Abstract
Background:
The objective of this paper was to describe the arterial supply of the uncus and quantify the branches directed to the anteromedial aspect of the human temporal cortex.
Methods:
We studied 150 human cerebral hemispheres identifying main afferent arteries supplying the anteromedial temporal cortex with particular attention to the uncus, determining the territory supplied by each artery through either cortical or perforating branches.
Results:
The uncus was supplied by 419 branches of the anterior choroidal artery (AChA), 210 branches of the internal carotid artery (ICA), 353 branches of the middle cerebral artery (MCA), and 122 branches of the posterior cerebral artery (PCA). The total of supplying vessels was 1104 among the 150 hemispheres studied, which corresponds to 7.36 arteries per uncus. The average of branches per hemisphere was as follows: 2.79 from AChA, 1.40 from ICA, 2.35 from MCA, and 0.81 from PCA. The relative contribution of each artery for the total of specimens studied was as follows: 38% from AChA, 19% from ICA, 32% from the MCA, and 11% from the PCA. We identified cortical anastomoses mostly between the MCA and PCA (27 cases).
Conclusion:
We described and quantified the uncus’ vascularization, including anatomical variations. This updated, detailed description of the mesial temporal vascularization is paramount to improve the treatment of neurosurgical conditions.
Keywords: Neuroanatomy, Temporal lobe, Uncus, Vascularization

INTRODUCTION
Neurosurgical lesions of the temporal lobe present numerous challenges in terms of approach, technique, and postoperative outcomes. Tumor, epilepsy, aneurysms, and arteriovenous malformations (AVMs) are some of the diseases presenting in this area. Particularly, surgeries for AVMs of the mesial temporal lobe often lead to permanent deficits.[1]
The uncus is the most medial and anterior portion of the parahippocampal gyrus. Its shape and positioning within the cranial vault gave rise to its name, which means “hook” in Latin. Functionally, the uncus is part of the limbic and olfactory cortices, corresponding to Brodmann’s area number 34. It is bound laterally by the rinal sulcus, representing the anteromedial surface of what Paul Broca named in 1878 as “the great limbic lobe.” Immediately inferior to the uncus lies the amygdaloid complex.[19,20] There are few papers that describe in great details the vascularization of the medial aspect of the human temporal lobe.[2,4,7,10,12,19]
The objective of this paper was to describe the arterial supply of the uncus, quantify the branches directed to the anteromedial aspect of the human temporal cortex, and relate the findings to neurosurgical pathologies. We sought to define the vascular pedicles from the contributing arteries (anterior choroidal artery [AChA], internal carotid artery [ICA], middle cerebral artery [MCA], and posterior cerebral artery [PCA]) and their anastomosis patterns, as well as the circulatory type of the arterial branches – either central (perforator) or cortical (peripherical).
MATERIALS AND METHODS
This study was conducted in the Neuroanatomy Laboratory of the Morphological Sciences Department of the Universidade Federal do Rio Grande do Sul. All anatomical specimens belong to the abovementioned laboratory and have been previously used for neuroanatomical studies of other cortical territories [Figure 1].
