Abstract
The incidence of atopic dermatitis (AD) has recently increased due to various factors. Its prevalence is higher among children and teenagers than in other age groups. Numerous methods to treat AD are available, including light ray therapy, which has been proposed as an alternative therapy for the treatment of AD. The present study aimed to evaluate the curative mechanism and optimal energy level of energy irradiation from a low-level laser (LLL) toward AD. AD was induced in BALB/c mice with dinitrochlorobenzene (DNCB) solution. The mice were divided into six groups, including one normal control (n=8), one AD control (n=10) and four AD experimental groups with LLL irradiation at 2 J/cm2 (n=10), 4 J/cm2 (n=10), 6 J/cm2 (n=9) and 8 J/cm2 (n=10). Following AD induction, an LLL was applied to the four AD experimental groups for 2, 4, 6, and 8 min, for two weeks (14 times in total) at a wavelength of 650 nm and an output of 50 mW. The effects of irradiation on AD were evaluated using a scratch test, a clinical skin severity test, immunoglobulin-E (IgE) analysis and measurements of numerous cytokine levels, including interleukin (IL)-4, IL-6, tumor necrosis factor (TNF)-α, and interferon-γ (IFN-γ), tissue thickness and mast cell count. The results demonstrated that serum IgE level in all irradiated groups was significantly decreased compared with that of the AD control group, and IL-4 level was significantly decreased in all irradiated groups apart from the 8 J/cm2 LLL irradiated group. IL-6 and TNF-α levels were also significantly decreased in all irradiated groups. The results from histological analysis revealed diminished epidermal thickness and mast cell counts in irradiated mice compared with those mice in the AD control group. In summary, these findings suggested that LLL irradiation may alleviate symptoms of AD and may be useful for restoring cytokines levels and tissues features to normal levels.
Keywords: low-level laser, atopic dermatitis, therapy, mice, dermatology
Introduction
Atopic dermatitis (AD) is a complex disease (1) caused by numerous environmental factors, such as stress from various types of environmental pollution, immunological factors (2), including increased serum immunoglobulin-E (IgE) levels and imbalance between T helper type 1 (Th1) and Th2 cells, and genetic factors (3,4). Th2 cells secrete cytokines, including interleukin (IL)-4, IL-5, IL-6, and IL-10, and are therefore involved in humoral immunity, while Th1 cells secrete interferon (IFN)-γ and IL-2, which are involved in cellular immunity. In AD, the levels of IL-4, IL-6 and tumor necrosis factor-α (TNF-α) tend to increase, whereas IFN-γ level tends to decrease (5). Furthermore, the number of Langerhans cells and activation of mast cells are increased in AD (6).
The main treatments for AD include local steroids, antihistamine creams and immunomodulators. Furthermore, IFN-γ-based drugs and immunosuppressants are typically administered. However, previous studies have focused on phototherapy, including UV phototherapy and infrared light emitting diode therapy (5,7-11).
Low-level laser (LLL) therapy (LLLT), which is a method using phototherapy, uses low power (≤500 mW) and produces minimal heat. Its therapeutic effect is mainly driven by the stimulation of cells with photo-energy (12,13). In addition, unlike sunlight, LLLT has a narrow wavelength bandwidth, allowing emission from a specific light source to be directed onto a focused and localized area. This technique is therefore effective for the treatment of a specific area.
The effects of LLLT in human tissues are mainly driven by the absorption of energy by specific photoreceptors, such as porphyrin and cytochrome c oxidase. Absorption of energy by these receptors promotes intracellular oxygen synthesis, mitochondrial ATP synthesis, chemiosmosis, DNA replication and infiltration of Ca2+ into the cell cytoplasm (14). These effects subsequently promote cell proliferation and migration, increase tissue oxygenation and control cytokine concentration, growth factors and inflammation (12,15). Furthermore, LLLT treatment increases blood flow to regenerate tissues, provides tension to the skin by promoting collagen production by fibroblasts, promotes cell division to stimulate cell growth, promotes bone regeneration and rearrangement, corrects abnormal hormones level and reduces pain (15-17). Based on these effects, numerous studies have reported that laser therapy displays some positive effects on several musculoskeletal diseases, rheumatoid arthritis, post-herpetic neuralgia, pain, inflammation, edema, cut wounds, nerve damage and neural regeneration (18-26).
Previous studies have examined the use of laser therapy in the treatment of AD (18,27-32). However, these studies used different types of laser and dosages and only demonstrated that laser therapy is clinically effective against AD. To the best of our knowledge, only few studies have thoroughly assessed the underlying mechanism of laser therapy therapeutic effect and suggested an optimal dosage.
The present study aimed to investigate the effects of LLLT on AD using clinical skin severity testing, scratch testing, total serum IgE and IL-4 level evaluation, as well as examined the gene expression of various cytokines (IL-4, IL-6, TNF-α, IFN-γ), epidermal thickness and mast cell counts.
Materials and methods
Animals
All experimental procedures and animal handling were performed following approval from the animal research ethics board of Sahmyook University (approval no. SYUIACUC2017-004). A total of 71 4-week-old BALB/c mice (weight, 16-18 g) were purchased from the laboratory animal center of Hallym Co. (Gyeonggi, South Korea). Mice were acclimatized for seven days in the animal room at the Center for Neurological Sciences, Sahmyook University. Animals had free access to food and water, and the temperature, humidity and day-night cycle were automatically controlled at 23±2˚C, 55±10% and a 12-h cycle, respectively.
