Abstract
In the present study, we investigated the potential role of insulin-like growth factor I (IGF-I) receptor (IGF-IR) in cell proliferation by overexpressing it in 32D myeloid progenitor cells. The overexpression of IGF-IR caused the transfectants to proliferate in response to IGF-I in the absence of insulin receptor substrate (IRS) expression. The activation of overexpressed wild-type IGF-IR, but not that of an ATP-binding mutant of IGF-IR, resulted in the increased tyrosine phosphorylation of several intracellular proteins, including SHC, Src homology 2-containing inositol-5-phosphatase, protein kinase C-δ, and Erk2. Grb2 association with SHC and mitogen-activated protein kinase (MAPK) activity was also enhanced in response to IGF-I stimulation. Interestingly, the stimulation of the IGF-IR transfectants with interleukin 4 (IL-4) also resulted in strong mitogenesis independent of IRS expression. Moreover, IGF-I and/or IL-4 induced long-term cell growth of the IGF-IR transfectants. IL-4 was able to synergize with IGF-I for DNA synthesis, even in the parental 32D cells and a pro-B-cell line, Baf3, indicating the physiological importance of the two growth factors in hematopoietic cell proliferation. IL-4 stimulation of the IGF-IR transfectants resulted in enhanced tyrosine phosphorylation of SHC, Erk2, and signal transducer and activator of transcription 6 (STAT6) proteins. Both IL-4 and IGF-I were able to induce c-myc early response gene expression, and this expression was maximal in the presence of both factors. Finally, we demonstrated that a MAPK kinase inhibitor was able to suppress mitogenesis of the IGF-IR transfectants in response to IGF-I and/or IL-4. Together, our results suggest that IL-4 synergizes with IGF-I for hematopoietic cell proliferation, likely through cross talk between SHC/Grb2/MAPK and STAT6 pathways and through c-myc gene up-regulation.
Insulin-like growth factor I (IGF-I) receptor (IGF-IR) is a type II receptor protein-tyrosine kinase and has approximately 70% sequence homology with the insulin receptor (IR) (41, 42). The binding of IGF-I to IGF-IR activates the intrinsic tyrosine kinase, resulting in receptor autophosphorylation and the presentation of suitable binding sites for substrates containing either Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains (3). The phosphorylated tyrosine residue 950 within the juxtamembrane domain of the IGF-IR β chain has been defined as the major interacting site for SHC and insulin receptor substrate 1 (IRS-1) and IRS-2 binding through their respective PTB domains (12, 14, 15, 30). Tyrosine phosphorylation of SHC and IRS molecules by the activated receptor subsequently stimulates downstream signaling molecules. While SHC activation has been directly linked to the Grb2/SOS/Ras/Raf/mitogen-activated protein kinase (MAPK) kinase (MEK)/MAPK cascade (7, 11), tyrosine phosphorylation of IRS molecules within different motifs is responsible for recruiting many signaling molecules and for their subsequent activation (47, 48). For example, the p85 subunit of phosphatidylinositol 3′ kinase (PI 3′K), protein tyrosine phosphatase 1D, Grb2, Lyn, and Nck have been shown to interact with tyrosine-phosphorylated IRS molecules through their respective SH2 domains. The activation of SHC/Grb2/SOS/Ras/Raf/MEK/MAPK and IRS/PI 3′K/Akt/p70S6K cascades has been implicated in IGF-IR signal transduction, leading to cell proliferation, differentiation, antiapoptosis, and tumor development (3).
Although the IR has great similarity to IGF-IR and most substrates are phosphorylated to a similar extent in response to both insulin and IGF-I stimulation, the biological responses resulting from the activation of these two receptor tyrosine kinases differ greatly (4). While stimulation of the IR pathway is mainly involved in glucose metabolism, the activation of IGF-IR is implicated in cell proliferation and transformation.
Using the 32D myeloid progenitor cell line as a model system, our group and others have previously attempted to understand the signal transduction of the IR leading to mitogenesis. The interleukin 3 (IL-3)-dependent 32D cells endogenously express the IR but lack the expression of IRS-1 and -2 molecules (40, 45). While the overexpression of the IR alone did not induce significant mitogenesis in response to insulin, the coexpression of the IR with either IRS-1 or IRS-2 rendered these double transfectants fully mitogenic in response to insulin (40, 45). Moreover, when the IL-4 receptor (IL-4R), a member of the cytokine receptor subfamily which lacks the intrinsic tyrosine kinase activity in its intracellular region (18), was coexpressed with IRS molecules in 32D cells, full mitogenesis was induced in response to IL-4 stimulation (45). On the other hand, ectopic expression of IRS-1 or -2 in 32D cells was not able to mediate mitogenesis in response to IL-4 or insulin (40, 45). These results clearly suggest that the overexpressed IR and IL-4R are able to utilize the IRS signaling pathway to initiate cell proliferation in the hematopoietic cell background (40, 43, 45).
Although IGF-IR has been demonstrated to be very critical for mitogenesis and cell transformation of cells of fibroblast origin (2, 3), its role in hematopoietic cell proliferation has not been thoroughly investigated. In this study, we overexpressed IGF-IR in 32D cells in order to test its potential role in myeloid proliferation. Our results showed that the IGF-IR transfectants were able to initiate DNA synthesis not only in response to IGF-I but also to IL-4 in the absence of IRS expression and activation. Furthermore, these two growth factors mediated long-term cell growth of the IGF-IR transfectants. IL-4 synergized with IGF-I to initiate DNA synthesis in both naive 32D and Baf3 hematopoietic cell lines. While PI 3′K activation appeared not to be involved in IGF-I- and IL-4-induced mitogenesis, stimulation of the SHC/Grb2/MAPK cascade was shown to play a pivotal role in both IGF-I- and IL-4-induced mitogenesis. Finally, c-myc gene up-regulation and enhanced SHC, Erk2, and signal transducer and activator of transcription 6 (STAT6) tyrosine phosphorylation correlated with IL-4-induced proliferation of the IGF-IR transfectants.
MATERIALS AND METHODS
Cell culture, transfection, and cDNAs.
32D and Baf3 hematopoietic cells were maintained in RPMI 1640 media containing 15% fetal calf serum (FCS) and 5% WEHI-3 cell culture supernatant as the source of IL-3. Transfection of different cDNAs into 32D cells has been previously described (27). The cloning of wild-type (WT) IGF-IR and NM1, a truncation mutant of IGF-IR, into pMEX vector was previously reported (17, 25, 26). The construction of an ATP binding site mutant of IGF-IR (IGF-IRKR) into pBPV vector was also described before (8). Since pBPV vector does not contain a drug-resistant gene, a pMEX vector was cotransfected with pBPV-IGF-IRKR in a molar ratio of 1 to 10, and neomycin-resistant cells were selected in the presence of 750 μg of G418 (Gibco BRL) per ml. The transfection and expression of IL-4R, IR, and IRS-1 into 32D cells have been documented previously (45).
Mitogenic assay.
Transfectants of 32D cells and 32D and Baf3 cell lines were washed twice with Dulbecco’s phosphate-buffered saline. The number of cells was determined with a cell counter (Coulter). A total of 2 × 105 cells were plated onto each well of 24-well plates in RPMI 1640 media containing only 15% FCS without IL-3. Human IGF-I (Intergen), murine IL-4 (Intergen), and human insulin (Upstate Biotechnology Inc. [UBI]) at a concentration of 100 ng/ml or at other concentrations were added to each well. Wortmannin (Calbiochem) and PD98059 (Calbiochem) were included at concentrations of 100 nM and 20 μM, respectively, in a mitogenic assay. After 44 h in culture, the cells were pulsed with 1 μCi of [3H]thymidine (TdR; Amersham) for another 4 h and harvested with a cell harvester (Skatron). Dried filters were soaked in scintillation liquid, and the number of counts per minute was measured with a beta counter (Beckman). The mean values from triplicate wells were calculated and plotted in each corresponding figure together with standard deviations.
Long-term cell growth.
Each 32D line was plated in a six-well Costar plate (2 × 105 cells/well) with RPMI 1640 media containing only 15% FCS in the absence or presence of various growth factors. Cell numbers were counted every other day till day 6 by using trypan blue exclusion staining.
Lysing of cells, immunoprecipitation, and immunoblot analysis.
