Abstract
Background
Dietary nitrate supplementation, usually in the form of beetroot juice, may improve exercise performance and endothelial function. We undertook a systematic review and meta-analysis to establish whether this approach has beneficial effects in people with respiratory disease.
Methods
A systematic search of records up to March 2021 was performed on PubMed, CINAHL, MEDLINE (Ovid), Cochrane and Embase to retrieve clinical trials that evaluated the efficacy of dietary nitrate supplementation on cardiovascular parameters and exercise capacity in chronic respiratory conditions. Two authors independently screened titles, abstracts and full texts of potential studies and performed the data extraction.
Results
After full-text review of 67 papers, eleven (two randomised controlled trials and nine crossover trials) involving 282 participants met the inclusion criteria. Three were single dose; seven short term; and one, the largest (n=122), done in the context of pulmonary rehabilitation. Pooled analysis showed that dietary nitrate supplementation reduced systolic blood pressure (BP), diastolic BP and mean arterial pressure (mean difference (95% CI), −3.39 mm Hg (−6.79 to 0.01); p=0.05 and –2.20 mm Hg (−4.36 to −0.03); p=0.05 and −4.40 mm Hg (−7.49 to −1.30); p=0.005, respectively). It was associated with increased walk distance in the context of pulmonary rehabilitation (standardised mean difference (95% CI), 0.47 (0.11 to 0.83), p=0.01), but no effect was identified in short-term studies (0.08 (−0.32 to 0.49).
Conclusion
Dietary nitrate supplementation may have a beneficial effect on BP and augment the effect of pulmonary rehabilitation on exercise capacity. Short-term studies do not suggest a consistent benefit on exercise capacity.
PROSPERO registration number
CRD42019130123.
Keywords: exercise, pulmonary rehabilitation
Key messages.
Why read on?
This review systematically evaluates the available evidence regarding the impact of dietary nitrate supplementation on exercise capacity and cardiovascular parameters in individuals with respiratory disease.
Introduction
Exercise limitation is a common feature in individuals with chronic respiratory disease (CRD) despite optimum medical treatment including pulmonary rehabilitation (PR) and pharmacotherapy.1–3 Factors contributing to breathlessness and reduced physical activity include altered pulmonary mechanics and cardiovascular function as well as skeletal muscle impairment.4–7 Nitric oxide (NO) is a ubiquitous signalling molecule with a key role in endothelial function, and a relationship between plasma nitrite (NO2-) levels and exercise performance has been identified.8 9 Dietary NO3- supplementation, which increases NO availability via a NO3-–NO2-–NO pathway, has therefore been proposed as a potential complementary approach to improve exercise capacity in people with cardiorespiratory disease.
In healthy adults, endurance exercise capacity increases following dietary NO3- supplementation10 11 and evidence suggests that NO3- supplementation with beetroot juice (BRJ) can reduce oxygen consumption (VO2) during submaximal exercise and increase the time to reach exhaustion during high-intensity training.12–14 Of note, dietary NO3- supplementation has been shown to improve exercise performance under hypoxic conditions,15 and there is evidence for an effect in some clinical conditions, for example, chronic obstructive pulmonary disease (COPD),16–19 peripheral vascular disease20 and heart failure.21 22
In addition to exercise limitation, vascular comorbidities are common in people with lung disease, and clinical guidelines highlight the need to identify and optimally treat them.23 If dietary NO3- supplementation can be shown to have a beneficial effect on exercise capacity and/or vascular comorbidities, it has the potential to improve outcomes in this patient group.
We therefore aimed to evaluate the available evidence regarding the impact of NO3- supplementation on exercise capacity and cardiovascular parameters in individuals with respiratory disease.
Methods
The review was registered in the International Prospective Register of Systematic Reviews database. It was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Search strategy
The first author (ASA) searched PubMed, CINAHL, MEDLINE (Ovid), Cochrane and Embase from inception to March 2021. The terms used in this search were ‘respiratory diseases’, ‘chronic obstructive pulmonary disease’, ‘COPD’, ‘chronic obstructive airways disease’, ‘emphysema’, ‘bronchitis’, ‘bronchiectasis’, ‘interstitial lung disease’, ‘ILD’, ‘cystic fibrosis’, ‘pulmonary hypertension’, ‘PHT’, ‘nitrate’, ‘beetroot’, ‘dietary nitrate’ and ‘nitrate supplementation’. A search filter was applied by using medical subject heading terms. This search strategy was conducted in collaboration with a librarian to ensure this review contained the appropriate and necessary keywords. Full search strategy from all databases is presented in online supplemental appendix 1.
bmjresp-2021-000948supp001.pdf (102.8KB, pdf)
Inclusion criteria
We included both randomised clinical trials and crossover studies.
The PICO format was used in our search strategy
P: Population included adults diagnosed with CRD such as COPD, interstitial lung disease (ILD), bronchiectasis or pulmonary hypertension (PHT) either undergoing usual care or taking part in PR.
I: Intervention was dietary NO3- supplementation delivered to patients with CRD.
C: Comparator was a placebo group for interventional studies.
O: Outcomes included both primary outcomes (exercise capacity and blood pressure) and secondary outcomes such as cardiovascular parameters: heart rate (HR), oxygen saturation (O2 sat), plasma NO3- and NO2- levels, peak and iso-time VO2, endothelial function (flow-mediated dilatation (FMD)) and fractional exhaled NO (FeNO).
Exclusion criteria
Studies examining children under 18 years.
Any papers written in a language other than English.
Conference abstracts or unpublished data.
