femoral neck in postmenopausal women: a meta-analysis of controlled clinical trials with individual patient data. American journal of obstetrics and gynecology 2006;194:760–767. [DOI] [PubMed] [Google Scholar]
- 21.Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. The journals of gerontology 2002;57:M599–604. [DOI] [PubMed] [Google Scholar]
- 22.Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 2008;43:521–531. [DOI] [PubMed] [Google Scholar]
- 23.Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC medicine 2010;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohrt WM, Ehsani AA, Birge SJ Jr. HRT preserves increases in bone mineral density and reductions in body fat after a supervised exercise program. J Appl Physiol 1998;84:1506–1512. [DOI] [PubMed] [Google Scholar]
- 25.Kohrt WM, Snead DB, Slatopolsky E, Birge SJ Jr. Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res 1995;10:1303–1311. [DOI] [PubMed] [Google Scholar]
- 26.Brown M, Ferreira JA, Foley AM, Hemmann KM. A rehabilitation exercise program to remediate skeletal muscle atrophy in an estrogen-deficient organism may be ineffective. Eur J Appl Physiol 2011. [DOI] [PubMed] [Google Scholar]
- 27.Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23:279–302. [DOI] [PubMed] [Google Scholar]
- 28.Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res 1984;1:405–411. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Taylor J, Gabriel R. Differential effectiveness of low-intensity exercise in young and old rats. The journals of gerontology 2003;58:B889–894. [DOI] [PubMed] [Google Scholar]
- 30.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 1996;18:37S–43S. [DOI] [PubMed] [Google Scholar]
- 31.Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcified tissue international 1988;43:179–183. [DOI] [PubMed] [Google Scholar]
- 32.Breen SA, Millest AJ, Loveday BE, Johnstone D, Waterton JC. Regional analysis of bone mineral density in the distal femur and proximal tibia using peripheral quantitative computed tomography in the rat In vivo. Calcified tissue international 1996;58:449–453. [DOI] [PubMed] [Google Scholar]
- 33.Wronski TJ, Schenck PA, Cintron M, Walsh CC. Effect of body weight on osteopenia in ovariectomized rats. Calcified tissue international 1987;40:155–159. [DOI] [PubMed] [Google Scholar]
- 34.Moayyeri A The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Annals of epidemiology 2008;18:827–835. [DOI] [PubMed] [Google Scholar]
- 35.Villareal DT, Binder EF, Yarasheski KE, et al. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. Journal of the American Geriatrics Society 2003;51:985–990. [DOI] [PubMed] [Google Scholar]
- 36.Kelley GA, Kelley KS. Efficacy of resistance exercise on lumbar spine and femoral neck bone mineral density in premenopausal women: a meta-analysis of individual patient data. Journal of women's health (2002) 2004;13:293–300. [DOI] [PubMed] [Google Scholar]
- 37.Martyn-St James M, Carroll S. Progressive high-intensity resistance training and bone mineral density changes among premenopausal women: evidence of discordant site-specific skeletal effects. Sports medicine (Auckland, NZ 2006;36:683–704. [DOI] [PubMed] [Google Scholar]
- 38.Jessop HL, Suswillo RF, Rawlinson SC, et al. Osteoblast-like cells from estrogen receptor alpha knockout mice have deficient responses to mechanical strain. J Bone Miner Res 2004;19:938–946. [DOI] [PubMed] [Google Scholar]
- 39.Chen JL, Yao W, Frost HM, Li CY, Setterberg RB, Jee WS. Bipedal stance exercise enhances antiresorption effects of estrogen and counteracts its inhibitory effect on bone formation in sham and ovariectomized rats. Bone 2001;29:126–133. [DOI] [PubMed] [Google Scholar]
- 40.Li CY, Jee WS, Chen JL, et al. Estrogen and "exercise" have a synergistic effect in preventing bone loss in the lumbar vertebra and femoral neck of the ovariectomized rat. Calcified tissue international 2003;72:42–49. [DOI] [PubMed] [Google Scholar]
- 41.Tromp AM, Bravenboer N, Tanck E, et al. Additional weight bearing during exercise and estrogen in the rat: the effect on bone mass, turnover, and structure. Calcified tissue international 2006;79:404–415. [DOI] [PubMed] [Google Scholar]
- 42.Honda A, Sogo N, Nagasawa S, Shimizu T, Umemura Y. High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as Sham rats. J Appl Physiol 2003;95:1032–1037. [DOI] [PubMed] [Google Scholar]
- 43.Peng Z, Tuukkanen J, Vaananen HK. Exercise can provide protection against bone loss and prevent the decrease in mechanical strength of femoral neck in ovariectomized rats. J Bone Miner Res 1994;9:1559–1564. [DOI] [PubMed] [Google Scholar]
- 44.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. American journal of physiology 2009;296:E854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J Bone Miner Res 2001;16:1937–1947. [DOI] [PubMed] [Google Scholar]
- 46.Lanyon L, Armstrong V, Ong D, Zaman G, Price J. Is estrogen receptor alpha key to controlling bones' resistance to fracture? J Endocrinol 2004;182:183–191. [DOI] [PubMed] [Google Scholar]
- 47.Zaman G, Jessop HL, Muzylak M, et al. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res 2006;21:1297–1306. [DOI] [PubMed] [Google Scholar]
- 48.Modder UI, Clowes JA, Hoey K, et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 2011;26:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008;283:5866–5875. [DOI] [PubMed] [Google Scholar]
- 50.Tu X, Rhee Y, Condon K, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechanical loading. Curr Osteoporos Rep 2011;9:76–82. [DOI] [PubMed] [Google Scholar]
- 52.Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 2009;24:1651–1661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison of bone mineral characteristics at the distal femoral metaphysis from the contralateral limbs of animals undergoing ovariectomy (OVX) + hindlimb unloading (HLU) + reambulation (REAMB) + exercise (EX).
Supplementary Table 2. Body Mass At Baseline and Sacrifice.


