Abstract
Liver Graft-versus-host disease (GVHD) is common in patients with post-transplant liver dysfunction following allogeneic hematopoietic stem cell transplantation (AHSCT). Oftentimes, the diagnosis is made clinically, and liver biopsy is deferred. Our objective was to evaluate the risk factors and clinical outcomes of liver GVHD among patients who developed post-transplant liver dysfunction. Additionally, we evaluated the feasibility of liver biopsy in this population. We compared outcomes between liver GVHD and a “non-liver GVHD” group, which consisted of other etiologies of post-transplant liver dysfunction. Between January 2003 and December 2010, 249 patients developed post-transplant liver dysfunction following AHSCT: 124 patients developed liver GVHD and 125 were in the “non-liver GVHD” group. The incidence of acute and chronic liver GVHD at one year was 15.7% and 31.0%, respectively. The competing risk analysis revealed full intensity conditioning regimen (Hazard ratio [HR], 1.76; P = .008) and related donor (HR, 1.68; P = .004) as independent risk factors for liver GVHD. The time-varying covariate Cox regression analysis with competing risk event, demonstrated that liver GVHD was independently associated with higher non-relapse mortality, and adverse relapse-free and overall survival. A total of 112 liver biopsies were performed in 100 patients. No major complications were observed. Liver biopsy confirmed prebiopsy hypotheses in 49% of cases, and led to treatment modification in 49% of patients. Our study shows that liver GVHD is associated with adverse survival. Liver biopsy is safe and often helps directing care in this setting.
1 |. INTRODUCTION
Post-transplant liver dysfunction occurs in approximately 50%−80% of patients following allogeneic hematopoietic stem cell transplantation (AHSCT).1–3 Although this often raises the suspicion of liver GVHD, other causes including chemotherapy, infection, sinusoidal obstruction syndrome (SOS), reactivation of hepatitis, and iron overload may play an important role in the etiology of these abnormalities.1,2,4–7 Understanding the cause is important as it affects the treatment and outcomes. Classic liver GVHD, the most common type, usually manifests with a cholestatic picture, including hyperbilirubinemia, and increase in alkaline phosphatase. These biochemical abnormalities associated with liver GVHD are often seen in many other conditions, making the attestation of the cause based on biochemical markers alone difficult. Traditionally bile duct damage and portal lymphocyte infiltration on liver biopsy are thought to be characteristic features of liver GVHD.8 However, often liver biopsy is deferred because of concern of bleeding complications from transplant related coagulopathy and thrombocytopenia and frequently, the diagnosis relies on clinical judgment. The current acute liver GVHD grading by Glucksberg, et al, accounts for only total bilirubin level, and identifies classic type of acute liver GVHD.9 Recently, a hepatitic-variant of liver GVHD has been described, which presents as an isolated AST or ALT elevation, more than 10 times normal.10 However, the Glucksberg scoring system, which does not take into account transaminase elevations, does not recognize hepatitic-variants, and may underestimate the true incidence of acute liver GVHD (1).
In the literature, only two studies have reported cumulative incidences of acute and chronic liver GVHD, at 6.7% and 5.8%, respectively.11,12 In general, the information on liver GVHD, and particularly its impact on survival is limited. Moreover, prior studies evaluating the safety of post-transplant liver biopsies in small numbers of patients have shown increased morbidity.13–15 However, given the significant advances made in the post-transplant patient management, imaging and liver biopsy techniques, we assume that liver biopsy could safely be performed in this population, and it may help direct further management. Our study evaluates incidence, risk factors and outcomes of liver GVHD among patients with post-transplant liver dysfunction. We also evaluated the safety and feasibility of liver biopsy in this patient population.
2 |. MATERIALS AND METHODS
We conducted a retrospective study of adult patients who underwent AHSCT at Karmanos Cancer Institute (KCI) for hematologic malignancies, between January 2003 and December 2010, and developed post-transplant liver dysfunction. Post-transplant liver dysfunction was defined as alanine aminotransferase (ALT), or aspartate aminotransferase (AST) levels above twice the normal upper limit, or total bilirubin level over 1.5 times the normal upper. The diagnosis of liver GVHD was made after excluding common etiologies including alcoholism, hepatitis (HBsAg, anti-hepatitis C antibody, herpes simplex, varicella zoster virus, HHV6, HHV8, parvovirus), CMV or EBV viremia. Also excluded were sepsis/systemic infection, drug-induced hepatotoxicity, iron overload, sinusoidal obstruction syndrome (SOS) and gallbladder pathology. Ultrasound of the abdomen was performed as a part of the work up. The patients were divided into two groups, namely liver GVHD and “non-liver GVHD”, and outcomes were compared in both groups. Acute and chronic GVHD classification and grading was as per physician discretion using standard criteria.9,16 Acute liver GVHD was defined as post-transplant liver dysfunction occurring within or around day +100 post-transplant. Chronic liver GVHD was defined as abnormalities in liver function beyond or around day +100 post-transplant. All patients received ursodiol starting a day prior to the preparative regimen and continued for the first three months. We reviewed patients’ records till last follow up or death. This study was approved by the Wayne State University Institutional Review Board.