Data extraction
Data were extracted into a standardised Microsoft Excel spreadsheet form (Microsoft Corp., Redmond, WA, USA). We contacted the corresponding authors for missing data. Two authors (ASA and AMA) independently screened titles and abstracts of potential studies. A third reviewer (NSH) was available to resolve any disagreements. The form included data about change in exercise capacity following dietary NO3- supplementation. Other data such as cardiovascular parameters (HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and O2 sat), VO2, FMD, FeNO, intervention protocol (eg, dose, duration and delivery method), exercise protocol (type and duration) and placebo were extracted.
Data analysis
The synthesis of results described the outcomes of interest, such as exercise capacity, VO2, exercise endurance time, plasma NO3- level, plasma NO2- level, FeNO, SBP, DBP, MAP, HR, O2 sat and FMD. Where appropriate, meta-analysis was conducted to estimate the pooled differences and 95% CIs in the outcomes of interests between NO3- and placebo conditions. For crossover studies, endpoint values were extracted from the end of the study after receiving the supplements (NO3- or placebo) and included in meta-analysis as if from a parallel designed trial.24 25 The random-effect model was applied because of the variety in several factors (eg, exercise protocol, dose and duration of NO3- supplementation). Continuous data are reported as the mean difference (MD) (Δ). Standardised mean difference (SMD) was used when the same outcome was assessed with different measures (eg, exercise capacity assessed using different walking tests). Heterogeneity among included studies was evaluating using the I2 value. The statistical analyses were performed using the Cochrane Collaboration’s Review Manager software (RevMan V.5.4.0).
Results
Selection of studies
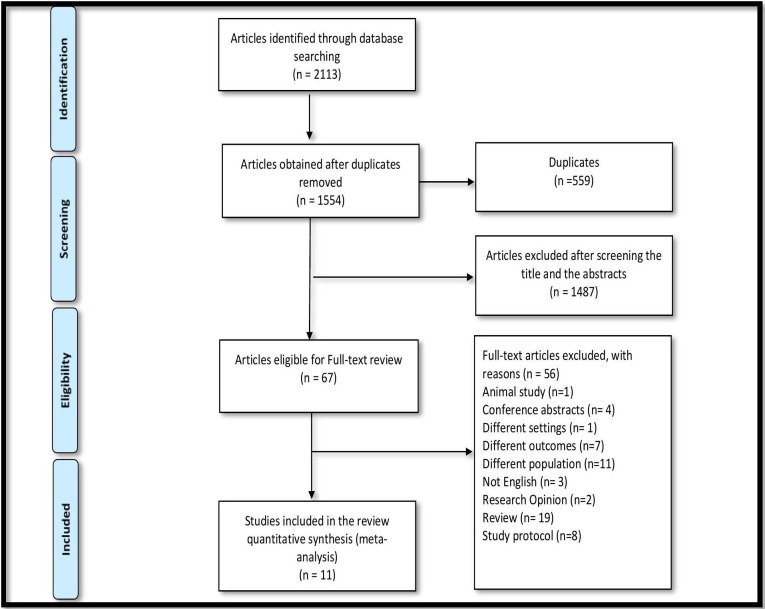
Initially, 2113 articles were identified through the database searches, 1554 after removing duplicates with 67 articles eligible for full-text review following title and abstract screening. Following full-text review, 11 studies met the inclusion criteria for the present systematic review (figure 1).
Figure 1.
Flowchart of study selection (Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram).
Study characteristics
Among the 11 randomised controlled trials (RCT), two used a parallel design, and nine used a crossover design. All 11 were published between 2015 and 2020. Eight studies were conducted in Europe, two in the USA and one in Australia. Studies were categorised based on the reported duration of NO3- supplementation as ‘acute effect’ (single dose of NO3- supplementation, 2.5–3 hours before exercise session) (n=3), ‘short term’ (less than 3 months in usual care) (n=7) or ‘during pulmonary rehabilitation’ (n=1). One study provided both acute effect and short-term data.26 The total number of participants was 282, including 15 with pulmonary arterial hypertension27 and 267 with COPD16–19 26 28–32 with sample sizes ranging from 8 to 122. Age of participants (mean±SD) was 66±3 years, and the majority (57%) were men. Ten trials used BRJ as the source of NO3- (n=10), and one used sodium NO3- (NaNO3-).26 The dose of NO3- used ranged from 6.45 mmol28 to 16 mmol27. A full description of the included studies is presented in table 1.
Table 1.
