Abstract
Background:
The purpose of this study was to evaluate the effectiveness of multiple hippocampal transections (MHT) in the treatment of drug-resistant mesial temporal lobe epilepsy.
Methods:
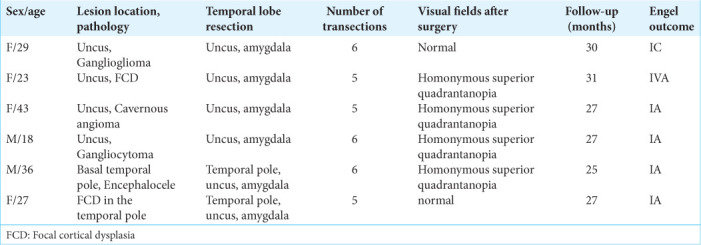
Six patients underwent MHT at Burdenko Neurosurgery Center in 2018. The age of the patients varied from 18 to 43 years. All patients suffered from refractory epilepsy caused by focal lesions of the mesial temporal complex or temporal pole in dominant side. Postoperative pathology revealed neuronal-glial tumors in two patients, focal cortical dysplasia (FCD) of the temporal pole – in two patients, cavernous angioma – in one patient, and encephalocele of the preuncal area – in one patient.
Results:
All patients underwent surgery satisfactorily. There were no postoperative complications except for homonymous superior quadrantanopia. This kind of visual field loss was noted in four cases out of six. During the follow-up period five patients out of six had Engel Class I outcome (83.3%). In one case, seizures developed after 1 month in a patient with FCD in the uncus (Engel IVA). After surgery, three out of six patients developed significant nominative aphasia. Two patients relative to the preoperative level demonstrated improvement in delayed verbal memory after MHT. Two patients showed a decrease level in delayed verbal memory. In preoperative period, visual memory was below the normal in one patient. Delayed visual memory in two cases impaired compared to the preoperative level.
Conclusion:
MHT can be considered as an effective method of drug-resistant mesial temporal lobe epilepsy caused by tumors of the medial temporal complex. At the same time, MHT makes it possible to preserve memory in patients with structurally preserved hippocampus. However, MHT do not guarantee the preservation of memory after surgery.
Keywords: Epilepsy surgery, Hippocampal transections, Memory, Multiple hippocampal transection, Neuronalglial tumor

INTRODUCTION
Resective surgery is an effective treatment for drug-resistant mesial temporal lobe epilepsy.[9,43] To achieve the treatment objective, two main methods are used: anteromedial temporal lobectomy and selective amygdalohippocampectomy. A necessary component of such interventions is the removal of the hippocampus along with the fimbria, parahippocampal gyrus, and amygdala.[8,9,25,31]
However, the consequences of resection of the hippocampus, where its function is still preserved, can be a decrease in verbal memory or visual-spatial memory, intelligence, emotional and speech performance, as well as cognitive disorders.[19,24] Even today, cognitive impairments that occur after hippocampectomy are sometimes neglected or are considered to be inevitable consequences of the surgical treatment.[2,13,37] In addition, the subventricular and subgranular zones of the hippocampus house progenitor cells that play an important role in the processes of neurogenesis throughout an individual’s life.[30]
To solve this problem, Shimizu et al.[36] in 2006 described the technique of the multiple hippocampal transection (MHT). The concept of MHT originated on the basis of multiple subpial transection in eloquent areas of the neocortex.[32] The principle of surgical treatment of medial temporal lobe epilepsy by multiple transverse transection of the hippocampus is the mechanical disruption of the longitudinal pathways of the hippocampus.
It is established that the hippocampus has two types of pathways: (1) trisynaptic pathways, which are located in parallel loops oriented orthogonally to the longitudinal axis of the hippocampus; and (2) longitudinal pathways that run along the long axis of the hippocampus.[3] Loops of trisynaptic pathways originating and ending in the entorhinal cortex are important for processing and stabilizing memory.[1] The longitudinal path of the hippocampus does not play an important physiological role; on the contrary, it facilitates the synchronization of pathological epileptic discharges and their propagation along the hippocampus and, further, to extrahippocampal structures, thus contributing to the development of a seizure.[41] For the pathological electrical activity in the hippocampal neurons to develop into an epileptic seizure, synchronization of the critical number of neurons – exceeding 5 mm thickness – located in the hippocampal segment is necessary. Therefore, if longitudinal horizontal interneuronal fibers along the axis of the hippocampus are separated with an intervals of 5 mm, then the pathological chain is broken and, as a result, an epileptic seizure does not occur.[40] Due to the fact that epileptic discharges in the hippocampus disrupt the normal function of the hippocampus, with the disappearance of seizures following transection, function of the hippocampus is restored with improved memory.[24]
This paper analyzes the results of six surgeries of patients with drug-resistant medial temporal lobe epilepsy.
