Abstract
Obesity is an increasingly prevalent state of energy imbalance that contributes to breast cancer risk and outcomes. The effects of obesity differ by breast cancer subtype and menopause. While most studies have focused on postmenopausal hormone receptor positive disease, less is known about the relationship between obesity and triple negative breast cancer (TNBC). Here we will review the observations linking obesity to TNBC, the socioeconomic disparities that contribute to obesity-related TNBC, and putative biologic mechanisms. Finally, we will consider the impact of obesity on surgical and medical treatment of TNBC and novel strategies to improve energy balance after cancer diagnosis.
Keywords: obesity, body fat, insulin, inflammation, metabolic syndrome, breast cancer, exercise, diet
Introduction: Obesity and cancer
The World Health Organization (WHO) defines obesity as a body mass index (BMI) above or equal to 30 kg/m2.1 In recent decades, the number of obese individuals in the United States has significantly increased with the prevalence of obesity being 43% in 2018 with numbers continuing to rise.2 This epidemic has steadily increased over the last two decades, with a rising prevalence (35% to 42%) in women between 2005 and 2018.3 There is a large, growing body of literature that suggests a connection between obesity and development of several different types of malignancies, including breast cancer.4-9 This observation is largely based on the use of anthropometric indices, such as BMI, to define obesity.10,11 In the Million Women Study, for example, obesity was estimated to contribute to approximately 5% of all cancers in postmenopausal women.12 Several studies have established obesity as a risk factor for postmenopausal breast cancer, specifically estrogen receptor-positive and triple negative phenotypes.13-16 In the post-diagnosis setting, higher BMI is a poor prognostic factor that is associated with an approximately 30% increased risk of recurrence or death in obese versus normal-weight women diagnosed with breast cancer.17,18
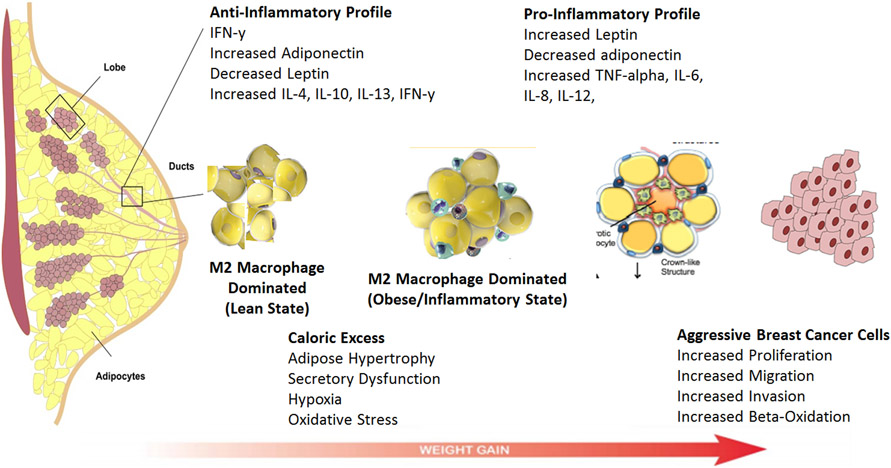
The underlying pathophysiology of the obesity-breast cancer link is complex and still under investigation, however evidence from observational and laboratory studies have suggested that local and systemic effects of obesity, altered levels of adipokines, circulating steroid hormones and local estrogen signaling, metabolic syndrome with insulin resistance, and adipose inflammation all play a role in the biologic impact of obesity on breast cancer (Figure 1).4 There is also recent evidence that suggests that a subset of women with normal BMI and excess body fat may be at increased risk of breast cancer. BMI is only a crude measure of body size that does not discriminate between adiposity and muscle and thus, individuals thought to be healthy by virtue of a normal BMI may in fact have metabolic obesity despite normal weight.19-23 Additionally, a minority of individuals with a BMI > 30 kg/m2 do not have abnormal metabolic profiles. These individuals are described as metabolically healthy obese and do not have insulin resistance, type 2 diabetes, dyslipidemia or hypertension.24-26 Thus, it is also important to consider metabolic health beyond BMI when assessing risk of obesity-related cancers.
FIGURE 1.
Pathophysiology of the obesity-breast cancer link.
While the link between postmenopausal obesity and estrogen receptor positive breast cancer is due in part to an increase in peripheral estrogen production by adipose tissue and subsequent stimulation of the estrogen receptor (ER), several hormone-independent mechanisms may drive the association between obesity and triple negative breast cancer (TNBC).27,28 Efforts to identify modifiable prognostic factors in TNBC are clinically urgent due to the less favorable outcomes of this breast cancer subtype. In this article, we review the biologic and clinical links between obesity and TNBC, as well as the impact of obesity on the treatment and prognosis of TNBC.
Biologic Links
The underlying pathophysiology linking obesity, adiposity and TNBC is complex and is under active investigation. The local and systemic effects of obesity on tumorigenesis, progression and metastasis involves altered levels of adipokines, insulin resistance, adipose inflammation and a pro-tumorigenic tissue microenvironment. These biologic alterations associated with obesity will be reviewed here.
