Abstract
Objectives:
This study aimed to evaluate the CT and MRI findings of focal splenic lesions and ascites in generalized lymphatic anomaly (GLA), kaposiform lymphangiomatosis (KLA), and Gorham-Stout disease (GSD).
Material and Methods:
Twenty-three patients (10 with GLA, 5 with KLA, and 8 with GSD) who underwent abdominal CT and/or MRI before treatment were included in this study, and their imaging findings were retrospectively evaluated.
Results:
Focal splenic lesions were observed in nine patients; these lesions were observed frequently in GLA (n = 5; 50%) or KLA (n = 3; 60%) compared with GSD (n = 1; 13%); however, no significant differences were found between the three groups (P = 0.190). On CT images among eight patients (4 with GLA, 3 with KLA, and 1 with GSD) with focal splenic lesions who underwent CT, the number of focal splenic lesions per patient ranged from 2 to 189 (mean, 42) and the maximum diameter of focal splenic lesions ranged from 2 to 39 mm (mean, 8 mm), while more than 30 focal splenic lesions per patient were observed in 2 (50%) GLA and focal splenic lesions with maximum diameters of ≥10 mm were observed in 4 (100%) GLA but not in KLA or GSD. Ascites was observed in five patients; significant differences were observed among KLA (n = 4; 80%), GLA (n = 1; 10%), and GSD (n = 0; 0%) (P < 0.01). Ascites was significantly more frequent in KLA than in GSD (P < 0.05).
Conclusion:
More than 30 focal splenic lesions per patient and/or focal splenic lesions with maximum diameters of ≥10 mm were observed only in GLA. Focal splenic lesions tended to be less frequent in GSD, whereas ascites tended to be frequent in KLA.
Keywords: Generalized lymphatic anomaly, Kaposiform lymphangiomatosis, Gorham-Stout disease, Spleen, Ascites
INTRODUCTION
Based on the International Society for the Study of Vascular Anomalies (ISSVA) guidelines, generalized lymphatic anomaly (GLA), kaposiform lymphangiomatosis (KLA), and Gorham-Stout disease (GSD) are classified as lymphatic anomalies (LAs). The most recent revision of the ISSVA guidelines was established in 2018 during the 22nd ISSVA workshop. According to the latest classification, GLA and GSD are categorized as lymphatic malformations, representing a subcategory of simple vascular malformations, whereas KLA is classified as a subtype of GLA.[1]
Patients with these diseases may suffer from serious complications, including pleural effusion (chylothorax) and bony lesion. Pleural effusion associated with LAs causes cough, wheeze, dyspnea, and decreasing exercise tolerance; it is regarded as a poor prognostic factor because of its high associated mortality and morbidity rates.[1-3] Hemorrhagic pericardial and pleural effusions are more frequent in KLA than GLA; therefore, patients with KLA demonstrate significantly poorer outcomes than those with GLA.[1] Meanwhile, locally aggressive lytic lesions with a loss of cortical bones are more frequent in patients with GSD, whereas systemic lytic lesions, sparing the cortical bones, are more frequent in those with GLA.[4,5] Bones affected by GSD are prone to pathological fractures, which can seriously impair patients’ quality of life. However, GSD had a significantly more favorable outcome than combined GLA and KLA.[1] Therefore, it is essential to distinguish subtypes in LAs for predicting prognosis and clinical outcomes.
To date, several original articles[1,3,5,6] and case reports[7-10] have reported focal splenic lesions associated with LAs. Meanwhile, ascites associated with LAs has been reported in a relatively fewer original articles[1,2] and case reports.[7,10] However, to the best of our knowledge, no study has presented a detailed investigation comparing the frequency of focal splenic lesions and ascites among GLA, KLA, and GSD or evaluated the number and size of focal splenic lesions among them. Although the diagnosis of GLA, KLA, or GSD is difficult because of overlapping disease entities, the investigation of abdominal imaging findings in this study may be useful for an accurate diagnosis or a prediction of disease state. Therefore, the present study aimed to evaluate the CT and MRI findings of focal splenic lesions and ascites in patients with GLA, KLA, or GSD.