Sensitization and challenge
The backs of all the mice were shaved clean and a 24-h recovery period was provided for the micro-wounds on the skin to heal. To induce AD, compound 1-chloro-2,4-di-nitrobenzene (DNCB; Sigma-Aldrich; Merck KGaA) solution (at 2.5%) was prepared by mixing acetone and olive oil in a 3:1 ratio to which DNCB was added. The compound DNCB solution (200 µl; volume given on day 1) was applied to the backs of the mice to induce immune responses. From day 3, 1.0% DNCB solution (150 µl) was applied once every three days, and eschar formation and more severe scratching were observed by the second application of the solution. The eschars started to fall off after the fourth application and had completely disappeared before the fifth application when AD was observed. At this time point, physical intervention with a LLL was initiated. To prevent natural healing while LLLT was performed, 1.0% DNCB solution (150 µl) was applied to the backs of the mice once every three days (33,34).
LLL irradiation
A diode laser therapy instrument (StraTek Co., Ltd.) at a wavelength of 650 nm and power of 50 mW was used for LLLT. The laser was emitted at a minimum intensity of 2 J/cm2 and a maximum intensity of 8 J/cm2. The scanning method was used for laser emission by setting the scanning speed to ‘fast’ in order to reproduce the same effect as that of a continuous laser emission.
An area of 3 cm2 on the back of each mouse was treated, and the energy density (J/cm2) was calculated from the laser output (W), duration (sec) and therapeutic area (J/cm2) as follows (35): Energy density=(laser output x duration)/therapeutic area.
There were six different groups in total. In the normal control group (n=8), only the solvent (acetone and olive oil mix in a 3:1 ratio; 200 µl) was applied to the backs of the mice. The five experiment groups were the following: One control group with induced AD (n=10) and four experimental groups with induced AD followed by treatment with LLLT at difference energy density [Laser 2 at 2 J/cm2 (n=10); Laser 4 at 4 J/cm2 (n=10); Laser 6 at 6 J/cm2 (n=9); and Laser 8 at 8 J/cm2 (n=10)]. Laser therapy was administered daily for two weeks (14 times in total).
Clinical skin severity test
Sensory evaluation is a clinical assessment involving physical examination. In total, two researchers performed this evaluation, and if there were differences, the results was determined via discussion. The outcome was represented as the sum of scores from five different categories. These five categories include erythema, pruritus and dry skin, edema and excoriation, erosion and lichenification. For each category, appropriate evaluation was performed and a score was assigned as follows: None (0), weak (1), intermediate (2) and severe (3). Subsequently, the final score ranged from 0 to 15 (36,37).
After five applications of DNCB solution (150 µl), a clinical skin severity test of the mice was performed. A total of 13 mice with sensory evaluation scores of ≤12 points were then excluded.
An LLL was emitted, at a wavelength of 650 nm and power of 50 mW, 14 times in total over a 2-week period. Each group were exposed to each particular duration only (for 2, 4, 6 and 8 min each time/day). Sensory evaluation (clinical skin severity test) and a scratch test were performed at the following time points: Before therapy and at days 1, 3, 7 and 14 of therapy. On day 14 of therapy, the experiment was complete, and the mice were sacrificed via cervical dislocation after anesthesia. The serum levels of IgE and IL-4 and the mRNA expression of IL-4, IL-6, TNF-α and IFN-γ were then evaluated. Finally, the tissues were stained using hematoxylin and eosin (H&E) and toluidine blue to evaluate the tissue condition and mast cell count.
Scratch test
Scratching behavior was observed and the numbers of scratching episodes counted for 14 days. Scratching was evaluated using a slightly modified version of the traditional method, which was performed for 1 h from 30 min before and after application of the test substance (37,38). The number of scratching movements made by the mice was measured during a 60-min time period before LLLT, a 30-min time period after LLLT, a 30-min time period after stabilization and a 30-min time period after 24 h on days 1, 3, 7 and 14 of LLLT.
Measurement of serum IgE and IL-4 levels
On the last day of the experiment, 0.6-0.8 ml of mouse blood was collected by cardiac puncture after euthanasia and left at room temperature to allow blood coagulation. The sample was centrifuged (-18˚C) at 1,000 x g for 7 min, and the separated serum was stored at -80˚C.
IgE and IL-4 concentrations in mouse serum were measured by ELISA. Mouse IgE and IL-4 ELISA kits (cat. nos. 555248 and 555232; BD OptEIA™; BD Biosciences) were provided by BD Pharmingen; BD Biosciences. The ELISA plates were coated with coating buffer that contains the capture antibody, one day before the experiment. On the day of the experiment, the plate was washed with washing buffer containing 0.05% Tween-20 in PBS. The plates were blocked with PBS containing 10% FBS (cat. no. FBS001-HI, Sigma-Aldrich; Merck KGaA). After additional washing with the washing buffer, 100 µl of standard IgE and mouse serum were added into each well. After incubation at room temperature for 2 h, the plate was washed, the detection antibody solution was added and the plate was incubated at room temperature for an additional 1 h for the reaction to occur. After the final wash with washing buffer, the substrate solution was added, and the plate was incubated in the dark. After 30 min, the color was confirmed and 2 N sulfuric acid was added to stop the reaction. The optical density was measured at 450 nm on an ELISA plate reader to determine the concentrations of IgE and IL-4.
Weight of spleen
At the end of the experiment, the mice were sacrificed by cervical dislocation and the spleen was extracted. The weight of the spleen was evaluated using an Electronic Scale (cat. no. CP224S; Sartorius AG) (33,39) and tissues were stored at -80˚C.