Two pathologists from our institution evaluated the liver biopsy samples using a scoring system based on our institutional criteria. At least 10 portal areas were evaluated for features of liver GVHD, including cholestasis, fibrosis, lobular inflammation, and iron deposition. Liver GVHD was classified as grade 1 with minimal lymphoplasmocytic infiltration of portal triads and/or lymphocytic infiltration of some of the bile ducts. Grade 2 is mild to moderate portal inflammation and/or involvement of most bile ducts. Grade 3 is moderate to severe portal inflammation and/or involvement of most bile ducts in moderate degree, plus increased portal fibrosis or bile duct loss or lobular inflammation. Iron deposition was classified as grade 0 (absent) as no stainable iron. Grade 1 (mild) as rare foci with positive staining involving less than 5% of macrophages or hepatocytes. Grade 2 (moderate) is positive staining in small foci involving less than 20% of macrophages or hepatocytes. Grade 3 (severe) is positive staining in confluent patches involving more than 20% of macrophages or hepatocytes.
2.1 |. Preparative regimen
Full intensity conditioning regimens included (1) busulfan (Bu) 130 mg/m2 (day −6 to −3),fludarabine (Flu) 30 mg/m2 (day −6 to −2); (2) BEAM ± R regimen consisted of carmustine 300 mg/m2 (day −7), etoposide (VP16), cytarabine both at 200 mg/m2/day (days −6 to −3), and melphalan 140 mg/m2 (day −2), and ± rituximab 375 mg/m2 (day −8); (3) Bu/CY consisted of Bu 16 mg/kg oral (day −7 to −4), cyclophosphamide (CY) 120 mg/kg daily (day −3 to −2); (4) VP16/TBI consisted of etoposide 60 mg/kg (day-4) followed by TBI 1200 cGY (day-2 to 0); (5) CY/TBI consisted of CY 120 mg/kg (day −6 to −5) and TBI 1200 cGY (day −3 to −1); (6) BAC consisted of Bu 4 mg/kg/day orally (day −8 to −5), Ara-C 2 g/M2 intravenously every 12 hours (day −4 and − 3) (a total of 4 doses) and cyclophosphamide 60 mg/kg/d IV (day −2 and − 1).
Reduced intensity regimens included (1) busulfan 130 mg/m2 (day −6 and − 5)/Flu 30 mg/m2 (day −6 to −2)/TBI 200 cGy (day 0), (2) Flu 30 mg/m2 (day −6 to −2)/melphalan 140 mg/m2 (day −2)/TBI 200 cGy (day 0) with or without rituximab, (3) Cy 60 mg/kg/day (day −5 & −4)/Flu 25 mg/m2 (day −6 to −4)/TBI 220 cGy twice daily (day −3 to −1), (4) Cy 60 mg/kg/day (day −4 and − 3)/Flu 30 mg/m2 (day −7 to −3).
2.2 |. Statistical methods
This is a single institution retrospective study among patients who developed liver dysfunction after AHSCT. The primary objectives were to estimate the impact of liver GVHD on overall survival (OS) and non-relapse mortality (NRM) compared to the “non-liver GVHD” group. Other time to event endpoints, such as relapse-free survival (RFS) and relapse were evaluated as well. As the liver GVHD events are post-transplant events, time-dependent covariates (TDC), the Cox model was used to take this into account. Acute and chronic liver GVHD were both modeled as TDC. The effect of acute and/or chronic liver GVHD on OS, or RFS, was assessed with the TDC Cox model, with starting time at AHSCT. For NRM, the effect of acute and/or chronic liver GVHD was assessed with the cause-specific TDC Cox model, with competing event of relapse. For relapse, the effect of acute and/or chronic liver GVHD was assessed with cause-specific TDC Cox with competing event of NRM. All Cox models were adjusted for the baseline conditioning regimen, disease risk index, donor type, and CMV serostatus.
Secondary objectives were to assess cumulative incidence of liver GVHD and its risk factors. As our population of interest, the post-transplant liver dysfunction patients were unidentifiable at the time of AHSCT. Our cumulative incidence of liver GVHD was descriptive, and the incidence would be higher compared to all patients who underwent AHSCT. The cumulative incidence of acute liver GVHD, and the cumulative incidence of chronic liver GVHD were calculated with disease relapse or NRM as competing risks. The risk factor for liver GVHD analyses was focused on association rather than prediction. This is similar to the baseline risk analysis for treatment responders/non-responders, who were unidentifiable pre-treatment. For baseline characteristics of the retrospective cohort, patients with vs without liver GVHD, were compared with Fisher’s exact test for categorical data, and Wilcoxon’s rank sum test for continuous data. Fine and Gray competing risks regression was used to assess the impact of baseline covariates on cumulative incidence of liver GVHD, where the competing risks were relapse or death. For patients who had both acute and chronic liver GVHD, the time of acute liver GVHD was used in the risk analysis.
Our tertiary objectives were to describe the effect of iron overload on OS among liver GVHD patients, who underwent liver biopsy and descriptive analyses on safety and utility of liver biopsy. The KM plot and log-rank test among the three iron overload stages, were performed with the starting time point of onset of acute or chronic liver GVHD, whichever came first. Median follow up for OS was calculated with the reverse KM method. The side-by-side boxplot and Bruskal-Wallis test were performed. They were for a simple correlation between the change of lab results from AHSCT, to the onset of liver dysfunction, such as ALT, AST, ALP, and total bilirubin, They were also to test clinical outcomes, such as NRM, relapse, and those alive without relapse, in non-liver GVHD and liver GVHD subgroups. All P values are 2-sided, and not adjusted for multiple testing, due to the nature of this exploratory study. Statistical analysis was performed using R version 3.3.2.