Description of the included studies
| Authors/design | Study sample | Nitrate (NO3-) dose | Placebo | Duration | Wash-out | Results (following NO3- vs placebo) |
| Behnia et al, 2018/RCT28 | N=25 GOLD stage 1–4 Age (y): 68±9 Sex (M/F): 13/12 |
70 mL BRJ + 180 mL black currant juice | 70 mL water + 180 mL black currant juice | 8 days | No |
|
| Beijers et al, 2018/RXT26 | N=18 GOLD stage 1–2 Age (y): 67±8 Sex (M/F): 13/5 FEV1%=69.2 |
Sodium NO3- (8 mmol) | NaCl ingestion | Acute and 7 days |
7 days |
|
| Berry et al, 2015/RXT16 | N=15 GOLD stage 1–2 Age (y): 70±9 Sex (M/F): 12/3 FEV1%=61.8 |
140 mL BRJ (7.58 mmol) | 163 mL prune juice (0.01 mmol NO3-) | Acute | 7 days |
|
| Curtis et al, 2015/RXT29 | N=21 GOLD stage 2–4 Age (y): 68±7 Sex (M/F): 16/5 FEV1%=50.1 |
140 mL BRJ (12.9 mmol) | 140 mL ND-BRJ | Acute | 7 days |
|
| Friis et al, 2015/RXT30 | N=15 GOLD stage 2–4 Age (y): 63±13 Sex (M/F): 9/6 FEV1%=44.7 |
140 mL BRJ | 140 mL ND-BRJ | 7 days | 7 days |
|
| Henrohn et al, 2018/RXT27 | N=15 Pulmonary hypertension, WHO group 1 Age (y): 60±15 Sex (M/F): 7/8 |
140 mL BRJ (16 mmol) | 140 mL ND-BRJ (0.118 mmol NO3-) | 7 days | 4–9 days |
|
| Kerley et al, 2015/RXT17 | N=11 GOLD stage 2–4 Age (y): 69±7 Sex (M/F): 5/6 FEV1%=43.4 |
140 mL BRJ + 200 mL black currant cordial | 140 mL water + 200 mL black currant cordial | Acute | 7 days |
|
| Kerley et al, 2019/RXT18 | N=8 GOLD stage 1–3 Age (y): 63±7 Sex (M/F): 5/3 FEV1%=55 |
140 mL BRJ (12.9 mmol) | 140 mL ND-BRJ (0.5 mmol NO3-) | 14 days | NA |
|
| Leong et al, 2015/RXT31 | N=19 GOLD stage 2 Age (y): 67±8 Sex (M/F): 5/14 FEV1%=62 |
140 mL BRJ (9.6 mmol) | 140 mL ND-BRJ (0.0056–0.020 mmol NO3-) | 3 days | 4 days |
|
| Pavitt et al, 2020/RCT During PR19 |
N=122 GOLD stage 2–4 Age (y): 68±10 Sex (M/F): 69/53 FEV1%=49 |
140 mL BRJ (12.9 mmol) | 140 mL ND-BRJ | 56 days | No |
|
| Shepherd et al, 2015/RXT32 | N=13 GOLD stage 1–2 Age (y): 65±8 Sex (M/F): NR FEV1%=57 |
140 mL BRJ (13.5 mmol) | 140 mL ND-BRJ (0.004 mmol NO3-) | 2.5 days | 7 days |
|
BRJ, beetroot juice; DBP, diastolic blood pressure; FeNO, fractional exhaled nitric oxide; FMD, flow-mediated dilatation; GOLD, Global Obstructive Lung Disease; ISWT, incremental shuttle walk test; MAP, mean arterial pressure; 6MWD, 6-minute walk distance; N, number of participants who completed the trial; NA, not available; NaCl, Sodium chloride; ND-BRJ, nitrate-depleted beetroot juice; NO2-, nitrite; NR, not reported; RCT, randomised controlled trial; RXT, randomised crossover trial; SBP, systolic blood pressure; VO2, oxygen consumption.;
Exercise capacity
Data on exercise capacity or endurance were reported in ten studies using different tests including the incremental shuttle walk test (ISWT) (n=3), 6-minute walk distance (6MWD) (n=3), endurance time during cycle ergometry (n=3) and endurance shuttle walk test (ESWT) (n=1) in individuals with COPD16–19 26 29–32 and PHT.27
The impact of NO3- supplementation on peak exercise capacity measured using walking tests (ISWT and 6MWD) in people with COPD is shown in (figure 2). Pooled analysis from five trials demonstrated an improvement in exercise capacity following NO3- supplementation compared with placebo (SMD (95% CI), 0.30 (0.03 to 0.57), p=0.03),17–19 30 32 although this effect was driven by the study in the context of PR. Thus, supplementation was associated with increased walk distance in the context of PR (SMD (95% CI), 0.47 (0.11 to 0.83), p=0.01), but no effect was identified in short-term studies (0.08 (−0.32 to 0.49). A single trial in 15 patients with PHT taking BRJ for 1 week did not show a significant effect on 6MWD.27
Figure 2.
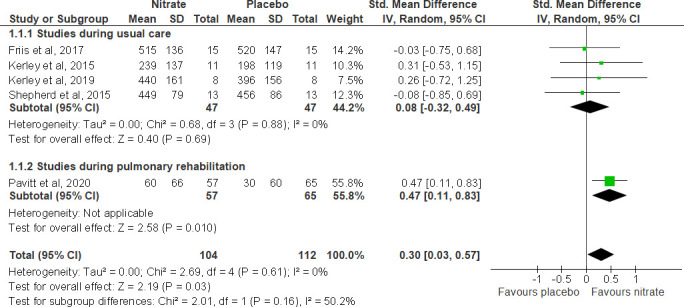
Forest plot for the effect of nitrate supplementation on peak exercise capacity measured with incremental shuttle walk test or 6-minute walk distance (in metres) in patients with chronic obstructive pulmonary disease.
Berry et al found an improvement in endurance exercise capacity during cycle ergometry at 75% maximal workload.16 In contrast, results from two trials providing NO3- supplementation (one using BRJ and one NaNO3-) did not demonstrate a significant improvement in endurance exercise time during cycle ergometry.26 29
Similarly, Leong et al investigated the effect of 3 days of BRJ on exercise endurance via ESWT at 85% VO2 max, among patients with stable moderate COPD, and found no difference in exercise endurance following BRJ compared with placebo.31
Effect of NO3- supplementation on physiological and cardiovascular parameters
Oxygen consumption
The impact of NO3- supplementation on peak VO2 was reported in six studies (figure 3).16 26–30 Pooled analysis from four trials demonstrated that peak VO2 did not change following NO3- supplementation compared with placebo (MD (95% CI), 0.09 mL/min/kg (−1.36 to 1.53), p=0.91).16 26 28 29 However, VO2 at iso-time was reported in two studies; Curtis et al report a significant decrease in iso-time VO2 after NO3- supplementation compared with a placebo (BRJ 16.6±6.0 mL/min/kg; placebo 17.2±6.0 mL/min/kg; p=0.043).29 Differently, Berry et al failed to find lower iso-time oxygen uptake (median+IQR; BRJ 14.1+5.4; placebo 13.4+5.5; p=0.099).16 We were unable to perform a meta-analysis of iso-time oxygen uptake due to incomplete data. Iso-time VO2 and other cardiopulmonary exercise parameters are provided in (table 2).
