Abstract
Objectives:
In breast cancer surgery, the combined use of the dye method and radioisotope (RI) method is recommended for identifying sentinel lymph nodes. However, the RI method is difficult to license, expensive, and difficult to introduce. Thus, we introduced computed tomography lymphography (CTLG) and investigated the characteristics and usefulness of CTLG.
Material and Methods:
Among breast cancer patients who underwent surgery during a 6-year period from January 2013 to December 2018, CTLG was performed on 141 patients with clinically negative lymph node metastasis. These cases were then retrospectively investigated. The number and location of lymph vessel, true sentinel lymph nodes, and the positional relationships with surrounding muscles and blood vessels were confirmed from the constructed 3D images. The actual surgeries were then performed using a dye method with indigo carmine based on images obtained using CTLG.
Results:
CTLG was able to identify lymph vessels and true sentinel lymph nodes in 131 of the 141 cases (92.91%). There were 97 patients in whom the first true sentinel lymph node reached from the breast was one node, 30 with two nodes, and 4 with three nodes. Moreover, there were three cases in which sentinel lymph nodes were present at Level II. During surgery, sentinel lymph nodes were identified in 131 patients (92.91%) using dye.
Conclusion:
CTLG has a high identification rate in sentinel lymph nodes, and it is considered a convenient and useful examination method because a lot of information, such as the number and position of sentinel lymph nodes, can be obtained.
Keywords: Breast neoplasms, Lymphography, Sentinel lymph node biopsy
INTRODUCTION
Recently, trends for breast cancer surgery have shifted greatly in the direction of shrinkage or minimal invasion. Sentinel lymph node biopsies have become the standard procedure worldwide rather than axillary lymph node dissection, particularly in patients who are considered clinically free of lymph node metastases.[1] Sentinel lymph nodes are defined as lymph nodes that are first reached through the lymph from the tumor. Cancer cells then metastasize to sentinel lymph nodes and then to other lymph nodes (non-sentinel lymph nodes) through lymphatic vessels. Thus, sentinel lymph nodes are believed to play a crucial role in regional lymph nodes.[2]
Two methods of sentinel lymph node identification are the dye method and radioisotope (RI) method. The dye method is used in most institutions because it is economical and convenient to search for dye stained lymphatic vessels after infusing the dye into the areola or similar areas and to visually identify dye stained lymph nodes.[3] The RI method involves injecting isotope particles into the areola or similar areas and using gamma probes to identify highly radioactive sentinel lymph nodes (hot spots).[4] The Japanese guideline clearly states that “The combination of blue dye and RI showed slightly better identification rates than either method used alone; therefore, the combined method is standard.”[5] However, medical law stipulates that a license is required to use RI in Japan; therefore, the introduction of the RI method is not easy. Moreover, the gamma probe used in the RI method is very expensive, costing >€15,000,[6] which makes the introduction more difficult. Only 28% of Japanese private hospitals that perform breast cancer surgery have a gamma probe.[7]
Ebina General Hospital is a medium-sized private hospital at which it has been difficult to introduce the RI method. There are ~50 breast cancer operations per year, of which ~30 are sentinel lymph node biopsies. It is difficult to obtain RI approval and introduce a new gamma prove due to financial concerns. Computed tomographic lymphography (CTLG) is an easy and useful method for identifying sentinel lymph nodes in general hospitals where it is difficult to introduce the RI method. This procedure was performed between 2012 and 2018.[8] In this study, we report the usefulness of CTLG and the anatomical features of the lymphatic vessels and the location of sentinel lymph nodes, which have not been documented to date. We then consider whether CTLG could be an alternative to the RI method.