32D cells and transfectants were serum and IL-3 starved for 2 h, stimulated with IGF-I and/or IL-4 (100 ng/ml) for 10 min, and lysed in a buffer containing Triton X-100 (21). The protein concentration was determined by using a kit from Bio-Rad. Equivalent amounts of cell lysates were immunoprecipitated with anti-phosphotyrosine (25 μl of anti-pTyr conjugated to protein A beads; UBI), polyclonal anti-SHC (1 μg per sample; Signal Transduction Laboratory), anti-SHIP (5 μl per sample; Santa Cruz), or anti-STAT6 (1 μg per sample; Santa Cruz) together with 40 μl of protein G beads (Pharmacia). Washed immunoprecipitates were separated by sodium dodecyl sulfate (SDS)–8% polyacrylamide gel electrophoresis (PAGE), and the proteins transferred onto Immobilon membranes (Millipore) were immunoblotted with anti-pTyr (UBI; 1 μg/ml), anti-SHC (1:1,000), anti-SHIP (1:200), anti-STAT6 (1:1,000), or anti-Erk2 (1:1,000; UBI). The protein bands were subsequently detected with the ECL Western blot detection system (Amersham). For direct immunoblot analysis, denatured protein samples (100 μg per sample) were directly subjected to SDS-PAGE, and transferred proteins were immunoblotted with anti-IGF-IR β chain (1:500; Santa Cruz).
PI 3′K activity assay.
Cells were similarly treated and lysed as described above. Equivalent amounts of cell lysates were immunoprecipitated with anti-pTyr and subjected to a PI 3′K activity assay by measuring the phosphorylation of PI to yield phosphatidylinositol phosphate (PIP) as previously reported (50).
MAPK activity assay.
Cells were similarly treated as above and lysed in a buffer which contained 20 mM HEPES (pH 7.5), 10 mM EGTA, 40 mM β-glycerophosphate, 1% Nonidet P-40, 2.5 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 20 μg of aprotinin per ml, and 20 μg of leupeptin per ml. Equivalent amounts of cell lysates were immunoprecipitated with anti-Erk2 antibody (Santa Cruz). Washed immunoprecipitates were incubated with a substrate buffer which contained 12.5 mM MOPS (morpholinepropanesulfonic acid) (pH 7.5), 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM sodium fluoride, 0.5 mM Na3VO4, 1 μCi of [γ-32P]ATP, 20 mM cold ATP, 3.3 μM dithiothreitol, and 1.5 mg of myelin basic protein (MBP) (Sigma) per ml at 30°C for 20 min. The reaction was stopped by adding 30 μl of a 2× sample buffer to the reaction mixture. The proteins were separated by SDS–12% PAGE, and the dried gel was autoradiographed.
Northern blot analysis.
Total RNA was isolated by using Trizol (Gibco BRL) according to the instructions from the company. Fifteen micrograms of total RNA was electrophoresed on a denaturing, 1.2% agarose gel. The fractionated RNAs were immobilized on a charged nylon membrane (Schleicher and Schuell) by capillary transfer, and the membrane was baked at 80°C. The 1.3-kb c-myc gene was isolated from the encoding plasmid (kindly provided by Frederick Mushinski) and labeled by random priming. The 40-mer β-actin probe (Oncogene Science) was end labeled with T4 polynucleotide kinase. Hybridization and washing procedures were performed as described previously (22).
Densitometric and statistical analyses.
The intensities of protein bands derived from enhanced chemiluminescence detection were quantified with the scan analysis program from Biosoft. Northern blot results were quantified by PhosphorImager (Molecular Dynamics). Data were analyzed by using one-factor analysis of variance (StatView 512) at 99 or 95% significant levels.
RESULTS
Overexpression of IGF-IR in 32D cells induces mitogenesis in response not only to IGF-I but also to IL-4.
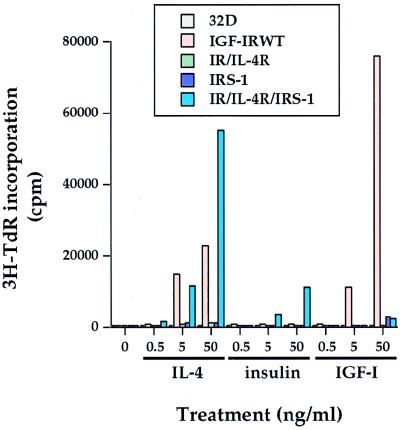
To investigate the potential role played by IGF-IR in hematopoietic cell proliferation, we overexpressed WT IGF-IR (IGF-IRWT), a constitutively activated IGF-IR (NM1), and an ATP binding site mutant of IGF-IR (IGF-IRKR) into 32D myeloid progenitor cells. Although 32D cells express some endogenous IGF-IR (see Fig. 6B and references 36 and 51), the stimulation of 32D cells with IGF-I did not induce any detectable [3H]TdR incorporation (Fig. 1). In striking contrast, the overexpression of IGF-IRWT induced IGF-I dose-dependent mitogenesis. As reported before (45), the expression of IL-4R or IR alone did not render these transfectants fully responsive to the corresponding ligands for mitogenesis. The expression of IRS-1 alone did not mediate any detectable mitogenesis, either. However, when IRS-1 was coexpressed with the receptors (IR/IL-4R/IRS-1), both insulin and IL-4 were able to induce mitogenesis (Fig. 1) (45). Although IGF-I was able to induce strong mitogenesis, insulin stimulation of the IGF-IR transfectants did not give rise to mitogenesis at the concentration utilized. Conversely, IGF-I did not elicit any detectable mitogenesis in the IR/IL-4R/IRS-1 transfectants. Unexpectedly, the addition of IL-4 to the IGF-IRWT transfectants induced a very strong mitogenic response in an IL-4 dose-dependent manner. The induction of mitogenesis of 32D/IGF-IR transfectants in response to both IGF-I and IL-4 was reproducibly detected by using the transfectants with different expression levels of IGF-IR (data not shown). Taken together, these results demonstrate that IGF-I can induce mitogenesis of 32D cells in the absence of IRS expression when sufficient amounts of IGF-IR are present, in sharp contrast to the IR in the same cell system (45). In addition, our results suggest that IL-4 can cooperate with overexpressed IGF-IR, which possesses a basal level of activation (see below) to mediate mitogenesis in the hematopoietic cell background.
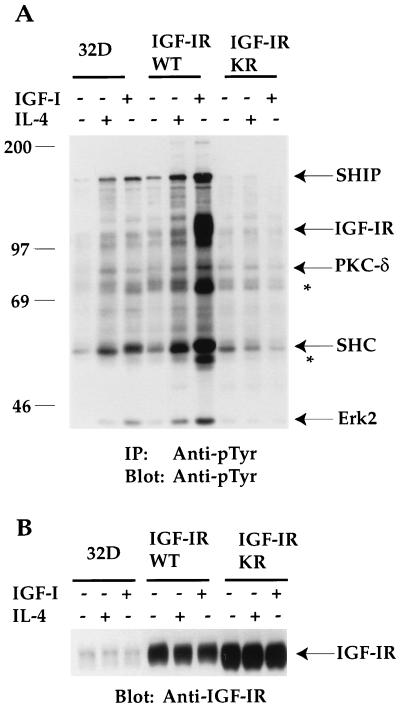
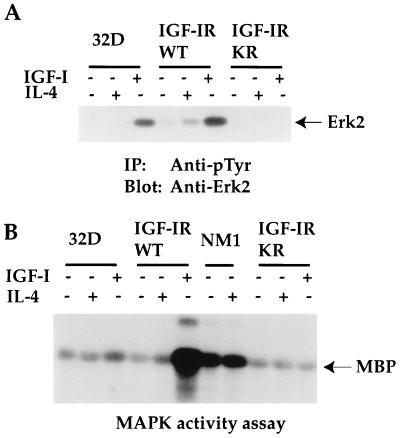
FIG. 6.
Overexpression of IGF-IRWT, but not that of the ATP binding mutant of IGF-IR (IGF-IRKR) leads to tyrosine phosphorylation of several intracellular proteins in response to IGF-I and IL-4 stimulation. The sizes of proteins (in kilodaltons) are given in numbers. (A) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-pTyr. Transferred proteins on Immobilon membranes were immunoblotted with the same antibody. Asterisks denote unknown proteins, whereas known proteins are indicated by their names. PKC-δ, protein kinase C-δ. (B) 32D cells and their transfectants were treated as described in the legend to panel A. Equivalent cell lysates (100 μg per lane) were subjected to SDS-PAGE, and transferred proteins were immunoblotted with anti-IGF-IR serum. The position of IGF-IR is indicated.
FIG. 1.
Overexpression of IGF-IR in 32D cells leads to IRS-independent mitogenesis in response not only to IGF-I but also to IL-4. 32D cells and transfectants were washed twice with Dulbecco’s phosphate-buffered saline and maintained in RPMI 1640 media containing 15% FCS in the presence of the various ligands at different doses. [3H]TdR was added after a period of 44 h in culture. Cells were harvested, and the number of counts per minute was measured. Duplicate wells were used in this particular mitogenic assay.
The mitogenic effects of IGF-I and IL-4 rely on the intrinsic tyrosine kinase activity of IGF-IR.