MATERIAL AND METHODS
Patients
The institutional review board of our hospital approved this retrospective study and waived informed consent. The electronic medical chart system of our hospital was reviewed, and 23 patients with GSD, GLA, or KLA (13 men and ten women; mean age, 20 years; age range, 5–46 years) who had undergone abdominal CT and/or MRI before treatment were identified between October 2004 and October 2020. Among them, vascular malformations associated with other anomalies, such as Klippel-Trenaunay, Parkes Weber, Servelle-Martorell, and Sturge-Weber syndrome, were not observed. Both CT and MRI were performed in eight patients, only CT was performed in 14 patients, only MRI was performed in the remaining one patient.
All the patients underwent histopathological examinations for the diagnosis of LAs using biopsy or surgically resected specimens obtained from the various sites; however, we could not obtain the pathological specimens of the focal splenic lesions in any patient. They were subsequently diagnosed with GSD, GLA, or KLA based on the following clinical, pathological, or radiological diagnostic criteria. The imaging criteria for GSD were defined as progressive osteolysis with cortical bone resorption, whereas those for GLA were discrete radiolucencies and an increasing number of affected bones over time without progressive osteolysis.[5,11] Histologically, although dilated, malformed lymphatic channels lined by a monolayer of endothelial cells are common to both GLA and KLA, the latter is also characterized by foci of patternless clusters of intra- or perilymphatic spindled cells associated with platelet microthrombi, extravasated red blood cells, hemosiderin, and some degree of fibrosis.[11,12] GLA was usually distinguished from KLA on the basis of pathological specimens; however, the clinical presentations of bleeding symptoms, thrombocytopenia, and disseminated intravascular coagulation associated with KLA were also considered in the differential diagnosis between the two pathologies.
Among the 23 study patients, ten were diagnosed with GLA (three men and seven women; mean age, 22 years; age range, 7–33 years), five were diagnosed with KLA (4 men and 1 woman; mean age, 11 years; age range, 5–18 years), and eight were diagnosed with GSD (6 men and 2 women; mean age, 22 years; age range, 7–46 years).
CT protocols
Twenty-two patients (9 patients with GLA, 5 with KLA, and 8 with GSD) were examined using multidetector-row CT. A 16-slice CT scanner (LightSpeed 16; GE Healthcare, Milwaukee, WI, USA) was used for three patients, a 64-slice CT scanner (Brilliance CT 64; Philips Medical Systems, Best, The Netherlands) for 9, and 64-detector CT scanner (Discovery CT 750HD; GE Healthcare, Milwaukee, WI, USA) for the remaining 10. Axial CT images were reconstructed at 5-mm section thickness and no overlap. Unenhanced CT images were obtained in all 22 patients. In ten patients (3 patients with GLA, 4 with KLA, and 3 with GSD), contrast-enhanced CT images were obtained 70–90 s after the intravenous injection of 2 mL/kg of nonionic iodine contrast material containing 300 mg iodine/mL at an injection rate of 1.5–3 mL/s.
MRI protocols
MRI was performed in nine patients (5 patients with GLA, 3 with KLA, and 1 with GSD) using a 1.5-T MRI system (Intera Achieva 1.5 T Pulsar, Philips Medical Systems, Best, The Netherlands). Axial T1-weighted gradient-echo (TR/ TE, 220–248/2.3 ms for opposed-phase and 220–248/4.6 ms for in-phase), respiratory-triggered fat-suppressed T2-weighted fast spin-echo (TR/TE, 1,600–4,869/80–90 ms), and T2-weighted single-shot fast spin-echo (TR/TE, 11,647– 12,773/150 ms) images were obtained at 5-mm section thickness and 1-mm intersection gap. In 4 patients (2 patients with GLA and 2 with KLA), axial gadolinium-enhanced breath-hold fat-suppressed three-dimensional T1-weighted gradient-echo (TR/TE, 4.7/2.3 ms; section thickness, 4 mm; overlap, 2 mm) were obtained 30, 60, and 180 s after the intravenous injection of 0.1 mmol/kg of gadolinium-based contrast agents at an injection rate of 1.5–3 mL/s.