3 |. RESULTS
A total of 249 patients developed post-transplant liver dysfunction during the study period. One hundred twenty-four patients were found to have acute and/or chronic liver GVHD, and 125 were in the “non-liver GVHD” group (Table 1). Median age of the population was 51 years. The most common indications for AHSCT were AML (44%), MDS (16%) NHL (13%), and ALL (9%). Peripheral blood was the most commonly used stem cell source (94%). Liver GVHD patients were more likely to receive matched related AHSCT compared to non-liver GVHD group (50% vs 34%; P = .015), while the non-liver GVHD group was more likely to undergo matched unrelated AHSCT (66% vs 50%). Liver GVHD patients more commonly received tacrolimus-mycophenolate (MMF) as GVHD prophylaxis, compared to the non-liver GVHD group (94% vs 85%, P = 004). The diagnosis of liver GVHD was made clinically in 44 patients, and pathologically through liver biopsy in 80 patients. The median follow-up of surviving patients was 7.1 years (95% CI, 6.5–7.6). The common causes of death were infections (27%), disease recurrence (22%), worsening chronic (18%) and acute (12%) GVHD, multiorgan failure (3%), SOS (3%) and others (15%).
TABLE 1.
Patient characteristics
| With liver GVHD (n = 124) | Non-liver GVHD (n = 125) | P valuea | |
|---|---|---|---|
| Age | 51 (14,70) | 52 (23,70) | 0.246 |
| Sex | 0.128 | ||
| Male | 73 (59) | 61 (49) | |
| Female | 51 (41) | 64(51) | |
| Race | 0.52 | ||
| Caucasian | 114 (92) | 111 (89) | |
| Black | 8(6) | 13 (10) | |
| Others | 2(2) | 1(1) | |
| Diagnosis | 0.913 | ||
| AML | 58 (47) | 53 (42) | |
| MDS | 19(15) | 21 (17) | |
| NHL | 16(13) | 16 (13) | |
| ALL | 13 (10) | 10(8) | |
| HKD | 0(0) | 2 (2) | |
| CLL | 5(4) | 8(6) | |
| AAA | 2(2) | 3 (2) | |
| PLL | 0(0) | 1(1) | |
| ANLL | 1(1) | 0 (0) | |
| MM | 1(1) | 2 (2) | |
| Myeloproliferative disorder | 9(7) | 9(7) | |
| Admit KPS | 80 (60100) | 80 (50100) | 0.008 |
| Comorbidity Index | 1 (0,5) | 1 (0,5) | 0.537 |
| Disease Risk Index | 0.472 | ||
| Low | 10 (8) | 8(6) | |
| Intermediate | 73 (59) | 65 (52) | |
| High | 36 (29) | 48 (38) | |
| Very High | 5 (4) | 4(3) | |
| Source of transplant | 0.137 | ||
| PBSC | 120 (97) | 115 (92) | |
| BM | 4(3) | 7(6) | |
| CB | 0(0) | 3 (2) | |
| Donor type | 0.015 | ||
| Allo Unrelated | 62 (50) | 82 (66) | |
| Allo Related | 62 (50) | 43 (34) | |
| Sex mismatch | 0.242 | ||
| Match | 73 (59) | 63 (50) | |
| M-F | 25 (20) | 36 (29) | |
| F-M | 26(21) | 24 (19) | |
| Unknown | 0(0) | 2(2) | |
| ABO mismatch | 0.863 | ||
| Major mismatch | 26(21) | 22 (18) | |
| Minor mismatch | 24 (19) | 27 (22) | |
| Matched | 63 (51) | 58 (46) | |
| Bidirectional | 3(2) | 4(3) | |
| Unknown | 8(6) | 14(11) | |
| HLA match | 0.074 | ||
| 8/8 | 123 (99) | 118 (94) | |
| 7/8 | 0 (0) | 2 (2) | |
| < 7/8 | 1 (1) | 5 (4) | |
| CMV serostatus | 0.98 | ||
| +/+ | 30 (24) | 28 (22) | |
| −/− | 39 (31) | 42 (34) | |
| +/− | 16(13) | 15 (12) | |
| −/+ | 39 (31) | 40 (32) | |
| Conditioning regimen | 0.071e | ||
| Full-intensity | |||
| R-BEAM/BEAM | 14(11) | 12 (10) | |
| Busulfan-based regimen b | 59 (48) | 51 (41) | |
| TBI based regimenc | 10 (8) | 14(11) | |
| BAC | 11(9) | 4(3) | |
| Reduced-intensity | |||
| Bu based regimen | 20 (16) | 29 (23) | |
| TBI based regimend | 9 (7) | 11 (9) | |
| Flu-ATG/FLU-CY/Flu-MEL-ATG | 0 (0) | 2 (2) | |
| Cy-Flu-ATG | 2 (2) | 3 (2) | |
| GVHD prophylaxis | 0.004 | ||
| Tacro/MMF | 117 (94) | 106 (85) | |
| Tacro/SIR/Thymo | 3(2) | 16 (13) | |
| CSA/MTX | 3(2) | 1 (1) | |
| Tacro/MMF/Thymo | 1(1) | 1 (1) | |
| Tacro | 0(0) | 1 (1) |
Note: Continuous data are presented as median (range); categorical data are expressed as the counts (percentage).
Fisher’s exact test or Wilcoxon’s rank sum test as appropriate.
Bu-Flu, Bu-Flu-ATG, BU-CY.