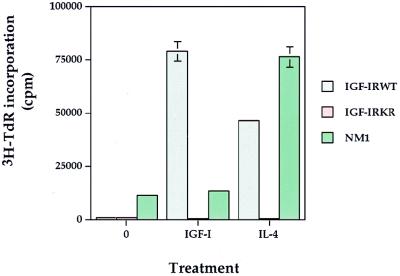
To determine whether IGF-I- and IL-4-induced mitogenesis of 32D/IGF-IR transfectants was dependent on the kinase activity of IGF-IR, we expressed either an ATP binding mutant of IGF-IR (IGF-IRKR) or a constitutively activated IGF-IR, designated NM1, in 32D cells and tested for their ability to induce mitogenesis in response to IGF-I and IL-4. The NM1 mutant was generated by deleting the entire extracellular domain of IGF-IR and fusing the remaining receptor with the N-terminal gag sequence of the avian sarcoma virus UR2 (25, 26). As seen in Fig. 2, IGF-IRWT transfectants were able to mediate mitogenesis in response to both IGF-I and IL-4 stimulation. In contrast, the expression of the IGF-IRKR mutant abolished mitogenesis from both IGF-I and IL-4, despite similar levels of IGF-IRKR and IGF-IRWT proteins expressed in the respective transfectants (see Fig. 6B). The basal level of [3H]TdR incorporation observed in NM1 transfectants reflected the constitutive activation of IGF-IR. As expected, NM1 transfectants did not respond to IGF-I for mitogenesis due to a deletion of the ligand-binding domain of IGF-IR. However, NM1 transfectants were still mitogenic to IL-4. These results clearly demonstrate that IGF-I-induced mitogenesis in 32D cells requires increased IGF-IR expression and that IL-4 can cooperate with the activated IGF-IR signaling pathway to induce mitogenesis.
FIG. 2.
The mitogenic effects of IGF-I and IL-4 rely on the intrinsic tyrosine kinase activity of overexpressed IGF-IR. The mitogenic assay was performed as described in the legend to Fig. 1, except that IL-4 and IGF-I were given at concentrations of 100 ng/ml. Standard deviations are shown by bars.
IGF-I and IL-4 are able to induce long-term cell growth of the IGF-IR transfectants.
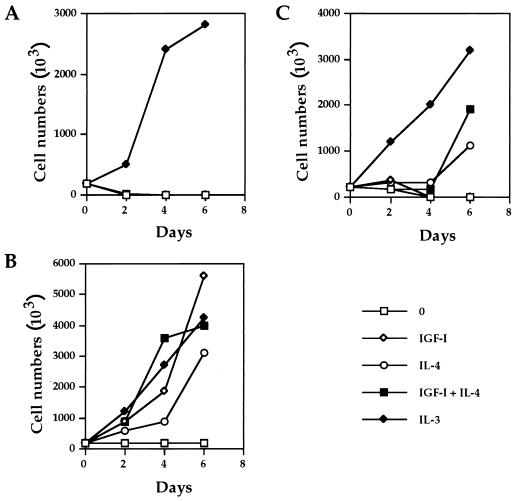
To correlate the DNA synthesis with cell proliferation in response to IGF-I and IL-4, we determined the level of long-term growth of the IGF-IR transfectants. As shown in Fig. 3A, IGF-I and/or IL-4 did not stimulate any cell proliferation of the parental 32D cells. As expected, the addition of IL-3 resulted in continuous proliferation and division of 32D cells. When IGF-IR was overexpressed, the addition of IGF-I and/or IL-4 supported long-term cell growth at rates similar to that of IL-3 (Fig. 3B). In the NM1 transfectants, IGF-I did not promote permanent growth due to the lack of IGF-I binding site within the NM1 construct (Fig. 3C). On the other hand, IL-4 alone or together with IGF-I caused these transfectants to proliferate. We reproducibly observed synergistic long-term growth as well as short-term DNA synthesis in response to IGF-I plus IL-4 in those IGF-IR transfectants which express relatively low levels of IGF-IR (data not shown). Together, the results demonstrate that IGF-I and IL-4 are able to mediate the long-term cell proliferation of 32D/IGF-IR transfectants in addition to short-term DNA synthesis.
FIG. 3.
IGF-I and IL-4 are able to mediate long-term proliferation of the IGF-IRWT transfectants. 32D (A), 32D/IGF-IRWT (B), and 32D/NM1 (C) cell lines were plated in six-well Costar plates and maintained in RPMI 1640 media containing 15% FCS with or without IGF-I (100 ng/ml), IL-4 (100 ng/ml), and IL-3 (5 ng/ml). Cell numbers were counted every other day until day 6.
IL-4 synergizes with IGF-I for hematopoietic cell mitogenesis.
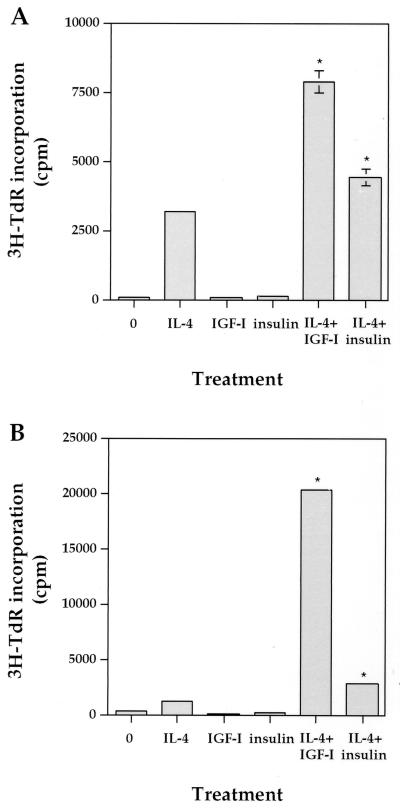
To further extend our study of IL-4 synergy with overexpressed IGF-IR in a more physiological condition, we tested their synergistic effect on DNA synthesis of two hematopoietic lines, 32D and Baf3. IL-4 stimulation of 32D cells resulted in very low [3H]TdR incorporation levels (about 3,000 cpm), while IGF-I and insulin alone did not induce any response (Fig. 4A). Interestingly, the coaddition of IL-4 and IGF-I induced DNA synthesis 2.5-fold higher than IL-4 alone, suggesting a synergistic effect of IL-4 and IGF-I on short-term mitogenesis. Insulin was also able to synergize with IL-4 to increase mitogenesis, but the effect was weaker than that of IGF-I.
FIG. 4.
IL-4 synergizes with IGF-I for hematopoietic cell mitogenesis. Mitogenic assays for 32D cells (A) and Baf3 cells (B) were performed as described in the legend to Fig. 1, and IL-4, IGF-I, and insulin were added at concentrations of 100 ng/ml. Standard deviations are shown by bars, and deviations for some samples are too small to be shown. Asterisks indicate statistical significance when compared to IL-4 alone (P < 0.01).
Like the 32D line, the pro-B-cell Baf3 line is also dependent on IL-3 for cell growth. When Baf3 cells were subjected to a mitogenic assay, weak [3H]TdR incorporation was also observed in response to IL-4 stimulation (Fig. 4B). Again, neither IGF-I nor insulin induced mitogenesis. Strikingly, the coaddition of IL-4 with IGF-I resulted in 16.5-fold more [3H]TdR uptake than that of IL-4 alone. On the other hand, the synergistic effect of insulin with IL-4 was much weaker. Taken together, our results demonstrate that IL-4 can synergize with IGF-I for DNA synthesis in two hematopoietic cell lines, providing evidence for the physiological interactions of these two factors in hematopoietic cell growth. The weaker synergistic effect of IL-4 with insulin than that of IGF-I on mitogenesis supports the concept that the IGF-I signaling pathway is more involved in cell proliferation.
PI 3′K activation is not responsible for IGF-I- and IL-4-induced mitogenesis of 32D/IGF-IR transfectants.