MATERIALS AND METHODS
Patients population
From March 2018 to June 2018, six patients underwent MHT at Burdenko Neurosurgery Center [Table 1]. The age of the patients varied from 18 to 43 years (median 28 years). All patients suffered from refractory epilepsy caused by focal lesions of the mesial temporal complex or temporal pole. The duration of epilepsy before surgery varied from 2 to 24 years (median 11 years). All surgeries were performed by the senior author of this study.
Table 1:
Main indicators in the hippocampal transection group.
Patient’s selection
In this group, we included the patients who suffered from drug-resistant epilepsy caused by focal lesions of the mesial temporal complex. Although, no clear signs of changes in the architecture and volume of the hippocampus were found on MRI scans. In all cases, the lesion was localized in the dominant left hemisphere. In all patients, memory was intact or slightly reduced. Final decision to perform the hippocampal transection was made intraoperatively – if epileptiform activity was recorded on the hippocampus following the resection of the lesion of the temporal lobe, transection was performed.
The decision to perform a surgical treatment was made at an interdisciplinary committee of physicians with the participation of neurosurgeons, neurologists, neurophysiologists, neuropsychologists, and neuroradiologists.
Patient’s examination
In all cases, video EEG showed seizures initiating from the left temporal lobe. Stereo EEG was not performed in any case. MRI revealed the lesions in the left dominant hemisphere but significant structural abnormalities did not extend to the hippocampus. Intraoperative electrocorticography (ECoG) of the basal temporal lobe and hippocampus was performed. ECoG recordings were performed during the background recording and after each stage of surgery (resection of the lesion, resection of the uncus with amygdala, and transection of the hippocampus). The recordings were made on an electroencephalograph “NicoletOne” (V44, USA).
The median follow-up after surgery comprised 27 months (from 25 to 31 months). Seizure outcome was evaluated using Engel scale.[10]
Neuropsychological tests before surgery and 6 months after surgery were obtained in all patients. Neuropsychological tests included the assessment of immediate and delayed verbal memory (evaluated using the Rey Auditory Verbal Learning Test - RAVLT), immediate and delayed visuospatial memory (evaluated using the Brief Visuospatial Memory Test–Revised - BVMT–R), motor memory, assessment of the preservation of executive functions (evaluated using the Frontal Assessment Battery - FAB) and spatial representations (evaluated using the Judgment of Line Orientation - JLO). In addition, a comprehensive, qualitative neuropsychological examination of higher mental functions was carried out to exclude specific disorders of gnosis, praxis, and speech. Before surgery, five out of six patients had normal values. In one patient (F29), there was a moderate decrease in scores on all the assessment scales.
All patients underwent an examination of the visual fields by means of automatic static perimetry, both before and after the surgery on the Humphrey II - 730 visual field analyzer.
The study was approved by the local ethical committee of Burdenko Neurosurgery Center. Informed consent was obtained from each patient.
Surgical technique
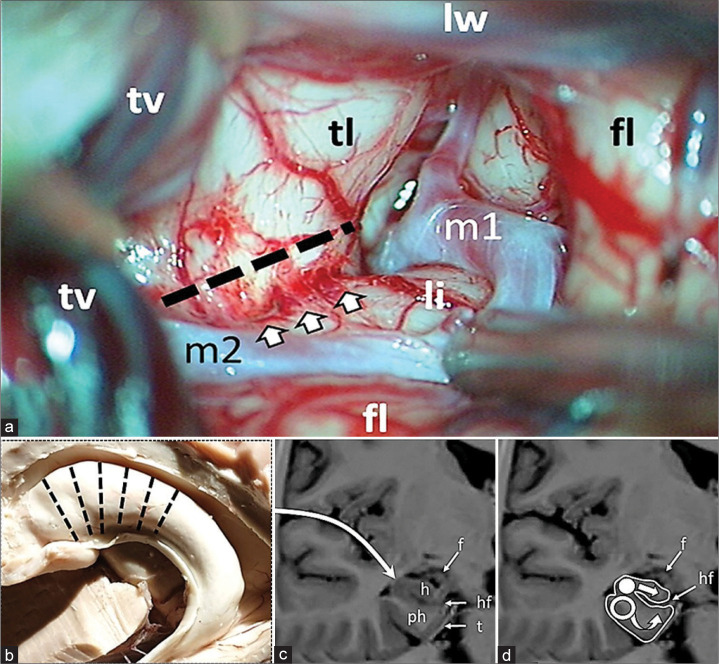
Under general anesthesia with minimal effective dosage of propofol 3–8 mg/kg/h, the patient is placed supine on the operating table with the head fixated on a MAYFIELD skull clamp and tilted about 45° to the opposite site of incision. Standard pterional craniotomy was performed in the left frontotemporal region. After the dura mater was opened an electrode was placed on a basal anterior temporal region and an ECoG was recorded from. The arachnoid of the sylvian fissure was dissected and the sylvian fissure was opened proximally about 3 cm [Figure 1a]. After the uncal region was exposed, piecemeal lesionectomy was performed.