Reverse transcription-quantitative (RT-q)PCR
The mRNA expression of IL-4, IL-6, TNF-α, and IFN-γ was evaluated in spleen tissues by RT-qPCR as previously described (40). Spleen tissues stored at -80˚C were thawed on ice and homogenized in an e-tube containing 1 ml Total RNA isolation solution (RiboEX™; GeneAll Biotechnology Co., Ltd.) using a homogenizer. Gene extraction solution (GeneAll®Hybrid-R™; GeneAll Biotechnology Co., Ltd.) was used to extract RNA from the tissues. The concentration of extracted RNA was measured with a NanoDrop ND-1,000 Spectrometer (NanoDrop products), and cDNA was obtained using AccuPower® CycleScript RT PreMix (Bioneer Corporation). Samples were stored at -80˚C until further analysis. RT-qPCR was run using SYBR® Green (SolGent Co., Ltd.). RT-qPCR was performed. The sequences of the primers used are presented in Table I. The relative expression levels were normalized to endogenous control and were expressed as 2-ΔΔCq (41).
Table I.
Sequence of the primers used for reverse transcription-quantitative PCR.
| Gene | Sequences |
|---|---|
| IL-4 | |
| Forward | 5'-AAGAACACCACAGAGAGTGAGCTC-3' |
| Reverse | 5'-TTTCAGTGATGTGGACTTGGACTC-3' |
| IL-6 | |
| Forward | 5'-CAAGAGACTTCCATCCAGTTGC-3' |
| Reverse | 5'-TTGCCGAGTAGATCTCAAAGTGAC-3' |
| TNF-α | |
| Forward | 5'-ATGAGCACAGAAAGCATGATC-3' |
| Reverse | 5'-TACAGGCTTGTCACTGGAATT-3' |
| IFN-γ | |
| Forward | 5'-GCCATCAGCAACAACATAAGCGTC-3' |
| Reverse | 5'-CCACTCGGATGAGCTCATTGAATG-3' |
| GAPDH | |
| Forward | 5'-GAGGGGCCATCCACAGTCTTC-3' |
| Reverse | 5'-CATCACCATCTTCCAGGAGCG-3' |
IL-4, interleukin-4; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α; IFN-γ, interferon-γ.
Histopathological analysis of mast cells and tissues
As soon as mice were sacrificed and at the end of the full protocol, mouse skin tissues from the lesions were biopsied from the backs of mice. To ensure that skin biopsies were performed at identical positions in all mice, the biopsies were collected from an area (1.5x1.5 cm2) immediately above the line dividing the back of the mouse in half. Fixed tissues in 10% formalin (18-20˚C, 2 days) were embedded in paraffin and 5 µm-thick blocks were prepared. H&E staining was subsequently performed to identify edema and measure the epidermal thickness in different tissues. Toluidine blue staining was also used to identify mast cells and to observe any potential infiltration of mast cells into the tissues. The number of mast cells that were stained by toluidine blue within one square of the grid lines (magnification, x40 and x100, HNM005 HiMaxthe®) was counted and the value was represented as cell count/mm2 (42). When mast cells are activated, substances contained in granules, such as histamine, are degranulated, and lipid mediators such as cytokines, prostaglandins and leukotriene, are newly synthesized and secreted. These substances cause symptoms such as vasodilation, extravasation of white blood cells and inflammatory reactions (43). Therefore, the current experiment observed the number of granulated cells and degranulated cells.
Statistical analysis
All data were presented as the means ± standard error of the mean. For evaluations involving temporal variables, including sensory evaluation and scratch test, two-way mixed repeated ANOVA was used followed by Bonferroni post hoc test. One-way ANOVA followed by Dunnett's post hoc test was used to compare the weights of the spleen and levels of IgE, IL-4, IL-6, TNF-α and IFN-γ between different groups. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using GraphPad Prism software version 5.02 (GraphPad Software, Inc.).
Results
Effects of LLLT on the clinical skin severity and scratch tests
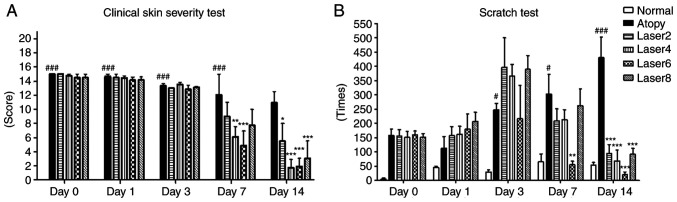
To understand the effects of LLL irradiation on sensory evaluation and scratching, mice were evaluated using a clinical skin severity test and a scratch test before LLLT and on days 1, 3, 7 and 14 of LLLT. From day 7 of LLLT, decrease in the clinical skin severity test in the Laser 4 (P<0.01) and Laser 6 groups (P<0.001) compared with the AD group was observed. Furthermore on day 7 of LLLT mice in the Laser 6 group showed significantly reduced scratching compared with the atopy group (P<0.01). By day 14, all experimental groups exhibited significant decrease in scratching and the clinical skin severity test compared with the AD group (P<0.001; Fig. 1).
Figure 1.
Effects of LLLT on the clinical skin severity and scratch tests. (A) Clinical skin severity test. (B) Scratch test. Day 0: Before Low Level Laser irradiation; Day 1: At days 1 of Low Level Laser irradiation; Day 3: At days 3 of Low Level Laser irradiation; Day 7: At days 7 of Low Level Laser irradiation; Day 14: At days 14 of Low Level Laser irradiation; Normal: Normal control group; Atopy: Only induced atopic dermatitis; Laser2 at 2 J/cm2 (n=10); Laser4 at 4 J/cm2 (n=10); Laser6 at 6 J/cm2 (n=9); and Laser8 at 8 J/cm2 (n=10). *P<0.05, **P<0.01, ***P<0.001 vs. Atopy; #P<0.05, ###P<0.001 vs. Normal.
