Abstract
Thyroid cancer is the most common malignancy of the endocrine system with a steadily rising incidence. The term thyroid cancer encompasses a spectrum of subtypes, namely papillary thyroid cancer, follicular thyroid cancer, anaplastic thyroid cancer, and medullary thyroid cancer. Each subtype differs histopathologically and in degrees of cellular differentiation, which may be in part due to signaling of the Notch pathway. The Notch pathway is an evolutionarily conserved signal transduction mechanism that regulates cell proliferation, differentiation, survival, stem cell maintenance, embryonic and adult development, epithelial-mesenchymal transition, and angiogenesis. Its role in cancer biology is controversial, as it has been shown to play both an oncogenic and tumor suppressive role in many different types of cancer. This discordance holds true for each subtype of thyroid cancer, indicating that Notch signaling is likely cell type and context dependent. Whether oncogenic or not, Notch signaling has proven to be significantly involved in the tumorigenesis of thyroid cancer and has thus earned interest as a therapeutic target. Advancement in the understanding of Notch signaling in thyroid cancer holds great promise for the development of novel treatment strategies to benefit patients.
Keywords: thyroid cancer, Notch signaling, Notch pathway, papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, anaplastic thyroid cancer, HDAC inhibitors, Notch signaling modulation
INTRODUCTION
Thyroid cancer encompasses a wide range of malignant subtypes that can differentially arise from various cell types within the thyroid gland. These subtypes include papillary, follicular, anaplastic, and medullary thyroid cancer. The incidence of these cancers is steadily increasing, with a parallel increase in mortality rates as well (Morris LGT 2013, Furuya-Kanamori L 2016, Mao Y 2016). Localized disease is often curable with surgery, but patients with metastatic disease have limited treatment options.
Since its discovery in the early 20th century, Notch signaling has proven to be a critical pathway in mammalian development by regulating cell fate decisions, proliferation, differentiation, and survival (Dexter JS 1914, Mohr OL 1919, Guruharsha KG 2012). Four transmembrane receptor isoforms termed Notch1–4 are capable of binding to 5 different ligands (Delta-like-1, −2, −4, Jagged1, Jagged2) in a juxtracrine manner (Takebe N 2014). This interaction initiates intracellular cleavage events that ultimately lead to the transcription of specific target genes (reviewed in other chapters).
The role of Notch signaling in cancer is particularly complex, as it acts as either an oncogene or a tumor suppressor. An oncogenic role of Notch signaling has been reported in: breast cancer (Reedijk M 2005), colon cancer (Sikandar SS 2010), T-cell acute lymphoblastic leukaemia (T-ALL) (Ellisen L 1991), chronic lymphocytic leukemia (CLL) (Fabbri 2011, Puente 2011), non-small cell lung cancer (Westoff 2009), pancreatic adenocarcinoma (Hanlon 2010), clear cell renal cell carcinoma (CCRCC) (Sjölund J 2008), and in gliomas (Dantas-Barbosa C 2015). On the other hand, Notch signaling has been shown to limit tumorigenicity. This effect was first described in keratinocytes (Nickoloff BJ 2002), and has since been observed in other cancers, including: prostate cancer (Shou J 2001), small cell lung cancer (Sriuranpong V 2001), pancreatic neuroendocrine cancer (Nakakura EK 2005, Kunnimalaiyaan M 2005), hepatocellular carcinoma (Qi R 2003, Viatour 2011), cervical cancer (Talora C 2002), B cell malignancies (Zweidler-McKay 2005), myeloid Leukemia (Klinakis A 2011), head and neck squamous cell carcinoma (Stransky N 2011), and neuroblastoma (Zage PE 2012).
Targeting the Notch pathway for cancer treatment continues to attract interest. In thyroid cancer, the importance of Notch signaling is only beginning to be understood. In this chapter, the diverse functions of Notch signaling in thyroid cancer will be discussed, along with current strategies used to target and modulate Notch signaling as a possible anti-cancer therapy.
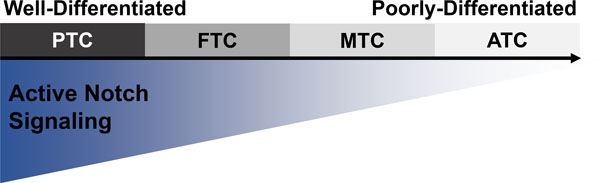
A SPECTRUM OF THYROID CANCER SUBTYPES (figure 1 here)
Figure 1. Hypothesized relationship between Notch signaling and thyroid cancer differentiation.