Figure 1:
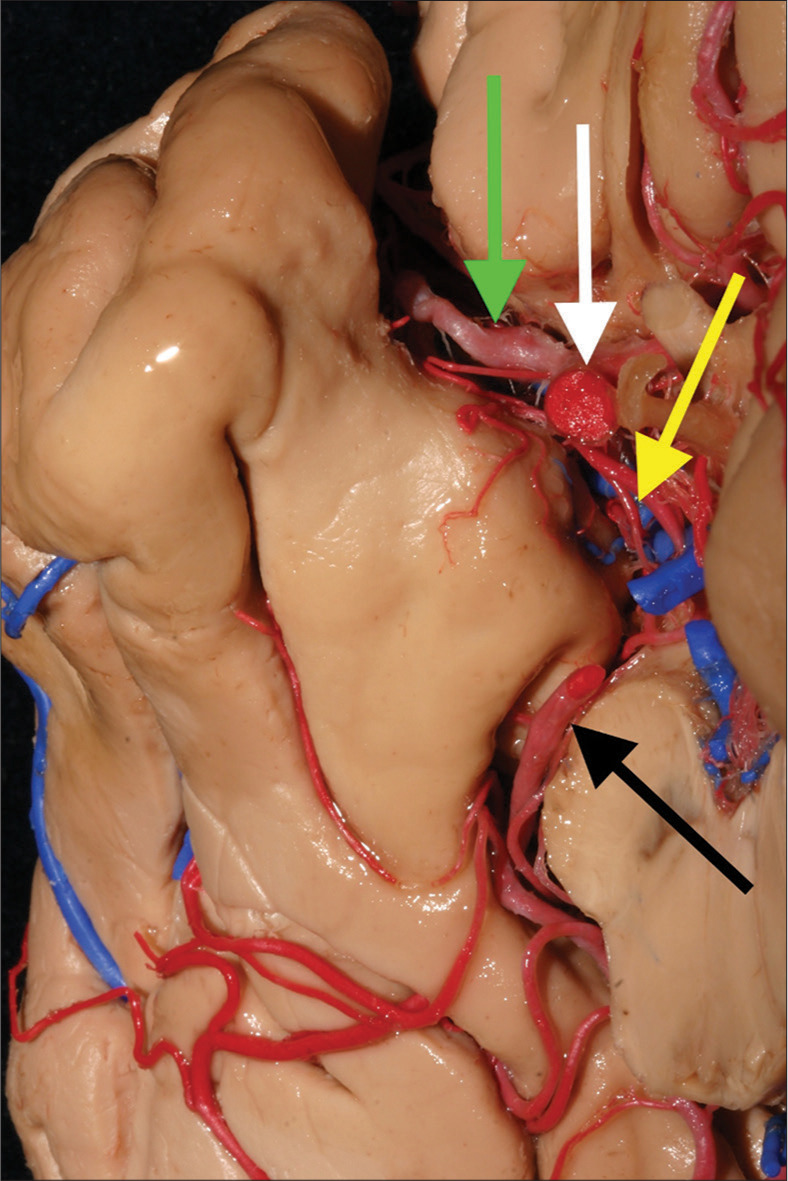
The uncus and its major supplying arteries. The uncus is the most medial an anterior part of the parahippocampal gyrus. It harbors many difficult mesial temporal lobe pathologies, such as arteriovenous malformations and tumors. The internal carotid, anterior choroidal, middle cerebral, and posterior cerebral arteries are the main trunks that provide blood supply to the uncus. In this medial view, we can identify these arteries and their syntopy. White arrow: Internal carotid artery; yellow arrow: Anterior choroidal artery; green arrow: Middle cerebral artery; black arrow: Posterior cerebral artery.
We studied 150 human cerebral hemispheres – males and females, 75 right and 75 left sided from cadavers whose deaths were not related to central nervous system lesions. After injection with colored jelly, latex, or Batson solution, we performed a complete microdissection of the uncus and parahippocampal gyrus. We identified main afferent arteries supplying the anteromedial temporal cortex with particular attention to the uncus, determining the territory supplied by each artery through either cortical or perforating branches. The uncus’ cortex was defined as the cortical portion of the amygdaloid complex.
The techniques described by Rodrigues (1998)[15] were employed to prepare the specimens, according to the following steps:
The encephalon was removed from the cranial vault conserving the arachnoid membranes. The ICA was sectioned next to the anterior clinoid process, the vertebral arteries, and the brainstem at the foramen magnum.
The ICAs and vertebral arteries were catheterized and the whole vascular system was washed with regular saline at room temperature within the first 6 h after step one.
Next, colored latex was injected for dissection and macroscopic studies.