Effects of LLLT on serum IgE and IL-4 concentrations
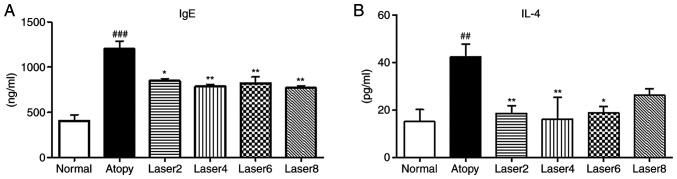
We measured the serum concentrations of IgE and IL-4 using ELISA in experimental mice with induced AD. In the AD control group, IgE concentration was significantly increased compared with normal control group (P<0.001). In addition, IgE concentration was significantly decreased in the experimental groups treated with LLLT compared with AD control group (Fig. 2A). Similarly, IL-4 concentration in the AD control group was significantly elevated compared with normal control group (P<0.01). Furthermore, IL-4 level was significantly decrease in all experimental groups treated with LLLT compared with AD control group (P<0.01; Fig. 2B).
Figure 2.
Effects of LLLT on serum IgE and IL-4 concentrations. (A) Effects of LLLT on serum IgE concentration. (B) IL4 concentration. Normal: Normal control group; Atopy: Only induced atopic dermatitis; Laser2 at 2 J/cm2 (n=10); Laser4 at 4 J/cm2 (n=10); Laser6 at 6 J/cm2 (n=9); and Laser8 at 8 J/cm2 (n=10). *P<0.05, **P<0.01 vs. Atopy; ##P<0.01, ###P<0.001 vs. Normal.
Weight of spleen
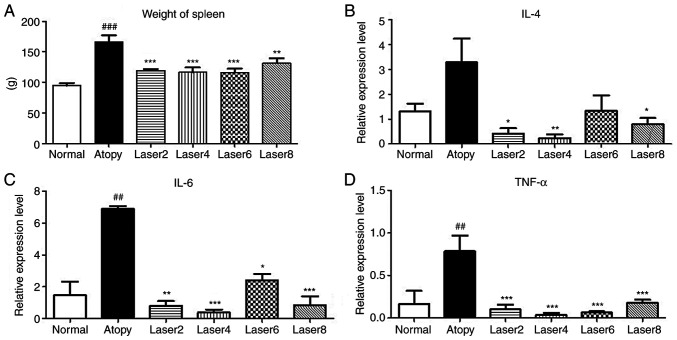
The weight of the spleen was measured at the end of the experiments to determine the effect of LLL on the immunization of laboratory animals caused by AD. The results demonstrated that the weight of spleen was significantly increased in the AD group compared with normal group (P<0.001). In addition, the experimental groups Laser 2 (P<0.001), Laser 4 (P<0.001), Laser 6 (P<0.001) and Laser 8 J/cm2 (P<0.01) exhibited significantly decreased spleen weight compared with AD control group (Fig. 3A).
Figure 3.
Weight of spleen and mRNA expression levels. (A) Effects of LLLT on weight of spleen. Effects of LLLT on mRNA expression levels of (B) IL4, (C) IL-6 and (D) TNF-α. Normal: Normal control group; Atopy: Only induced atopic dermatitis; Laser2 at 2 J/cm2 (n=10); Laser4 at 4 J/cm2 (n=10); Laser6 at 6 J/cm2 (n=9); and Laser8 at 8 J/cm2 (n=10). *P<0.05, **P<0.01, ***P<0.001 vs. Atopy; ##P<0.01, ###P<0.001 vs. Normal. IL-4, interleukin-4; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Effects of LLLT on the mRNA expression of IL-4, IL-6, TNF-α and IFN-γ
The expression levels of IL-4, IL-6, TNF-α and IFN-γ in experimental mice with induced AD were assessed using RT-qPCR. IL-6 and TNF- α expression levels in AD group were significantly increased compared with normal group. Moreover, IL-4 expression levels in AD group were not significantly increased compared with normal group, but IL-4 expression was significantly decreased in all experimental groups compared with AD group, except Laser 6 (Fig. 3B). IL-6 and TNF-α expression was significantly decreased in all experimental groups compared with AD group (Fig. 3C and D).
Effects of LLLT on skin tissue
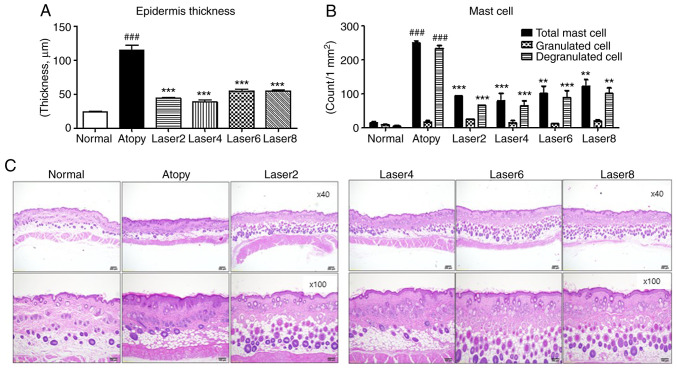
Changes in epidermal thickness, structure and number of mast cells in mouse tissues are presented in Fig. 4. The epidermal thickness was determined in all tissues. The results revealed a significant increase in epidermal thickness in the AD control group compared with the normal group (P<0.001). Furthermore, all experimental groups treated with LLLT exhibited significantly reduced epidermal thickness compared with the AD group (P<0.001). Furthermore, according to results from H&E staining, the AD group showed increased epidermal and dermal thickness, as well as markedly increased hyperkeratinization near the lesion, compared with the normal group. Furthermore, the experimental groups treated with LLLT showed decreased epidermal and dermal thickness similar to that of the normal group, and the hyperkeratinization was improved.