Figure 3.
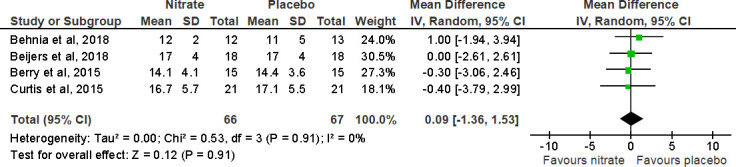
Forest plot for the effect of nitrate supplementation on peak oxygen consumption (mL/min/kg) in patients with chronic obstructive pulmonary disease.
Table 2.
Iso-time oxygen saturation and other cardiopulmonary exercise parameters
| Study | Parameter | Placebo | BRJ | P value |
| Berry et al, 201516 | HR | 112 (99, 124) | 110 (97, 123) | 0.300 |
| SBP | 167.1 (151.7, 182.4) | 160.1 (147.8, 172.5) | 0.137 | |
| DBP | 86.3 (79.6, 92.9) | 79.9 (72.8, 86.9) | 0.001 | |
| VO2* | 13.4+5.5 | 14.1+5.4 | 0.099 | |
| O2 saturation | 95.1 (94.0, 96.2) | 95.1 (93.9, 96.2) | 0.895 | |
| Curtis et al, 201529 | HR | 122 (17) | 121 (20) | 0.30 |
| VO2 | 17.2 (6.0) | 16.6 (6.0) | 0.043 | |
| O2 saturation | 92 (4) | 93 (4) | 0.15 |
Berry et al, 2015; Curtis et al, 2015: Data are presented as mean (SD).
*Non-normally distributed variables are presented as medians and IQRs. Normally distributed values are presented as means and 95% CIs.
BRJ, beetroot juice; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; VO2, oxygen comsumption.
Blood pressure
SBP and DBP were reported in all included studies, while MAP was reported in three studies.17 19 29 Meta-analysis for systolic, diastolic and mean arterial blood pressure in people with COPD found significant reductions compared with placebo (figure 4) (MD (95% CI) was −3.39 mm Hg (−6.79 to 0.01), p=0.05, for SBP; −2.20 mm Hg (−4.36 to −0.03), p=0.05, for DBP; and −4.40 mm Hg (−7.49 to −1.30), p=0.005, for mean arterial blood pressure).16–19 26 28–32 However, in one study in individuals with PHT, SBP and DBP did not significantly change following NO3- supplementation compared with placebo.27
Figure 4.
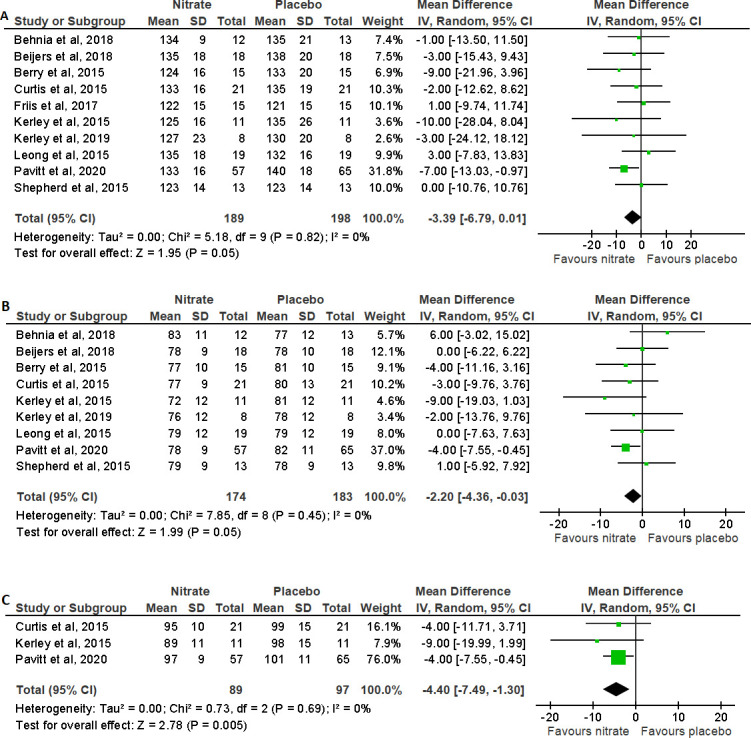
Forest plot for the effect of nitrate supplementation on (A) systolic blood pressure (mm Hg), (B) diastolic blood pressure (mm Hg) and (C) mean arterial blood pressure (mm Hg) in patients with chronic obstructive pulmonary disease.
Heart rate
The impact of NO3- supplementation on HR was reported in seven studies.16 17 26–30 Pooled analysis from four trials of HR at rest and at peak of exercise in people with COPD is shown in figure 5. Following the meta-analysis, the MD (95% CI) was 0.23 (−3.58 to 4.03), p=0.91, for HR at rest and 0.22 (−5.80 to 6.24), p=0.94, for HR at peak of exercise, showing no change in HR following NO3- supplementation compared with placebo.17 26 28 29
Figure 5.