Adipokines are bioactive hormones produced and secreted by adipose tissue. The production of these hormones are modulated by several stimuli, including insulin, estrogens and inflammatory mediators.4,29 Leptin is an important adipokine which rises with increasing BMI and it is known to activate the JAK/STAT, MAPK/ERK and PI3K/AKT signaling pathways. These activated signaling pathways lead to increased cell migration, invasion, cell survival, tumor growth and metastasis in TNBC through the upregulation of multiple factors such as Serpine 1, SNAI2, IL-6, TWIST1 and others that promote cancer cell migration.30,31 Adiponectin, another adipokine, is also involved in the association of obesity and breast cancer with levels being inversely correlated with obesity. In contrast to leptin, adiponectin is protective against tumor growth. Multiple studies have demonstrated that women with low levels of adiponectin have an increased risk of breast cancer with various mechanisms being responsible for this association. 32-34
In TNBC specifically, insulin signaling is an important mediator of obesity-related cancer growth. The insulin-like growth factor (IGF) system is involved in tumorigenesis and the proliferation, survival and migration of tumor cells.35-38 Saxena et al reported that leptin directly increases activity of the IGF-1 receptor and IGF-1 reciprocally increased activity of the leptin receptor.39 This bidirectional crosstalk promotes proliferation and migration of TNBC cells and is compounded by the observation that IGF-1 receptor is expressed at higher levels in TNBC.40 Hyperinsulinemia is also associated with increased synthesis of IGF-1 which subsequently activates signaling proteins such as MAPK and Akt. Davison et al specifically demonstrated that the IGF signal transduction pathway is active in TNBC cells and that its activation increases cell proliferation and promotes cell survival. Insulin has been shown to stimulate the overexpression of leptin as well as activate the Akt/mTOR signaling pathway. This pathway plays a pivotal role in cell growth, proliferation, and survival and activation of this signaling predicts a poor prognosis in women with TNBC.41-44 In addition, rapidly growing TNBCs are glucose dependent and generate energy via aerobic glycolysis which Akt/mTOR signaling pathway promotes. This increase in glycolysis and glucose uptake supplies anabolic precursors for rapid growth and promotes mitochondrial dysfunction that leads to cancer cell apoptosis resistance.45-47
Obesity is recognized to be a state of chronic inflammation with increased circulating levels of inflammatory cytokines including interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α and C-reactive protein (CRP).48,49 These cytokines promote tissue inflammation and can directly stimulate cancer cell growth.6 Locally, inflammation of breast white adipose tissue (WAT) has been associated with increased breast cancer risk50 and worse outcomes for women with breast cancer51 Excess adiposity and adipocyte cell death lead to macrophage infiltration and the development of crown-like structures (CLS), which are comprised of a dead or dying adipocyte surrounded by activated macrophages.52 The presence of CLS are associated with the activation of NF-κB, and increased expression and activity of aromatase, the rate limiting enzyme in estrogen biosynthesis.53 While these findings suggest that WAT inflammation promotes breast tumor growth through estrogen signaling, hormone-independent pathways may link WAT inflammation to the growth of TNBCs. For example, WAT inflammation is associated with the metabolic syndrome, which compromises a number of clinical disorders such as obesity, insulin resistance and dyslipidemia. These states of energy imbalance can promote tumor growth through dysregulated adipokine and insulin signaling as discussed above. Furthermore, WAT inflammation has been associated with estrogen-independent cancers such as high grade prostate tumors54 and shortened survival for patients with squamous cell carcinoma of the oral tongue55 Importantly, excess body fat has is associated with adipocyte hypertrophy and WAT inflammation in a subset of women with normal BMI.56 Our group previously reported that approximately one third of normal weight women have WAT inflammation and its associated local and systemic alterations that promote cancer growth56. In a subsequent study, we reported that normal weight women with high levels of body fat have an increased risk of invasive breast cancer of all subtypes21. Further studies are needed to better understand the impact of normal weight obesity on TNBC specifically.
Clinical Links
Several population-based studies link obesity to TNBC; key studies will be reviewed here and are presented in Table 1. Obesity is prevalent in patients diagnosed with TNBC. In a retrospective study including 680 Caucasian women in West Virginia, obesity was present in 49.6% of patients with TNBC but only 35.8% of those with other breast cancer subtypes.57 In another study, Ademuyiwa et al reported that 31.1% of patients with TNBC were overweight and 39.2% were obese, compared to 29.7% who were normal weight.58 In addition to BMI, obesity may be diagnosed using waist/hip ratio (WHR), which is to be a more significant measure of visceral adiposity. The World Health Organization classifies a WHR of ≥0.85 as high risk for metabolic disorders.59 In the Carolina Breast Cancer Study, WHR was used to investigate an association between obesity and TNBC. Across all women, there was an increased risk (OR of 2.3, CI 1.4-3.6) for developing TNBC with higher WHR, and this effect was observed in pre and postmenopausal women.40,60 Notably, BMI was not associated with TNBC risk in this study, which underscores the greater sensitivity of WHR for identifying obesity-related disorders.