Figure 1:
Intraoperative figure and schematic representation of multiple hippocampal transection. (a) Proximal part of the sylvian fissure is opened. The dotted line indicates the site of the temporal stem dissection parallel to the inferior peri-insular sulcus (arrows). fl: Frontal lobe; li: Limen of insule; lw: Lesser wing of sphenoid bone; m1, m2: Segments of middle cerebral artery; tl: Temporal lobe; tv: Temporal vein. (b) Transection locations along hippocampus; (c) Approach is performed through the Sylvian fissure, after lesionectomy and dissection of the limen insula the temporal horn of the lateral ventricle is opened; (d) Hippocampus (h) is intersected in the orthogonal plane to its longitudinal axis with a blunt silver knife (white circle) up to the hippocampal fissure (hf) with preservation of fimbria (f); following transection of hippocampus the parahippocampal gyrus is transected with a blunt silver knife (hollow circle) in the same plane as the hippocampus up to the tentorium (t) and to the arachnoid membrane of the ambient cistern inferomedially and to the hippocampal fissure (hf) dorsally. h: Hippocampus; ph: Parahippocampus; f: Fimbria; hf: Hippocampal fissure; t: Tentorium.
The amygdala was also resected, and the temporal horn of the left lateral ventricle was opened and the anterior part of the head of the hippocampus was exposed. The inferior peri-insular sulcus was identified, and the temporal lobe was dissected along the sulcus at a distance of 15 mm, and thus the surface of the hippocampus, including its tail, was widely exposed. A 4-contact electrode was placed on the surface of the hippocampus from the head to its tail, and ECoG activity was recorded over the hippocampus for 5 min. Registration of characteristic epileptic discharges and spike-wave complexes indicated the involvement of the hippocampus in the generation of seizures, and a decision was made in favor of its transection. Subsequently, the surgery was performed according to the presented scheme [Figure 1b-d].
The alveus consisted of dense fibers is sharply incised with micro-scissors. The transverse incision lines are 5 mm apart and perpendicular to the hippocampal axis [Figure 2a]. After the incisions are made on alveus, the hippocampus is dissected along these incisions up to the hippocampal fissure, where the vessels supplying the hippocampal formation are located. The incisions are made using 4 mm round, blunt silver loop [Figure 2b and c]. After hippocampal incisions, transverse incisions of the parahippocampal gyrus are made with a blunt bayonet ring-shaped silver loop (diameter - 4 mm) from the lateral margin of hippocampus to the arachnoid membrane of the ambient cistern medially and to the vascular layer of the hippocampal fissure dorsally. After the transaction was completed, ECoG was repeated over the entire hippocampus. If there was absence in spiking activity, the transactions were completed. If activity was detected in an untreated area, transection was extended posteriorly. The total number of transections comprised 5 to 6, depending on the presence of pathological activity on ECoG. After reliable hemostasis was achieved, the surgery was completed and wound was closed. After waking up, patients were transferred from ICU department to patient room at the same day. CT scan underwent all patients before discharge and after 3–6 months an MRI scan was performed [Figure 3].
Figure 2:

(a) View of the head and the body of the hippocampus after transection. (b and c) Special silver knives 4 mm in diameter for hippocampal transection (lower) parahippocampal gyrus (upper).
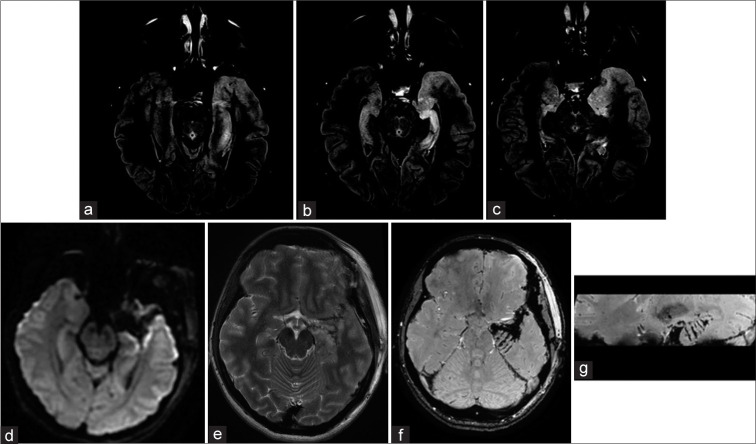
Figure 3:
Multiple hippocampal transections Case #F29. Focal cortical dysplasia, in the left temporal pole is visualized on MRI T2 weighted image. FCD does not spread to the hippocampus and it’s assize and structure were normal. MRI after FCD with the temporal pole resection and hippocampal transection. SWAN, axial and sagittal planes (b and c). Multiple hippocampal transactions. Patient #F27. Preoperative MRI scans on FLAIR reveals focal cortical dysplasia of the temporal pole and uncus. In addition, hippocampal sclerosis without its obvious reduction in volume can be observed on MRI. MRI scans 6 months after resection of the temporal pole, uncus and multiple hippocampal transections. There are no obvious signs of hippocampal infarction on MRI images in DWI (d) and FLAIR (e) sequences; In axial (f) and sagittal (g) MRI images in SWAN sequence, can be clearly visualized transections of hippocampus and parahippocampal gyrus (arrows).