Although the role of Notch signaling is controversial, a majority of investigative efforts have shown that as thyroid cancer cells lose differentiation, Notch signaling also decreases. The directionality of this relationship is not clear, but the canonical role of Notch signaling in mammals suggests that a loss of Notch signaling would contribute to a reduction in cellular differentiation.
Thyroid cancer is the most common malignancy of the endocrine system with an increasing global incidence (Zhang 2017, Seib CD 2019, Schneider DF 2013, Powers AE 2019). The heterogenous clinical presentations and genetic profiles of thyroid cancer can make this disease complex in nature. The term “thyroid cancer” encompasses a range of subtypes that originate from different cell types within the thyroid- namely follicular thyrocytes and the parafollicular C-cells. Across the various thyroid cancer subtypes, the degree of cellular differentiation has a strong influence on disease progression, treatment strategies, and overall patient survival (Jung CW 2017, Yu XM 2016, Yuan L 2019). As these cancers de-differentiate, they tend to become more aggressive and gain lethality (Ragazzi M 2014, Cooper DS 2006, Gallo 2018).
Well-differentiated tumors of the thyroid fall on one end of the spectrum and include thyrocyte-derived papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC). These subtypes account for over 90% of thyroid cancer cases and are generally associated with good prognoses and high survival rates (Yu XM 2016, Zhang 2017, Yamashita 2013, Xiao X 2009, Jung CW 2017, Choi D 2016). FTC tends to be more aggressive than PTC; although local and distant metastases have been reported in both subtypes, leading to poor clinical outcomes (Xiao X 2009, Lin JD 2004).
The other end of the subtype spectrum includes less-differentiated thyroid cancers, meaning that the cells lack thyroid cell-specific characteristics. One of the most lethal human cancers, anaplastic thyroid cancer (ATC) falls within this group. ATC causes over 50% of thyroid cancer deaths and carries a 1-year survival rate of less than 20% (Chen J 2008, Hsu 2014, Smallridge RC 2010). Another subtype that lacks differentiation is medullary thyroid cancer (MTC). This subtype originates from the parafollicular C-cells of the thyroid and is classified as a neuroendocrine neoplasm (Jaskula-Sztul 2011, Lou I 2017, Roy M 2013). Localized cases of MTC are often curable, but patients with distant metastases have a 5-year survival rate less than 50% (Lou I 2017).
There is an evident and urgent need to improve patient outcomes by developing effective therapeutic options for each thyroid cancer subtype. Among each of them, the Notch signaling pathway consistently emerges as a therapeutic target due to its frequently observed dysregulation (Hsu 2014, Takebe N 2014). In this chapter, the highly controversial role and current breadth of research available regarding Notch signaling in each subtype of thyroid cancer will be summarized.
NOTCH SIGNALING IN PAPILLARY THYROID CANCER
Papillary thyroid cancer (PTC) is the most common subtype of thyroid cancer and is considered to be well-differentiated. The role of Notch signaling in PTC is not clearly defined, as it has been discordantly reported as both oncogenic and tumor suppressive.
In 2011, Park et al. analyzed tissues from patients with PTC and found that the IHC expression of the Notch1 receptor correlated with the increased presence of nodal metastases, extrathyroidal extension, and greater tumor size. However, they found no correlation between the presence of the Notch3 receptor and clinic-pathological factors. Therefore, the authors concluded that in PTC, Notch1 expression is correlated with poor prognostic factors, but Notch3 expression is not. That same year, Geer et al. 2011 found that Notch1 is expressed in normal thyrocytes and has even higher expression in PTC. In support of the notion that PTC overexpresses Notch receptors, additional studies showed that the upregulation of Notch1 expression was observed in both human PTC tissue and a transgenic mouse model of PTC (Yamashita 2013, Gallo 2018). Likewise, greater Notch1 and Notch2 expression was also observed in a PTC cell line when compared to normal thyrocytes (Gallo 2018). Interestingly, the Notch3 and Notch4 isoforms were reported to have low, variable expression levels in PTC cell lines (Gallo 2018). Taken together, these studies conclude that PTC overexpresses the Notch1 and Notch2 isoforms, but the expression of the Notch3 and Notch4 isoforms in PTC is variable. The higher levels of Notch1 and Notch2 in PTC appear to be correlated with more aggressive disease factors.