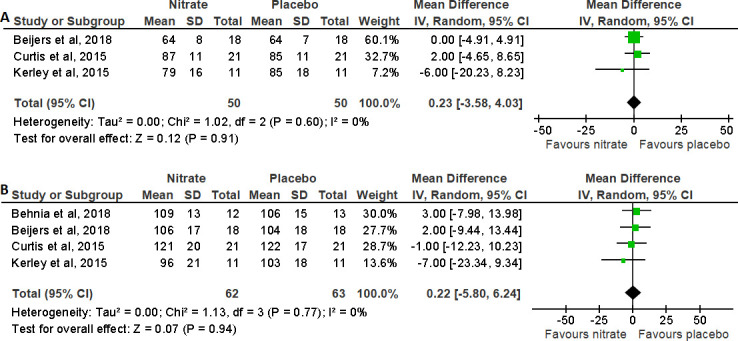
Forest plot for the effect of nitrate supplementation on (A) heart rate at rest (bpm) and (B) heart rate at peak (bpm) in patients with chronic obstructive pulmonary disease.
Endothelial function
Pavitt et al assessed the impact of NO3- supplementation during PR on endothelial function using brachial artery flow-mediated dilatation,19 finding an improvement (increase) in FMD in the treatment group (n=10) compared with placebo (n=10) (median (IQR) percent change: +6.6% (0.6 to 17.6), placebo: −4.7% (−21.5 to 11.8), and estimated treatment effect: −20.3% (95% CI −33.8 to 3.4); p=0.046).
O2 saturation
The impact of dietary NO3- supplementation on O2 sat was reported in three studies.16 17 29 Pooled analysis from two trials for oxygen saturation at rest and at peak exercise in COPD is shown in figure 6. The MD (95% CI) was 0.20 (−1.72 to 2.12)%, p=0.84, for oxygen saturation at rest and −0.37 (−2.88 to 2.14)%, p=0.77, for oxygen saturation at peak of exercise, showing no effect on oxygen saturation following NO3- supplementation compared with placebo.17 29 Curtis et al did demonstrate a reduction in area under the curve for oxygen saturation during exercise with NO3- supplementation compared with placebo.29 Of note, this study excluded individuals with resting hypoxia.
Figure 6.
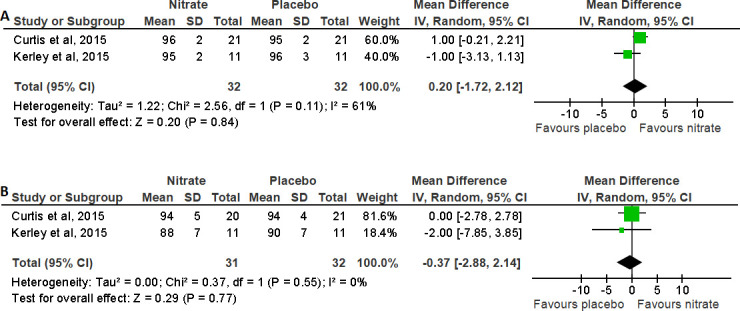
Forest plot for the effect of nitrate supplementation on (A) oxygen saturation at rest (%) and (B) oxygen saturation at peak (%) in patients with chronic obstructive pulmonary disease.
Plasma NO3- and NO2- levels
Plasma NO3- and NO2- levels were measured in seven studies.16–18 26 27 29 32 Pooled analysis from six trials for plasma NO3- and NO2- levels in COPD individuals is shown in figure 7. Following the meta-analysis, the MD (95% CI) was 445.61 (254.69 to 636.53), p<0.00001, for plasma NO3- level and 367.07 (232.87 to 501.27), p<0.00001, for plasma NO2- level, showing that levels of plasma NO3- and NO2- significantly increased following NO3- supplementation compared with placebo.16–18 26 29 32
Figure 7.
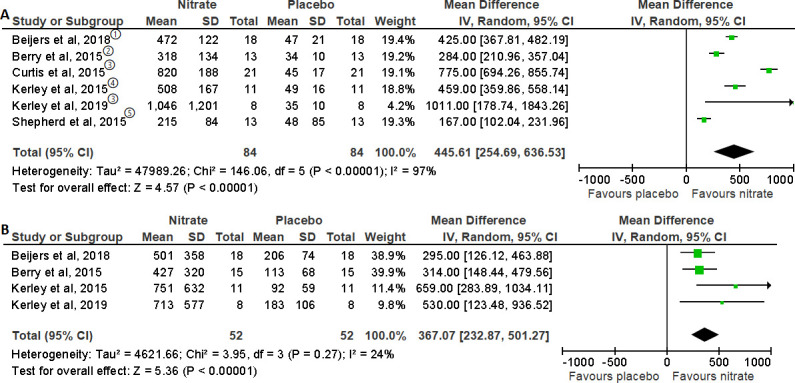
Forest plot for the effect of nitrate supplementation on (A) plasma nitrate (µM) and (B) plasma nitrite (nM) levels in patients with chronic obstructive pulmonary disease. (1) sodium nitrate (8 mmol); (2) beetroot juice (BRJ) (7.58 mmol); (3) BRJ (12.9 mmol); (4) 140 mL of BRJ + 200 mL black currant cordial; (5) BRJ (6.75 mmol).
Fractional exhaled NO
The impact of NO3- supplementation on FeNO was measured in three trials two conducted in individuals with COPD18 28 and one with PHT.27 Pooled analysis from two trials for FeNO in COPD individuals is shown in figure 8. Following the meta-analysis, the MD (95% CI) was 17.23 (−3.35 to 37.80) ppb, p=0.10, for FeNO, showing no consistent effect on FeNO following NO3- supplementation compared with placebo.18 28 However, Henrohn et al (2018) found that the level of FeNO in individuals with PHT increased at all flow rates (50–300 mL/s) following NO3- supplementation compared with placebo, at a flow rate of 50 mL/s (median of differences 18, 95% CI 11 to 26, p<0.0010).27
Figure 8.
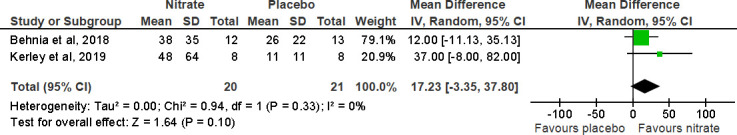
Forest plot for the effect of nitrate supplementation on fractional exhaled nitric oxide (ppb) in patients with chronic obstructive pulmonary disease.