MATERIAL AND METHODS
Patients
This study was conducted as a single-center, retrospective observational study of CTLG. Among the breast cancer patients who underwent surgery at Ebina General Hospital during the 6 years from January 2013 to December 2018, CTLG was performed on patients with clinically negative lymph node metastasis. Moreover, CTLG was performed in patients who received pre-operative drug therapy, but only in those patients who were clinically judged to have no lymph node metastasis before and after drug therapy. CTLG was not performed in patients with suspected contrast agent allergy or who did not agree to the test. A total of 141 patients (four of them had cN0 breast cancers in both breasts, which were handled as separate patients with disease in either the left or right breast) underwent CTLG 1–2 days before surgery. All of these patients were included in the study. The medical records of these patients were retrospectively reviewed. Information on age, sex, pre-operative medication, tumor size (Tis, T0–T4), and breast surgery (total or partial mastectomy) was then obtained.
Identification of sentinel lymph node using CTLG
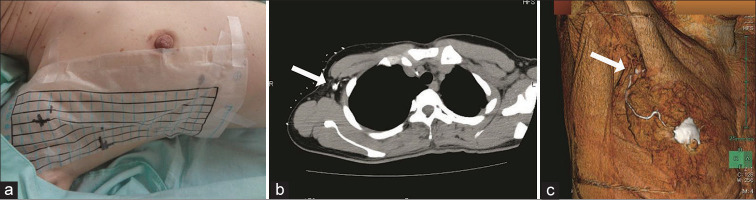
CTLG is a method in which computed tomography (CT) scans are obtained after local injection of a mixture of iodine contrast and local anesthetic into the areola to identify contrast-enhanced lymphatic vessels and sentinel lymph nodes. CTLG was performed 1–2 days before surgery. A 64-slice multidetector CT scanner (Aquilion; Toshiba, Tokyo, Japan) was then used to obtain CT scans. The patient’s position at the time of CT imaging was the same as that at the time of surgery, with the affected upper limb rotated at 90°. However, the elbow was flexed at 90° because the patient had to pass through the dome of the CT scanner. A radiopaque grid was placed in the axilla [Figure 1a] and ~1.5–3 ml of a total of 3 ml mixture of iodine contrast medium iopamidol (2 ml) and 1% lidocaine (1 ml) was intradermally injected into the areola. CT scans were taken in 2 mm sized slices after 2 min. Contrast-enhanced sentinel lymph nodes were confirmed [Figure 1b], and the position was marked on the body surface using a laser pointer and grid. Three-dimensional volume rendered images were constructed from the resulting volume images [Figure 1c]. Using this image, information on the number and location of lymph vessels and sentinel lymph nodes was confirmed.
Figure 1:
Identification of sentinel lymph nodes using CTLG. (a) Affixing a grid that is visible on computed tomography scans to the axilla. (b) Confirmation of contrast-enhanced sentinel nodes (arrow). (c) Construction of three-dimensional images. The sentinel lymph node position is confirmed in a 3D image (arrow).
Identification of sentinel lymph nodes by dye method in surgery
During surgery, 1.5–3 ml of dye was injected into the same site. Sentinel lymph node biopsy was then performed, relying on the images and markings obtained on CTLG. Indigo carmine was used as the dye. If sentinel lymph nodes could not be identified, lymph node dissection or sampling was performed.
Pathological diagnosis
Sentinel lymph nodes removed by surgery could be rapidly diagnosed by frozen section during surgery, and the presence or absence of metastases was diagnosed.
Ethical approval
In Japan, the use of iodine contrast media is not covered by insurance other than use for angiography and urography; therefore, the Institutional Ethics Committee provided approval, and each patient provided written informed consent. Approval Number: 101 and Approval Date: December 14, 2012. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki of 1975.
Statistical analysis
Statistical analysis was performed using Fisher’s exact test to assess the correlation between multiple sentinel lymph nodes and lymph node metastasis in CTLG. Moreover, we evaluated the correlation of lymph node metastasis using multiple lymph vessels. P < 0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
Table 1 lists the characteristics of 141 patients. One of the 141 patients was a male patient. Ten patients had undergone chemotherapy or hormone therapy before surgery, and three patients had advanced cancer with T3 (>5 cm).