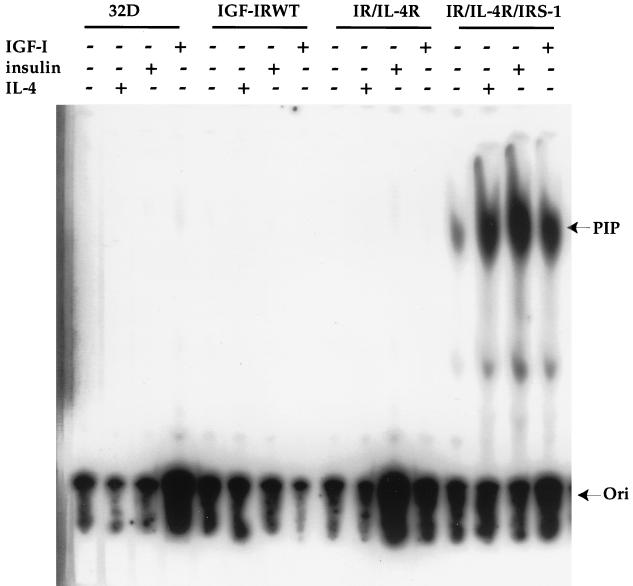
Recently, a role for PI 3′K in the IR-induced and IRS-dependent mitogenic response was established by mutating tyrosine residues within the IRS-1 molecule (28). Since 32D cells do not endogenously express IRS-1 (45), -2 (40), and -4 (data not shown), we tested whether the overexpression of IGF-IR in 32D cells would bypass IRS to activate PI 3′K and subsequently mediate mitogenesis. Therefore, a PI 3′K activity assay was performed to measure the PIP from all the transfectants in response to various stimuli. As shown in Fig. 5, the coexpression of IR and IL-4R with IRS-1 (IR/IL-4R/IRS-1) resulted in PI 3′K activation in response to both insulin and IL-4. In the same transfectants, IGF-I stimulation of endogenous IGF-IR also induced relatively high PI 3′K activity. On the other hand, no detectable PI 3′K activity from anti-pTyr immunoprecipitates was observed in both IGF-IRWT and IR/IL-4R transfectants, although the former was capable of inducing mitogenesis in response to both IGF-I and IL-4. Since endogenous IGF-IR induced PI 3′K activity with transfected IRS-1 but did not mediate mitogenesis (Fig. 1) and overexpressed IGF-IR induced mitogenesis without PI 3′K activation, we conclude that PI 3′K is not likely to be involved in overexpressed IGF-IR-mediated cell proliferation in response to both IGF-I and IL-4. This conclusion is substantiated by the inability of a PI 3′K inhibitor to suppress IGF-I- and IL-4-induced mitogenesis (see Fig. 10B). Certainly, our data do not exclude the possibility that the activation of PI 3′K may still be required for IL-4- and insulin-driven mitogenesis through IRS-1 tyrosine phosphorylation in the IR/IL-4R/IRS-1 transfectants.
FIG. 5.
PI 3′K activation is not responsible for IGF-I- and IL-4-induced mitogenesis of the IGF-IR transfectants. 32D cells and transfectants were serum starved for 2 h and stimulated with IL-4, insulin, or IGF-I for 10 min. Equivalent cell lysates were immunoprecipitated with anti-pTyr and subjected to PI 3′K assay as previously described (50). The origin (Ori) of the loading and PIP products are marked by arrows.
FIG. 10.
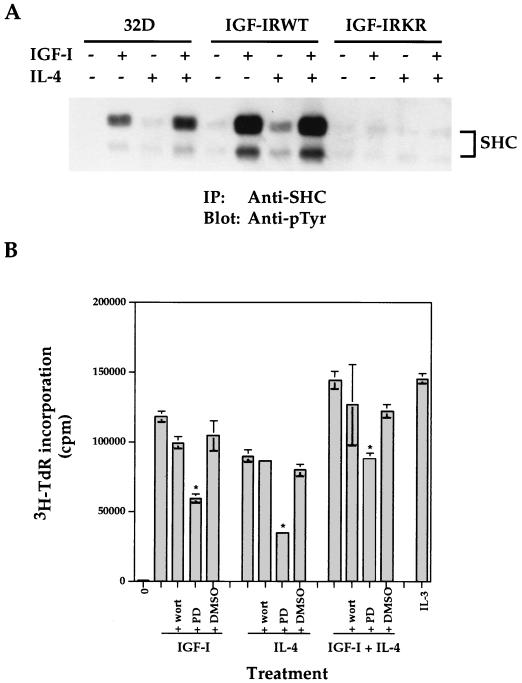
IL-4 synergizes with IGF-I for activation of the SHC/MAPK pathway. (A) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I and/or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-SHC. Transferred proteins were immunoblotted with anti-pTyr. The position of tyrosine-phosphorylated SHC is indicated. (B) The IGF-IRWT transfectants were subjected to a mitogenic assay in the presence of IGF-I (100 ng/ml) and/or IL-4 (100 ng/ml) with or without 100 nM wortmannin (wort), 20 μM PD98059 (PD), or 1 μl of DMSO per well. IL-3 (5 ng/ml) was included as a positive control. Asterisks indicate statistical significance of inhibition by PD98059 (P < 0.01) when compared to the addition of ligand alone. Error bars indicate standard deviations.
Overexpression of IGF-IRWT leads to enhanced tyrosine phosphorylation of intracellular proteins.
To seek insight into the potential mechanisms leading to IGF-IR-mediated cell proliferation other than IRS/PI 3′K activation, we first tested whether tyrosine phosphorylation of intracellular proteins would be affected by the expression of the WT or the ATP binding site mutant of IGF-IR. As shown in Fig. 6A, the stimulation of 32D cells with IGF-I and IL-4 led to tyrosine phosphorylation of intracellular proteins of 145, 110, 80, 72, 52, and 42 kDa. IGF-I or IL-4 stimulation of IGF-IRWT transfectants increased tyrosine phosphorylation of these proteins, compared to that of the 32D parental line. In addition, tyrosine phosphorylation of a 100-kDa protein was observed in response to IGF-I in the IGF-IRWT transfectants. On the other hand, the expression of IGF-IRKR in 32D cells suppressed tyrosine phosphorylation of most proteins detected in 32D cells and those enhanced in the IGF-IRWT transfectants, indicating a dominant inhibitory effect of the mutant molecule in suppressing endogenous IGF-IR activation. Subsequently, it was demonstrated that the 145-, 100-, 80-, 52-, and 42-kDa proteins represent SH2-containing inositol-5-phosphatase (SHIP) (Fig. 7), IGF-IR (data not shown), protein kinase C-δ (21), SHC (Fig. 7), and Erk2 of MAPK (see Fig. 9A), respectively. The identities of the other tyrosine phosphoproteins remain to be determined (Fig. 6A).
FIG. 7.
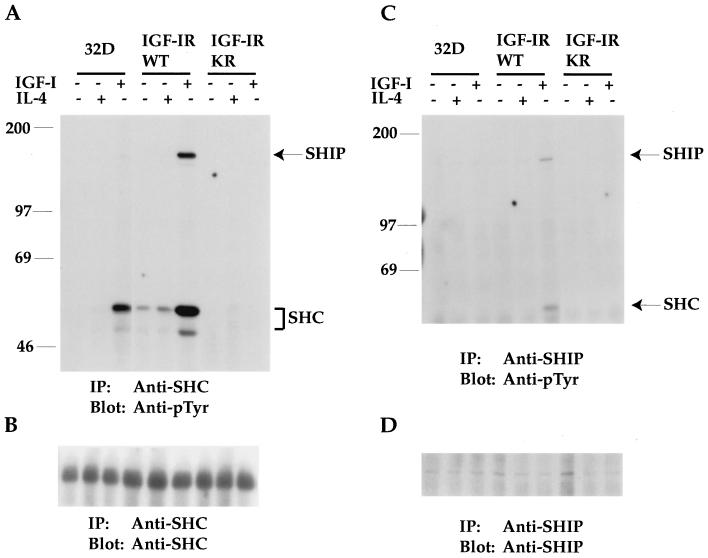
SHIP is tyrosine phosphorylated and associated with SHC in response to IGF-I stimulation of 32D/IGF-IR transfectants. (A) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-SHC. Transferred proteins were immunoblotted with anti-pTyr. The positions of SHC and pp145 SHIP are indicated. (B) The same Immobilon membrane shown in panel A was reblotted with anti-SHC serum. (C) Cells were similarly treated as described in the legend to panel A. Equivalent cell lysates were immunoprecipitated with an anti-SHIP antibody followed by anti-pTyr immunoblot analysis. (D) The same Immobilon membrane shown in panel C was reblotted with anti-SHIP serum. The sizes of proteins (in kilodaltons) are given in numbers in panels A and C.
FIG. 9.
Tyrosine phosphorylation of Erk2 and MAPK activity are greatly enhanced in response to IGF-I stimulation in the IGF-IR transfectants. (A) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-pTyr. Transferred proteins were immunoblotted with anti-Erk2 serum. The position of the 42-kDa Erk2 protein is indicated. (B) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated with an anti-Erk2 antibody. Washed immunoprecipitates were subjected to an in vitro MAPK activity assay with MBP as a substrate. The position of phosphorylated MBP is indicated.
Immunoblot analysis with anti-IGF-IR serum indicates that IGF-IR protein levels were increased by 6.8- and 9.2-fold, respectively, in the IGF-IRWT and IGF-IRKR transfectants, compared to endogenous IGF-IR (Fig. 6B). Taken together, our results demonstrate that the overexpression of IGF-IRWT, but not that of the KR mutant, leads to both IGF-I- and IL-4-dependent tyrosine phosphorylation of several intracellular substrates which may play positive roles in IGF-I- and IL-4-induced proliferation.
SHIP is tyrosine phosphorylated in response to IGF-I stimulation.