Risk of bias and evidence quality assessment
Using the Cochrane risk-of-bias assessment tool,33 the included studies showed considerable variation in the risk of bias, but most were limited by a lack of allocation concealment, blinding and incomplete reporting of data (figure 9).
Figure 9.
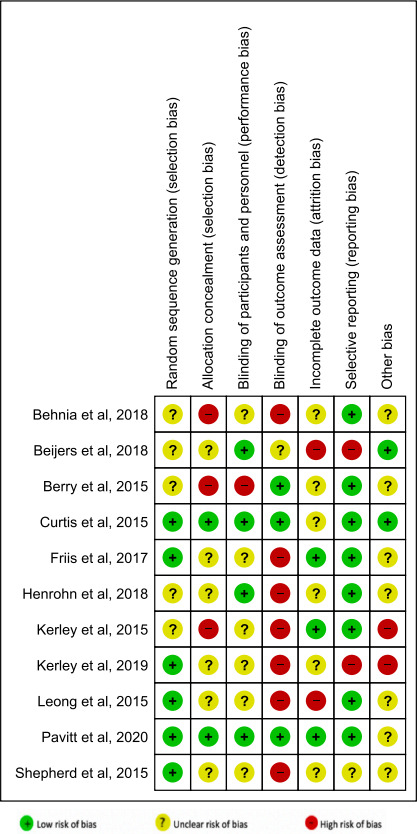
Risk of bias summary: review authors’ judgements about each risk-of-bias item for each included study.
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE)34 criteria were used to assess the overall evidence around specific outcomes (eg, exercise capacity and blood pressure endpoints). For exercise capacity, the majority of studies were small and short term, which limit the precision of estimates. Studies did not focus clearly on disease severity, limiting the directness of the evidence to COPD phenotypes, particularly hypoxic patients. Further, studies used a variety of interventions (eg, doses, duration and delivery method) and outcome measures contributing to heterogeneity or inconsistency. Therefore, the quality of evidence by GRADE to support an effect of NO3- supplementation on exercise capacity in people with lung disease is low. In the specific context of PR, only a single high-quality RCT, the largest and the longest of the included studies, was identified. Therefore, the total evidence by GRADE to support the impact of NO3- supplementation on exercise capacity in the context of PR is moderate. Regarding blood pressure endpoints, studies were consistent, although the longest duration study is only 8 weeks, so taken together, the evidence to support an impact of NO3- supplementation on blood pressure is moderate.
Discussion
The main findings of this review into the effects of dietary NO3- supplementation in people with CRD are that, although it can augment the effects of PR on exercise performance, a consistent short-term effect on exercise capacity in the absence of exercise training has not so far been demonstrated. However, studies to date do suggest that dietary NO3- supplementation can lower blood pressure, perhaps by improving endothelial function, which is potentially important given the high prevalence of cardiovascular disease in people with COPD, especially if a single intervention could address both issues.
Significance of findings
NO is a vital physiological mediator in the body. It is produced in two different ways: by an endogenous pathway (oxygen-dependent) via the L-arginine NO synthase system and by an exogenous pathway (oxygen-independent) via the reduction of dietary NO3- via NO2- to NO.35 36 In the human diet, the main source of NO3- is green leafy vegetables, which have high concentrations of NO3-. NO3- reduction to NO is favoured by conditions found in exercising muscle, in particular hypoxia, acidosis and the presence of deoxyhaemoglobin and myoglobin. Effects on exercise in people with respiratory disease could be mediated through improved muscle mitochondrial efficiency or through effects on vascular endothelium in either the systemic or pulmonary circulation. Endothelial effects of NO are also likely to underpin the effects on blood pressure that were observed.
Regarding the impact of dietary NO3- supplementation on exercise capacity, the current meta-analysis includes studies in COPD, which found an improvement in exercise capacity,16–19 and others that did not.26 28–32 Studies included heterogeneous COPD populations (eg, COPD severity and age) and used different exercise protocols (eg, ISWT, 6MWD and endurance time during cycle ergometry). Furthermore, hypoxic patients who required oxygen supplementation, a patient phenotype that might be expected to benefit most given that NO2- to NO conversion is enhanced in hypoxic conditions, were typically excluded. The duration of treatment and doses of NO3- used in trials also differed. The results from trials indicate that longer-term studies in specific patient phenotypes are needed to see if NO3- supplementation can improve exercise capacity in the absence of a training stimulus. Likewise, although dietary NO3- supplementation was associated with a greater increase in walk distance during PR, it is not clear how long this benefit might be sustained for—the 8-week ON-EPIC trial19 is the longest study to date of this intervention in people with CRD.
Physiological parameters at peak exercise including VO2 did not change significantly with NO3- supplementation compared with placebo,16 26–30 32 although one study found a significant reduction in VO2 at iso-time during cycle exercise in patients with COPD.29 Again, these negative results could be due to an absence of effect or a result of using insufficient dose or duration of supplementation. A dose–response effect for reduction in VO2 during exercise has previously been described in healthy individuals.37
Dietary NO3- supplementation has been shown to reduce blood pressure in individuals who are either normotensive38 or hypertensive.39 We found an overall effect to lower systolic, diastolic and mean arterial blood pressure in the studies reviewed here. People with lung disease are at high risk of cardiovascular disease, and this includes damage to the pulmonary vascular bed, which can lead to PHT.40 There is also interesting data in individuals with idiopathic PHT, which demonstrate that a low level of plasma NO3- is associated with increased mortality risk making it a potential prognostic indicator for PHT.41 Although one study with PHT was identified by our search strategy to be small, we advise against overinterpreting it, and further studies are needed.