Table 1:
Patient characteristics.
| Characteristics | |
|---|---|
| Age, y | |
| Median | 68.0±11.9 |
| Range | 37–88 |
| Sex | |
| Female | 140 (99.30%) |
| Male | 1 (0.71%) |
| Pre-operative therapy | |
| No | 131 (92.91%) |
| Neoadjuvant chemotherapy | 7 (4.96%) |
| Neoadjuvant hormone therapy | 3 (2.13%) |
| Tumor size | |
| cT0 | 2 (1.42%) |
| cT1 | 78 (55.32%) |
| cT2 | 45 (31.91%) |
| cT3 | 3 (2.13%) |
| cT4 | 0 (0.00%) |
| cTis | 13 (9.22%) |
| Surgery on the breast | |
| Partial mastectomy | 53 (37.59%) |
| Total mastectomy | 88 (62.41%) |
Identification of sentinel lymph nodes
A flowchart showing our procedure is shown in Figure 2. CTLG was able to identify sentinel lymph nodes in 131 of the 141 cases, resulting in an identification rate of 92.9%. Regardless of CTLG identification, dye was used to identify sentinel lymph nodes during surgery. Moreover, the dye was able to identify sentinel lymph nodes in 131 cases, resulting in an identification rate of 92.9%. Of the 131 cases in which sentinel lymph nodes were identified by dye, 16 cases had metastases in the frozen section, 13 cases were macrometa, and 3 cases were micrometa.
Figure 2:
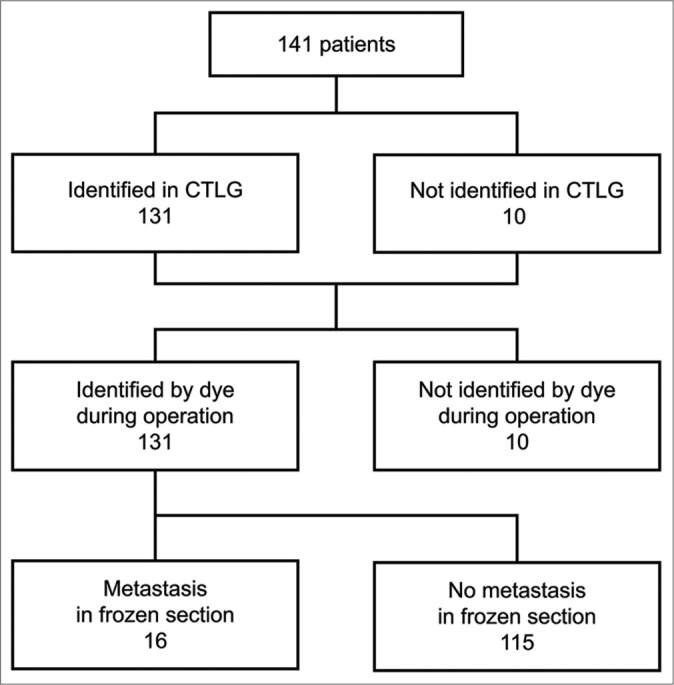
Flowchart showing the process for sentinel lymph node identification.
Characteristics of lymphatic vessels and sentinel lymph nodes in CTLG
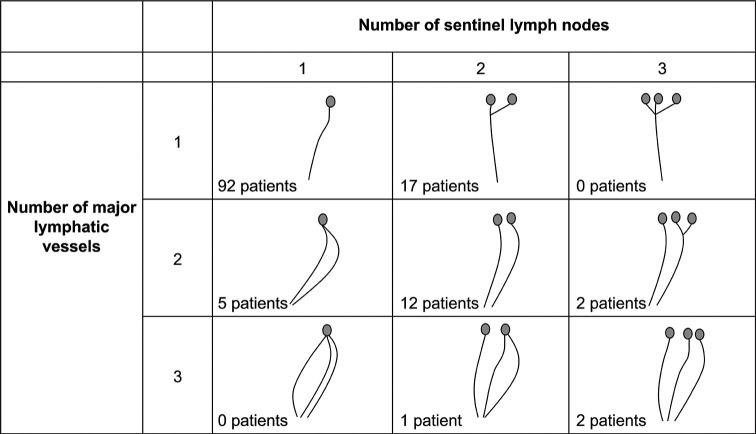
Figure 3 shows the relationship between the main lymphatic vessels and true sentinel lymph nodes in 131 cases. The percentage of multiple sentinel lymph nodes was 26.0%, and 16.8% had multiple main lymphatic vessels. Most sentinel lymph nodes were located in the axillary lymph node Level I (outside the pectoralis minor muscle), but three cases were found in the axillary lymph node Level II (dorsal pectoralis muscle) [Figure 4a]. In certain cases, sentinel lymph nodes were present in contact with the axillary vein [Figure 4b].