RESULTS
All patients underwent surgery satisfactorily and were discharged from the hospital on average 4 days after surgery. There were no postoperative complications except for homonymous superior quadrantanopia as a consequence of Meyer’s loop injury during temporal lobe stem dissection. This kind of visual field loss was noted in four cases out of six [Table 1].
Control of postoperative seizures
During the follow-up period of 25–31 months (median 27 months), five patients out of six had Engel Class I outcome (83.3%). In one case, seizures developed after 1 months in a patient with focal cortical dysplasia (FCD) in the uncus (Engel IVA outcome). In the Engel I group, one patient developed rare epileptic seizures 26 months after surgery (Engel IC). During the follow-up period five patients (F29, F23, F43, M18, and M36) are continuing anticonvulsant therapy, and three of these reduced the dose of anticonvulsants (M36, F43, and M18).
Intraoperative ECoG
Despite the resection of lesion, the amygdala, and even the temporal pole in two cases, when recording biopotentials from the hippocampus, sharp wave forms of epileptiform activity were detected in all patients. In two cases (F23 and F27), continued spike activity was recorded. In one patient (M18), activity was minimal, revealed as rare sharp waves.
After hippocampal transections, spikes either completely disappeared in all patients, or single low-amplitude sharp waves were recorded asynchronously under different electrodes (F43). In one case (F23), after hippocampal transections, low-amplitude sharp waves, synchronously conducted from the cortex, were recorded under all electrodes of the strip.
Neuropsychological outcome
According to results of preoperative neuropsychological evaluation, all patients demonstrated normal indicators on all evaluation scales, except for one patient, F29, where all indicators were decreased.
Immediately after surgery, three out of six patients (M36, F29, and F23) developed significant nominative aphasia which later demonstrated a certain regression; however, they still persisted 6 and 12 months after surgery.
Evaluation of verbal memory in two cases revealed worsening compared to preoperative level [Figure 4].
Figure 4:
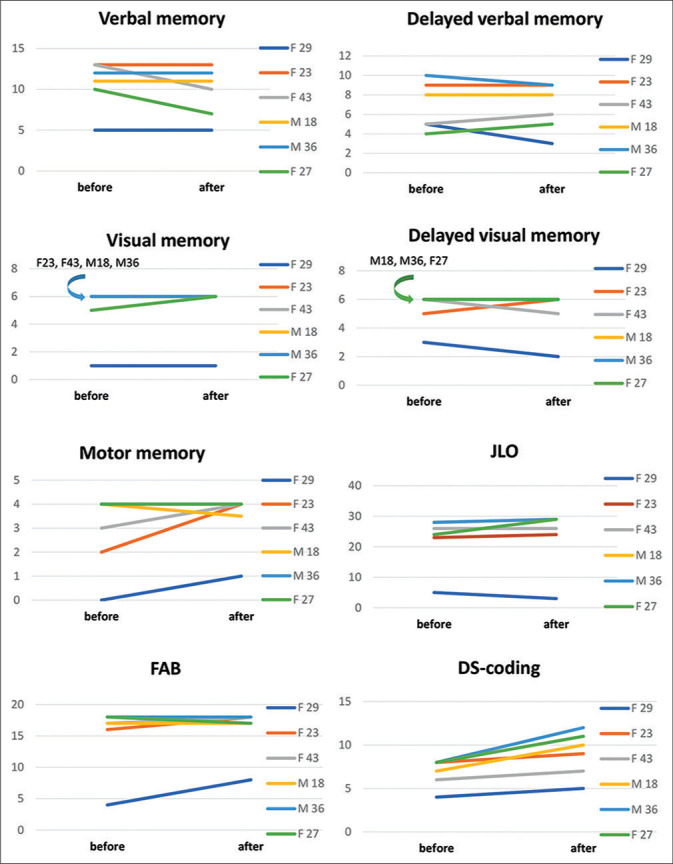
Evaluation of neurocognitive function in patients before and 6 months after MHT. The charts on the right show the gender and age of the patients.
Delayed verbal memory decreased in two patients (F29 and M36). Improvement was noted in two patients (F43 and F27).