A median sagittal cut was employed to enable visualization of the medial cortex, including dissection and measurements. From this point on, we used the D.F. Vasconcellos Surgical Microscope (São Paulo, Brazil) with 12.5× to carry out the dissection.
We defined the territories of the ICA, AChA, MCA, and PCA with photographic documentation of the vascular anatomy. The vascularization pattern – either cortical or perforating – was defined through subarachnoid dissection of the branches from their origins in the parent arteries. For the perforators, the dissection proceeded until their penetration into the brain.
Statistical analyses
Statistical analyses were conducted through Chi-squared tests for categorical variables and Fisher’s exact test for continuous variables. P < 0.05 was considered statistically significant.
RESULTS
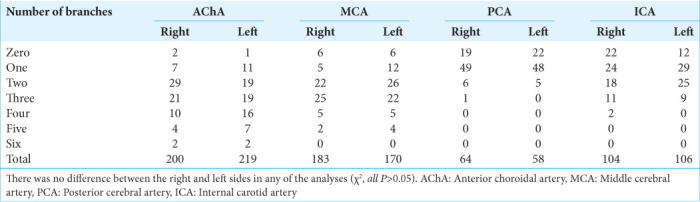
In the dissection of the 75 right-sided cerebral hemispheres, we identified 551 arterial supplying branches – 200 from the AChA, 104 from the ICA, 183 from the MCA, and 64 from the PCA. In the 75 left-sided hemispheres, we identified 553 arterial supplying branches – 219 from the AChA, 106 from the ICA, 170 from the MCA, and 58 from the PCA. The vessels originated from the PCA were dissected in the posteroanterior direction through the medial temporal cortex until they reached the uncus, where they anastomosed with branches derived from the other three main trunks. There was no statistical difference in the distribution of arterial vessels between the right and left sides (χ2, P = 0.68). The distribution of frequencies of branches arising from each of the four main vessels is detailed in [Table 1]. There was no statistical difference between the right and left sides regarding how many branches originated from neither AChA (χ2, P = 0.47), MCA (χ2, P = 0.63), PCA (χ2, P = 0.72), nor ICA (χ2, P = 0.31).
Table 1:
Number of branches from each artery.
Regarding the MCA, 174 branches originated directly from its trunk (84 in right-sided hemispheres and 90 in left-sided hemispheres). Conversely, they originated indirectly from the orbitofrontal artery in 99 right-sided hemispheres and 80 left-sided hemispheres. The prefrontal artery was excluded from the analysis because its territory of irrigation includes only the lateral aspect of the hemispheres. There was no difference on the origin of the branches between the right and left hemispheres (χ2, P = 0.21).
Regarding the PCA, 90 branches derived from the P1 segment (55 in right hemispheres and 35 in left-sided hemispheres), whereas they originated from the anterior temporal artery in nine right-sided hemispheres and 23 left-sided hemispheres. This difference was statistically significant (χ2, P < 0.01). Table 2 summarizes the specifics of MCA and PCA [Figure 2].
Table 2:
Origin of the branches (MCA and PCA).