Image interpretation
A radiologist with 20 years of post-training experience who was blinded to the patient information reviewed all the CT and MRI images and qualitatively evaluated them for the presence of focal splenic lesions, ascites, peritoneal lesions, hepatic lesions, pancreas lesions, and renal lesions. Peritoneal lesions were defined as peritoneal thickening or peritoneal nodular lesions.
If focal splenic lesions existed in patients who underwent CT, their number, size, shape, margin, CT attenuation, and contrast enhancement were assessed. If focal splenic lesions existed in patients who underwent MRI, their shape, margin, signal intensity, and contrast enhancement were also assessed.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 (SPSS, Inc, an IBM Company, Chicago, Illinois, USA) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan). The Fisher exact test was performed to compare the frequencies of focal splenic lesions, ascites, and peritoneal lesions among GLA, KLA, and GSD. When the two-sided P < 0.05 of the test for the three groups was observed, we concluded that there was a difference in the frequency between the groups. Post hoc pairwise comparisons among the groups were performed only if the Fisher exact test for three groups was statistically significant. P values were corrected according to the Bonferroni method for pairwise comparisons. The Welch test and Tukey/Games-Howell post hoc test were used to compare the maximum diameters of focal splenic lesions among GLA, KLA, and GSD.
RESULTS
CT and MRI findings of GLA, KLA, and GSD are summarized in Tables 1-3. Focal splenic lesions were observed in nine patients, namely, 5 (50%) patients with GLA, 3 (60%) with KLA, and 1 (13%) with GSD. The frequency of focal splenic lesions tended to be higher in patients with GLA or KLA than in patients with GSD; however, no significant differences were found between the three groups (P = 0.190).
Table 1:
CT and MRI findings of GLA, KLA, and GSD.
| Patient number/age/sex | Diagnosis | Exam | Number of FSLs on CT | MD of FPLs on CT | Ascites | |
|---|---|---|---|---|---|---|
| Mean (mm) | Range (mm) | |||||
| 1/7/M | GLA | uCT/uMR | 10 | 5 | 2−18 | − |
| 2/12/M | GLA | uCT | 0 | NA | NA | − |
| 3/13/F | GLA | uCT/eMR | 0 | NA | NA | − |
| 4/15/F | GLA | uCT/uMR | 16 | 19 | 7−39 | − |
| 5/25/F | GLA | eCT | 189 | 8 | 3−25 | − |
| 6/25/F | GLA | eCT | 0 | NA | NA | − |
| 7/27/F | GLA | uCT/eMR | 61 | 9 | 4−22 | − |
| 8/31/F | GLA | uCT | 0 | NA | NA | − |
| 9/32/M | GLA | uMR | NA | NA | NA | − |
| 10/33/F | GLA | eCT | 0 | NA | NA | + |
| 11/5/F | KLA | eCT/eMR | 13 | 3 | 2−4 | + |
| 12/7/M | KLA | uCT/uMR | 0 | NA | NA | − |
| 13/8/M | KLA | eCT | 21 | 3 | 2−5 | + |
| 14/15/M | KLA | eCT | 0 | NA | NA | + |
| 15/18/M | KLA | eCT/eMR | 2 | 4 | 4 | + |
| 16/7/M | GSD | eCT | 20 | 4 | 2−6 | − |
| 17/8/M | GSD | eCT | 0 | NA | NA | − |
| 18/19/M | GSD | uCT | 0 | NA | NA | − |
| 19/19/F | GSD | uCT/uMR | 0 | NA | NA | − |
| 20/19/F | GSD | eCT | 0 | NA | NA | − |
| 21/21/M | GSD | uCT | 0 | NA | NA | − |
| 22/39/M | GSD | uCT | 0 | NA | NA | − |
| 23/46/M | GSD | uCT | 0 | NA | NA | − |
GLA: Generalized lymphatic anomaly, KLA: Kaposiform lymphangiomatosis, GSD: Gorham-Stout disease, FSL: Focal splenic lesion, MD: maximum diameter, uCT: Unenhanced CT, eCT: Enhanced CT, uMR: Unenhanced MRI, eMR: Enhanced MRI, NA: Not available
Table 3:
Quantitative CT findings of GLA, KLA, and GSD with focal splenic lesions.