In preoperative period, visual memory was below the normal in one patient (F29). In one case, memory improvement was observed (F27). Delayed visual memory in two cases impaired compared to the preoperative level (F29 and F43).
Preoperative evaluation showed that motor memory was impaired in two patients (F23 and F29); after surgery, one of them showed an improvement relative to preoperative values. In the remaining patients, these indicators remained within the normal range.
When assessing the regulatory component-using block of methods FAB, DS-coding, in the overall preoperative evaluation, regulatory capabilities were impaired in one patient (F29). In post-operative period – relative to preoperative evaluation, three patients showed improvement and three patients remained unchanged.
In the study of visuospatial synthesis, using the JLO technique, only one patient revealed impairment in preoperative evaluation, four patients in postoperative period showed improvement.
DISCUSSION
Significance of MHT and seizure control
Based on recent publications, there is now a consensus that radical excision of neuronal-glial tumors located in the lateral temporal lobe is quite sufficient and hippocampal resection is not necessary for achievement of seizure control.[11,15,38] On the contrary, when a neuronal-glial tumor is located in the mesial temporal lobe complex, removal of the tumor with additional hippocampal excision leads to seizure free period much more often than only radical tumor removal sparing the hippocampus.[12,16,23,44]
The same can be stated about treatment of FCD in the temporal lobe pole; its surgical treatment involves resection of the temporal pole along with the amygdala and hippocampus.[6]
Considering the above mentioned, it can be stated that surgical resection of the hippocampus contributes to effective treatment and management of seizures. However, excision of a normal functioning hippocampus can lead to serious impairment of verbal memory, visual memory, behavior, and emotions.[4,7,17,20]
Presurgical predictors of memory impairment after amygdalohippocampectomy are normal architecture and hippocampal volume on MRI scans and normal or slightly reduced memory on neuropsychological testing.[18,19,39]
Milner[29] first described the high frequency of verbal memory impairment, after hippocampal resection in patients with intact hippocampus in the 1950s, and later other authors stated same.[21,26,28,39] Baxendale et al.[5] reported that partial excision of the hippocampus gives the patient a chance to regain memory after surgery.
The above stated facts indicate the importance of preserving the hippocampus in cases where the patient’s memory is not affected or is slightly impaired. For this purpose, Shimizu et al.[36] proposed a MHT technique. The theoretical basis for this type of surgery rested on the previous experiments on monkeys and on subpial incision technique for epilepsy treatment.[32,45] At present, there is not much experience of MHTs in the world, with a total of just 139 operations. These surgeries were performed in four hospitals in Japan and the USA. The research results are given in [Table 2].
Table 2:
Results of hippocampal transection.
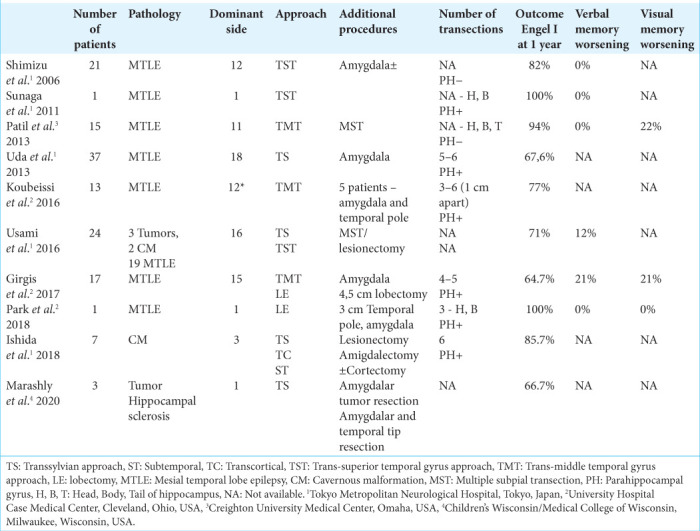
Hippocampal transection was mainly performed in cases of hippocampal sclerosis (126 cases). Only in 13 cases, hippocampal transections were performed for medial temporal space occupying lesions. Of these, cavernous malformations were the cause of epilepsy in nine cases, and neuronal-glial tumors in four cases (three gangliogliomas and 1 DNET).[22,27,42] In this group of patients, MHT was used simultaneously with resection of the medial temporal lesions in cases when intraoperative ECoG showed epileptic discharges coming from the hippocampus after tumor removal. Our approach is consistent with the paradigm described by Ishida et al.[22] Hippocampal transections were performed only in those cases when epileptiform activity remained on the hippocampal surface, despite removal of lesion. In most cases when resecting the tumors of the medial temporal complex, we did not perform hippocampal transections if there were no epileptic discharges recorded over the hippocampus.