A recent meta-analysis of 421 patients with PTC conducted by Yuan et al. revealed a significant correlation between an upregulation of Notch1 signaling and the presence of nodal metastasis, tumor size, clinical stage, and capsular invasion (Yuan L 2019). Importantly, the authors concluded that greater Notch1 signaling in PTC could contribute to a poor prognosis for patients, emphasizing an oncogenic role of Notch signaling in PTC. A separate study performed an extensive immunohistochemical analysis of Notch1 expression in 106 thyroid neoplasms and similarly discovered that Notch1 expression was associated with PTC; however, somewhat contrary to the previously mentioned studies, this study found that Notch1 expression was seen exclusively in the tumor cells, highlighted by results that showed normal thyroid tissues were consistently negative for Notch1 expression (Piana 2019).
In contrast to a possible oncogenic role of Notch signaling in PTC that was been discussed in this chapter thus far, several studies have shown that a re-activation, or an overexpression, of the Notch pathway mediates a tumor suppressive effect by suppressing the growth of PTC cells. Such studies showed that Notch1 is highly expressed in normal human thyroid tissue, but minimally present in both resected human metastatic PTC tissue and metastatic PTC cells in vitro (Xiao X 2009, Yu XM 2016). Furthermore, a significantly higher rate of PTC recurrence was observed in patients with low levels of Notch1 expression (Yu XM 2016). In a study conducted by Xiao X and colleagues, the pharmacological induction of Notch1 protein expression resulted in a dose-dependent growth reduction of PTC cells in vitro. In accordance with these observations, the re-activation of Notch signaling in PTC has been speculated as a therapeutic strategy.
The role of Notch signaling in PTC lacks a definitive consensus, thereby demanding further investigation. This disparity suggests that the mechanisms of Notch signaling in PTC are contextually-dependent, with the degree of cellular differentiation playing a potentially critical role in the determination of how Notch signaling affects cells. Additionally, it appears that the individual Notch receptor isoforms can have different implications on PTC cells, although the direct mechanisms are yet to be resolved.
NOTCH SIGNALING IN FOLLICULAR THYROID CANCER
Follicular thyroid cancer (FTC) carries a similar degree of well-differentiation as PTC, although these two subtypes are histopathologically different. The primary behavior of Notch signaling in FTC has been documented as tumor suppressive. Current research has shown that in comparison to normal thyroid tissue, FTC cells have a lower level of Notch receptor expression, along with lower levels of the Notch signaling target gene, Hes1, thus suggesting minimal pathway activity (Feretti 2008, Xiao 2009, Somnay 2017). In fact, one study discovered that the expression level of the Notch3 receptor was highest in both normal human thyroid tissue and in normal thyroid cells grown in vitro, when compared against resected human FTC tissue and FTC cells grown in vitro (Somnay 2017). Moreover, it has also been shown that metastatic FTC expresses even lower levels of Notch1 than primary FTC, even when comparing metastatic versus primary cells taken from the same patient (Xiao 2009). In summary, these studies demonstrate that normal thyroid cells retain expression of the Notch1 and Notch3 receptors, but FTC lacks expression of Notch1 and Notch3 (Notch2 and Notch4 have yet to be investigated in FTC). These findings have led to the hypothesis that upregulating Notch signaling in FTC could cause tumor suppression.
In order to modulate Notch pathway activity in FTC for an anti-cancer effect, efforts have been made to increase, or reinstate, Notch signaling in these cells. Overexpressing the intracellular domain of either Notch1 or Notch3 by in vitro plasmid transfection demonstrated the ability to reduce FTC growth while simultaneously increasing markers of thyrocyte differentiation (Feretti 2008, Somnay 2017). In a complimentary fashion, knocking down the expression of the Notch3 intracellular domain in FTC cells using silencing ribonucleic acid (siRNA) led to an expected increase in cell migration and reduction of thyrocyte differentiation markers (Somnay 2017). This tumor suppressive effect was also observed after pharmacological modulation of Notch signaling. Several compounds classified as histone deacetylase (HDAC) inhibitors, which result transcriptional upregulation due to chromatin relaxation, have been employed to increase the expression of the Notch1 receptor (Xiao 2009). A higher level of Notch1 expression in FTC was subsequently linked to various anti-cancer effects. This included a reduction in FTC cell proliferation in vitro, growth inhibition by cell cycle arrest, and increased apoptosis exemplified by the induction of apoptotic markers (Xiao 2009, Yu 2016).