| GLA (n=4) |
KLA (n=3) |
GSD (n=1) |
|
|---|---|---|---|
| Number | |||
| Mean | 69 | 12 | 20 |
| Median | 39 | 13 | 20 |
| Range | 10–189 | 2–21 | 20 |
| Maximum diameter | |||
| Mean | 9 | 3 | 3 |
| Median | 8 | 3 | |
| Range | 2–39 | 2–5 | 2–6 |
GLA: Generalized lymphatic anomaly, KLA: Kaposiform lymphangiomatosis, GSD: Gorham-Stout disease
Table 2:
Qualitative CT and MRI findings of GLA, KLA, and GSD.
| GLA (n= 10) |
KLA (n= 5) |
GSD (n= 8) |
P value | |
|---|---|---|---|---|
| Focal splenic lesions | 5 (50%) | 3 (60%) | 1 (13%) | 0.190 |
| Ascites | 1 (10%) | 4 (80%) | 0 (0%) | 0.003* |
| Peritoneal lesions | 1 (10%) | 1 (20%) | 0 (0%) | 0.684 |
| Hepatic lesions | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Pancreas lesions | 0 (0%) | 0 (0%) | 0 (0%) | NA |
| Renal lesions | 0 (0%) | 0 (0%) | 0 (0%) | NA |
GLA: Generalized lymphatic anomaly, KLA: Kaposiform lymphangiomatosis, GSD: Gorham-Stout disease, NA: Not available. *The frequency in KLA is higher than in GLA or GSD (P< 0.05).
On CT images, a total of 332 focal splenic lesions were observed in eight patients who underwent CT (4 GLA, 3 KLA, and 1 GSD). All the eight patients had multiple focal splenic lesions, and the number of focal splenic lesions per patient ranged from 2 to 189 (mean, 42 lesions; median, 18 lesions). The number of focal splenic lesions per patient ranged from 10 to 189 in patients with GLA (mean, 69 lesions; median, 39 lesions), whereas this number ranged from 2 to 21 in patients with KLA (mean, 12 lesions; median, 13 lesions). Twenty splenic lesions were identified in the single patient with GSD. More than 30 focal splenic lesions per patient were observed in two patients with GLA, but such findings were not observed in any patient with KLA or GSD.
On CT images, the maximum diameter of the 332 focal splenic lesions ranged from 2 to 39 mm (mean, 8 mm; median, 7 mm); of note, it was significantly greater in patients with GLA (mean, 9 mm; median, 8 mm, range, 2–39 mm) than in patients with KLA (mean, 3 mm; median, 3 mm, range, 2–5 mm) or GSD (mean, 3 mm; median, 3 mm, range, 2–6 mm) (P = 0.000) [Figures 1-3]. Focal splenic lesions with maximum diameters of ≥10 mm were observed in 4 patients with GLA but not in any patient with KLA or GSD.