Figure 3:
Relationship between major lymphatic vessels and true sentinel lymph nodes.
Figure 4:
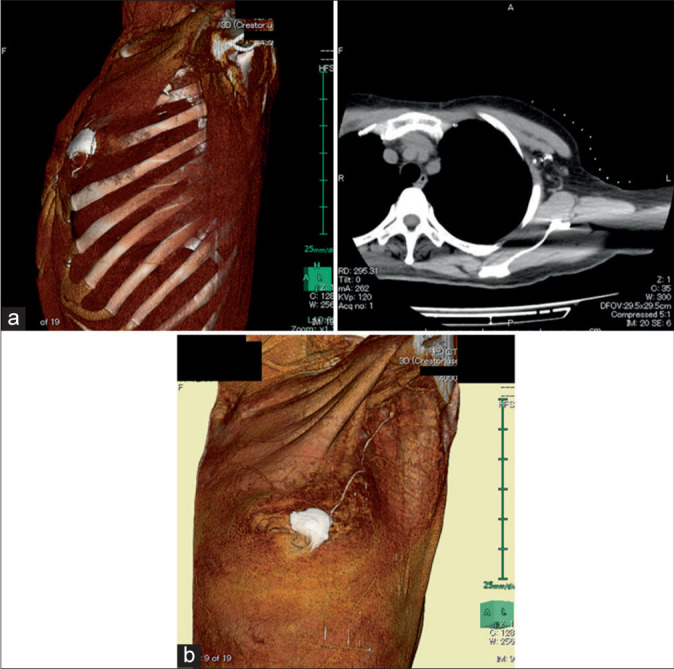
Different locations of rare sentinel lymph nodes. (a) Sentinel nodes present in Level II (arrow). (b) Sentinel lymph nodes located close to the axillary veins (arrow).
Moreover, no sentinel lymph nodes were present in the parasternal lymph node region. Multiple sentinel lymph nodes were not associated with metastasis, and multiple primary lymphatic vessels did not correlate with metastasis [Table 2].
Table 2:
Correlation between sentinel lymph nodes, lymphatic vessels, and lymph node metastasis.
| Single sentinel lymph node | Multiple sentinel lymph nodes | P value | |
|---|---|---|---|
| Metastasis (+) | 11 | 5 | 0.56 |
| Metastasis (−) | 86 | 29 | |
| Single lymphatic vessel | Multiple lymphatic vessels | P value | |
| Metastasis (+) | 12 | 4 | 0.47 |
| Metastasis (−) | 97 | 18 |
DISCUSSION
Sentinel lymph node biopsies have become the standard procedure worldwide rather than axillary lymph node dissection; however, biopsying non-sentinel lymph nodes as if they were sentinel lymph nodes detracts from the purpose of this surgical procedure. There is a requirement to reliably identify true sentinel lymph nodes and to confirm the presence or absence of metastases. In patients with multiple sentinel lymph nodes, these must be biopsied with certainty. In fact, there was only one patient with axillary lymph node recurrence despite no metastases on sentinel lymph node biopsy. This case may have failed to remove the true sentinel lymph node.