TABLE 1.
Population-Based Studies Linking Obesity to TNBC
| Publication Year | Study Years | Number of Subjects | ||
|---|---|---|---|---|
| Case-Control | ||||
| Phipps et al | 2008 | 1997-1999, 2000-2004 | 1,524 | |
| Dolle et al | 2009 | 1983-1990, 1990-1992 | 1,728 | |
| Trivers et al | 2009 | 1990-1992 | 1,017 | |
| Berstad et al | 2010 | 1994-1998 | 8,038 | |
| Gaudet et al | 2011 | 1980-1982 | 3,532 | |
| Phipps et al | 2011 | 1993-1998 | 1,51,982 | |
| Dawood et al | 2012 | 1990-2010 | 2,311 | |
| Mowad et al | 2013 | 1998-2011 | 183 | |
| Cakar et al | 2015 | 2005-2010 | 2,900 | |
| Bandera et al | 2015 | Consortium of Studies | 3,174 | |
| Kawai et al | 2016 | 2004-2006 | 20,090 | |
| Nagrani et al | 2016 | 2009-2013 | 1,633 | |
| Case-Case | ||||
| Millikan et al | 2008 | 1993-1996, 1996-2001 | 1,385 | |
| Phipps et al | 2008 | 1997-1999, 2000-2004 | 1,124 | |
| Vona-Davis et al | 2008 | 1999-2004 | 512 | |
| Kwan et al | 2009 | 1997-2000, 2006-2009 | 2,517 | |
| Maiti et al | 2009 | 2004-2009 | 176 | |
| Dolle et al | 2009 | 1983-1990, 1990-1992 | 881 | |
| Stead et al | 2009 | 1998-2006 | 403 | |
| Trivers et al | 2009 | 1990-1992 | 474 | |
| Gaudet et al | 2011 | 1980-1982 | 855 | |
| Yang et al | 2011 | Consortium of Studies | 11,356 | |
| Lara-Medina et al | 2011 | 1998-2008 | 2,074 | |
| Phipps et al | 2011 | 1993-1998 | 2,898 | |
| Ademuyiwa et al | 2011 | 1996-2010 | 418 | |
| Dawood | 2012 | 1990-2010 | 2,311 | |
| Chen et al | 2013 | 2004-2012 | 2,659 | |
| Tait et al | 2014 | 2006-2010 | 501 | |
| Hao et al | 2015 | 2002-2012 | 1,106 | |
| Bonsang-Kitzis | 2015 | 2002-2012 | 326 | |
| Chen et al | 2016 | 2006-2015 | 206 | |
| Meta-Analysis | ||||
| Suzuki et al | 2009 | 1970-2007 | 3,672 | 16 studies |
| Yang 2011 | 2011 | 1998-2009 | 47,184 | 34 Studies |
| Pierobon et al | 2013 | 2008-2012 | 3,845 | 11 Studies |
| Mei et al | 2018 | 2011-2017 | 4,412 | 9 Studies |
Menopausal status is emerging as an important mediator of obesity-related breast cancer risk. A systematic review and meta-analysis evaluating the associations among TNBC, obesity and menopausal status suggested that premenopausal obese (BMI ≥30 kg/m2) women have a 42% higher risk of developing TNBC compared to non-obese women, though this study is limited by the low incidence of TNBC in this cohort.14 Chen et al corroborated these observations, reporting that obese premenopausal women had an 82% increased risk of TNBC compared to women with normal BMI. Among postmenopausal women in this study, obesity was associated with reduced risks of TNBC (OR 0.72, CI 0.54-1.00).61 However, another meta-analysis of prospective cohorts and case-control studies indicated that among premenopausal women, obesity was associated with a 20% lower risk of hormone receptor positive tumors, but no association with other tumor subtypes.62 In the postmenopausal setting, obesity is a well-established risk factor for hormone receptor positive breast cancer.62 Excess adipose tissue after menopause may increase endogenous estrogen production and may help explain the association between body weight and risk of hormone-dependent breast cancer in the postmenopausal setting. Collectively, epidemiologic data suggest that premenopausal obesity is associated with a mildly protective effect against hormone receptor positive breast cancer and may be associated with an increased risk of TNBC, whereas obesity is clearly associated with an increased risk of postmenopausal hormone receptor positive breast cancer.
Disparities contributing to Obesity-related disorders
Obesity is generally more prevalent in urban areas and in populations with lower education and income levels.63,64 The ability to purchase healthy foods, adequate time for physical activity, and access to quality health care are all potential contributors to the disparate prevalence of obesity. Additional nutritional factors that contribute to the correlation between poverty and obesity include lower cost of calorie-dense, highly processed foods, food deserts in low-income neighborhoods with limited access to supermarkets, and neighborhood stress (e.g., safety) which may discourage inhabitants from engaging in physical activity.65 And, disparities in income have a particular effect on African-Americans and Hispanics.66 African-American and Hispanic families in particular are also more likely to live in food deserts compared to non-Hispanic women of European or Asian descent.44,67,68 Additionally, African American populations living in urban environments have been found to be less likely to engage in physical activity, which is associated with lack of access to public parks and green spaces.69 Taken together, the inability to afford healthy food, compounded with the lack of availability of healthy food choices and environments that make regular physical activity challenging, increases the risk of obesity-related disorders for underserved communities.