Figure 1:
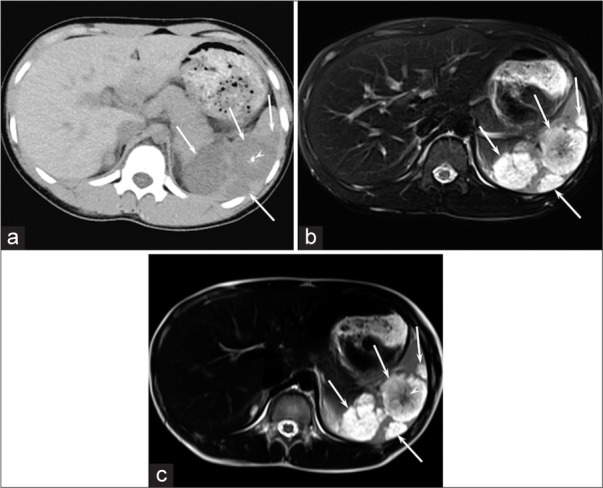
A 15-year-old girl with GLA (Patient 3). (a) Unenhanced CT image shows hypodense focal splenic lesions larger than 10 mm (arrows) with central calcification (arrowhead). (b and c) Respiratory-triggered fat-suppressed T2-weighted fast spin-echo (TR/TE, 1,600/80 ms) (b) and T2-weighted single-shot fast spin-echo (TR/TE, 12,146/150 ms) (c) images show hyperintense focal splenic lesions larger than 10 mm (arrows) with central hypointense areas (arrow heads).
Figure 3:
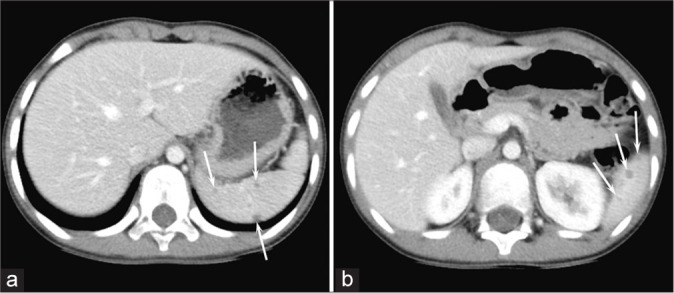
A 7-year-old boy with GSD (Patient 14). (a and b) Enhanced CT images show hypodense focal splenic lesions smaller than 10 mm (arrows).
Most of the focal splenic lesions included in the present study exhibited spherical or elliptic shapes, well-demarcated margins, hypodensity on CT images, hypointensity on T1-weighted images, and hyperintensity on T2-weighted images. On T2-weighted images, a majority of focal splenic lesions exhibited marked hyperintensity similar to cerebrospinal fluid, whereas some focal splenic lesions exhibited mild hyperintensity. However, a focal splenic lesion with the maximum diameter of 36 mm in a patient with GLA (Patient 3) had central calcification on CT images and central hypointense areas on T2-weighted images [Figure 1]. Hyperintense areas on T1-weighted images were observed only in two patients with GLA (Patient 3 and 6). Among six patients with focal splenic lesions who underwent contrast-enhanced CT and/or MRI, contrast enhancement of large focal splenic lesions was not observed, while that of small focal splenic lesions, especially those below the section thickness, could not be accurately evaluated.
Ascites was observed in five patients, namely, 1 (10%) patient with GLA, 4 (80%) with KLA, and none (0%) with GSD, which suggests that significant differences in ascites were observed among GLA, KLA, and GSD (P < 0.01) [Figure 2]. Ascites was significantly more frequent in patients with KLA than in patients with GSD (P = 0.021); however, no significant differences in ascites were found between GLA and KLA (P = 0.051) and between GLA and GSD (P > 0.99).
Figure 2:
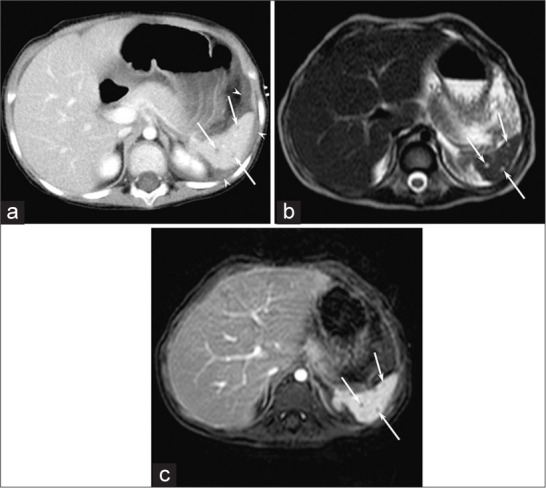
A 5-year-old girl with KLA (Patient 10). (a) Enhanced CT image shows hypodense focal splenic lesions smaller than 10 mm (arrows) with small amounts of ascites around the spleen (arrowhead). (b) T2-weighted single-shot fast spin-echo (TR/TE, 12,338/150 ms) image shows hyperintense focal splenic lesions smaller than 10 mm (arrows). (c) Gadolinium-enhanced breath-hold fat-suppressed three-dimensional T1-weighted gradient-echo (TR/TE, 4.7/2.3 ms) image shows unenhanced focal splenic lesions smaller than 10 mm (arrows).