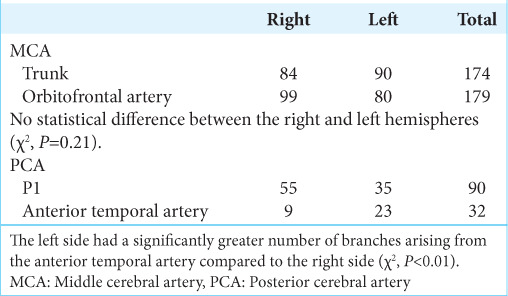
Figure 2:
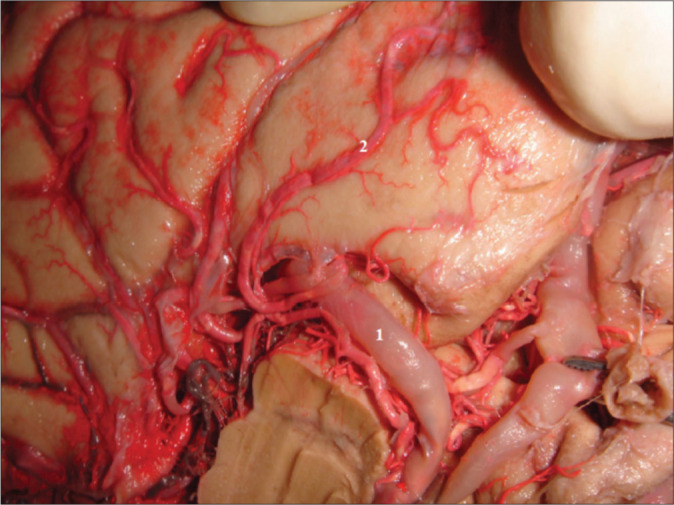
Cortical branch from the posterior cerebral artery (PCA) to the uncus. The PCA is a previously underrecognized supplier of the uncus. With an average 0.8 branches per hemisphere, it corresponded to 11% of all branches in our study. 1: PCA; 2: Cortical branch.
Most of the branches irrigating the uncus came from the AChA [Figure 3]. There were also some anatomical variations: this artery presented itself as a double artery in 4 cases (2.6%) [Figure 4]; it presented itself as a branch of posterior communicating artery (PComm) in 3 cases (2%) and as having a common origin with the PComm in 2 cases (1.3%).
Figure 3:
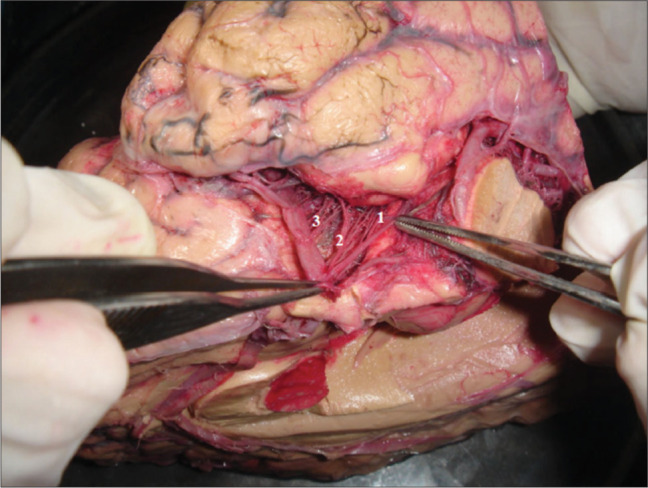
Branches from the anterior choroidal artery (AChA), internal carotid artery (ICA), and middle cerebral artery (MCA) to the uncus. The majority of the arterial inflow to the uncus comes from the anterior circulation. The AChA is the main supplier, with an average 2.8 branches per hemisphere, accounting for 38% of all branches that rich the uncus. 1: AChA branches; 2: ICA branches; 3: MCA branches.
Figure 4:
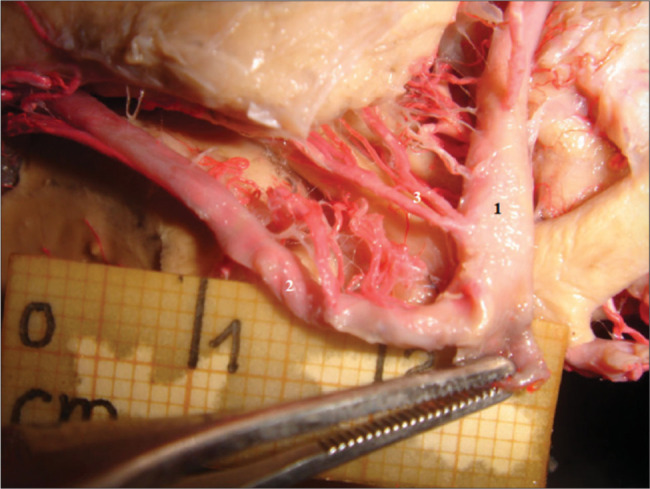
Duplicated anterior choroidal artery (AChA) in an embryonic circle of Willis. The AChA is the main source of arterial blood to the uncal region. A duplicated AChA is shown in a specimen presenting an embryonic circle of Willis. Recognition of such variations is key to perform safer surgeries in this region. 1: Middle cerebral artery; 2: Posterior cerebral artery; 3: Duplicated AChA.