Japanese guidelines recommend a combination of staining and RI methods to identify sentinel lymph nodes. However, the introduction of RI is difficult, as previously described, and we only intraoperatively perform the dye technique and identify sentinel lymph nodes by performing pre-operative CTLG. The identification rate of sentinel lymph nodes with CTLG during the 6-year period evaluated in this study was 92.9%. In many studies of clinical trials comparing dye and RI methods and the combination method with both, the rate of identification was >90%.[9,10] Our results were comparable in this study; if sentinel lymph nodes were not initially imaged after injecting a contrast medium into the areola, the procedure was performed again with an additional massage. However, few lymph nodes were imaged with the addition of the massage. After improvements were added and injections were performed again in patients not imaged from 2016, patients in whom lymph nodes were imaged increased. This resulted in an identification rate of 87.7% in 57 patients during the first 3 years (2013–2015) compared with 96.4% in 84 patients during the second 3 years (2016–2018). This improvement allowed for even higher identification rates. Thus, identification rates with CTLG are not considered to be inferior compared with other methods.
Lymphatic vessels and true sentinel lymph nodes can be reliably identified using CTLG imaging. In this study, 97 (74.0%) patients had one sentinel lymph node, and 34 (26.0%) patients had 2 or 3. Yamamoto et al.[11] and Minohata et al.[12] similarly investigated contrast-enhanced patterns from CTLG and reported that 30% of patients had multiple sentinel lymph nodes. When the sentinel lymph node biopsy is performed using only the dye method, there is a risk that the second and third sentinel lymph nodes cannot be biopsied. This is because the running of lymphatic vessels and the number and position of sentinel lymph nodes cannot be known in advance. Moreover, the combined use of CTLG appeared to resolve such issues. Furthermore, obtaining the location of true sentinel lymph nodes was an interesting result. We believed that sentinel lymph nodes would be located in the area of Level I axillary lymph nodes and that metastases in Level I would spread to Level II. However, there were 3 (2.3%) patients in whom the sentinel lymph nodes themselves were already present in Level II in our study. To the best of our knowledge, there have been no reports of such sentinel lymph node locations.
This study retrospectively investigated the identification rate and characteristics of sentinel lymph nodes using CTLG. The limitation of this study is that it cannot be compared using the RI method. It is difficult to compare the CTLG and RI methods because they are completely different methods. The RI method is a procedure performed in real time during surgery, whereas CTLG is a pre-operative examination. Even if CTLG can identify a sentinel lymph node before surgery, it does not necessarily identify and remove it during surgery.
Several other alternatives have been reported for identifying sentinel lymph nodes. The most notable method being the fluorescent dye method using indocyanine green, which is also introduced as a new method in the Japanese guidelines.[5] This method may be superior to CTLG in that it enables real-time lymphatic drainage and true sentinel lymph nodes. However, it is imperative to use appropriate fluorescent dyes, such as the Photodynamic Eye System (PDE; Hamamatsu Photonics, Hamamatsu, Japan), which is considerably expensive and may cost approximately €50,000.[6] Alternatively, a superparamagnetic oxide may also be used, but the magnetometer probe is also relatively expensive.[6] CTLG only requires CT and contrast media. Considering the fact that a CT device may be at most hospitals, this may be the most cost-effective method that does not necessarily require additional expenditure. It can be introduced immediately even in developing countries with hospitals having substandard facilities.
Nakagawa et al.[13] reported new possibilities for CTLG. CTLG was performed in 228 breast cancer patients without clinically confirmed lymph node metastases, and the presence or absence of sentinel lymph node metastases reportedly could be predicted as per contrast-enhanced patterns. In terms of nodal enhancement, if contrast-enhanced features, such as partial staining of sentinel lymph nodes, stagnant lymph vessels, dilated lymph vessels, or detoured lymph vessels, were observed, the possibility of metastases was considered to be high. This method was used to diagnose the presence or absence of sentinel lymph node metastases with a reported accuracy of 89.0%, sensitivity of 92.5%, and specificity of 88.6%. This is a very interesting report that suggests the possibility of omitting dissection based only on CTLG results. Moreover, we believe that it is worth collecting more cases for consideration.