Plasma NO3- and NO2- levels have potential to be used as a biomarker for NO availability.42 As expected, the available evidence showed that plasma NO3- and NO2- levels increased following dietary nitrate supplementation. The FeNO has been used as a diagnostic test for asthma.43 44 However, studies describing the FeNO level in people with COPD are inconclusive. In this review, two studies showed that FeNO level increased following NO3- supplementation,27 28 while another study had a negative result,18 so further work is needed to clarify this. Further work is needed to establish if FeNO can be used as a biomarker to monitor compliance in therapeutic trials of NO3- supplementation or even to adjust dose in individuals.
Strengths and limitations
A variety of lessons can be learnt from this review. First, most of the trials covering the effect of dietary NO3- supplementation on exercise capacity in people with respiratory disease have focused on COPD, with only one on PHT. Most trials were short term. Second, trials involved a variety of study designs, outcome measures, clinical phenotypes (severity of the disease and of hypoxia in particular), exercise protocols and dose and duration of NO3- supplementation. Third, it will be important to define whether there are different COPD phenotypes or subpopulations that can be categorised as NO3- responders or non-responders. Fourth, in most studies, BRJ was used as the source of NO3-. It is possible that other bioactive compounds in the juice that have antioxidant and anti-inflammatory properties including vitamin C, carotenoids, phenolics and betalains could contribute to beneficial effects. Many but not all studies have used NO3--depleted BRJ as a control, which is ideal for identifying effects of NO3- itself but runs the risk of underestimating the effect of BRJ itself if these other components have a role. Finally, none of the trials we identified for this review evaluate the effect of dietary NO3- supplementation on exercise capacity or cardiovascular parameters in people with rarer lung diseases such as ILD and cystic fibrosis.
Conclusion
Dietary NO3- supplementation has potential to reduce cardiovascular risk by lowering blood pressure in people with COPD as well as improving exercise capacity, though evidence for the latter is largely in the context of PR. Importantly, further work is needed to understand whether it is the rehab setting that is giving the benefit (ie, combining supplementation with exercise) or whether it is purely due to the fact that participants were followed for a longer duration than in other non-PR studies. To date, no data exist that might support dietary NO3- supplementation for lung diseases other than COPD. The data support trials of dietary NO3- supplementation in patients with COPD to address vascular endpoints, and we would suggest that exercise capacity also be measured in such trials to see whether a ‘dual benefit’ can be elicited. Outside the context of PR, further trials are indicated to evaluate the value of dietary NO3- supplementation on exercise capacity in COPD and specifically to identify the phenotypes most likely to profit from this intervention.
Footnotes
Contributors: NSH, ASA and MIP conceived the study. ASA, AMA, SMA performed the systematic review, ASA produced the first draft of the paper to which all authors contributed. All authors have reviewed and approved the final version. NSH is the guarantor.
Funding: The main author disclosed receipt of the following financial support for the research, authorship and/or publication of this article. This study was supported by a scholarship from Jazan University in Saudi Arabia.
Competing interests: NSH is chair of Action on Smoking and Health and medical director of the British Lung Foundation. Other authors have no conflict of interest to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Kelly JL, Bamsey O, Smith C, et al. Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration 2012;84:193–9. 10.1159/000336549 [DOI] [PubMed] [Google Scholar]
- 2.Elbehairy AF, Quint JK, Rogers J, et al. Patterns of breathlessness and associated consulting behaviour: results of an online survey. Thorax 2019;74:814–7. 10.1136/thoraxjnl-2018-212950 [DOI] [PubMed] [Google Scholar]
- 3.Philip K, Gaduzo S, Rogers J, et al. Patient experience of COPD care: outcomes from the British Lung Foundation Patient Passport. BMJ Open Respir Res 2019;6:e000478. 10.1136/bmjresp-2019-000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006;3:219–32. 10.1080/15412550600977478 [DOI] [PubMed] [Google Scholar]
- 5.Maddocks M, Shrikrishna D, Vitoriano S, et al. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur Respir J 2014;44:1188–98. 10.1183/09031936.00066414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012;40:1115–22. 10.1183/09031936.00170111 [DOI] [PubMed] [Google Scholar]
- 7.Jackson AS, Shrikrishna D, Kelly JL, et al. Vitamin D and skeletal muscle strength and endurance in COPD. Eur Respir J 2013;41:309–16. 10.1183/09031936.00043112 [DOI] [PubMed] [Google Scholar]
- 8.Rassaf T, Lauer T, Heiss C, et al. Nitric oxide synthase-derived plasma nitrite predicts exercise capacity. Br J Sports Med 2007;41:669–73. 10.1136/bjsm.2007.035758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreissigacker U, Wendt M, Wittke T, et al. Positive correlation between plasma nitrite and performance during high-intensive exercise but not oxidative stress in healthy men. Nitric Oxide 2010;23:128–35. 10.1016/j.niox.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 10.McMahon NF, Leveritt MD, Pavey TG. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: a systematic review and meta-analysis. Sports Med 2017;47:735–56. 10.1007/s40279-016-0617-7 [DOI] [PubMed] [Google Scholar]
- 11.Van De Walle GP, Vukovich MD. The effect of nitrate supplementation on exercise tolerance and performance: a systematic review and meta-analysis. J Strength Cond Res 2018;32:1796–808. 10.1519/JSC.0000000000002046 [DOI] [PubMed] [Google Scholar]
- 12.Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 2009;107:1144–55. 10.1152/japplphysiol.00722.2009 [DOI] [PubMed] [Google Scholar]