Cy-TBI, VP16-TBI.
Flu-MEL-TBI, R-Flu-MEL-TBI, CY-Flu-TBI, R-BU-Flu-TBI.
P value for testing difference between full-intensity and reduced intensity.
3.1 |. Cumulative incidence of liver GVHD
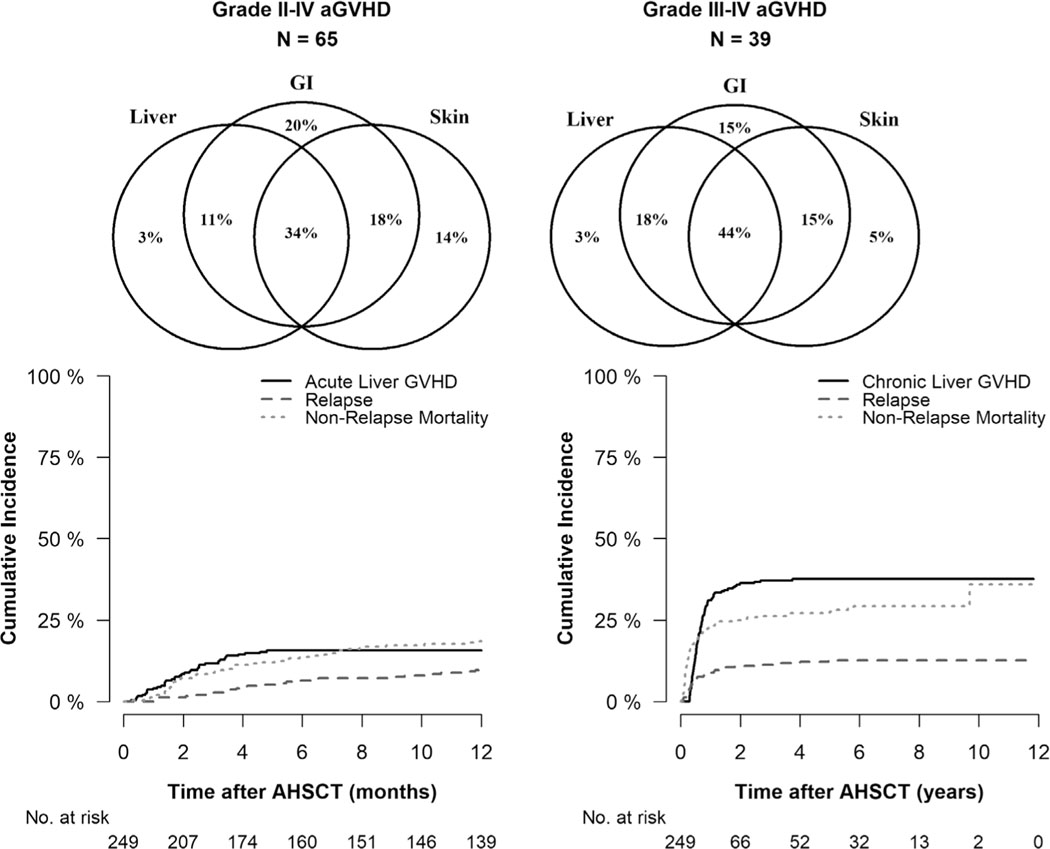
Thirty-nine patients developed acute liver GVHD at a median of 57 days (range, 7–146) post-transplant. The cumulative incidence of acute liver GVHD was 15.7% (95% CI, 11.5% −20.5%) at 6 months (Figure 1). As per Glucksberg criteria, stage 1, stage 2, stage 3 and stage 4 acute liver GVHD was observed in 18%, 13%, 18%, and 28% patients, respectively. Twenty-three percent (n = 9) patients with acute liver GVHD, did not have bilirubin elevation and the pathological diagnosis was made in 60% of these patients. The rates of grade II-IV and III-IV aGVHD of entire liver GVHD group were 52%, and 31%, respectively. Table S1 shows distribution of grade, stage and organ involvement of aGVHD. Isolated liver GVHD was observed in 3.1% and 2.6% in grade II-IV and III-IV aGVHD, respectively (Figure 1). Ninety-three patients developed chronic liver GVHD at a median of 210 days (range, 103–1366) post-transplant, and one-year cumulative incidence was 31.0% (95% CI, 25.3–36.8%), Figure 1. Eighty four out of 93 patients (90%) had extensive and 9 (10%) had limited chronic GVHD. Four patients (3%) were diagnosed as hepatitic-variant liver GVHD, and liver biopsy in three patients revealed evidence of GVHD. The kinetics of AST, ALT, ALP, and total bilirubin in the liver GVHD and non-liver GVHD group is shown in Tables 2–6. Liver GVHD patients had significantly higher values of AST, ALT, ALP, and total bilirubin compared to non-liver GVHD group.
FIGURE 1.
Venn diagram showing Liver, GI, and Skin GVHD involvement in Grade II-IV Acute GVHD (left) and Grade III-IV Acute GVHD (right) for liver GVHD group. Acute Liver GVHD with relapse or NRM as competing risks (bottom left). Chronic Liver GVHD with relapse or NRM as competing risks (bottom right)
TABLE 2.