In general, taking into account all published data (except for publications describing single observations), after hippocampal transection, seizure control (Engel I) was achieved – from 64.7% to 94.7% of cases during a follow-up period of 12–60 months.[14,34,36,40,42] Seizure control rate is also high in the group of patients with medial temporal lobe lesions sparing the hippocampus. In the study of Usami et al.,[42] the Engel I outcome was achieved in 100% of cases by 2 years and 60% of cases by 5 years. According to Ishida et al.,[22] Engel 1 outcome was achieved in 85.7% of cases over an average follow-up period of 63 months (20–119 months).
In our series of patients, four out of six patients had lesions in the medial temporal lobe and two patients in the temporal pole. None of the patients revealed any changes of hippocampus volume or architecture confirmed on MRI scans. After hippocampal transection, an Engel I outcome was achieved in five patients (83.3%) within 27 months after surgery (25–31 months). One patient, with FCD of the temporal pole, developed seizures after 3 months of surgery. In this patient, seizure frequency was much lower than before surgery. Apparently, in this case, the area of initiation of abnormal activity was beyond the temporal lobe, which is not uncommon in FCD.
Neuropsychological outcome
The advantage of MHT lies in its ability to preserve cognitive function, particularly auditory verbal memory on the dominant hemisphere and visuospatial memory on the non-dominant.[14,36,40]
Analysis of few published studies showed that hippocampal transection preserves or even improves verbal and visuospatial memory.[27,34,36,40] There is a slight memory impairment in rare cases.[35,42]
According to Shimizu et al.,[36] verbal memory was not affected in all eight patients who underwent neuropsychological evaluation after MHT. In another series of nine cases,[33] after MHT, improved verbal memory was confirmed in seven patients, with no changes in two cases. Visual memory improved in four cases, worsened in two cases and remained stable in three cases. Uda et al.[40] reported differences based on the surgical side: MHT on the non-dominant side resulted in significant improvements in verbal but not visual memory, whereas MHT on the dominant side did not lead to significant changes in verbal or visual memory.
According to Ishida et al.,[22] out of seven cases, MHT, simultaneously with removal of the cavernous malformation of the temporal lobe with refractory epilepsy, did not worsen postoperative memory or memory was reduced by no more than 25%.
In our series, among six patients, two patients relative to the preoperative level demonstrated improvement in delayed verbal memory after MHT. One patient showed a decrease level in delayed verbal memory, and two patients remained unchanged. It is important to note that despite decrease in delayed verbal memory in some patients, it still remains within normal range.
However, it is worth paying attention that in the postoperative period, three patients had nominative difficulties, with only one of them having decreased cognitive abilities in the auditory-verbal link both before and after evaluation. In two other patients with nominative difficulties, auditory-verbal memory indicators remained within the normal range.
This being said, it is important to note that MHT does not impair regulatory abilities, nor does it impair optical-spatial analysis and synthesis, which is a significant indicator of the choice of this type of treatment for patients with initially high cognitive levels.
Limitations
Our study has several important limitations. First, it is a retrospective study with a small number of patients. Second, the outcomes of surgical treatment of different pathologies were evaluated. Third, at the preoperative stage, the patients did not undergo stereo EEG monitoring, which seems to have been the cause of a diagnostic error in patient F29, who suffered from postoperative refractory seizures. Furthermore, the assessment of seizure freedom in the reported patient population cannot be construed as an evaluation of MHT success.
CONCLUSION
MHTs can be considered as an effective method of drug-resistant mesial temporal lobe epilepsy caused by tumors of the medial temporal complex. At the same time, MHT makes it possible to preserve memory in patients with structurally preserved hippocampus. However, MHT do not guarantee the preservation of memory after surgery.
Footnotes
How to cite this article: Pitskhelauri D, Kudieva E, Kamenetskaya M, Kozlova A, Vlasov P, Dombaanai B, et al. Multiple hippocampal transections for mesial temporal lobe epilepsy. Surg Neurol Int 2021;12:372.
Contributor Information
David Pitskhelauri, Email: dav@nsi.ru.
Elina Kudieva, Email: kudieva@nsi.ru.
Maria Kamenetskaya, Email: mkamenetskaya@nsi.ru.
Antonina Kozlova, Email: akozlova@nsi.ru.
Pavel Vlasov, Email: pvlasov@nsi.ru.
Baiyr Dombaanai, Email: bdombaanay@nsi.ru.
Natalia Eliseeva, Email: neliseeva@nsi.ru.
Lyudmila Shishkina, Email: lshishkina@nsi.ru.
Alexander Sanikidze, Email: asanikidse@nsi.ru.
Evgeniy Shults, Email: eshults@nsi.ru.
Dmitriy Moshev, Email: moshev@nsi.ru.