Figure 4.
Effects of LLLT on skin tissue. (A) Changes in epidermal thickness. (B) Number of mast cells in the tissue. (C) Histopathological analyses of mast cells and tissues (magnification, x40 x100). Normal: Normal control group; Atopy: Only induced atopic dermatitis; Laser2 at 2 J/cm2 (n=10); Laser4 at 4 J/cm2 (n=10); Laser6 at 6 J/cm2 (n=9); and Laser8 at 8 J/cm2 (n=10). **P<0.01, ***P<0.001 vs. Atopy; ###P<0.001 vs. Normal.
The total number of mast cells, granulated and degranulated cells within an area of 1 mm2 of the tissues was subsequently assessed in all tissues. The results demonstrated that the total number of mast cells in the experimental groups treated with LLLT was significantly lower compared with that in the AD group (P<0.001). The number of granulated cells in the AD control group was slightly higher than that in the normal group. The experimental groups treated with LLLT presented similar numbers of granulated cells to that in the AD group. The number of degranulated cells in the AD group was significantly increased compared with the normal group (P<0.001). In addition, the experimental groups treated with LLLT exhibited a significantly decreased number of degranulated cells compared with the AD control group (P<0.001).
Discussion
The effects of LLLT on relieving the symptoms of dermatitis have been previously demonstrated in clinical settings (17,44). The present study attempted therefore to confirm the curative effect of a ‘designed’ energy irradiation dose of LLL towards AD.
The wavelength used in the present study was 650 nm, which is within the range of suggested wavelengths (630-660 nm) for inflammation therapy (45). It has been reported that LLL at 1-6 J/cm2 is effective against acute and subacute inflammations, while LLL at 4-8 J/cm2 is effective against chronic inflammations, such as atopic dermatitis and arthritis (35). According to the World Association for Laser Therapy, the red wavelength doses used for superficial diseases tend to be ~4 J/cm2 (32). The present study used therefore laser outputs of 2, 4, 6 and 8 J/cm2.
In the present study, the results from sensory evaluation, which evaluates the therapeutic effectiveness by assigning scores to different symptoms of AD, demonstrated that after 7 days of LLLT, the experimental groups treated with LLLT exhibited significantly decreased symptoms compared with the AD group.
In the present study, a scratch test was performed following treatment with LLLT. The results demonstrated that the AD group exhibited increased scratching from day 3. All experimental groups treated with LLLT exhibited increased scratching on day 3, followed by significantly decreased scratching on days 7 and 14 compared with the AD group. The smallest decrease in scratching was observed in the Laser 6 group.
In the present study, the onset of AD was confirmed by changes in serum IgE and IL-4 concentrations. In previous studies, it was demonstrated that LLLT can suppress the induction of AD (1,32,36). In addition, IL-4 serum concentration in mice with induced AD was significantly elevated compared with that in mice in the normal group. These findings were similar to previous studies, which demonstrated that the level of the Th2 cell cytokine IL-4 is elevated in patients with AD (1,6,36). In the present study, except for the Laser 8 group, all experimental groups treated with LLLT, showed significant reduction in serum IL-4 concentration compared with the AD group. The Laser 4 group exhibited the most efficient reduction. These findings indicated that laser therapy was effective for relieving symptoms of AD.
As described in previous studies (33,39), the weight of spleen in the present study was measured and the immune response in mice with AD was analyzed. The results demonstrated that mice in the AD groups had increased spleen weight. However, the weight of spleen decreased significantly in all laser irradiation groups compared with than in the AD group, Increased spleen weight is associated with increased T lymphocytes. However, a decreased weight means a decrease in inflammatory reactions by T lymphocytes (39). Thus, these findings suggested that LLLT may have an effect on immunity.
Subsequently, the expression of some mRNA was assessed in spleen tissues. In humans, ~10-15% of the lymphocytes in the blood, ~20-25% of the lymphocytes in the lymph nodes and ~40-45% of the lymphocytes in the spleen cells are B cells (46); the spleen has therefore the largest amount of lymphocytes. If taken from the blood, cytokines, represented by white blood cells, may also be sensitive to differences in blood collection or sample manipulation methods (47). Quantitative analysis of cytokine mRNA expression levels suggests that these cytokines can be considered as potential sensitive markers for determination of the state of immune cell activation (48). In present study, total RNA was extracted from the spleen tissues of each group, and the mRNA expression of IL-4, IL-6 and TNF-α (factors of Th 2 immune response) and IFN-γ (factor of Th1 immune response) was evaluated. Th1 and Th2 cells maintain immune balance, where IL-4 suppresses the Th1 type reaction and IFN-γ suppresses the Th2 type reaction (1). T-cells in patients with AD have decreased ability to produce IFN-γ and are highly reactive to IL-4, which is thought to be the main reason for immune response by type Th2 cells in AD (1). The present study assessed the mRNA expression of IL-4, IL-6, TNF-α and IFN-γ (data not shown) after LLLT treatment. The expression levels of IL-4, IL-6 and TNF-α were significantly increased in the AD group, which has been previously described (3-6). Since IL-4 is the cytokine responsible for IgE release in AD, patients with AD exhibit elevated levels of IL-4. In addition, increased IL-6 and TNF-α levels in AD have been reported in several studies (1,6,8,9,45,49,50). Subsequently, the increased mRNA expression of these cytokines described in the present study indicated that AD was successfully induced. Furthermore, following LLLT treatment, the expression level of IL-4 was significantly decreased in all experimental groups except for the Laser 6 group. Among these groups, the Laser 4 group exhibited the lowest IL-4 mRNA expression. In addition, the IL-6 expression level was decreased in all experimental groups treated with LLLT, with Laser 4 group presenting the lowest IL-6 expression level. Furthermore, the mRNA expression of TNF-α in all experimental groups treated with LLLT was significantly decreased compared with that in the AD group. Once again, the Laser 4 group showed the lowest expression level of TNF-α. Atopic dermatitis is caused by abnormal activation of IL4, the Th2 cytokine (1). These findings suggested that LLLT may suppress the immune response from Th2 cells, subsequently reducing the inflammatory response in AD.