Embryonic circle of Willis
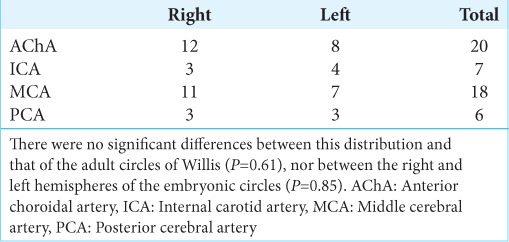
Throughout the study, we identified seven hemispheres in which the ACA, MCA, and PCA originated from the ICA – corresponding to 4.6% of our samples. In those cases, the right-sided unci received 12 branches from the AChA, 3 from the ICA, 11 from the MCA, and 3 from the PCA, adding to a total of 29 branches. The left-sided unci received eight branches from the AChA, four from the ICA, seven from the MCA, and three from the PCA. The distribution of vessels arising from the embryonic circles of Willis was not statistically different from typical adult circles (P = 0.61), and there was also no difference between the left- and right-sided hemispheres (P = 0.85). [Table 3] summarizes the information of the embryonic circles of Willis.
Table 3:
Origin of the branches in the embryonic circles of Willis.
Cortical anastomosis
We identified cortical anastomoses between the different vessels that supply the uncus. Most of them connected branches of the MCA and PCA (27 cases) followed by anastomoses of AChA and MCA branches (19 cases). To a lesser extent, we found AChA-MCA, AChA-ICA, and ICAPCA branches anastomosing in three cases each. Only one anastomosis was identified between ICA and MCA branches [Figure 5].
Figure 5:
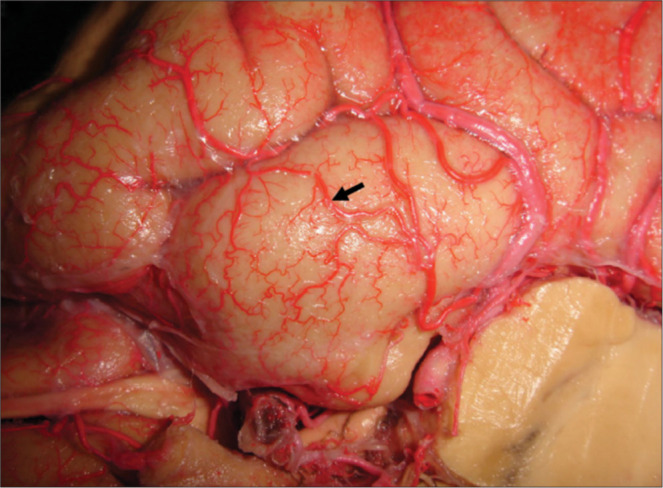
Cortical anastomosis between branches of the middle and posterior cerebral arteries. Cortical anastomoses play an important role in the arterial supply of the uncus. The solid black arrow points do an anastomosis between branches of the middle and posterior cerebral arteries, evidence of a mixed vascularization from both the anterior and posterior circulations. This network could be important in protecting the structures in the case of thrombotic or ischemic events.
General irrigation of the uncus
Since there was no statistical difference between the right and left hemispheres, we were able to compile the results from all 150 hemispheres to provide a broader qualitative and quantitative perspective of the vascularization of the uncus.