Armen Melikyan, Email: pronin@nsi.ru, melikian@nsi.ru.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Acsády L, Káli S. Models, structure, function: The transformation of cortical signals in the dentate gyrus. Prog Brain Res. 2007;163:577–99. doi: 10.1016/S0079-6123(07)63031-3. [DOI] [PubMed] [Google Scholar]
- 2.Alonso Vanegas MA, Lew SM, Morino M, Sarmento SA. Microsurgical techniques in temporal lobe epilepsy. Epilepsia. 2017;58(Suppl 1):10–8. doi: 10.1111/epi.13684. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 4.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 5.Baxendale S, Thompson P, Kitchen N. Postoperative hippocampal remnant shrinkage and memory decline: A dynamic process. Neurology. 2000;55:243–9. doi: 10.1212/wnl.55.2.243. [DOI] [PubMed] [Google Scholar]
- 6.Dührsen L, Sauvigny T, House PM, Stodieck S, Holst B, Matschke J, et al. Impact of focal cortical dysplasia Type IIIa on seizure outcome following anterior mesial temporal lobe resection for the treatment of epilepsy. J Neurosurg. 2018;128:1668–73. doi: 10.3171/2017.2.JNS161295. [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott RE, Bollo RJ, Berliner JL, Silverberg A, Carlson C, Geller EB, et al. Anterior temporal lobectomy with amygdalohippocampectomy for mesial temporal sclerosis: Predictors of long-term seizure control. J Neurosurg. 2013;119:261–72. doi: 10.3171/2013.4.JNS121829. [DOI] [PubMed] [Google Scholar]
- 9.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J., Jr Update on surgical treatment of the epilepsies, Summary of the second international palm desert conference on the surgical treatment of the epilepsies 1992. Neurology. 1993;43:1612–7. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- 11.Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012;53:51–7. doi: 10.1111/j.1528-1167.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- 12.Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70:921–8. doi: 10.1227/NEU.0b013e31823c3a30. discussion 928. [DOI] [PubMed] [Google Scholar]
- 13.Georgiadis I, Kapsalaki EZ, Fountas KN. Temporal lobe resective surgery for medically intractable epilepsy: A review of complications and side effects. Epilepsy Res Treat. 2013;2013:752195. doi: 10.1155/2013/752195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girgis F, Greil M, Fastenau P, Sweet J, Lüders H, Miller JP. Resection of temporal neocortex during multiple hippocampal transections for mesial temporal lobe epilepsy does not affect seizure or memory outcome. Oper Neurosurg (Hagerstown) 2017;13:711–7. doi: 10.1093/ons/opx031. [DOI] [PubMed] [Google Scholar]
- 15.Giulioni M, Galassi E, Zucchelli M, Volpi L. Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurg. 2005;102(Suppl 3):288–93. doi: 10.3171/ped.2005.102.3.0288. [DOI] [PubMed] [Google Scholar]
- 16.Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R, et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: Lesionectomy compared with tailored resection. J Neurosurg. 2009;111:1275–82. doi: 10.3171/2009.3.JNS081350. [DOI] [PubMed] [Google Scholar]
- 17.Gleissner U, Helmstaedter C, Schramm J, Elger C. Memory outcome after selective amygdalohippocampectomy: A study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- 18.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–32. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter C, Petzold I, Bien CG. The cognitive consequence of resecting nonlesional tissues in epilepsy surgery-results from MRI-and histopathologynegative patients with temporal lobe epilepsy. Epilepsia. 2011;52:1402–8. doi: 10.1111/j.1528-1167.2011.03157.x. [DOI] [PubMed] [Google Scholar]
- 20.Helmstaedter C. Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disord. 2013;15:221–39. doi: 10.1684/epd.2013.0587. [DOI] [PubMed] [Google Scholar]
- 21.Hermann BP, Wyler AR, Bush AJ, Tabatabai FR. Differential effects of left and right anterior temporal lobectomy on verbal learning and memory performance. Epilepsia. 1992;33:289–97. doi: 10.1111/j.1528-1157.1992.tb02318.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishida W, Morino M, Matsumoto T, Casaos J, Ramhmdani S, Lo SL. Hippocampal transection plus tumor resection as a novel surgical treatment for temporal lobe epilepsy associated with cerebral cavernous malformations. World Neurosurg. 2018;119:e209–15. doi: 10.1016/j.wneu.2018.07.108. [DOI] [PubMed] [Google Scholar]
- 23.Jooma R, Yeh HS, Privitera MD, Rigrish D, Gartner M. Seizure control and extent of mesial temporal resection. Acta Neurochir (Wien) 1995;133:44–9. doi: 10.1007/BF01404946. [DOI] [PubMed] [Google Scholar]