The current study also measured the expression of IFN-γ and observed lower expression level of IFN-γ; however, the difference was not significant. Since IFN-γ is the cytokine that induces immune responses from Th1 cells (51), these findings indicated that AD treatment with LLLT for two weeks was not sufficient to correct the immune imbalance between Th1 and Th2 cells.
The epidermal thickness of AD lesions was assessed by H&E staining. The results showed a significant reduction in epidermal thickness in the experimental groups treated with LLLT, particularly in the Laser 4 group that exhibited the greatest reduction, compared with the AD group. Furthermore, the number of mast cells after toluidine blue staining in the AD groups was significantly increased compared with the normal group. In addition, the number of degranulated cells in the AD control group was significantly higher than in all experimental groups treated with LLLT. This observation was due to the larger number of mast cells in this group. These findings suggested that LLLT may be considered as an effective treatment option for AD.
The wavelength of LLL used in this study (650 nm) could effectively recover inflammation Although 1.0% DNCB solution was frequently applied to prevent natural healing of AD, all experimental groups treated with LLLT exhibited decreased IL-4, IL-6, and TNF-α levels. Immunomodulation by the Th1 and Th2 cells might have accounted for the elevated IFN-γ expression level in the AD group compared with that in the other experimental groups treated with LLLT. However, the present results indicated that gene expression of IFN-γ in the AD control group did not differ from that in the other experimental groups treated with LLLT (data not shown). Therefore, the AD improvement observed in this study did not result from immunomodulation by the Th1 and Th2 cells, but rather from the intracellular activity promoted by LLLT to induce recovery of inflammation and skin restoration (52) and to reduce the mRNA expression of IL-4, IL-6 and TNF-α. Future investigation on immunomodulation by the Th1 and Th2 cells is therefore required.
The findings from the present study suggested that LLLT may be considered as an effective treatment for AD since it may promote intracellular activation to reduce the release of inflammatory cytokines from Th2 cells. Furthermore, comparisons of the different treatment group outcomes suggested that the 4 J/cm2 laser output was more effective at improving the symptoms of AD and reducing the levels of cytokines compared with other output values.
The present study evaluated the changes in clinical skin severity and scratching for 14 days. Further investigation will therefore investigate IgE and IL-4 levels evolution over time. IgE was evaluated because it is directly related to inflammation. Furthermore, histamine is released when allergy antigens bind to IgE antibodies that bind to obese cells. Increased IgE leads to histamine release, causing inflammatory reactions. Histamine is one of the organic substances that the body secretes for rapid defense against external stimulation (stress). In other words, it is a substance that causes inflammation, in which the wound swells red and causes pain (53). Therefore, IgE overproduction may be associated with the release of high levels of histamine. The present study identified how LLL irradiation affects IgE and cytokines (IL-4, IL-6 and TNF-α) in the skin that caused atopic dermatitis. Future investigation will therefore examine the release of histamine in this model.
Since the number of experimental animals used in this study was relatively low, Clinical research, including a large number of animal trials, will be needed. Taken together, the findings from the present study may help determining clinically appropriate energy outputs for LLLT against AD.
Acknowledgements
The authors would like to thank Dr Miyung Kim, Dr June Bryan, Dr Irene Joy, Dr Chrislean Jun and Dr Raly James (Uimyung Research Institute. Sahmyook University) for their assistance preparing animal experiuments and their support on this paper.