The higher prevalence of obesity-related disorders in underserved communities may also extend to cancers associated with obesity. The incidence of TNBC is highest in women with germline BRCA1 mutations and in premenopausal African American women.60,70-73 For example, in the Carolina Breast Cancer Study, TNBC occurred in 39% of premenopausal African American women compared to 14% of postmenopausal African American women and 16% of postmenopausal non-Hispanic European American women.60 In this cohort, higher WHR was associated with increased risk of TNBC among pre- and postmenopausal women. Data from the Women’s Circle Health Study similarly indicated that increased WHR was associated with an increased risk of premenopausal breast cancer in African Americans after adjustment for BMI.74,75
Pooled data from the African American Breast Cancer Epidemiology and Risk (AMBER) consortium has also helped to better understand TNBC patterns in African Americans. The AMBER consortium is a collaboration of four studies: The Carolina Breast Cancer Study, the Women’s Circle of Health Study, the Black Women’s Health Study and the Multiethnic Cohort Study.60,75-78 This consortium was formed in attempts to investigate the inconsistencies of results across the individual studies that evaluated the potential associations between obesity and TNBC in African American women. In this pooled dataset, the effect of general and central obesity (BMI vs WHR) varied by menopausal status and hormone receptor subtype. Specifically, in premenopausal women, increased BMI was associated with a decreased incidence of ER+ cancer with no associations with TNBC, however higher WHR was associated with increased risk of ER+ cancers. Postmenopausal women with high BMIs had an increased risk of ER+ cancers (OR 1.31 95% CI 1.02-1.67) and a reduced risk of TNBC (OR 0.60 95% CI 0.39-0.93). There was an elevated risk with higher WHR for each breast cancer subtype in postmenopausal women, with strongest risk for TNBC (OR 1.73 95% CI 1.02-2.91).75 Future studies that more accurately classify body composition, for example through radiographic assessment, may provide new insights into the impact of adiposity on TNBC risk in pre- and postmenopausal populations – particularly among various racial groups where body fat distribution may not be adequately characterized by anthropometric indices such as BMI and WHR.
Other Populations
The predictive utility of BMI and WHR for breast cancer risk varies by race/ethnicity, menopausal status and tumor subtype. Among East Asian populations, ER+ tumors incidence is highest in premenopausal women, and elevated BMI has not been associated with an increased risk in this population.79-82 A higher level of body fat per unit BMI in Asians compared to other ethnic groups and differences in distribution of adipose tissue may lead to this underestimation of breast cancer risk by BMI. Our group previously reported that WAT inflammation occurs in Taiwanese women and is associated with elevated BMI, increased body fat and alterations in circulating metabolic and inflammatory factors. When compared to Caucasian women, Taiwanese woman had larger breast adipocytes despite lower BMI, with adipocyte size correlating with total body fat. Women with hormone receptor positive breast cancers had the highest levels of body fat compared to other subtypes of cancer. This association between total body fat and WAT inflammation indicate that women with excess body fat have distinct alterations within the breast microenvironment that likely predisposes them to carcinogenesis.79
Obesity rates are also high among Hispanic/Latina populations with breast cancer, which is reflective of the overall Hispanic/Latina population in the United States.83 Compared with non-Hispanic white women, Hispanic/Latina women are more likely to be diagnosed with more advanced TNBCs, which have poor survival rates.84 Conflicting observations have been reported regarding prognosis in Hispanic women, which may be confounded by the high rates of obesity in this population. In a cohort of Hispanic/Latina women, our group previously reported that breast WAT inflammation was present in nearly half of women in this cohort, which is a higher prevalence than previously reported in predominantly Caucasian study populations . The prevalence and severity of inflammation were strongly associated with higher BMI in this cohort.84 Patients with severe WAT inflammation also had significantly larger adipocytes, which is consistent with previous studies, and suggests that obesity-associated adipocyte hypertrophy leads to immune cell recruitment and WAT inflammation.85 As described above, WAT inflammation is associated with adverse breast cancer risk and outcomes. Collectively, these findings suggest that the higher prevalence of obesity and obesity-related inflammation could contribute to worse breast cancer risk and outcomes in Hispanic/Latina populations.
The Appalachian population in rural West-Virginia is a unique cohort that is 95% Caucasian, ranked sixth highest in the United States for the percent of the population that is below the poverty line and ranked fourth in the nation for the prevalence of obesity.86,87 In this population, TNBC was observed to be more prevalent in obese patients compared to those who had a BMI < 30 (49.6% vs 35.8%, p = 0.01). Interestingly, the age of TNBC diagnosis was closer to that reported in African American populations than the later age at presentation reported in other cohorts of White women; 44.5% of West Virginian White women with TNBC were diagnosed with breast cancer at <50 years old compared to 26.7% of those with non-triple negative tumors. These findings suggest that socioeconomic factors are likely to be key mediators of cancer presentation and may exert stronger effects in obesity-related cancers, particularly in underserved and/or minority populations.