- 24.Koubeissi MZ, Kahriman E, Fastenau P, Bailey C, Syed T, Amina S, et al. Multiple hippocampal transections for intractable hippocampal epilepsy: Seizure outcome. Epilepsy Behav. 2016;58:86–90. doi: 10.1016/j.yebeh.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Labate A, Aguglia U, Tripepi G, Mumoli L, Ferlazzo E, Baggetta R, et al. Long-term outcome of mild mesial temporal lobe epilepsy: A prospective longitudinal cohort study. Neurology. 2016;86:1904–10. doi: 10.1212/WNL.0000000000002674. [DOI] [PubMed] [Google Scholar]
- 26.LoGalbo A, Sawrie S, Roth DL, Kuzniecky R, Knowlton R, Faught E, et al. Verbal memory outcome in patients with normal preoperative verbal memory and left mesial temporal sclerosis. Epilepsy Behav. 2005;6:337–41. doi: 10.1016/j.yebeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Marashly A, Koop J, Loman M, Kim I, Maheshwari M, Lew SM. Multiple hippocampal transections for refractory pediatric mesial temporal lobe epilepsy: Seizure and neuropsychological outcomes. J Neurosurg Pediatr. 2020;26:1–10. doi: 10.3171/2020.4.PEDS19760. [DOI] [PubMed] [Google Scholar]
- 28.Martin RC, Kretzmer T, Palmer C, Sawrie S, Knowlton R, Faught E, et al. Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Arch Neurol. 2002;59:1895–901. doi: 10.1001/archneur.59.12.1895. [DOI] [PubMed] [Google Scholar]
- 29.Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958;36:244–57. [PubMed] [Google Scholar]
- 30.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 31.Miserocchi A, Cascardo B, Piroddi C, Fuschillo D, Cardinale F, Nobili L, et al. Surgery for temporal lobe epilepsy in children: Relevance of pre-surgical evaluation and analysis of outcome. J Neurosurg Pediatr. 2013;11:256–67. doi: 10.3171/2012.12.PEDS12334. [DOI] [PubMed] [Google Scholar]
- 32.Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: A new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231–9. doi: 10.3171/jns.1989.70.2.0231. [DOI] [PubMed] [Google Scholar]
- 33.Patil AA, Andrews R. Long term follow-up after multiple hippocampal transection (MHT) Seizure. 2013;22:731–4. doi: 10.1016/j.seizure.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Patil AA, Chamczuk AJ, Andrews RV. Hippocampal transections for epilepsy. Neurosurg Clin N Am. 2016;27:19–25. doi: 10.1016/j.nec.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Patil AA, Andrews RV. Nonresective hippocampal surgery for epilepsy. World Neurosurg. 2010;74:645–9. doi: 10.1016/j.wneu.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu H, Kawai K, Sunaga S, Sugano H, Yamada T. Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. J Clin Neurosci. 2006;13:322–8. doi: 10.1016/j.jocn.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Sindou M, Guenot M, Isnard J, Ryvlin P, Fischer C, Mauguière F. Temporo-mesial epilepsy surgery: Outcome and complications in 100 consecutive adult patients. Acta Neurochir (Wien) 2006;148:39–45. doi: 10.1007/s00701-005-0644-x. [DOI] [PubMed] [Google Scholar]
- 38.Tomita T, Volk JM, Shen W, Pundy T. Glioneuronal tumors of cerebral hemisphere in children: Correlation of surgical resection with seizure outcomes and tumor recurrences. Childs Nerv Syst. 2016;32:1839–48. doi: 10.1007/s00381-016-3140-0. [DOI] [PubMed] [Google Scholar]
- 39.Trenerry M, Jack CJ, Ivnik R, Sharbrough FW, Cascino GD, Hirschorn KA, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- 40.Uda T, Morino M, Ito H, Minami N, Hosono A, Nagai T, et al. Transsylvian hippocampal transection for mesial temporal lobe epilepsy: Surgical indications, procedure, and postoperative seizure and memory outcomes. J Neurosurg. 2013;119:1098–104. doi: 10.3171/2013.6.JNS13244. [DOI] [PubMed] [Google Scholar]
- 41.Umeoka SC, Lüders HO, Turnbull JP, Koubeissi MZ, Maciunas RJ. Requirement of longitudinal synchrony of epileptiform discharges in the hippocampus for seizure generation: A pilot study. J Neurosurg. 2012;116:513–24. doi: 10.3171/2011.10.JNS11261. [DOI] [PubMed] [Google Scholar]
- 42.Usami K, Kubota M, Kawai K, Kunii N, Matsuo T, Ibayashi K, et al. Long-term outcome and neuroradiologic changes after multiple hippocampal transection combined with multiple subpial transection or lesionectomy for temporal lobe epilepsy. Epilepsia. 2016;57:931–40. doi: 10.1111/epi.13374. [DOI] [PubMed] [Google Scholar]
- 43.Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 44.Zaghloul KA, Schramm J. Surgical management of glioneuronal tumors with drug-resistant epilepsy. Acta Neurochir (Wien) 2011;153:1551–9. doi: 10.1007/s00701-011-1050-1. [DOI] [PubMed] [Google Scholar]
- 45.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–9. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]