Biochemical variables at allogeneic transplant
| With liver GVHD | Non-liver GVHD | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | Median (min, max) | n | Median (min, max) | P valuea | |
| AST | 124 | 27(8118) | 123 | 28 (0.4402) | 0.445 |
| ALT | 123 | 32 (12135) | 121 | 28 (10350) | 0.109 |
| ALP | 123 | 84 (43337) | 121 | 88 (35866) | 0.194 |
| Total bilirubin | 124 | 0.4 (0.1,3.4) | 123 | 0.4 (0.1,2.4) | 0.726 |
Wilcoxon’s rank sum test.
TABLE 6.
Biochemical variables at peak of liver dysfunction between liver biopsy and non-liver biopsy group
| Liver biopsy | Non-liver biopsy | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | Median (min, max) | n | Median (min, max) | P valuea | |
| AST | 80 | 231 (645404) | 99 | 116 (333345) | <0.001 |
| ALT | 80 | 352.5 (7017225) | 99 | 143 (263500) | <0.001 |
| ALP | 80 | 414.5 (732499) | 99 | 181 (551819) | <0.001 |
| Total bilirubin | 80 | 2.85 (0.4,50.4) | 99 | 1 (0.1,42.4) | <0.001 |
Wilcoxon’s rank sum test.
3.2 |. Risk factors of liver GVHD
Admit Karnofsky Performance Score (KPS) (P = .008), donor type (P = .015), and GVHD prophylaxis (0.0004) (Table 1) were risk factors based on univariate analysis. The multivariate analysis demonstrated that full intensity conditioning regimen (HR = 1.76, P = .008), and matched related donor AHSCT (HR = 1.68 P = .004) were associated with liver GVHD (Table S2).
3.3 |. Infectious complications
There was no difference in infectious complications between the liver GVHD and the non-liver GVHD group. Seventy-four of 124 patients (60%) with liver GVHD had positive systemic infections, compared to 78 of 125 patients (62%) in the non-liver GVHD group. No episode of CMV or EBV reactivation was noted at the time of onset of liver GVHD, or liver dysfunction in the non-liver GVHD group. There was no difference in the rate of CMV or EBV reactivations in both groups. Twenty-five patients with liver GVHD had CMV reactivation, with a median CMV PCR of 2713 (range, 321–58 500), while 36 patients with non-liver GVHD group developed CMV reactivation with a median CMV PCR of 3421 (range, 258–146 000). Five patients with liver GVHD had EBV reactivation with a median EBV PCR of 589 (range, 293–2749), while 11 patients with non-liver GVHD had EBV reactivation with a median EBV PCR of 432 (range, 205–1883).
3.4 |. Impact of liver GVHD on survival, RFS, relapse and NRM
Both acute and chronic liver GVHD were independent risk factors for adverse NRM. The adverse impact of acute liver GVHD was predominantly observed in grade III-IV aGVHD patients. Patients with grade III-IV aGVHD with liver involvement had poor OS (HR 3.65, P < 0.001), RFS (HR 3.62, P < 0.001) and NRM (HR 4.03, P < 0.001), compared to grade III-IV aGVHD without liver involvement (Table 7). Liver involvement does not moderate the effect of chronic GVHD on OS or NRM. However, among patients who had both grade III-IV aGVHD and chronic GVHD, those with liver involvement had worse OS (HR 4.67, P < 0.001), RFS (HR 4.43, P < 0.001) and NRM (6.16, P < 0.001), than without liver involvement. No adverse impact of liver GVHD on relapse was noticed.
TABLE 7.
Results of cox model for OS, RFS, Relapse, and NRM
| OS | RFS | Relapse | NRM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Acute GVHD* | |||||||||
| No acute GVHD | 93 | Reference | Reference | Reference | Reference | ||||
| l-llaGVHD& Liver (−) | 79 | 0.87 (0.57–1.33) | 0.517 | 0.89 (0.59–1.36) | 0.599 | 1.67 (0.82–3.41) | 0.161 | 0.60 (0.35–1.04) | 0.068 |
| l-llaGVHD& Liver (+) | 14 | 1.12 (0.52–2.40) | 0.778 | 1.09 (0.51–2.34) | 0.819 | 0.45 (0.06–3.50) | 0.444 | 1.37 (0.60–3.14) | 0.459 |
| lll-IV aGVHD & Liver (−) | 38 | 1.44 (0.87–2.40) | 0.160 | 1.41 (0.85–2.33) | 0.187 | 0.77 (0.22–2.73) | 0.687 | 1.63 (0.93–2.85) | 0.088 |
| lll-IV aGVHD & Liver (+) | 25 | 5.26 (3.15–8.76) | <0.001 | 5.10 (3.07–8.47) | <0.001 | 0.88 (0.11–6.80) | 0.901 | 6.57 (3.82–11.30) | <0.001 |
| Contrast lll-IV aGVHD & Liver (+) vs lll-IV aGVHD & Liver (−) | 3.65 (2.00–6.65) | <0.001 | 3.62 (1.99–6.61) | <0.001 | 1.14 (0.12–11.09) | 0.910 | 4.03 (2.14–7.61) | <0.001 | |
| Chronic GVHD* | |||||||||
| No chronic GVHD | 73 | Reference | Reference | Reference | Reference | ||||
| cGVHD & Liver (−) | 83 | 1.17 (0.63–2.18) | 0.620 | 1.39 (0.76–2.57) | 0.288 | 1.03 (0.37–2.84) | 0.954 | 1.67 (0.77–3.63) | 0.192 |
| cGVHD & Liver (+) | 93 | 1.50 (0.78–2.88) | 0.225 | 1.71 (0.89–3.27) | 0.108 | 0.70 (0.22–2.26) | 0.551 | 2.56 (1.14–5.73) | 0.022 |
| Contrast cGVHD & Liver (+) vs cGVHD & Liver (−) |
1.28 (0.80–2.05) | 0.303 | 1.22 (0.77–1.95) | 0.395 | 0.68 (0.27–1.69) | 0.405 | 1.53 (0.88–2.66) | 0.135 | |
| Contrast III-IV aGVHD & Liver (+) and cGVHD & Liver (+) vs III-IV aGVHD & Liver (−) and cGVHD & Liver (−) |
4.67 (2.15–10.13) | <0.001 | 4.43 (2.05–9.60) | <0.001 | 0.77 (0.07–9.16) | 0.839 | 6.16 (2.62–14.49) | <0.001 |
We plotted the distribution of the change of AST, ALT, ALP and total bilirubin in liver GVHD and non-liver GVHD groups, from AHSCT to onset of liver dysfunction. We divided patients into three groups: alive without relapse, relapse and non-relapse mortality. Total bilirubin level was significantly associated with higher NRM compared to AST, ALT and ALP in both groups (Figure S1 and S2).