Funding Statement
Funding: This study was supported by a grant from Sahmyook University in 2017.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YLK and HSL carry out the experiments. YLK and HSL analyzed and interpreted the data regarding the cytokines. YLK and SML made substantial contributions to conception and design of the study, and confirmed the authenticity of all the raw data. All authors read and approved the final manuscript
Ethics approval and consent to participate
This study was approved by the Animal Research Ethics Board of Sahmyook University (approval no. SYUIACUC2017-004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandstrom MH, Faergemann J. Prognosis and prognostic factors in adult patients with atopic dermatitis: A long-term follow-up questionnaire study. Br J Dermatol. 2004;150:103–110. doi: 10.1111/j.1365-2133.2004.05711.x. [DOI] [PubMed] [Google Scholar]
- 3.Youssef DM, Elbehidy RM, El-Shal AS, Sherief LM. T helper 1 and T helper 2 cytokines in atopic children with steroid-sensitive nephrotic syndrome. Iran J Kidney Dis. 2015;9:298–305. [PubMed] [Google Scholar]
- 4.Hanzlikova J, Ulcova-Gallova Z, Malkusova I, Sefrna F, Panzner P. TH1-TH2 response and the atopy risk in patients with reproduction failure. Am J Reprod Immunol. 2009;61:213–220. doi: 10.1111/j.1600-0897.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 1994;131:348–353. doi: 10.1111/j.1365-2133.1994.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 6.Jung JH, Kim GJ. Anti-inflammatory effects of herbal medicines (Rubus coreanus, Rehmanniae Radix, Houttuynia cordata, Betulae cortex) EtOH extract on acute atopic dermatitis mice. J Korean Med Ophthalmol Otolaryngol Dermatol. 2015;28:68–84. [Google Scholar]
- 7.Lim YY, Kim HM, Jang WS, Seo SH, Ahn HH, Kim Mn, Kim BJ. Study on tests of skin safety and inhibition of atopic dermatitis using a StoneTouch® infrared scanner in a mouse model. Korean J Dermatol. 2011;49:217–226. [Google Scholar]
- 8.Lim YY, Jang WS, Kim HM, Kim IS, Lee JW, Kim MN, Kim BJ. Therapeutic effects of light emitting diode on atopic dermatitis-like lesions in NC/Nga mice. Korean J Asthma Allergy Clin Immunol. 2011;31:207–214. [Google Scholar]
- 9.Kwon TR. UV-LED 310-nm and 340-nm of phototherapy in the treatment of atopic dermatitis. PhD Thesis, Chung-Ang University, Seoul, Korea, 2015. [Google Scholar]
- 10.Garritsen FM, Brouwer MW, Limpens J, Spuls PI. Photo(chemo)therapy in the management of atopic dermatitis: An updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501–513. doi: 10.1111/bjd.12645. [DOI] [PubMed] [Google Scholar]
- 11.Jekal SJ, Park MS, Kim DJ. The combined effects of curcumin administration and 630 nm LED phototherapy against DNCB-induced atopic dermatitis-like skin lesions in BALB/c mice. Korean J Clin Lab Sci. 2017;49:150–160. [Google Scholar]
- 12.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(7000417) doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W, Naim JO, Lanzafame RJ. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med. 1997;20:56–63. doi: 10.1002/(sici)1096-9101(1997)20:1<56::aid-lsm9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Barber AJ, Luger E, Karpfetal A. Advances in laser therapy for bone repair. Laser Ther. 2000;13:84–85. [Google Scholar]
- 15.Hamblin MR, Demidova TN. Mechanisms of low level light therapy. In: Mechanisms for Low-Light Therapy. Hamblin MR, Waynant RW and Anders J (eds). Proceedings of SPIE. Vol 6140. 1-12, 2006. [Google Scholar]
- 16.Kim KU. Effects of low level laser irradiation with 904 nm pulsed diode laser on the extraction wound. J Korean Academy of Oral Med. 1998;23:301–309. [Google Scholar]
- 17.Marei MK, Abdel-Meguid SH, Mokhtar SA, Rizk SA. Effect of low-energy laser application in the treatment of denture-induced mucosal lesions. J Prosthet Dent. 1997;77:256–264. doi: 10.1016/s0022-3913(97)70182-3. [DOI] [PubMed] [Google Scholar]
- 18.Joon YH, Sung YJ, Gon KD, Yong LJ. The effects of low level laser therapy on decrease of atopic dermatitis symptoms. Korean J Pediatr Med. 2009;23:193–206. [Google Scholar]
- 19.Clokie C, Bentley KC, Head TW. The effects of the helium-neon laser on postsurgical discomfort: A pilot study. J Can Dent Assoc. 1991;57:584–586. [PubMed] [Google Scholar]
- 20.Kert J. Low level laser therapy used pre-operatively. Laser News. 1992;4:26–32. [Google Scholar]
- 21.Roynesdal AK, Bjornland T, Barkvoll P, Haanaes HR. The effect of soft-laser application on postoperative pain and swelling. A double-blind, crossover study. Int J Oral Maxillofac Surg. 1993;22:242–245. doi: 10.1016/s0901-5027(05)80646-0. [DOI] [PubMed] [Google Scholar]
- 22.Pick RM, Powell GL. Laser in dentistry: Soft tissue procedure. Dent Clin North Am. 1993;37:281–296. [PubMed] [Google Scholar]
- 23.Miller M, Truhe T. Lasers in dentistry: An overview. J Am Dent Assoc. 1993;124:32–55. doi: 10.14219/jada.archive.1993.0034. [DOI] [PubMed] [Google Scholar]
- 24.Hall J, Clarke AK, Elvins DM, Ring EF. Low level laser therapy is ineffective in the management of rheumatoid arthritic finger joints. Br J Rheumatol. 1994;33:142–147. doi: 10.1093/rheumatology/33.2.142. [DOI] [PubMed] [Google Scholar]
- 25.Basford JR. Laser therapy: Scientific basis and clinical role. Orthopedics. 1993;16:541–547. doi: 10.3928/0147-7447-19930501-06. [DOI] [PubMed] [Google Scholar]
- 26.Beckerman H, de Bie RA, Bouter LM, De Cuyper HJ, Oostendorp RA. The efficacy of laser therapy for musculoskeletal and skin disorders: A criteria-based meta-analysis of randomized clinical trials. Phys Ther. 1992;72:483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- 27.Morita H, Kohno J, Hori M, Kitano Y. Clinical application of low reactive level laser therapy (LLLT) for atopic dermatitis. Keio J Med. 1993;42:174–176. doi: 10.2302/kjm.42.174. [DOI] [PubMed] [Google Scholar]