In this study, we found that the uncus was supplied by 419 branches of the AChA, 210 branches of the ICA, 353 branches of the MCA, and 122 branches of the PCA. The total of supplying vessels was 1104 among the 150 hemispheres studied, which corresponds to 7.36 arteries per uncus. Thus, the average of branches per hemisphere was as follows: 2.79 from AChA, 1.40 from ICA, 2.35 from MCA, and 0.81 from PCA. The relative contribution of each artery for the total of specimens studied was as follows: 38% from AChA, 19% from ICA, 32% from the MCA, and 11% from the PCA.
DISCUSSION
Overview
This study identified and quantified the supplying branches of the uncus, which arise from the AChA, ICA, MCA, and PCA [Figure 6]. Overall, there was a predominance of vessels derived from the AChA, There were similar percentual contributions from MCA and ICA branches, but we found a more significant contribution from the PCA compared to previously existing data.[2,4,7,19]
Figure 6:
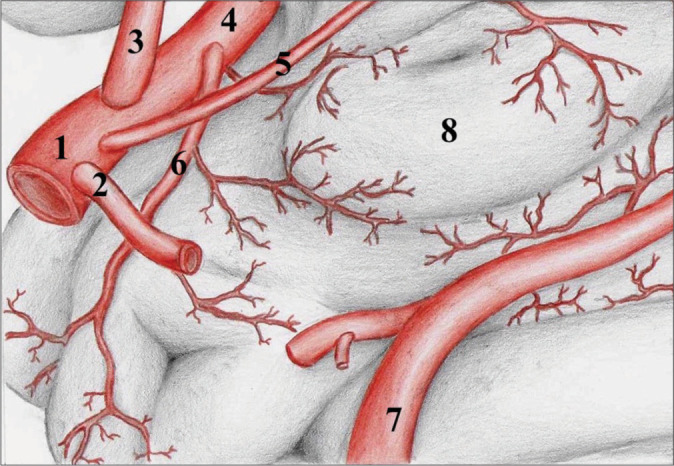
Schematic drawing of the major suppliers to the uncus. Schematic drawing summarizing the major uncal suppliers. 1: Internal carotid artery; 2 Posterior communicating artery (removed for better visualization); 3: Anterior cerebral artery; 4: Middle cerebral artery; 5: Anterior choroidal artery; 6: Temporal polar artery; 7: Posterior cerebral artery; 8: Uncus.
Branches of the AChA were closely related to the anteromedial aspect of the uncus. This artery gave rise to the largest number of irrigating branches, both perforating and superficial. Some high-quality studies evaluated the origin, anatomical variations, and vascular territories supplied by the AChA, but those refer mainly to branches directed to the basal ganglia and choroidal plexuses.[15] Those studies date back to the works of Cooper (1954)[3] who investigated the ligation of AChA for the treatment of Parkinson’s disease. Otomo (1965)[14] described the AChA in 778 hemispheres of consecutive autopsies, defining the origin of the choroidal arteries as 99.2% originating from the ICA, but his works did not describe the AChA’s branches to the uncus.
Our findings state that the AChA provided most of the branches that supply the uncus, with an average of 2.79 per hemisphere or 38% of all the branches that reach this particular anatomical structure. We also encountered two cases in which the AChA shared a common origin with the PComm (1.3%), three occasions in which AChA was a branch of PComm (2%), and four cases of a duplicated AChA (2.7%).
The MCA was the second largest contributor of branches to the uncus, with the ramifications arising from either the artery’s trunk itself or from the orbitofrontal artery.[1,5] Some authors state that uncus’ suppliers also arise from the prefrontal artery, but this is largely debatable. We did not find any such branches – all vessels arising from the prefrontal artery follow a path to the basal portion of the frontal lobe.[9]
Some of the blood supply to the uncus also came from the ICA – in our study, 1.4 per hemisphere or 19% of all uncus’ suppliers. Those were generally short, straight branches that reach the brain close to the ICA itself, having a very similar length and diameter among them. They mostly penetrate the parenchyma as perforating branches but may sometimes appear as cortical vessels.