3.5 |. Safety and utility of liver biopsy
One hundred twelve liver biopsies were performed during the study period in 100 patients. Ninety patients underwent one biopsy, eight patients had two biopsies and two had three liver biopsies, respectively for liver dysfunction not improving after initial treatment. Biopsy approach was transjugular (n = 106), percutaneous (n = 3) or laproscopic (n = 3). The median time between AHSCT to liver biopsy was 175 days (range, 21–1366). The median platelet count prior to biopsy was 121 000 per microliter (range, 10 000–418 000 per microliter). Seven patients had platelets <50 000 per microliter, 36 had platelets between 50 000 and 100 000 per microliter and 67 had platelets >100 000 per microliter at the time of biopsy. Twenty-three patients received platelet transfusions with a median of 15 units (range, 1–40), whereas 10 patients received fresh frozen plasma with a median of four units (range, 1–16) during the peri-biopsy period. Three patients experienced hematoma and pneumothoraces following liver biopsy, and all had platelet counts above 100 000 per microliter at the time of biopsy. No mortality was observed.
A pathological diagnosis was made in all patients. Primary diagnoses were following: liver GVHD (n = 80), iron overload (n = 11), nonalcoholic steatohepatitis (n = 7), acute non-viral hepatitis (n = 5), SOS (n = 3), cholestasis (n = 5), viral hepatitis (n = 1), drug related liver injury (n = 1), extrahepatic biliary obstruction (n = 1), and fibrosis (n = 2). Seventy-six patients out of 80 with liver GVHD had evidence of iron overload, and four patients had more than one pathology present in the specimen. Following histologic stages of liver GVHD were noted in liver biopsy specimens (n = 80 patients): stage I (n = 22, 28%), stage II (n = 43, 54%), and stage III (n = 15, 19%). Grade I, II, and III iron overload was noted in 19%, 36%, 40% patients, respectively (Table S3). The log rank test did not show any survival difference among patients with grade I, II, and III iron overload (P = .57) (Figure S3). In general, 1–2 hypotheses were generated prior to liver biopsy, and the hypothesis was correct in 49% of cases. In 49% of cases (55 out of 112 biopsies) biopsy results led to modification of the treatment. Based on biopsy results, immunosuppressive medications were escalated in 14 patients and discontinued in four patients, antiviral medications were started in two patients, phlebotomy was started in 32 patients. Both phlebotomy initiation and immunosuppressive medication escalation was performed in three patients. Systemic steroid use was frequent and prolonged in patients with liver GVHD. The median number of immunosuppressive therapies was two in liver GVHD group. Ninety-five patients (77%) with liver GVHD responded at a median of 35 days (range, 0–3706). The remaining 28 patients who did not respond are deceased. One patient without response is alive.
4 |. DISCUSSION
In this retrospective study, we present cumulative incidence, risk factors, and clinical outcomes of liver GVHD among post-transplant liver dysfunction patients. We evaluated safety and utility of liver biopsy as well. To date, our study is one of the largest to evaluate outcomes of both acute and chronic liver GVHD. Following important findings can be made: (1) the incidence of acute and chronic liver GVHD was 15.7% and 31.0%, respectively among patients with post-transplant liver dysfunction, (2) liver biopsy is safe in post-transplant period and modified treatment in 49% of cases, (3) full intensity conditioning regimen and related donors were risk factors for liver GVHD and (4) acute and chronic liver GVHD led to adverse NRM and OS.
We observed higher incidences of acute and chronic liver GVHD. In a study by Arai et al, the cumulative incidence of acute liver GVHD was 6.7%, whereas Chen et al reported chronic liver GVHD incidence rate at 5.8%.11,12 The difference in these rates could be attributed to different populations. Our rates reflect incidences among patients with post-transplant liver dysfunction, whereas above mentioned studies included an entire cohort of transplant patients. We think that stem cell source and GVHD prophylaxis could have also contributed to this difference. Bone marrow was a predominant source of stem cells (>50% of patients) in the Arai et al study, compared to peripheral blood stem cells (97% of patients) in our study. Tacrolimus and mycophenolate was a frequently used GVHD prophylaxis regimen. We have previously demonstrated that use of tacrolimus and mycophenolate was associated with relatively higher incidences of severe acute and chronic GVHD, in matched related and unrelated donors with AHSCT.17 Hepatitic-variant liver GVHD was less frequently noticed in our cohort: 3% in our study vs 36% in others.18 Although the information on transplant characteristics of the prior study is not available, the reason for the discrepancy could be the difference in conditioning regimen or supportive care. Isolated acute liver GVHD was noted in 2.6–3.1% of patients in our cohort, which was in line with prior studies.