Current evidence heavily supports a tumor suppressive role of Notch signaling in FTC. Studies that investigated Notch signaling in tumor samples from patients with FTC found that lower expression levels of Notch receptors were associated with more aggressive, less-differentiated disease and that Notch expression could potentially be used as a prognostic marker to predict patient outcomes (Yu 2016, Somnay 2017).
NOTCH SIGNALING IN MEDULLARY THYROID CANCER
The third subtype of thyroid cancer is medullary thyroid cancer (MTC), a malignancy of the neuroendocrine system. This subtype can be characterized by hormone secretions that cause debilitating side effects in patients (Greenblatt DY 2007, Cook M 2010). Unlike the controversial role of Notch signaling described in PTC, the role of Notch signaling in MTC has been consistently reported to be tumor suppressive. Many studies have shown that the expression of Notch1 is down-regulated in both tumor tissue from patients with MTC and in MTC cell lines (Cook M 2010, Jaskula-Sztul R 2011, Ning L 2008). More specifically, MTC features an upregulation of achaete-scute homolog-1 (ASCL1), a transcription factor critical for normal development of parafollicular cells and is transcriptionally repressed by Hes1, a target gene of the Notch pathway (Jaskula-Sztul R 2011, Ning L 2008).
To investigate the effects of Notch1, and subsequently ASCL1 expression, a doxycycline-inducible-Notch1-intracellular domain model was developed in vitro to study MTC (Jaskula-Sztul R 2011). The artificial induction of Notch1 resulted in a dose-dependent down-regulation of ASCL1 expression, a decrease in MTC cell proliferation, and a reduction in the secretion level of a hormone named calcitonin (Jaskula-Sztul R 2011). This effect was also observed in vivo, where MTC xenografts that underwent Notch1 induction by doxycycline had an average reduction in tumor volume by 57% when compared to MTC xenografts without Notch1 induction (Jaskula-Sztul R 2011).
Interestingly, the extent of MTC growth inhibition was directly proportional to the amount of Notch1 protein present. The mechanism of MTC cell growth inhibition upon Notch1 activation has been speculated to occur through cell cycle arrest at the G1/S phase due to the upregulation of p21, phosphor-Cdc2, and cyclin D1 (Jaskula-Sztul R 2011). Accordingly, the mechanism by which ASCL1 protein decreases upon Notch1 activation may be due to transcriptional silencing of ASCL1, likely by Hes1, which is known to transcriptionally and translationally inhibit ASCL1 (Chen H 1997, Sriuranpong V 2002, Sueda R 2019, Jaskula-Sztul R 2011). Notably, the overexpression of the Notch1 intracellular domain was also correlated with a dose-dependent decrease in chromogranin-A (CgA), a hormone known to be widely expressed in neuroendocrine neoplasms (Jaskula-Sztul R 2011). Therefore, one could conclude that activating Notch signaling through the Notch1 receptor in MTC, where Notch signaling is innately low, has the potential to decrease the oncogenic neuroendocrine phenotype.
In addition to the Notch1 receptor, the Notch3 receptor has also been identified a potential therapeutic target in MTC. In 2017, Lou I and colleagues created a doxycycline-inducible MTC cell line to specially overexpress the Notch3 intracellular domain. When this model was tested in vivo, induction of Notch3 did not prevent tumor formation, but did show an anti-proliferative effect in which led authors to conclude that activating Notch signaling through the Notch3 receptor could serve as a potential treatment option for patients with metastatic MTC (Lou I 2017). In summary, currently available research has demonstrated that Notch signaling is diminished in MTC and upregulating the pathway can reduce the oncogenic attributes of MTC.
NOTCH SIGNALING IN ANAPLASTIC THYROID CANCER
Anaplastic thyroid cancer (ATC) is the least differentiated subtype and harbors the least favorable patient outcomes out of all thyroid cancer subtypes. Most of the currently available research reports the Notch pathway as tumor suppressive in ATC. This section will describe what is known about Notch signaling in ATC.
The first step in elucidating the role of Notch signaling in ATC warrants an exploration into the basal expression levels of the various Notch receptor isoforms. To this extent, multiple studies report a loss of Notch1 expression in ATCs (Feretti 2008, Patel P 2014, Yu XM 2013). Moreover, it has been shown that as thyroid cancer cells become less differentiated, they also lose Notch1 expression (Feretti 2008, Somnay 2017, Piana 2019). Such evidence has led to the conclusion that a lack of Notch signaling is correlated with more aggressive thyroid tumors, such as ATC. In further support of this conclusion, Notch1 has been experimentally overexpressed in ATC cells with results showing a reduction in cell proliferation and migration, along with an increase, or reinstitution, of thyrocyte differentiation markers (Feretti 2008, Yu XM 2013, Patel P 2014). These studies that have described a tumor suppressive mechanism of Notch signaling in ATC suggest that the Notch pathway may not be solely responsible for causing ATC progression, but it definitely acts to suppress growth and is involved with cellular differentiation. Therapeutically activating Notch signaling has been thought to be a potentially effective treatment strategy for ATC, which is currently considered to be an incurable disease.