Obesity and TNBC Outcomes
There is a substantial body of evidence that links obesity to prognostic outcomes among women with breast cancer.17,18,88 One of the largest studies that included patients with ER negative and lymph node negative breast cancer was the NSABP-14 trial, which demonstrated that contralateral breast cancer and overall mortality were increased in obese patients, however insufficient data regarding HER2 expression precluded analysis by tumor subtype. In a systematic review including 391 breast cancer studies, obesity was associated with poorer overall and breast cancer-specific survival in both pre and post-menopausal women with hormone-receptor positive subtypes.89 However, the prognostic impact of obesity in TNBC is mixed.40,90 In one of the largest retrospective studies including 2311 women with TNBC, there was no differences in disease-free survival (DFS) and overall survival (OS) across BMI groups at diagnosis.91 Tait et al, Sparano et al and Ademuyaiwa et al observed similar trends in their retrospective reviews.92 In a Turkish cohort, Cakar et al also reported no differences in survival among normal weight, overweight and obese patients with TNBC, even when comparing tumor size, lymph node status and Ki-67 index among the 3 BMI subgroups.93 In contrast, among 518 patients with TNBC in the Shanghai Breast Cancer Survival study, elevated BMI at one year prior to breast cancer diagnosis was associated with shortened survival.94 Similarly, others have reported that high BMI in Chinese populations is an independent predictor of worse survival after TNBC diagnosis. 95 High BMI has also been associated with poor treatment response, manifested as decreased pathologic complete response rates after neoadjuvant therapy for TNBC.95,96 Taken together these mixed observations indicate that the relationship between obesity and outcomes after TNBC diagnosis is complex and that race is a key mediator. Further studies are needed to better characterize body composition and adiposity to advance our understanding of the role adiposity plays in response to breast cancer treatment and ultimately survival.
Impact of Obesity on treatment of TNBC
Surgical
The management of TNBC typically includes surgical, medical and radiation treatments. The presence of obesity and/or metabolic dysfunction can impact all components of treatment plans. Obesity increases post-surgical complications after mastectomy with or without reconstruction. For example, after reconstructive surgical procedures, obese women were more likely to experience wound dehiscence, wound infection, seromas, and flap failure in autologous reconstruction.97-100 Additionally, obese women are more likely to suffer post-operative medical conditions such as deep venous thrombosis (DVT), pulmonary embolism (PE) and pneumonia.101-103 Lymphedema is also much more prevalent in obese women after breast surgery, with risk estimates up to 5.5-fold increase or higher than non-obese women.4,104
Medical
Chemotherapy is currently the most commonly used systemic treatment option for TNBC. Compared with hormone receptor positive subtypes, pathologic complete response (pCR) rates are higher after neoadjuvant chemotherapy for TNBC.105,106 Attaining pCR Patients who experience pCR after neoadjuvant therapy for TNBC have improved survival outcomes compared to those who have residual disease.91,105 The effect of obesity on response rates to chemotherapy has been examined. In a study of 1,169 patients diagnosed with invasive breast cancer, obesity was associated with lower pCR rates after neoadjuvant chemotherapy (OR = 0.67, CI 0.45-0.99), and obese patients were more likely to have hormone receptor negative and later stage tumors. 40,107 Consistently, in a pooled analysis of neoadjuvant trials, higher BMI was associated with lower pCR rates and shorter disease free survival in patients with TNBC. 93,96 In light of these poor prognostic findings, it should be noted that the American Society of Clinical Oncology (ASCO) guidelines recommend administration of full weight-based chemotherapy dose for obese patients, based upon the observation that worsened survival in obese patients may be related to under-dosing of cytotoxic therapies.108-110
Radiation
Less is known about the impact of obesity on response to radiation therapy. Larger breast volume in obese women has been associated with higher incidence of skin toxicity, late complications and poor cosmetic outcomes in patients treated with adjuvant breast radiotherapy.111-113 Treatment discontinuation due to toxicity could potentially have an adverse impact on recurrence rates, particularly for more proliferative breast cancer subtypes such as TNBC.