Full intensity conditioning regimen and matched related donors were risk factors for the development of liver GVHD. Busulfan based full intensity conditioning regimen was predominantly used in our cohort. Studies have shown that busulfan is a potent hepatotoxic agent. Higher incidence of veno-occlusive disease and chronic GVHD was noted in patients treated with busulfan compared to TBI.19 Moreover, myeloablative regimens are shown to cause higher rate of grade II-IV acute GVHD and chronic GVHD. This effect could partly be related to cytokine storm arising from the pronounced GI mucosal injury.20 Matched related donor was emerged as a risk factor for liver GVHD. The precise mechanism is unclear. However, similar finding was noticed in a study by Arai et al.11 Our study also reveals that liver GVHD is an independent risk factor for adverse NRM and survival. This effect was pronounced in grade III-IV aGVHD, while a positive trend was noted in chronic liver GVHD. Unlike our study, few previous reports showed adverse prognosis of acute liver GVHD in all grades of aGVHD.11,21,22 This difference could be related to relatively smaller sample size in our study. The adverse NRM and survival could be related to prolonged use of systemic steroids and immunosuppressive medications.
Post-transplant liver dysfunction is common after AHSCT and reflects underlying liver injury. Majority of the times, physicians tend to assign diagnosis based on clinical suspicion. However, in cases with no clear diagnosis based on clinical, laboratory or radiologic imaging results, liver biopsy was performed. Our study revealed a different diagnosis than the one hypothesized before liver biopsy in half of the cases and liver biopsy led to change in management in 49% of cases. In an another study evaluating liver biopsy results, the correlation between pre- and post-liver biopsy diagnosis was noted in 34% of cases and treatment modification was made in 65% of cases following liver biopsy.23 Few other studies have reported treatment modifications in 37% to 66% of cases following liver biopsy.13–15,24 This indicates that liver biopsy should be considered when uncertainty in diagnosis prevails. Except for bleeding complications occurring in a minority of patients (2.7%), no major complications were observed. No bleeding complications occurred in patients with platelets below 50 000 per microliter, which indicates that liver biopsy can be safely performed despite lower platelet counts.25,26 This could be due to transjugular liver biopsies which is associated with less complications.27 Conversely, previous reports have noted severe hemorrhages, subcapsular bleeding, cardiac arrythmia, ICU admissions and procedure related deaths.13–15 Another study reported 3% complication rate following percutaneous liver biopsies.28
In conclusion, liver GVHD is the most common etiology of post-transplant liver dysfunction and associated with adverse survival. Liver biopsy is safe and helpful in determining accurate etiology, which can have therapeutic implications.
Supplementary Material
TABLE 3.
Biochemical variables at peak between liver GVHD and non-liver GVHD group
| With Liver GVHD | Non-Liver GVHD | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | Median (min, max) | n | Median (min, max) | P valuea | |
| AST | 123 | 201 (375404) | 118 | 129 (334447) | 0.003 |
| ALT | 123 | 274 (2317225) | 118 | 162 (263500) | 0.001 |
| ALP | 123 | 359 (732499) | 118 | 193 (552402) | <0.001 |
| Total bilirubin | 123 | 2.1 (0.3,50.4) | 118 | 1.1 (0.1,42.4) | <0.001 |
Wilcoxon’s rank sum test.
TABLE 4.
Biochemical variables at allogeneic transplant between liver biopsy and non-liver biopsy group
| Liver biopsy | Non-liver biopsy | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | Median (min, max) | n | Median (min, max) | P valuea | |
| AST | 99 | 27(11,71) | 147 | 28 (0.4124) | 0.483 |
| ALT | 97 | 31 (14120) | 146 | 29 (10350) | 0.259 |
| ALP | 97 | 85 (43337) | 146 | 86 (35304) | 0.448 |
| Total bilirubin | 99 | 0.4 (0.1,3.4) | 147 | 0.4 (0.1,2.4) | 0.802 |
Wilcoxon’s rank sum test.
TABLE 5.
Biochemical variables at the time of onset of liver dysfunction between liver biopsy and non-liver biopsy group
| Liver biopsy | Non-liver biopsy | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | Median (min, max) | n | Median (min, max) | P valuea | |
| AST | 85 | 173 (181425) | 98 | 107.5 (173345) | <0.001 |
| ALT | 85 | 223 (381998) | 98 | 136.5 (183500) | <0.001 |
| ALP | 85 | 273 (691229) | 97 | 168 (221871) | <0.001 |
| Total bilirubin | 85 | 1.3 (0.2,26.8) | 98 | 0.85 (0.1,45) | 0.026 |
Wilcoxon’s rank sum test.