To identify the substrates with enhanced phosphorylation in response to IGF-I in the IGF-IRWT transfectants, we first tested for SHC tyrosine phosphorylation. SHC has been defined as a substrate in addition to IRS in the IGF-IR signaling pathway and is known to play an important role in IGF-IR-mediated cell proliferation and transformation (6, 38). Tyrosine phosphorylation of SHC was detected by the immunoprecipitation of cell lysates with an anti-SHC serum, followed by anti-pTyr immunoblot analysis from 32D cells in response to IGF-I stimulation (Fig. 7A). Some constitutive phosphorylation of SHC was observed from IGF-IRWT transfectants, indicating that overexpressed IGF-IR may possess some constitutive kinase activity. This phosphorylation was greatly enhanced in response to IGF-I stimulation. In contrast, the expression of the IGF-IRKR mutant abolished tyrosine phosphorylation of SHC in response to IGF-I.
A 145-kDa tyrosine-phosphorylated protein was also detected from anti-SHC immunoprecipitates of 32D cells in response to IGF-I stimulation (Fig. 7A). The phosphorylation of this protein was increased in the IGF-IRWT transfectants, which possessed the strongest tyrosine phosphorylation of SHC. On the other hand, pp145 was not detected from anti-SHC immunoprecipitates of IGF-IRKR transfectants. These results suggest that pp145 is a substrate of IGF-IR and can associate with SHC in an IGF-I-dependent fashion. Subsequent reblotting of the same membrane with an anti-SHC antibody demonstrated that similar amounts of SHC proteins were immunoprecipitated and loaded on the gel (Fig. 7B).
Several proteins, including SHIP (10, 23) and IRS-4 (20), are within the size range of 140 to 160 kDa and are potentially tyrosine phosphorylated in vivo. Since IRS-4 is not expressed in 32D cells (data not shown), we tested whether SHIP could be pp145. As shown in Fig. 7C, immunoprecipitated SHIP protein from 32D cells was weakly tyrosine phosphorylated in response to IGF-I and this phosphorylation was greatly enhanced in IGF-IRWT transfectants. More importantly, a pp52 protein was associated with tyrosine-phosphorylated SHIP in response to IGF-I from IGF-IRWT transfectants (Fig. 7C). It was subsequently confirmed that this SHIP-associating protein was SHC (data not shown). Reblotting the membrane shown in Fig. 7C with SHIP indicated similar loading among the samples (Fig. 7D). Together, these results confirm that SHC is a major substrate of IGF-IR, which becomes highly phosphorylated upon IGF-IR overexpression. We also provide the first evidence that SHIP is tyrosine phosphorylated by IGF-IR activation and associates with SHC in vivo in an IGF-I-dependent manner. Much weaker tyrosine phosphorylation of SHIP by anti-SHIP immunoprecipitation than anti-pTyr immunoprecipitation may be due to the lower affinity of the anti-SHIP serum (Fig. 6A and 7C).
Activation of SHC by IGF-IR results in Grb2 association in vivo.
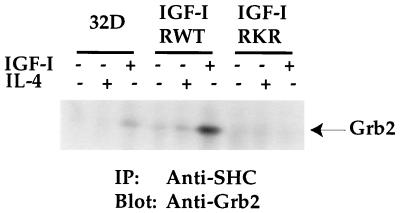
Grb2 has been defined as a downstream signaling molecule of SHC activation through interactions of its SH2 domain with the tyrosine-phosphorylated SHC (16, 39). To show a direct association of SHC with Grb2, equivalent amounts of proteins from each cell line after various treatments were immunoprecipitated with anti-SHC serum followed by anti-Grb2 immunoblot analysis. As shown in Fig. 8, Grb2 was weakly detectable from SHC immunoprecipitates of 32D cells after IGF-I stimulation. Again, some constitutive association of these two molecules was detected in the IGF-IRWT transfectants. However, IGF-I stimulation resulted in the maximal association of these two molecules in the IGF-IRWT transfectants. The expression of the IGF-IRKR mutant fully suppressed this association. Taken together, these results demonstrate that maximal SHC tyrosine phosphorylation and Grb2 association occur in the IGF-IRWT transfectants, which correlates with IGF-I-induced mitogenesis in the same transfectants.
FIG. 8.
Activation of SHC by IGF-IR results in Grb2 association in an IGF-I-dependent manner. 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-SHC. Transferred proteins were immunoblotted with anti-Grb2 serum. The position of Grb2 is indicated.
MAPK activity in IGF-IR transfectants is increased in response to IGF-I stimulation.
To search for the downstream signaling pathway leading to IGF-I-induced mitogenesis, we tested for MAPK activation, a known signaling molecule downstream of the SHC/Grb2/Ras/Raf/MEK cascade. As shown in Fig. 9A, Erk2 tyrosine phosphorylation was strongly detected in 32D cells in response to IGF-I but was detected only weakly in response to IL-4 stimulation. The basal phosphorylation of Erk2 in the IGF-IRWT transfectants was 9.7-fold higher than that of 32D cells, indicating the constitutive activation of IGF-IR due to its overexpression. IGF-I stimulation of the IGF-IRWT transfectants resulted in maximal tyrosine phosphorylation of Erk2. IL-4 stimulation of the IGF-IRWT transfectants also caused a 3.3-fold increase in phosphorylation when compared to the basal level in the same transfectants. Again, Erk2 phosphorylation was inhibited by the expression of the IGF-IRKR mutant.
A MAPK activity assay was subsequently performed to correlate with the results of Erk2 tyrosine phosphorylation (Fig. 9B). Basal phosphorylation of MBP was detected from 32D cells, and this phosphorylation was slightly increased in response to IGF-I but not to IL-4 stimulation. The MAPK activity of the IGF-IRWT transfectants was increased at least fivefold in response to IGF-I stimulation. A slight increase in MBP phosphorylation was also observed in the same transfectants in response to IL-4 stimulation. The overexpression of NM1 caused a constitutive activation of MAPK, although this activity was not as high as that of IGF-IRWT transfectants stimulated by IGF-I. Interestingly, a protein of 25 kDa was coimmunoprecipitated with anti-Erk2 and was strongly phosphorylated in response to IGF-I stimulation. In contrast to both IGF-IRWT and NM1 transfectants, MBP phosphorylation was significantly inhibited in the IGF-IRKR transfectants. Taken together, the activation of MAPK in response to IGF-I stimulation correlates with the increased tyrosine phosphorylation of SHC and SHIP and the enhanced association of Grb2 with SHC in the IGF-IR transfectants.
Activation of MAPK pathway is required for IL-4 to induce mitogenesis of the IGF-IR transfectants.
Having demonstrated that IL-4 induced mitogenesis and long-term growth of the IGF-IR transfectants and that IL-4 synergized with IGF-I for 32D DNA synthesis, we determined if IL-4 would also utilize the SHC/MAPK pathway for cell proliferation. As shown in Fig. 10A, although SHC was only weakly tyrosine phosphorylated in 32D cells in response to IL-4, the coaddition of IGF-I and IL-4 to 32D cells resulted in 1.7-fold-more phosphorylation than that of IGF-I alone. Furthermore, the stimulation of the IGF-IRWT transfectants with IL-4 increased SHC tyrosine phosphorylation 3.6-fold when compared to the nonstimulating condition in the same transfectants. As expected, the expression of the IGF-IRKR mutant diminished SHC phosphorylation.
To further explore the involvement of the MAPK pathway and exclude PI 3′K activation in IGF-I- and IL-4-induced mitogenesis, PD98059 and wortmannin, which specifically inhibit the MEK and PI 3′K pathways, respectively, were utilized in a mitogenic assay. As shown in Fig. 10B, wortmannin (100 nM) was not able to inhibit IGF-I- and/or IL-4-induced mitogenesis of the IGF-IRWT transfectants, although the same dose of it fully suppressed IL-3-induced Akt activation in 32D cells (data not shown). In striking contrast, inhibition of the MAPK pathway by PD98059 significantly (P < 0.01) suppressed IGF-I- and/or IL-4-mediated mitogenesis. The solvent dimethyl sulfoxide (DMSO) did not affect ligand-induced mitogenesis. The coaddition of IGF-I and IL-4 resulted in increased mitogenesis compared to that of each ligand alone, which reached a similar level as that induced by the saturated amounts of IL-3. We conclude that the SHC/Grb2/MAPK cascade plays an important role in both IGF-I- and IL-4-induced mitogenesis of the IGF-IR transfectants and further excludes the involvement of PI 3′K in the process.
STAT6 activation in response to IL-4 is enhanced in the IGF-IRWT transfectants.