We found a more substantial contribution from PCA branches than were previously described – 0.81 per hemisphere, representing 11% of all supplying branches. Previous works perhaps underreported those vessels because of their posteroanterior direction or because they often arise from proximal ramifications of the PCA (i.e., anterior temporal artery). Thus, the use of a modern surgical microscope together with contrast/coloring solution injection might have enabled us to identify those structures better. We also identified branches arising from the main trunk, the P2 segment, and the anterior and middle temporal arteries – those last two being derived from the lateral occipital artery, the first branch of the PCA.[9,11]
As the four arteries that supply the uncus are not terminal vessels, it was no surprise that we found anastomoses connecting their territories. They occur most frequently between branches of the MCA and PCA or AChA and PCA. To the best of our knowledge, such anastomoses have described in the literature only once.[8]
Practical relevance and applications
In early reports of surgical techniques for excision of uncal region AVMs, the MCA is placed as a “minor contributor.”[6,17] As stressed by Marinkovic et al., precise determination of the vascular pattern of the uncal and parahippocampal arteries is of uttermost importance to occlude the AVMs feeding arteries.[12] However, the same authors reported a much lower contribution of the MCA to this region, in terms of mean number of branches.[12] We have shown that, in fact, the MCA is the second largest supplier of this territory, with a much larger number of branches. With the vast anastomoses present in this region, it is possible that ignoring these MCA branches might play a role in the still very high rates of postoperative deficits, as reported in 2011 by Boström et al.[1]
Visual field deficits also arise from those procedures, either from direct lesion of optic pathways or vascular supply damage. The magnitude of those deficits varies widely in the literature, but reports go as high as 60% of patients with permanent visual deficits.[8,13,16,18] Therefore, a detailed understanding of this region’s vasculature cannot be overstated.
CONCLUSION
The four arteries that contribute to the arterial supply of the uncus are, from the most to the least frequent branches: AChA, MCA, ICA, and PCA. The MCA and PCA provide a more significant contribution than previously recognized. This updated, detailed description of the mesial temporal vascularization is paramount to improve the treatment of neurosurgical conditions.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
How to cite this article: Isolan GR, Marrone AC, Marrone LC, Stefani MA, da Costa JC, Telles JP, et al. Vascularization of the uncus – Anatomical study and clinical implications. Surg Neurol Int 2021;12:393.
Contributor Information
Gustavo Rassier Isolan, Email: gisolan@yahoo.com.br.
Antônio Carlos Huf Marrone, Email: achmarrone@gmail.com.
Luiz Carlos Porcellos Marrone, Email: lcpmarrone@gmail.com.
Marco Antonio Stefani, Email: mstefani@vortex.ufrgs.br.
Jaderson Costa da Costa, Email: jcc@pucrs.br.
Joao Paulo Mota Telles, Email: joao.telles@fm.usp.br.
Gil Goulart Choi, Email: gil.choi@fm.usp.br.
Saul Almeida da Silva, Email: alm.saul@gmail.com.
Nícollas Nunes Rabelo, Email: nicollasrabelo@hotmail.com.