Adjunct treatments targeting obesity
Physical activity and weight loss have been observed to have an inverse relationship with breast cancer risk and recurrence. In the Women’s Healthy Eating and Living (WHEL) randomized control trial, consumption of a diet high in fruits and vegetables plus increased physical activity (equivalent of walking 30 minutes per day, 6 days per week) was associated with a 46% reduction in mortality (HR = 0.56, CI 0.31-0.98). However, when stratified by tumor subtype, the effect of the intervention was only detected in patients with hormone receptor positive tumors, while no significant effect was observed in the TNBC subgroup (p = 0.40).114 These findings have been corroborating in a meta-analysis that included 12,108 patients across 6 studies. The pooled analyses indicated that post-diagnosis physical activity was associated with reduced breast cancer deaths (HR = 0.50 CI 0.34-0.74) for patients with ER+ tumors with no significant effects in the ER-negative subgroup.115 In an analysis that included 2,987 women from the Nurses Health Study, Holmes and colleagues reported an association between increased physical activity and reduced risk of death in patients with ER+ breast cancer, however no significant effect was observed in patients with TNBC.116
In addition to exercise, dietary interventions have been studied in patients diagnosed with breast cancer. In the Women’s Intervention Nutrition Study (WINS) which included 2,437 women diagnosed with breast cancer, patients randomized to the dietary intervention group were counseled to reduce calories from fat to 15%. When stratified by breast cancer subtype, a trend towards reduction in recurrence events was observed in the intervention arm for hormone receptor negative cancers compared to hormone receptor positive cancers, though this was not a statistically significant difference (p = 0.15).117 An ongoing clinical trial, the Breast Cancer Weight Loss (BWEL) study, will test the effect of a diet and exercise counseling intervention on recurrence and mortality for patients diagnosed with HER2-negative breast cancer. This is a phase III randomized trial evaluating the effect of a weight loss program on cancer recurrence among 3,136 overweight and obese women with stage II to II breast cancer in the United States and Canada.118,119 Participants are randomly assigned to a 2-year weight loss program or to a control group, and study results are anticipated in approximately 2024. While the trial does not specifically focus on patients with TNBC, this subtype is included in the study.
To date, the impact of physical activity and diet on TNBC outcomes has been extrapolated from epidemiologic observations and subgroup analyses that are largely underpowered. Future studies of lifestyle interventions are needed that target TNBC and that are powered to address this cancer subtype specifically. This approach could help to clarify mixed observations from population studies and could provide low-cost, low-toxicity strategies to potentially improve treatment response in TNBC.
Conclusions and Future Directions
The disruption of energy homeostasis, which classically manifests as obesity, leads to the development of multiple metabolic disorders and several cancers. A growing body of evidence indicates that the effects of obesity and energy imbalance on the development and progression of cancer are complex and vary by cancer, tumor subtype, menopausal status, socioeconomic characteristics, and other factors. Breast cancer is one of the most common female cancers and is a leading cause of death worldwide. The identification of modifiable factors that could reduce the incidence and mortality of breast cancer is a major public health priority, and obesity is a leading candidate factor. To date, most studies that aim to interrogate the relationship between obesity and breast cancer remain focused on hormone receptor positive tumors in postmenopausal women (i.e., the most common subtype). Stratification of population data by tumor subtype coupled with subgroup analyses of a limited number of lifestyle modification trials in breast cancer have raised the intriguing hypothesis that treating obesity and improving energy balance may optimize TNBC risk and outcomes. Despite mixed results reported thus far, the preponderance of evidence supports the need for studies that are adequately powered to investigate associations among obesity, adiposity, metabolic disorders and TNBC. Such data would inform the design of energy balance intervention trials such as lifestyle modification (e.g. diet, exercise) or use of metabolically active medications in TNBC. Reducing the risk of developing TNBC or reducing the risk of advanced disease by targeting obesity through low-cost, low-toxicity lifestyle interventions would represent a major clinical advance and thereby warrants continued research efforts in order to reduce the mortality burden of TNBC.
Financial Support:
This work was supported by the Breast Cancer Research Foundation, the Kat’s Ribbon of Hope Foundation, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Competing Interests:
Dr. Iyengar receives consulting fees from Novartis and Seattle Genetics.
References
- 1.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998;6Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 2.Adult Obesity Facts. Center for Disease Control and Prevention. [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 4.Argolo DF, Hudis CA, Iyengar NM. The Impact of Obesity on Breast Cancer. Curr Oncol Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 6.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting 2013:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011;29:4561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control 2002;13:325–32. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005;23:1370–8. [DOI] [PubMed] [Google Scholar]
- 10.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body size and risk of breast cancer. Am J Epidemiol 1997;145:1011–9. [DOI] [PubMed] [Google Scholar]
- 12.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Bmj 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleary MP. Impact of obesity on development and progression of mammary tumors in preclinical models of breast cancer. J Mammary Gland Biol Neoplasia 2013;18:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137:307–14. [DOI] [PubMed] [Google Scholar]