- 28.Nistico SP, Saraceno R, Capriotti E, Felice CD, Chimenti S. Efficacy of monochromatic excimer light (308 nm) in the treatment of atopic dermatitis in adults and children. Photomed Laser Surg. 2008;26:14–18. doi: 10.1089/pho.2007.2116. [DOI] [PubMed] [Google Scholar]
- 29.Baltas E, Csoma Z, Bodai L, Ignacz F, Dobozy A, Kemeny L. Treatment of atopic dermatitis with the xenon chloride excimer laser. J Eur Acad Dermatol Venereol. 2006;20:657–660. doi: 10.1111/j.1468-3083.2006.01495.x. [DOI] [PubMed] [Google Scholar]
- 30.Brenninkmeijer EE, Spuls PI, Lindeboom R, van der Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. Clobetasol propionate 0.05% ointment in prurigo form of atopic dermatitis: A randomized controlled trial, a pilot. Br J Dermatol. 2010;163:823–831. doi: 10.1111/j.1365-2133.2010.09858.x. [DOI] [PubMed] [Google Scholar]
- 31.Syed S, Weibel L, Kennedy H, Harper JI. A pilot study showing pulsed-dye laser treatment improves localized areas of chronic atopic dermatitis. Clin Exp Dermatol. 2008;33:243–248. doi: 10.1111/j.1365-2230.2007.02644.x. [DOI] [PubMed] [Google Scholar]
- 32.Stich AN, Rosenkrantz WS, Griffin CE. Clinical efficacy of low-level laser therapy on localized canine atopic dermatitis severity score and localized pruritic visual analog score in pedal pruritus due to canine atopic dermatitis. Vet Dermatol. 2014;25:464–474. doi: 10.1111/vde.12144. [DOI] [PubMed] [Google Scholar]
- 33.Lee KS, Jeong ES, Hea SH, Seo JH, Jeong DG, Choi YK. A novel model for human atopic dermatitis: Application of repeated DNCB patch in BALB/c mice, in comparison with NC/Nga mice. Lab Anim Res. 2010;26:95–102. [Google Scholar]
- 34.Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. NC/Nga mice: A mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999;120 (Suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH. Phototherapy. Hanlddlag, Gunggido, p198, 2005. [Google Scholar]
- 36.Suzuki R, Shimizu T, Kudo T, Ohtsuka Y, Yamashiro Y, Oshida K. Effects of n-3 polyunsaturated fatty acids on dermatitis in NC/Nga mice. Prostaglandins Leukot Essent Fatty Acids. 2002;66:435–440. doi: 10.1054/plef.2002.0370. [DOI] [PubMed] [Google Scholar]
- 37.Lee GS, Jung HM, Oh SK, Cheong JH, Kang TJ. Effects of herbal complex on atopic dermatitis in BALB/c mice. Kor J Pharmacogn. 2012;43:59–65. [Google Scholar]
- 38.Umeda K, Noro Y, Murakami T, Tokime K, Sugisaki H, Yamanaka K, Kurokawa I, Kuno K, Tsutsui H, Nakanishi K, Mizutani H. A novel acoustic evaluation system of scratching in mouse dermatitis: Rapid and specific detection of invisibly rapid scratch in an atopic dermatitis model mouse. Life Sci. 2006;79:2144–2150. doi: 10.1016/j.lfs.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Ahn JY, Lee RI, Kim JH, Park JH, Kim DK, Lee YM. Effects of rumecis radix water extract on development of atopic dermatitis in BALB/c Mice. Korean J Pharmacogn. 2009;40:218–223. [Google Scholar]
- 40.Kim M, Custodio RJ, Botanas CJ, de la Peña JB, Sayson LV, Abiero A, Ryoo ZY, Cheong JH, Kim HJ. The circadian gene, Per2, influences methamphetamine sensitization and reward through the dopaminergic system in the striatum of mice. Addict Biol. 2019;24:946–957. doi: 10.1111/adb.12663. [DOI] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Choy DF, Hsu DK, Seshasayee D, Fung MA, Modrusan Z, Martin F, Liu FT, Arron JR. Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J Allergy Clin Immunol. 2012;130:1335–1343.e5. doi: 10.1016/j.jaci.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owen J, Punt J, Stratford S. Kuby Immunology. 7th edition. W.H. Freemand and Company, New York, NY, USA, p372, 2013. [Google Scholar]
- 44.Moreira MS, Velasco IT, Ferreira LS, Ariga SK, Abatepaulo F, Grinberg LT, Marques MM. Effect of laser phototherapy on wound healing following cerebral ischemia by cryogenic injury. J Photochem Photobiol B. 2011;105:207–215. doi: 10.1016/j.jphotobiol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Cooper D, Hales J, Camp R. IgE-dependent activation of T cells by allergen in atopic dermatitis: Pathophysiologic relevance. J Invest Dermatol. 2004;123:1086–1091. doi: 10.1111/j.0022-202X.2004.23484.x. [DOI] [PubMed] [Google Scholar]
- 46.Abbas AK, Andrew H. Lichtmann, Shiv Pillai: Cellular and Molecular Immunology. 6 edition. Saunders Elsevier, Philadelphia, p50, 2007. [Google Scholar]
- 47.Hartel C, Bein G, Muller-Steinhardt M, Kluter H. Ex vivo induction of cytokine mRNA expression in human blood samples. J Immuno Methods. 2001;249:63–71. doi: 10.1016/s0022-1759(00)00334-3. [DOI] [PubMed] [Google Scholar]
- 48.Whiteside TL. Cytokines and cytokine measurements in clinical laboratory. Clin Diagn Lab Immunol. 1994;1:257–266. doi: 10.1128/cdli.1.3.257-260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniuchi S, Kojima T, Hara Mt K, Yamamoto A, Sasai M, Takahashi H, Kobayashi Y. Increased serum nitrate levels in infants with atopic dermatitis. Allergy. 2001;56:693–695. doi: 10.1034/j.1398-9995.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 50.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–189. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 51.Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. Bummoon-Education, Seoul, pp320-321, 1998. [Google Scholar]
- 52.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy-an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.