Peritoneal lesions were observed in two patients, namely, 1 (10%) patient with GLA, 1 (20%) with KLA, and none (0%) with GSD, which suggests that no significant differences were observed in peritoneal lesions among GLA, KLA, and GSD. Hepatic lesions, pancreas lesions, and renal lesions were not observed among GLA, KLA, and GSD.
DISCUSSION
Focal splenic lesions associated with LAs have been described in previous research. Lala et al. identified splenic or hepatic cysts in patients with GLA (19/32, 63%) and with GSD (4/19, 21%).[5] Croteau et al. reported splenic involvement in patients with KLA (10/20, 50%), which was mainly an incidental finding on imaging elucidated typically with multifocal hypoechoic cystic lesions.[3] Kotecha et al. reported that patients with GSD (6/8, 75%) had splenic involvement, exhibiting multiple, hypodense, rounded lesions on CT; rounded lesions with hypointensity on T1-weighted images; hyperintensity on proton density-weighted images; and partial rim enhancement on contrast-enhanced MRI images.[6] Several case reports have reported multiple splenic cysts or nodules in patients with GLA,[8] KLA,[7,9] or GSD.[10] However, the number and size of the focal splenic lesions were mainly not revealed in these articles. In addition, no study has been reported the histopathological findings of the focal splenic lesions.
In the present study, focal splenic lesions were observed in patients with GLA, KLA, or GSD. The frequency of focal splenic lesions tended to be higher in patients with GLA (5/10, 50%) or KLA (3/5, 60%) than in patients with GSD (1/8, 13%); however, no significant differences were found between the three groups. These findings were consistent with those of previous reports by Lala et al.[5] and Croteau et al.[3] In contrast, Kotecha et al.[6] reported that patients with GSD frequently (6/8, 75%) had focal splenic lesions; however, patients with GLA or KLA may have been included in the study population because the distinct diagnostic criteria of GSD were not considered. Further investigation is required to completely understand the frequency of focal splenic lesions.
In the present study, more than 30 focal splenic lesions per patient and/or focal splenic lesions with maximum diameters of ≥10 mm were observed only in patients with GLA. To the best of our knowledge, this is the first study to evaluate the number and size of focal splenic lesions associated with LAs. Similar to the findings of the present study, patients with GLA[8] presented with relatively larger focal splenic lesions, whereas patients with KLA[7,9] or GSD[10] presented with relatively smaller focal splenic lesions on CT or MRI images, as reported in several case reports.
In the present study, a majority of the focal splenic lesions exhibited marked hyperintensity similar to cerebrospinal fluid on T2-weighted images, and contrast enhancement was not observed in large focal splenic lesions. Focal splenic lesions associated with LAs have been previously reported as “splenic cysts.”[3,5,8,10] Similarly, although we wanted to label the lesions as “splenic cysts,” we used the term “focal splenic lesions” because contrast-enhanced imaging was not performed in all the patients in the present study. In addition, contrast enhancement of small focal splenic lesions, especially those below the section thickness, could not be accurately evaluated because of the partial volume effect.