Eberval Gadelha Figueiredo, Email: ebgadelha@yahoo.com.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Boström A, Schaller K, Seifert J, Schramm J. The place for surgical treatment for AVM involving the temporal lobe. Acta Neurochir (Wien) 2011;153:271–8. doi: 10.1007/s00701-010-0885-1. [DOI] [PubMed] [Google Scholar]
- 2.Caplan L, Babikian V, Helgason C, Hier DB, deWitt D, Patel D, et al. Occlusive disease of the middle cerebral artery. Neurology. 1985;35:975–82. doi: 10.1212/wnl.35.7.975. [DOI] [PubMed] [Google Scholar]
- 3.Cooper I. Surgical occlusion of the anterior choroidal artery in parkinsonism. Surg Gynecol Obstet. 1954;92:207–9. [PubMed] [Google Scholar]
- 4.Erdem A, Yaşargil MG, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993;79:256–65. doi: 10.3171/jns.1993.79.2.0256. [DOI] [PubMed] [Google Scholar]
- 5.Hale AR, Reed AF. Studies in cerbral circulation. Methods for the qualitative and quantitative study of human cerebral blood vessels. Am Heart J. 1963;66:226–42. doi: 10.1016/0002-8703(63)90039-5. [DOI] [PubMed] [Google Scholar]
- 6.Heros RC. Arteriovenous malformations of the medial temporal lobe. Surgical approach and neuroradiological characterization. J Neurosurg. 1982;56:44–52. doi: 10.3171/jns.1982.56.1.0044. [DOI] [PubMed] [Google Scholar]
- 7.Huther G, Dörfl J, van der Loos H, Jeanmonod D. Microanatomic and vascular aspects of the temporomesial region. Neurosurgery. 1998;43:1118–36. doi: 10.1097/00006123-199811000-00065. [DOI] [PubMed] [Google Scholar]
- 8.Kucukyuruk B, Richardson RM, Wen HT, Fernandez-Miranda JC, Rhoton AL. Microsurgical anatomy of the temporal lobe and its implications on temporal lobe epilepsy surgery. Epilepsy Res Treat. 2012;2012:769825. doi: 10.1155/2012/769825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazorthes G, Gouazé A, Salamon G. 1976. Vascularisation et Circulation de L’encéphale: Anatomie Descriptive et Fontionnelle. [Google Scholar]
- 10.Marinković S, Milisavljević M, Puškaš L. Microvascular anatomy of the hippocampal formation. Surg Neurol. 1992;37:339–49. doi: 10.1016/0090-3019(92)90001-4. [DOI] [PubMed] [Google Scholar]
- 11.Marinkovic SV, Kovacevic MS, Marinkovic JM. Perforating branches of the middle cerebral artery. Microsurgical anatomy of their extracerebral segments. J Neurosurg. 1985;63:266–71. doi: 10.3171/jns.1985.63.2.0266. [DOI] [PubMed] [Google Scholar]
- 12.Marinkovic SV, Milisavljevic MM, Vuckovic VD. Microvascular anatomy of the uncus and the parahippocampal gyrus. Neurosurgery. 1991;29:805–14. doi: 10.1097/00006123-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Morioka T, Matsukado K, Natori Y, Sasaki T. Retrospective analysis of the surgically treated temporal lobe arteriovenous malformations with focus on the visual field defects and epilepsy. Surg Neurol. 2006;66:50–5. doi: 10.1016/j.surneu.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Otomo E. The anterior choroidal artery. Arch Neurol. 1965;13:656–8. doi: 10.1001/archneur.1965.00470060092009. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues H. 2nd ed. Vitória: Arte Visual; 1998. Técnicas Anatômicas. [Google Scholar]
- 16.Schramm J, Aliashkevich AF. Surgery fortemporal mediobasal tumors. Neurosurgery. 2007;60:285–95. doi: 10.1227/01.NEU.0000249281.69384.D7. [DOI] [PubMed] [Google Scholar]
- 17.Stein BM. Arteriovenous malformations of the medial cerebral hemisphere and the limbic system. J Neurosurg. 1984;60:23–31. doi: 10.3171/jns.1984.60.1.0023. [DOI] [PubMed] [Google Scholar]
- 18.Uribe JS, Vale FL. Limited access inferior temporal gyrus approach to mesial basal temporal lobe tumors. J Neurosurg. 2009;110:137–46. doi: 10.3171/2008.4.17508. [DOI] [PubMed] [Google Scholar]
- 19.Wen HT, Rhoton AL, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe: Part 1: Mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45:549–92. doi: 10.1097/00006123-199909000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Yaşargil MG, Türe U, Yaşargil DCH. Impact of temporal lobe surgery. J Neurosurg. 2004;101:725–38. doi: 10.3171/jns.2004.101.5.0725. [DOI] [PubMed] [Google Scholar]