Footnotes
CONFLICT OF INTEREST
None.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Ho GT, Parker A, MacKenzie JF, Morris AJ, Stanley AJ. Abnormal liver function tests following bone marrow transplantation: aetiology and role of liver biopsy. Eur J Gastroenterol Hepatol. 2004;16(2):157–162. [DOI] [PubMed] [Google Scholar]
- 2.Tomas JF, Pinilla I, Garcia-Buey ML, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant. 2000;26(6):649–655. [DOI] [PubMed] [Google Scholar]
- 3.Kim BK, Chung KW, Sun HS, et al. Liver disease during the first post-transplant year in bone marrow transplantation recipients: retrospective study. Bone Marrow Transplant. 2000;26(2):193–197. [DOI] [PubMed] [Google Scholar]
- 4.Strasser SI, Sullivan KM, Myerson D, et al. Cirrhosis of the liver in long-term marrow transplant survivors. Blood. 1999;93(10):3259–3266. [PubMed] [Google Scholar]
- 5.El-Sayed MH, El-Haddad A, Fahmy OA, Salama II, Mahmoud HK. Liver disease is a major cause of mortality following allogeneic bone-marrow transplantation. Eur J Gastroenterol Hepatol. 2004;16(12):1347–1354. [DOI] [PubMed] [Google Scholar]
- 6.Kusumi E, Kami M, Kanda Y, et al. Hepatic injury following reduced intensity unrelated cord blood transplantation for adult patients with hematological diseases. Biol Blood Marrow Transplant. 2006;12(12):1302–1309. [DOI] [PubMed] [Google Scholar]
- 7.Ozdogan O, Ratip S, Ahdab YA, et al. Causes and risk factors for liver injury following bone marrow transplantation. J Clin Gastroenterol. 2003;36(5):421–426. [DOI] [PubMed] [Google Scholar]
- 8.Snover DC, Weisdorf SA, Ramsay NK, McGlave P, Kersey JH. Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology. 1984;4(1):123–130. [DOI] [PubMed] [Google Scholar]
- 9.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 10.Akpek G, Boitnott JK, Lee LA, et al. Hepatitic variant of graft-versushost disease after donor lymphocyte infusion. Blood. 2002;100(12):3903–3907. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y, Kanda J, Nakasone H, et al. Risk factors and prognosis of hepatic acute GvHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51(1):96–102. [DOI] [PubMed] [Google Scholar]
- 12.Chen CT, Liu CY, Yu YB, et al. Characteristics and risk of chronic graft-versus-host disease of liver in allogeneic hematopoietic stem cell transplant recipients. PLoS One. 2017;12(9):e0185210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chahal P, Levy C, Litzow MR, Lindor KD. Utility of liver biopsy in bone marrow transplant patients. J Gastroenterol Hepatol. 2008;23(2):222–225. [DOI] [PubMed] [Google Scholar]
- 14.Oshrine B, Lehmann LE, Duncan CN. Safety and utility of liver biopsy after pediatric hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2011;33(3):e92–e97. [DOI] [PubMed] [Google Scholar]
- 15.Shulman HM, Gooley T, Dudley MD, et al. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation. 1995;59(7):1015–1022. [DOI] [PubMed] [Google Scholar]
- 16.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Kadhimi Z, Gul Z, Chen W, et al. High incidence of severe acute graft-versus-host disease with tacrolimus and mycophenolate mofetil in a large cohort of related and unrelated allogeneic transplantation patients. Biol Blood Marrow Transplant. 2014;20(7):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma SY, Au WY, Lie AK, et al. Liver graft-versus-host disease after donor lymphocyte infusion for relapses of hematologic malignancies post allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34(1):57–61. [DOI] [PubMed] [Google Scholar]
- 19.Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia Nordic Bone Marrow Transplantation Group. Blood. 1999;93(7):2196–2201. [PubMed] [Google Scholar]
- 20.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–185. [DOI] [PubMed] [Google Scholar]
- 21.Pidala J, Chai X, Kurland BF, et al. Analysis of gastrointestinal and hepatic chronic graft-versus-host [corrected] disease manifestations on major outcomes: a chronic graft-versus-host [corrected] disease consortium study. Biol Blood Marrow Transplant. 2013;19(5):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robin M, Porcher R, de Castro R, et al. Initial liver involvement in acute GVHD is predictive for nonrelapse mortality. Transplantation. 2009;88(9):1131–1136. [DOI] [PubMed] [Google Scholar]
- 23.Ruggiu M, Bedossa P, Rautou PE, et al. Utility and safety of liver biopsy in patients with undetermined liver blood test anomalies after allogeneic hematopoietic stem cell transplantation: a monocentric retrospective cohort study. Biol Blood Marrow Transplant. 2018;24(12):2523–2531. [DOI] [PubMed] [Google Scholar]
- 24.Carreras E, Granena A, Navasa M, et al. Transjugular liver biopsy in BMT. Bone Marrow Transplant. 1993;11(1):21–26. [PubMed] [Google Scholar]
- 25.McAfee JH, Keeffe EB, Lee RG, Rosch J. Transjugular liver biopsy. Hepatology. 1992;15(4):726–732. [DOI] [PubMed] [Google Scholar]
- 26.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165–173. [DOI] [PubMed] [Google Scholar]
- 27.Dohan A, Guerrache Y, Boudiaf M, Gavini JP, Kaci R, Soyer P. Transjugular liver biopsy: indications, technique and results. Diagn Interv Imaging. 2014;95(1):11–15. [DOI] [PubMed] [Google Scholar]
- 28.Maximova N, Gregori M, Barbieri F, Pizzol A, Sonzogni A. Safety and utility of percutaneous liver biopsy in hematopoietic stem cell transplant pediatric recipients: a retrospective study. BMC Cancer. 2016;16:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.