CONCLUSION
The identification rate of sentinel lymph nodes with CTLG was confirmed to be high. Note that 26% of patients were reported to have multiple sentinel lymph nodes, and certain patients had sentinel lymph nodes in Level II. Although CTLG is not a confirmation test performed in real time during surgery, the procedure is convenient and is considered useful because a lot of information can be obtained.
Acknowledgments
The authors thank Crimson Interactive Pvt. Ltd. (Ulatus) www.ulatus.jp for their assistance in manuscript translation and editing.
Footnotes
How to cite this article: Kamata A, Miyamae T, Koizumi M, Kohei H, Sarukawa H, Nemoto H, et al. Using computed tomography lymphography for mapping of sentinel lymph nodes in patients with breast cancer. J Clin Imaging Sci 2021;11:43.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gemignani ML. Trends in breast cancer treatment: Striving to deliver optimal cancer treatment while avoiding morbidity. Clin Obstet Gynaecol. 2016;59:649–50. doi: 10.1097/GRF.0000000000000245. [DOI] [Google Scholar]
- 2.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–66. doi: 10.1002/1097-0142(197702)39:2<456::AID-CNCR2820390214>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–50. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 4.Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer-a multicenter validation study. N Engl J Med. 1998;339:941–6. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 5.Inokuchi M, Kutomi G, Kijima Y, Sakai T, Sawaki M, Shien T, et al. The Japanese breast cancer society clinical practice guidelines for surgical treatment of breast cancer. Breast Cancer. (2018 edition) 2020;27:4–8. doi: 10.1007/s12282-019-01030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal A. New technologies for sentinel lymph node detection. Breast Care. 2018;13:349–53. doi: 10.1159/000492436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda T, Sugie T, Shimizu A, Toi M. Patterns of clinical practice for sentinel lymph node biopsy in women with node-negative breast cancer: The results of a national survey in Japan. Breast Cancer. 2017;24:341–4. doi: 10.1007/s12282-016-0720-5. [DOI] [PubMed] [Google Scholar]
- 8.Kamata A, Kano T, Sarukawa H, Yagi A, Miyamae T, Koizumi M, et al. Case report of 3D-CT lymphography of sentinel lymph node biopsies from breast cancer patients. Kanagawa Igakkai Zasshi. 2013;40:132–5. [Google Scholar]
- 9.Nakamura S, Tsugawa K, Iwata H, Ono S, Akiyama F, Motumura K, et al. A multicenter-based phase 2 study on the safety of sentinel lymph node biopsy for primary breast cancer without clinical axillary lymph node metastases. Jpn J Breast Cancer. 2009;24:271–7. [Google Scholar]
- 10.Morrow M, Rademaker AW, Bethke KP, Talamonti MS, Dawes LG, Clauson J, et al. Learning sentinel node biopsy: Results of a prospective randomized trial of two techniques. Surgery. 1999;126:714–22. doi: 10.1016/S0039-6060(99)70127-3. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Maeda J, Tamesa M, Nagachima Y, Oka M, Kan K, et al. Sentinel lymph node biopsies in breast cancer based on 3D-CT lymphography findings: Necessity of identifying true sentinel lymph nodes. Shujutsu. 2006;10:1597–602. [Google Scholar]
- 12.Minohata J, Tatsumi K, Yoshida A, Ishikawa Y, Wakuya J. Sentinel lymph node biopsy using CT-lymphography with ICG fluorescence navigation in breast cancer. Jpn J Breast Cancer. 2009;24:183–8. [Google Scholar]
- 13.Nakagawa M, Morimoto M, Takechi H, Tadokoro Y, Tangoku A. Preoperative diagnosis of sentinel lymph node (SLN) metastasis using 3D CT lymphography (CTLG) Breast Cancer. 2016;23:519–24. doi: 10.1007/s12282-015-0597-8. [DOI] [PubMed] [Google Scholar]