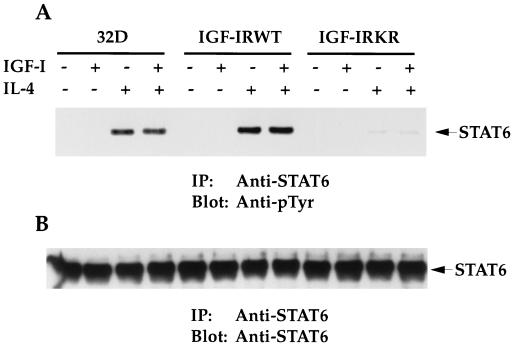
STAT6 has been shown to be a major and specific IL-4 transcriptional factor, which plays a pivotal role in IL-4-induced cell proliferation (18). When endogenous STAT6 was immunoprecipitated, its tyrosine phosphorylation was detectable in the parental 32D line (Fig. 11A). Very interestingly, the stimulation of the IGF-IRWT transfectants with IL-4 reproducibly increased STAT6 phosphorylation, when compared to that of 32D cells (1.4-fold more). In striking contrast, STAT6 phosphorylation in the IGF-IRKR transfectants was reduced to 4% of that in 32D cells. Although IL-4 induced higher levels of STAT6 phosphorylation in the IGF-IRWT transfectants than in 32D cells, the costimulation of the IGF-IRWT transfectants with IGF-I and IL-4 did not further enhance the phosphorylation. When the membrane used in Fig. 11A was reblotted with anti-STAT6 serum, no differences in STAT6 protein levels were detected among the cell lines (Fig. 11B), indicating that the changes in STAT6 phosphorylation are not due to the influence of IGF-IR on STAT6 protein expression. Together, the results suggest that STAT6 tyrosine phosphorylation in response to IL-4 is enhanced in the IGF-IRWT transfectants, which correlates with increased cell proliferation.
FIG. 11.
Tyrosine phosphorylation of STAT6 in response to IL-4 is enhanced in the IGF-IRWT transfectants. (A) 32D cells and transfectants were serum starved and either left untreated or stimulated with IGF-I and/or IL-4 for 10 min. Equivalent cell lysates were immunoprecipitated (IP) with anti-STAT6. Transferred proteins were immunoblotted with anti-pTyr. The position of tyrosine-phosphorylated STAT6 is indicated. This experiment was performed three times with the same results. (B) The same Immobilon membrane shown in panel A was reblotted with an anti-STAT6 antibody. The position of the STAT6 protein is indicated.
IL-4 synergizes with IGF-I to induce c-myc early response gene up-regulation.
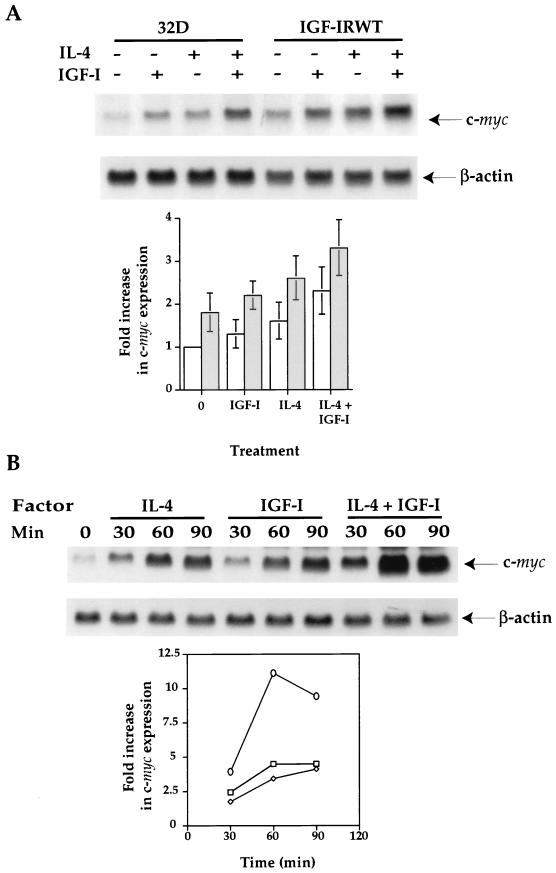
To further understand the mechanisms underlying the synergistic effect of IL-4 and IGF-I on myeloid cell proliferation, we tested for expression of the early response gene c-myc, which had been shown to be important for cell proliferation mediated by many growth factors (1, 9, 37). When Northern blot analyses were performed in three separate experiments by using c-myc probes and normalizing for RNA loading by β-actin controls, both IGF-I and IL-4 stimulation of 32D cells resulted in c-myc gene up-regulation of 1.3- and 1.6-fold, respectively, when compared to the nonstimulated 32D control (Fig. 12A). The addition of IGF-I and IL-4 together in 32D cells caused a 2.3-fold increase in c-myc gene expression (statistically significant [P < 0.05]). The synergistic effect of c-myc up-regulation correlated with DNA synthesis of 32D cells in response to both factors (Fig. 4A). More interestingly, the basal c-myc expression level was already increased in 32D/IGF-IR transfectants by 1.8-fold. The treatment of the transfectants with IGF-I and IL-4 induced c-myc up-regulation by 2.2- and 2.6-fold, respectively, similar to the extent of induction achieved by simultaneous IL-4 and IGF-I stimulation of 32D cells. The costimulation of cells with IL-4 and IGF-I in the 32D/IGF-IR transfectants resulted in the maximal 3.3-fold induction of c-myc expression.
FIG. 12.
IL-4 synergizes with IGF-I to induce c-myc early response gene up-regulation. (A) 32D cells and transfectants were serum starved for 5 h in the presence of 0.1% bovine serum albumin and subsequently stimulated with IL-4 (100 ng/ml), IGF-I (100 ng/ml), or IL-4 plus IGF-I (100 ng/ml each) for 30 min. The reaction was stopped by adding cold Dulbecco’s modified Eagle medium to the reaction mixture, and total RNA was isolated. Fifteen micrograms of total RNA from each sample was fractionated and hybridized with the c-myc probe. The same membrane was stripped and rehybridized with the β-actin probe. Three Northern blot analyses were performed. After normalization for RNA loading by a β-actin control, the fold increases in c-myc gene expression were obtained by comparing them with the basal c-myc levels in nonstimulated 32D cells. White and shaded bars are c-myc levels of 32D cells and IGF-IRWT transfectants, respectively. Standard deviations are shown by bars. (B) 32D cells were similarly starved as described above and stimulated with IL-4 (square), IGF-I (diamond), and IL-4 plus IGF-I (circle) for the indicated periods of time. The fold increases in c-myc expression were obtained by normalizing for RNA loading with the β-actin probe and by comparing it with the basal c-myc levels in nonstimulated 32D cells.
A time course experiment demonstrated that both IL-4 and IGF-I were able to induce c-myc gene up-regulation in the periods of incubation of 30, 60, and 90 min (Fig. 12B). Again, the synergistic effect of the two factors on c-myc expression was detected in all the periods analyzed, with the maximal induction at 60 min after stimulation. Taken together, our results demonstrate that c-myc gene up-regulation correlates with mitogenesis induced by IGF-I or IL-4 alone in the 32D/IGF-IR transfectants (Fig. 1 to 3) and by both factors in the parental 32D cells (Fig. 4A).
DISCUSSION
In the present study, we provide evidence that the stimulation of overexpressed IGF-IR with IGF-I led to mitogenesis independent of IRS molecule expression and activation. More interestingly, IL-4 stimulation of the IGF-IRWT and NM1 transfectants also resulted in cell proliferation. In contrast, expression of an ATP binding mutant of IGF-IR did not initiate any detectable mitogenesis. Both IGF-I and IL-4 were able to mediate long-term growth of the IGF-IR transfectants. Furthermore, IGF-I and IL-4 synergized to induce 32D and Baf3 DNA synthesis, demonstrating the physiological importance of this synergy in hematopoietic cell proliferation. Our results are further substantiated by the recent finding that IGF-I was also able to cooperate with erythropoietin for human IL-3-dependent, erythroleukemia cell proliferation (29).
The results of IGF-IR-induced cell proliferation presented differ from the previous data in which IL-4 and insulin were dependent on IRS-1 or -2 expression and activation for mitogenesis in the same cell background (Fig. 1). Stimulation of the IR pathway also resulted in Ras/Raf/MAPK activation even in the 32D-IR transfectants (13). It is possible that a threshold level of IR expression may be necessary to initiate mitogenic signals solely through the activation of the SHC/Grb2/Ras/Raf/MAPK cascade independent of IRS expression. We are currently testing this possibility by generating new IR transfectants with higher expression levels. On the other hand, the preferential synergy between IGF-I versus insulin and IL-4 for 32D and Baf3 DNA synthesis clearly indicates that IGF-I plays a more important role than insulin in hematopoietic cell proliferation (Fig. 4).
Our data strongly suggest that the SHC/Grb2/MAPK cascade plays an important role in IGF-IR-mediated cell proliferation of 32D cells overexpressing IGF-IR. The tyrosine phosphorylation of SHC and Erk2, the association of Grb2 with SHC, and MAPK activity were all increased in the IGF-IR transfectants in response to IGF-I. The expression of the KR mutant of IGF-IR fully suppressed the endogenous IGF-IR-mediated signal transduction of this cascade. NM1 overexpression caused much weaker spontaneous cell proliferation than that of IGF-IRWT transfectants stimulated by IGF-I (Fig. 2). This correlated well with the lower levels of MAPK activation (Fig. 9B) and weaker tyrosine phosphorylation of several cellular proteins (22a) in NM1 transfectants than those in IGF-IRWT transfectants, substantiating the role of the MAPK cascade in IGF-IR-mediated cell proliferation. Finally, the strong inhibition by PD98059, but not that by wortmannin, demonstrates the absolute importance of the MAPK pathway in IGF-I-induced mitogenesis of the IGF-IR transfectants.