In general, hyperintense areas on T1-weighted images are not rare in lymphatic malformations, but they were observed only in two patients with GLA in this study. On T1-weighted images, hyperintense areas of multiple focal splenic lesions in a patient with GLA (Patient 6) may be caused by hemorrhage. Central areas of focal splenic lesion with the maximum diameter of 36 mm in a patient with GLA (Patient 3) showed hyperintensity on T1-weighted images and hypointensity on T2-weighted images; thus, the splenic lesion did not appear as a cystic lesion. However, because the surrounding splenic lesions seemed to be cystic, the atypical lesion with calcification would be caused by similar mechanism. Possible mechanism for central hypointense areas on T2-weighted images may be thought to be microcystic and honeycomb structure within multilocular lesion. In addition, hemorrhage or infection might occur within the cyst because the frequent cause of cyst wall calcification is hemorrhage or infection.
In the present study, ascites was significantly more frequent in patients with KLA (4/5, 80%) than in patients with GSD (0/8, 0%) (P < 0.05). Goyal et al.[2] reported that ascites was observed in patients with KLA (4/20, 20%) at presentation; however, during the course of the disease, ascites developed in more patients (9/20, 45%). Similar to ascites, pleural effusion has previously been identified in 50% (16/32)[5] and 25% (1/4)[13] patients with GLA and 85% (17/20)[2] and 67% (2/3)[13] patients with KLA. The high frequency of ascites and pleural effusion in patients with KLA might lead to advanced disease and poor prognosis. Although the cause of ascites is still unclear, it can be estimated that leakage of lymphatic fluid due to abnormal lymphatic tissue or peritoneal involvement of LAs may cause ascites.
The differential diagnosis of multiple lesions of the spleen includes abscess, sarcoidosis, lymphangioma, hemangioma, hamartoma, lymphoma, and metastasis [Table 4].[14] Among them, abscess and lymphangioma may predominantly appear as cystic lesions. Focal splenic lesions associated with LAs tended to be multiple and small; thus, they may be similar to microabscesses on CT and MRI images. However, clinical manifestations are important, because splenic microabscesses are frequent in HIV-infected patients with prolonged fever.
Table 4:
Differential diagnosis of benign and malignant focal splenic lesions.
| Non-neoplasms | Neoplasms |
|---|---|
| Trauma | Benign |
| Infection/Inflammation | Hamartoma |
| Pyogenic abscess | Hemangioma |
| Fungal abscess | SANT |
| Tuberculosis | Littoral cell angioma |
| Parasitic cyst | IMT |
| Sarcoidosis | Malignant |
| Inflammatory pseudotumor | Metastasis |
| Vascular | Lymphoma |
| Infarction | Angiosarcoma |
| Hematoma | Hemangioendothelioma |
| Lymphatic malformation | |
| Arteriovenous malformation | |
| Hematologic disorders | |
| Extramedullary hematopoiesis | |
| Others | |
| Epidermoid cyst | |
| Pseudocyst | |
| Gamna-Gandy body | |
| SANT: Sclerosing angiomatoid nodular transformation, IMT: Inflammatory myofibroblastic tumor | |
The present study has several limitations that should be highlighted. First, the present study enrolled relatively small cohort, because the investigation was conducted at a single institution and the introduced LAs are extremely rare. Second, the contrast-enhanced CT and MRI findings were not available for all the patients because of the retrospective nature of this study. Third, although some patients underwent diffusion-weighted imaging, the signal intensities on diffusion-weighted images and apparent diffusion coefficient values could not be evaluated accurately due to the small size of the focal splenic lesions and low spatial resolution of diffusion-weighted imaging. Therefore, we excluded the assessment of diffusion-weighted images from this study. Finally, the radiological–pathological correlation could not be established because the pathological specimens of the focal splenic lesions could not be obtained.
CONCLUSION
Focal splenic lesions tended to be frequent in patients with GLA or KLA than in patients with GSD. Although focal splenic lesions tended to be multiple and small, more than 30 focal splenic lesions per patient and/or focal splenic lesions with maximum diameters of ≥10 mm were observed only in patients with GLA. Ascites tended to be frequent in patients with KLA than in patients with GLA or GSD. These differences in imaging findings may contribute to the clinical differential diagnosis among GLA, KLA, and GSD.