- 13.Breese BC, McNarry MA, Marwood S, et al. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol 2013;305:R1441–50. 10.1152/ajpregu.00295.2013 [DOI] [PubMed] [Google Scholar]
- 14.Lansley KE, Winyard PG, Fulford J, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 2011;110:591–600. 10.1152/japplphysiol.01070.2010 [DOI] [PubMed] [Google Scholar]
- 15.Masschelein E, Van Thienen R, Wang X, et al. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol 2012;113:736–45. 10.1152/japplphysiol.01253.2011 [DOI] [PubMed] [Google Scholar]
- 16.Berry MJ, Justus NW, Hauser JI, et al. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 2015;48:22–30. 10.1016/j.niox.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerley CP, Cahill K, Bolger K, et al. Dietary nitrate supplementation in COPD: an acute, double-blind, randomized, placebo-controlled, crossover trial. Nitric Oxide 2015;44:105–11. 10.1016/j.niox.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Kerley CP, James PE, McGowan A, et al. Dietary nitrate improved exercise capacity in COPD but not blood pressure or pulmonary function: a 2 week, double-blind randomised, placebo-controlled crossover trial. Int J Food Sci Nutr 2019;70:222–31. 10.1080/09637486.2018.1492521 [DOI] [PubMed] [Google Scholar]
- 19.Pavitt MJ, Tanner RJ, Lewis A, et al. Oral nitrate supplementation to enhance pulmonary rehabilitation in COPD: ON-EPIC a multicentre, double-blind, placebo-controlled, randomised parallel group study. Thorax 2020;75:547–55. 10.1136/thoraxjnl-2019-214278 [DOI] [PubMed] [Google Scholar]
- 20.Kenjale AA, Ham KL, Stabler T, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 2011;110:1582–91. 10.1152/japplphysiol.00071.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggebeen J, Kim-Shapiro DB, Haykowsky M, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 2016;4:428–37. 10.1016/j.jchf.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamani P, Rawat D, Shiva-Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015;131:371–80. 10.1161/CIRCULATIONAHA.114.012957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopkinson NS, Molyneux A, Pink J, et al. Chronic obstructive pulmonary disease: diagnosis and management: summary of updated NICE guidance. BMJ 2019;366:l4486. 10.1136/bmj.l4486 [DOI] [PubMed] [Google Scholar]
- 24.Elbourne DR, Altman DG, Higgins JPT, et al. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–9. 10.1093/ije/31.1.140 [DOI] [PubMed] [Google Scholar]
- 25.Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: www.cochrane-handbook.org
- 26.Beijers RJHCG, Huysmans SMD, van de Bool C, et al. The effect of acute and 7-days dietary nitrate on mechanical efficiency, exercise performance and cardiac biomarkers in patients with chronic obstructive pulmonary disease. Clin Nutr 2018;37:1852–61. 10.1016/j.clnu.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 27.Henrohn D, Björkstrand K, Lundberg JO, et al. Effects of oral supplementation with nitrate-rich beetroot juice in patients with pulmonary arterial hypertension—results from BEET-PAH, an exploratory randomized, double-blind, placebo-controlled, crossover study. J Card Fail 2018;24:640–53. 10.1016/j.cardfail.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 28.Behnia M, Wheatley CM, Avolio A, et al. Influence of dietary nitrate supplementation on lung function and exercise gas exchange in COPD patients. Nitric Oxide 2018;76:53–61. 10.1016/j.niox.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 29.Curtis KJ, O’Brien KA, Tanner RJ, et al. Acute dietary nitrate supplementation and exercise performance in COPD: a double-blind, placebo-controlled, randomised controlled pilot study. PLoS One 2015;10:e0144504. 10.1371/journal.pone.0144504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friis AL, Steenholt CB, Løkke A, et al. Dietary beetroot juice–effects on physical performance in COPD patients: a randomized controlled crossover trial. Int J Chron Obstruct Pulmon Dis 2017;12:12. 10.2147/COPD.S135752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong P, Basham JE, Yong T, et al. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm Med 2015;15:1–9. 10.1186/s12890-015-0057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepherd AI, Wilkerson DP, Dobson L, et al. The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: a double blind placebo controlled, randomised control trial. Nitric Oxide 2015;48:31–7. 10.1016/j.niox.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochrane training. GRADE approach 2021.
- 35.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001;357:593–615. 10.1042/bj3570593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008;7:156–67. 10.1038/nrd2466 [DOI] [PubMed] [Google Scholar]
- 37.Wylie LJ, Kelly J, Bailey SJ, et al. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 2013;115:325–36. 10.1152/japplphysiol.00372.2013 [DOI] [PubMed] [Google Scholar]
- 38.Hobbs DA, Kaffa N, George TW, et al. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br J Nutr 2012;108:2066–74. 10.1017/S0007114512000190 [DOI] [PubMed] [Google Scholar]
- 39.Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015;65:320–7. 10.1161/HYPERTENSIONAHA.114.04675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaouat A, Bugnet A-S, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:189–94. 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Wang X-J, Zhang H-D, et al. Profiling nitric oxide metabolites in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2016;48:1386–95. 10.1183/13993003.00245-2016 [DOI] [PubMed] [Google Scholar]
- 42.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 2003;35:790–6. 10.1016/S0891-5849(03)00406-4 [DOI] [PubMed] [Google Scholar]
- 43.Deykin A, Massaro AF, Drazen JM, et al. Exhaled nitric oxide as a diagnostic test for asthma: online versus offline techniques and effect of flow rate. Am J Respir Crit Care Med 2002;165:1597–601. 10.1164/rccm.2201081 [DOI] [PubMed] [Google Scholar]
- 44.Smith AD, Cowan JO, Filsell S, et al. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med 2004;169:473–8. 10.1164/rccm.200310-1376OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-000948supp001.pdf (102.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.