SHIP belongs to a subgroup of the inositol polyphosphate-5-phosphatase family. It possesses 5-phosphatase activity towards Ins(1,3,4,5)P4 and PtdIns(3,4,5)P3 but not Ins(1,4,5)P3 or PtdIns(4,5)P2. The protein was originally cloned for its ability to bind the PTB domain of SHC in the yeast two-hybrid system (23) and for its association with the SH3 domain of Grb2 from the IL-3-stimulated murine hematopoietic line, B6SUtA1 (10). In addition to the N-terminal SH2 domain, SHIP also contains a proline-rich domain and two PTB motifs (NPXY) at the C terminus. Tyrosine phosphorylation of SHIP has been documented mainly in hematopoietic cells in response to cytokines, such as IL-3, colony-stimulating factor 1, and erythropoietin (10, 23, 24). Although the interaction of SHIP with SHC and Grb2 has been established, the exact domains or motifs in SHIP and its interacting molecules required for binding and activation have not been fully elucidated. Similarly, the functional role of SHIP in vivo in cell signaling remains elusive. An inhibitory role in hematopoietic cell proliferation and B-cell signaling has been attributed to SHIP, most likely due to its phosphatase activity toward PIP3 (5, 31). Although the role of SHIP in IGF-I- and IL-4-induced mitogenesis in the 32D cell system needs to be further investigated, our results provide the first evidence for SHIP phosphorylation by activated IGF-IR and for its association with SHC in an IGF-I-dependent manner.
IL-4 is a pleiotropic cytokine, which induces B-lymphocyte differentiation, immunoglobulin class switching and major histocompatibility complex expression. It is also a proliferating factor for T lymphocytes. IL-4R is composed of a unique α chain responsible for ligand binding and a common subunit (γc) shared by receptors for IL-2, IL-7, IL-9, and IL-15 (18, 34). IL-4 stimulation induces tyrosine phosphorylation of the α chain, which may be due to the activation of Janus kinases (JAK). Subsequently, STAT6 is tyrosine phosphorylated, translocates into the nucleus, and activates the transcriptional machinery. IL-4 can also induce IRS tyrosine phosphorylation through JAK1 activation (44) and initiates a mitogenic response in 32D cells when both IRS and IL-4R are coexpressed (19, 45). While the biological effects of IL-4 are mainly restricted to hematopoietic cells, recent observations from our laboratory showed that IL-4 was able to synergize with the platelet-derived growth factor for JAK1 and STAT6 tyrosine phosphorylation, STAT6 transcriptional activity, and mitogenesis of fibroblasts (32, 33). These results suggest that IL-4 has a cooperative or synergistic role in mitogenesis mediated by tyrosine kinase receptors. In our study, IL-4 stimulation of the IGF-IR transfectants caused more than a 30-fold increase in mitogenesis (Fig. 1, 2, and 10B). Moreover, the synergy between IGF-I and IL-4 was also observed in the parental 32D and Baf3 cells (Fig. 4). These results, together with the facts that both IL-4R and IGF-IR are ubiquitously expressed and that IGF-I is a major mitogen existing in the serum, suggest a functional relevance of their synergy in hematopoietic cell proliferation.
The mechanisms underlying IL-4 synergy with IGF-I for cell proliferation are still not fully understood. IL-4 has been shown to activate the MAPK cascade (46). Tyrosine phosphorylation of SHC and Erk2 was detected in response to IL-4 in IGF-IR transfectants (Fig. 7 and 9A). SHC tyrosine phosphorylation was induced maximally by both IGF-I and IL-4 in 32D cells (Fig. 10A). Combined with the inhibition of mitogenesis by an MEK inhibitor, these results demonstrate the potential importance of the MAPK pathway in IL-4-induced mitogenesis. In addition, STAT6 tyrosine phosphorylation was increased in the IGF-IRWT transfectants in response to IL-4 when compared to that in 32D cells (Fig. 11A). However, the costimulation of the transfectants with both IL-4 and IGF-I did not further enhance this increased phosphorylation. It is possible that IGF-IR can enhance JAK1 activation stimulated by IL-4, leading to increased STAT6 phosphorylation in the IGF-IRWT transfectants. On the other hand, IL-4-induced translocation of STAT6 into the plasma membrane may increase when some basal IGF-IR activity is present. Although we do not yet know the mechanisms governing the regulation of STAT6 phosphorylation by IGF-IR, the increased MAPK and STAT6 activation in response to IL-4 may cooperate to initiate mitogenesis of the IGF-IR transfectants.
The induction of early response genes, including c-jun, c-fos, and c-myc has been reported in response to many growth factors and cytokines (9, 37). The stimulation of IGF-IR and IL-4R pathways can induce c-myc gene expression, which is thought to play a role in G1-S-phase transition and DNA synthesis. For example, IL-4 was able to induce keratinocyte proliferation and c-myc up-regulation. A specific tyrosine kinase inhibitor, genistein, blocked both IL-4-induced cell proliferation and c-myc induction (49). In 32D cells, c-fos and egr-1 expression was demonstrated to be dependent on the levels of IR and the activation of the MAPK pathway initiated from overexpressed IR (13). However, c-fos and egr-1 expression does not seem to be required for IR-mediated cell proliferation through IRS-1. On the other hand, c-myc gene expression correlated with mitogenesis induced by coexpressing human IL-4R and IRS-1 (35). In our experiments, c-myc up-regulation was reproducibly detected even in the parental 32D cells in response to either IL-4 or IGF-I (Fig. 12). The synergistic effect of IGF-I and IL-4 on c-myc expression parallels the synergy induced by these factors for mitogenesis in 32D cells (Fig. 4). Moreover, the ability of IL-4 alone to induce mitogenesis in 32D/IGF-IR transfectants coincided with the strong up-regulation of c-myc in the same transfectants. Currently, we do not know how the c-myc gene is up-regulated by both IGF-I and IL-4. It is speculated that the MAPK pathway induced by IGF-I and the STAT6 pathway activated by IL-4 may finally target the c-myc promoter for its up-regulation. Nevertheless, our results point to the importance of c-myc in IGF-I- and IL-4-induced hematopoietic cell proliferation.
In summary, the present studies demonstrate that the stimulation of the IGF-IR signaling pathway through SHC/SHIP/Grb2/MAPK, but not that through IRS/PI 3′K, induces 32D cell proliferation when sufficient levels of IGF-IR are present. Moreover, IL-4 can synergize with IGF-I for mitogenesis, which may occur through cross talk between the MAPK and STAT6 pathways and c-myc gene up-regulation.