- 15.Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 2009;20:1071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev 2011;20:454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2014;25:1901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–35. [DOI] [PubMed] [Google Scholar]
- 19.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–9. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Ambrosi J, Silva C, Galofre JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) 2012;36:286–94. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar NM, Arthur R, Manson JE, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol 2019;5:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes 1998;47:699–713. [DOI] [PubMed] [Google Scholar]
- 23.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes care 2004;27:2222–8. [DOI] [PubMed] [Google Scholar]
- 24.Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism 2016;65:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann W, Reuter W, Schutz C, Lindhofer HG, Wurzberger G, Schneider P. [The behavior of specific parameters of lipid and lipoprotein metabolism in metabolically healthy and obese subjects]. Z Gesamte Inn Med 1982;37:43–50. [PubMed] [Google Scholar]
- 26.Bluher S, Schwarz P. Metabolically healthy obesity from childhood to adulthood - Does weight status alone matter? Metabolism 2014;63:1084–92. [DOI] [PubMed] [Google Scholar]
- 27.Stoll BA. Adiposity as a risk determinant for postmenopausal breast cancer. Int J Obes Relat Metab Disord 2000;24:527–33. [DOI] [PubMed] [Google Scholar]
- 28.Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res 2013;184:253–9. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med 2015;66:281–96. [DOI] [PubMed] [Google Scholar]
- 30.Sabol RA, Bowles AC, Cote A, et al. Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res 2019;21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Esposito V, Liguoro D, Ambrosio MR, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016;7:24495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 2007;92:1041–8. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clinical cancer research : an official journal of the American Association for Cancer Research 2003;9:5699–704. [PubMed] [Google Scholar]
- 34.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 2006;237:109–14. [DOI] [PubMed] [Google Scholar]
- 35.Davison Z, de Blacquiere GE, Westley BR, May FE. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia 2011;13:504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998;351:1393–6. [DOI] [PubMed] [Google Scholar]
- 37.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2005;14:699–704. [DOI] [PubMed] [Google Scholar]
- 38.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer 2006;13:273–8. [DOI] [PubMed] [Google Scholar]
- 39.Saxena NK, Taliaferro-Smith L, Knight BB, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res 2008;68:9712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer 2012;2012:809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massihnia D, Galvano A, Fanale D, et al. Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 2016;7:60712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iqbal J, Thike AA, Cheok PY, Tse GM, Tan PH. Insulin growth factor receptor-1 expression and loss of PTEN protein predict early recurrence in triple-negative breast cancer. Histopathology 2012;61:652–9. [DOI] [PubMed] [Google Scholar]
- 43.Ueng SH, Chen SC, Chang YS, et al. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int J Clin Exp Pathol 2012;5:806–13. [PMC free article] [PubMed] [Google Scholar]
- 44.Dietze EC, Chavez TA, Seewaldt VL. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am J Pathol 2018;188:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 2006;66:8927–30. [DOI] [PubMed] [Google Scholar]
- 46.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 2004;64:3892–9. [DOI] [PubMed] [Google Scholar]
- 47.Robey RB, Hay N. Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 2009;19:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris PG, Zhou XK, Milne GL, et al. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev Res (Phila) 2013;6:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter JM, Hoskin TL, Pena MA, et al. Macrophagic "Crown-like Structures" Are Associated with an Increased Risk of Breast Cancer in Benign Breast Disease. Cancer Prev Res (Phila) 2018;11:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin Cancer Res 2016;22:2283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–55. [DOI] [PubMed] [Google Scholar]
- 53.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gucalp A, Iyengar NM, Zhou XK, et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic Dis 2017;20:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyengar NM, Ghossein RA, Morris LG, et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer 2016;122:3794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyengar NM, Brown KA, Zhou XK, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res (Phila) 2017;10:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vona-Davis L, Rose DP, Hazard H, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev 2008;17:3319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ademuyiwa FO, Groman A, O'Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 2011;117:4132–40. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. [Google Scholar]
- 60.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Cook LS, Tang MT, et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat 2016;157:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer 2009;124:698–712. [DOI] [PubMed] [Google Scholar]
- 63.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 65.US Department of Agriculture.
- 66.Urban Institute.
- 67.Beaulac J, Kristjansson E, Cummins S. A systematic review of food deserts, 1966-2007. Prev Chronic Dis 2009;6:A105. [PMC free article] [PubMed] [Google Scholar]
- 68.Cummins S, Clary C, Shareck M. Enduring challenges in estimating the effect of the food environment on obesity. Am J Clin Nutr 2017;106:445–6. [DOI] [PubMed] [Google Scholar]
- 69.US Department of Health and Human Services. [DOI] [PubMed]
- 70.Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 2015;15:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;113:357–70. [DOI] [PubMed] [Google Scholar]