Footnotes
How to cite this article: Nakamura F, Kato H, Ozeki M, Matsuo M. CT and MRI findings of focal splenic lesions and ascites in generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham-Stout disease. J Clin Imaging Sci 2021;11:44.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This work was supported by Health Labour Sciences Research Grant, Research on Measures for Intractable Diseases (20FC1031).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and gorham-stout disease. Pediatr Blood Cancer. 2016;63:832–8. doi: 10.1002/pbc.25914. [DOI] [PubMed] [Google Scholar]
- 2.Goyal P, Alomari AI, Kozakewich HP, Trenor CC, 3rd, Perez-Atayde AR, Fishman SJ, et al. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol. 2016;46:1282–90. doi: 10.1007/s00247-016-3611-1. [DOI] [PubMed] [Google Scholar]
- 3.Croteau SE, Kozakewich HP, Perez-Atayde AR, Fishman SJ, Alomari AI, Chaudry G, et al. Kaposiform lymphangiomatosis: A distinct aggressive lymphatic anomaly. J Pediatr. 2014;164:383–8. doi: 10.1016/j.jpeds.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger MT, Garg N, Olsen BR. Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone. 2014;63:47–52. doi: 10.1016/j.bone.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly-clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42:917–24. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham's disease. Clin Radiol. 2012;67:782–8. doi: 10.1016/j.crad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes VM, Fargo JH, Saini S, Guerrera MF, Marcus L, Luchtman-Jones L, et al. Kaposiform lymphangiomatosis: Unifying features of a heterogeneous disorder. Pediatr Blood Cancer. 2015;62:901–4. doi: 10.1002/pbc.25278. [DOI] [PubMed] [Google Scholar]
- 8.Joshi M, Phansalkar DS. Simple lymphangioma to generalized lymphatic anomaly: Role of imaging in disclosure of a rare and morbid disease. Case Rep Radiol. 2015;2015:603859. doi: 10.1155/2015/603859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radzikowska E, Blasinska-Przerwa K, Szolkowska M, Miasko A, Kupis W, Wiatr E. Kaposiform lymphangiomatosis with human papillomavirus infection. Am J Respir Crit Care Med. 2017;195:e47–8. doi: 10.1164/rccm.201612-2421IM. [DOI] [PubMed] [Google Scholar]
- 10.Bargagli E, Piccioli C, Cavigli E, Scola M, Rosi E, Lavorini F, et al. Gorham-stout disease management during pregnancy. AJP Rep. 2017;7:e226–9. doi: 10.1055/s-0037-1615259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, Ozeki M, Fukao T, Matsuo M. MR imaging findings of vertebral involvement in Gorham-Stout disease, generalized lymphatic anomaly, and kaposiform lymphangiomatosis. Jpn J Radiol. 2017;35:606–12. doi: 10.1007/s11604-017-0674-3. [DOI] [PubMed] [Google Scholar]
- 12.Safi F, Gupta A, Adams D, Anandan V, McCormack FX, Assaly R. Kaposiform lymphangiomatosis, a newly characterized vascular anomaly presenting with hemoptysis in an adult woman. Ann Am Thorac Soc. 2014;11:92–5. doi: 10.1513/AnnalsATS.201308-287BC. [DOI] [PubMed] [Google Scholar]
- 13.Kato H, Ozeki M, Fukao T, Matsuo M. Chest imaging in generalized lymphatic anomaly and kaposiform lymphangiomatosis. Pediatr Int. 2018;60:667–8. doi: 10.1111/ped.13593. [DOI] [PubMed] [Google Scholar]
- 14.Kamaya A, Weinstein S, Desser TS. Multiple lesions of the spleen: Differential diagnosis of cystic and solid lesions. Semin Ultrasound CT MR. 2006;27:389–403. doi: 10.1053/j.sult.2006.06.004. [DOI] [PubMed] [Google Scholar]