REFERENCES
- 1.Amati B, Littlewood T D, Evan G I, Land H. The c-Myc protein induces cell cycle progression and apoptosis through dimerization with Max. EMBO J. 1993;12:5083–5087. doi: 10.1002/j.1460-2075.1993.tb06202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth. Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 3.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 4.Blakesley V A, Scrimgeour A, Esposito D, LeRoith D. Signaling via the insulin-like growth factor-I receptor: does it differ from insulin receptor signaling? Cytokine Growth Factor Rev. 1996;7:153–159. doi: 10.1016/1359-6101(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 5.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 6.Boney C M, Smith R M, Gruppuso P A. Modulation of insulin-like growth factor I mitogenic signaling in 3T3-L1 preadipocyte differentiation. Endocrinology. 1998;139:1638–1644. doi: 10.1210/endo.139.4.5920. [DOI] [PubMed] [Google Scholar]
- 7.Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci P G. Not all Shc’s roads lead to Ras. Trends Biochem Sci. 1996;21:257–261. [PubMed] [Google Scholar]
- 8.Burgaud J L, Resnicoff M, Baserga R. Mutant IGF-I receptors as dominant negatives for growth and transformation. Biochem Biophys Res Commun. 1995;214:475–481. doi: 10.1006/bbrc.1995.2311. [DOI] [PubMed] [Google Scholar]
- 9.Conover C A, Bale L K. Insulin-like growth factor I induction of c-myc expression in bovine fibroblasts can be blocked by antecedent insulin receptor activation. Exp Cell Res. 1998;238:122–127. doi: 10.1006/excr.1997.3815. [DOI] [PubMed] [Google Scholar]
- 10.Damen J E, Liu L, Rosten P, Humphries R K, Jefferson A B, Majerus P W, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downward J. Control of ras activation. Cancer Surv. 1996;27:87–100. [PubMed] [Google Scholar]
- 12.Gustafson T A, He W, Craparo A, Schaub C D, O’Neill T J. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995;15:2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada S, Smith R M, Smith J A, White M F, Jarett L. Insulin-induced egr-1 and c-fos expression in 32D cells requires insulin receptor, Shc, and mitogen-activated protein kinase, but not insulin receptor substrate-1 and phosphatidylinositol 3-kinase activation. J Biol Chem. 1996;271:30222–30226. doi: 10.1074/jbc.271.47.30222. [DOI] [PubMed] [Google Scholar]
- 14.He W, Craparo A, Zhu Y, O’Neill T J, Wang L M, Pierce J H, Gustafson T A. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J Biol Chem. 1996;271:11641–11645. doi: 10.1074/jbc.271.20.11641. [DOI] [PubMed] [Google Scholar]
- 15.He W, O’Neill T J, Gustafson T A. Distinct modes of interaction of SHC and insulin receptor substrate-1 with the insulin receptor NPEY region via non-SH2 domains. J Biol Chem. 1995;270:23258–23262. doi: 10.1074/jbc.270.40.23258. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara H, Sasaoka T, Ishiki M, Takata Y, Imamura T, Usui I, Langlois W J, Sawa T, Kobayashi M. Functional importance of Shc tyrosine 317 on insulin signaling in Rat1 fibroblasts expressing insulin receptors. J Biol Chem. 1997;272:9581–9586. doi: 10.1074/jbc.272.14.9581. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Chan J L-K, Zong C S, Wang L-H. Effect of tyrosine mutations on the kinase activity and transforming potential of an oncogenic human insulin-like growth factor I receptor. J Biol Chem. 1996;271:160–167. doi: 10.1074/jbc.271.1.160. [DOI] [PubMed] [Google Scholar]
- 18.Keegan A D, Nelms K, Wang L M, Pierce J H, Paul W E. Interleukin 4 receptor: signaling mechanisms. Immunol Today. 1994;15:423–432. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 19.Keegan A D, Nelms K, White M, Wang L M, Pierce J H, Paul W E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 20.Lavan B E, Fantin V R, Chang E T, Lane W S, Keller S R, Lienhard G E. A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J Biol Chem. 1997;272:21403–21407. doi: 10.1074/jbc.272.34.21403. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Jiang Y-X, Zhang J, Soon L, Flechner L, Kapoor V, Pierce J H, Wang L-H. Protein kinase C-δ is an important signaling molecule in insulin-like growth factor I receptor-mediated cell transformation. Mol Cell Biol. 1998;18:5888–5898. doi: 10.1128/mcb.18.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Yu J-C, Michieli P, Beeler J F, Ellmore N, Heidaran M A, Pierce J H. Stimulation of the platelet-derived growth factor β receptor signaling pathway activates protein kinase C-δ. Mol Cell Biol. 1994;14:6727–6735. doi: 10.1128/mcb.14.10.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Li, W. Unpublished observations.
- 23.Lioubin M N, Algate P A, Tsai S, Carlberg K, Aebersold A, Rohrschneider L R. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 24.Lioubin M N, Myles G M, Carlberg K, Bowtell D, Rohrschneider L R. Shc, Grb2, Sos1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Rutter W J, Wang L-H. Enhancement of transforming potential of human insulin-like growth factor I receptor by N-terminal truncation and fusion to avian sarcoma virus. J Virol. 1992;66:374–385. doi: 10.1128/jvi.66.1.374-385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, Rutter W J, Wang L-H. Modulatory effects of the extracellular sequence of the human insulin-like growth I receptor on its transforming and tumorigenic potential. J Virol. 1993;67:9–18. doi: 10.1128/jvi.67.1.9-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ and not protein kinase C-βII, -γ, -ζ, and -η. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 28.Myers M G, Jr, Zhang Y, Aldaz G A, Grammer T, Glasheen E M, Yenush L, Wang L M, Sun X J, Blenis J, Pierce J H, White M F. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol Cell Biol. 1996;16:4147–4155. doi: 10.1128/mcb.16.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okajima Y, Matsumura I, Nishiura T, Hashimoto K, Yoshida H, Ishikawa J, Wakao H, Yoshimura A, Kanakura Y, Tomiyama Y, Matsuzawa Y. Insulin-like growth factor-I augments erythropoietin-induced proliferation through enhanced tyrosine phosphorylation of STAT5. J Biol Chem. 1998;273:22877–22883. doi: 10.1074/jbc.273.36.22877. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill T J, Craparo A, Gustafson T A. Characterization of an interaction between insulin receptor substrate 1 and the insulin receptor by using the two-hybrid system. Mol Cell Biol. 1994;14:6433–6442. doi: 10.1128/mcb.14.10.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono M, Bolland S, Tempst P, Ravetch J V. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 32.Patel B K, Wang L M, Lee C C, Taylor W G, Pierce J H, LaRochelle W J. Stat6 and Jak1 are common elements in platelet-derived growth factor and interleukin-4 signal transduction pathways in NIH 3T3 fibroblasts. J Biol Chem. 1996;271:22175–22182. doi: 10.1074/jbc.271.36.22175. [DOI] [PubMed] [Google Scholar]
- 33.Patel B K R, Pierce J H, LaRochelle W J. Regulation of interleukin 4-mediated signaling by naturally occurring dominant negative and attenuated forms of human Stat6. Proc Natl Acad Sci USA. 1998;95:172–177. doi: 10.1073/pnas.95.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul W E. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found Symp. 1997;204:208–216. doi: 10.1002/9780470515280.ch14. [DOI] [PubMed] [Google Scholar]
- 35.Pernis A, Witthuhn B, Keegan A D, Nelms K, Garfein E, Ihle J N, Paul W E, Pierce J H, Rothman P. Interleukin 4 signals through two related pathways. Proc Natl Acad Sci USA. 1995;92:7971–7975. doi: 10.1073/pnas.92.17.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prisco M, Hongo A, Rizzo M G, Sacchi A, Baserga R. The insulin-like growth factor I receptor as a physiologically relevant target of p53 in apoptosis caused by interleukin-3 withdrawal. Mol Cell Biol. 1997;17:1084–1092. doi: 10.1128/mcb.17.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roussel M F, Cleveland J L, Shurtleff S A, Sherr C J. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991;353:361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- 38.Sasaoka T, Ishiki M, Sawa T, Ishihara H, Takata Y, Imamura T, Usui I, Olefsky J M, Kobayashi M. Comparison of the insulin and insulin-like growth factor 1 mitogenic intracellular signaling pathways. Endocrinology. 1996;137:4427–4434. doi: 10.1210/endo.137.10.8828504. [DOI] [PubMed] [Google Scholar]
- 39.Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 40.Sun X J, Wang L M, Zhang Y, Yenush L, Myers M G, Jr, Glasheen E, Lane W S, Pierce J H, White M F. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 41.Ullrich A, Gray A, Tam R W, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, Jacobs S, Franke U, Ramachandran J, Fujita-Yamaguchi Y. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–211. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 43.Wang L-M, Keegan A D, Li W, Lienhard G E, Pacini S, Gutkind J S, Myers M G, Jr, Whiter M F, Aaronson S A, Paul W E, Pierce J H. Common elements in interleukin 4 and insulin signaling pathways in factor-dependent hematopoietic cells. Proc Natl Acad Sci USA. 1993;90:4032–4036. doi: 10.1073/pnas.90.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L M, Keegan A, Frankel M, Paul W E, Pierce J H. Signal transduction through the IL-4 and insulin receptor families. Stem Cells (Dayton) 1995;13:360–368. doi: 10.1002/stem.5530130407. [DOI] [PubMed] [Google Scholar]
- 45.Wang L M, Myers M G, Jr, Sun X J, Aaronson S A, White M, Pierce J H. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993;261:1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- 46.Wery S, Letourneur M, Bertoglio J, Pierre J. Interleukin-4 induces activation of mitogen-activated protein kinase and phosphorylation of shc in human keratinocytes. J Biol Chem. 1996;271:8529–8532. doi: 10.1074/jbc.271.15.8529. [DOI] [PubMed] [Google Scholar]
- 47.White M F. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl. 2):S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 48.White M F, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Yoo H M, Choi I, Pyun K H, Byun S M, Ha H. Interleukin 4-induced proliferation in normal human keratinocytes is associated with c-myc gene expression and inhibited by genistein. J Investig Dermatol. 1996;107:367–372. doi: 10.1111/1523-1747.ep12363346. [DOI] [PubMed] [Google Scholar]
- 50.Yu J-C, Gutkind J S, Mahadevan D, Li W, Meyer K A, Pierce J H, Heidaran M A. Biological function of PDGF-induced PI-3K activity: its role in αPDGF receptor-mediated mitogenic signaling. J Cell Biol. 1994;127:479–487. doi: 10.1083/jcb.127.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou-Li F, Xu S Q, Dews M, Baserga R. Co-operation of simian virus 40 T antigen and insulin receptor substrate-1 in protection from apoptosis induced by interleukin-3 withdrawal. Oncogene. 1997;15:961–970. doi: 10.1038/sj.onc.1201265. [DOI] [PubMed] [Google Scholar]