- 72.Stead LA, Lash TL, Sobieraj JE, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res 2009;11:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 2010;116:4926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev 2013;22:1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bandera EV, Chandran U, Hong CC, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 2015;150:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berstad P, Coates RJ, Bernstein L, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev 2010;19:1532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972) 1995;50:56–8. [PubMed] [Google Scholar]
- 78.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iyengar NM, Chen IC, Zhou XK, et al. Adiposity, Inflammation, and Breast Cancer Pathogenesis in Asian Women. Cancer Prev Res (Phila) 2018;11:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin CH, Liau JY, Lu YS, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol Biomarkers Prev 2009;18:1807–14. [DOI] [PubMed] [Google Scholar]
- 81.Huang CS, Lin CH, Lu YS, Shen CY. Unique features of breast cancer in Asian women--breast cancer in Taiwan as an example. J Steroid Biochem Mol Biol 2010;118:300–3. [DOI] [PubMed] [Google Scholar]
- 82.Chen MJ, Wu WY, Yen AM, et al. Body mass index and breast cancer: analysis of a nation-wide population-based prospective cohort study on 1 393 985 Taiwanese women. Int J Obes (Lond) 2016;40:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenlee H, Shi Z, Hibshoosh H, et al. Obesity-associated Breast Inflammation among Hispanic/Latina Breast Cancer Patients. Cancer Prev Res (Phila) 2019;12:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 2015;45:2446–56. [DOI] [PubMed] [Google Scholar]
- 86.Proctor BDDJ. US Census Bureau. Poverty in the United States: 2002. Washington (DC). [Google Scholar]
- 87.Ahluwalia IB, Mack KA, Murphy W, Mokdad AH, Bales VS. State-specific prevalence of selected chronic disease-related characteristics--Behavioral Risk Factor Surveillance System, 2001. MMWR Surveill Summ 2003;52:1–80. [PubMed] [Google Scholar]
- 88.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep 2014;3:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres-de la Roche LA, Steljes I, Janni W, Friedl TWP, De Wilde RL. The Association between Obesity and Premenopausal Breast Cancer According to Intrinsic Subtypes - a Systematic Review. Geburtshilfe Frauenheilkd 2020;80:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 2010;7:683–92. [DOI] [PubMed] [Google Scholar]
- 91.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer 2012;12:364–72. [DOI] [PubMed] [Google Scholar]
- 92.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst 2012;104:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cakar B, Muslu U, Erdogan AP, et al. The Role of Body Mass Index in Triple Negative Breast Cancer. Oncol Res Treat 2015;38:518–22. [DOI] [PubMed] [Google Scholar]
- 94.Bao PP, Cai H, Peng P, et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control 2016;27:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen HL, Ding A, Wang ML. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. Springerplus 2016;5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat 2015;150:127–39. [DOI] [PubMed] [Google Scholar]
- 97.Xing L, Culbertson EJ, Wen Y, Robson MC, Franz MG. Impaired laparotomy wound healing in obese rats. Obes Surg 2011;21:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scheflan M, Kalisman M. Complications of breast reconstruction. Clin Plast Surg 1984;11:343–50. [PubMed] [Google Scholar]
- 99.Pierpont YN, Dinh TP, Salas RE, et al. Obesity and surgical wound healing: a current review. ISRN Obes 2014;2014:638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panayi A, Agha RA, Sieber BA, Orgill DP. Impact of obesity on outcomes in breast reconstruction: A systematic review protocol. Int J Surg Protoc 2016;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeevan R, Browne JP, Pereira J, et al. Socioeconomic deprivation and inpatient complication rates following mastectomy and breast reconstruction surgery. Br J Surg 2015;102:1064–70. [DOI] [PubMed] [Google Scholar]
- 102.Fischer JP, Nelson JA, Kovach SJ, Serletti JM, Wu LC, Kanchwala S. Impact of obesity on outcomes in breast reconstruction: analysis of 15,937 patients from the ACS-NSQIP datasets. J Am Coll Surg 2013;217:656–64. [DOI] [PubMed] [Google Scholar]
- 103.Fischer JP, Tuggle CT, Au A, Kovach SJ. A 30-day risk assessment of mastectomy alone compared to immediate breast reconstruction (IBR). J Plast Surg Hand Surg 2014;48:209–15. [DOI] [PubMed] [Google Scholar]
- 104.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500–15. [DOI] [PubMed] [Google Scholar]
- 105.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–81. [DOI] [PubMed] [Google Scholar]
- 106.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–34. [DOI] [PubMed] [Google Scholar]
- 107.Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26:4072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2012;30:1553–61. [DOI] [PubMed] [Google Scholar]
- 109.Colleoni M, Li S, Gelber RD, et al. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet 2005;366:1108–10. [DOI] [PubMed] [Google Scholar]
- 110.Rosner GL, Hargis JB, Hollis DR, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1996;14:3000–8. [DOI] [PubMed] [Google Scholar]
- 111.Dore M, Hennequin C. [Late sequelae and cosmetic outcome after radiotherapy in breast conserving therapy]. Cancer Radiother 2012;16:462–9. [DOI] [PubMed] [Google Scholar]
- 112.Verbelen H, Gebruers N, Beyers T, De Monie AC, Tjalma W. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: a systematic review. Breast Cancer Res Treat 2014;147:463–71. [DOI] [PubMed] [Google Scholar]
- 113.Goldsmith C, Haviland J, Tsang Y, Sydenham M, Yarnold J, Group FT. Large breast size as a risk factor for late adverse effects of breast radiotherapy: is residual dose inhomogeneity, despite 3D treatment planning and delivery, the main explanation? Radiother Oncol 2011;100:236–40. [DOI] [PubMed] [Google Scholar]
- 114.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 2007;25:2345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 2011;28:753–65. [DOI] [PubMed] [Google Scholar]
- 116.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479–86. [DOI] [PubMed] [Google Scholar]
- 117.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767–76. [DOI] [PubMed] [Google Scholar]
- 118.Ligibel JA, Basen-Engquist K, Bea JW. Weight Management and Physical Activity for Breast Cancer Prevention and Control. Am Soc Clin Oncol Educ Book 2019;39:e22–e33. [DOI] [PubMed] [Google Scholar]
- 119.Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 2017;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]