The investigation into Notch signaling in ATC is far from complete. Despite the evidence previously described, one paper has reported an oncogenic role of the Notch pathway in ATC. This paper contradicts the aforementioned ATC studies in two ways. The first is by stating that ATC expresses higher levels of both the Notch1 receptor and the active Notch1 intracellular domain when compared to PTC (Kim 2016). The second is that the knockdown of Notch1, and consequently Notch signaling, reduced ATC cell proliferation and migration (Kim 2016). When Notch1 expression was knocked down, the reduction of ATC cell growth and migration could be confounded by the reported greater amount of cell death. The majority of available literature dedicated to understanding the role of Notch signaling in ATC shows agreement with each other. There is however a single publication that directly opposes the studies that have shown that the overexpression of Notch1 was significantly associated with decreased cell growth and migration in ATC cells (Feretti 2008, Yu XM 2013, Patel P 2014). This discordance necessitates further investigation of Notch signaling in ATC.
MODULATING NOTCH SIGNALING IN THYROID CANCER USING NATURAL COMPOUNDS
Although the role of Notch signaling in thyroid cancer has yet to be clearly defined, it clearly holds significance. As previously discussed, many studies have shown that Notch signaling is lower in thyroid cancer cells and the re-activation of the pathway yields a tumor-suppressive effect. Based on this observation, various natural compounds have been identified that induce Notch signaling in thyroid cancer. In this section, several different compounds shown to modulate Notch signaling as an anti-cancer mechanism will be discussed in detail.
The first compound that will be discussed herein is thiocoraline, a thiodepsipeptide bisintercalator found in marine bacteria. This compound has demonstrated cytotoxic effects in different cancers, including lung, breast, colon, renal, and melanoma (Romero F 1997, Erba E 1999, Negri A 2007, Wyche TP 2014). The precise mechanism of action in which thiocoraline works is not fully elucidated, but several studies have shown that it can induce G1 cell cycle arrest. However, it has also been shown to activate the Notch pathway in MTC cells as demonstrated by elevated levels of Notch1 and Notch2 after treatment (Rashid FA 2017, Tesfazghi S 2013). This activation was further supported by increased mRNA levels of the downstream targets: Hey1, Hey2, Hes1, and Hes2 (Rashid FA 2017, Tesfazghi S 2013). Conclusively, thiocoraline treatment lead to anti-proliferative effects on MTC cells in vitro, as well as the downregulation of markers correlated with poor prognoses, which could be attributed to the activation of the Notch signaling pathway (Tesfazghi S 2013).
Another compound that has demonstrated a Notch-activating effect is resveratrol, a polyphenolic compound found naturally in grapes and berries, along with other plants (Yu XM 2013). Resveratrol has primarily been studied for Notch pathway induction in ATC cells. In vitro studies revealed that it was capable of suppressing growth in ATC cells through cell cycle arrest in addition to causing apoptosis (Yu XM 2013). Moreover, resveratrol activated Notch1 signaling but no there was no apparent change in the active forms of Notch2 or Notch3, suggesting that resveratrol is likely specific to regulating the Notch1 isoform. Resveratrol also increased the transcription of the thyrocyte differentiation markers: TTF1, TTF2, PAX8, and the sodium/iodide symporter (NIS). Finally, resveratrol was tested in vivo and significantly reduced the tumor volume of ATC xenografts as compared to untreated control groups (Yu XM 2013).
In the context of ATC, these cells were also sensitive to induction of Notch signaling upon treatment with a compound named hesperetin (Patel P 2014). Hesperetin is a naturally occurring flavanone found in citrus fruits. Similar to resveratrol, hesperetin has been shown to activate Notch1 signaling, cause cellular apoptosis, and induce thyrocyte differentiation. More specifically, in vitro studies on an ATC cell line resulted in growth inhibition after treatment with hesperetin. Analysis of apoptotic markers suggested that the primary mechanism of the observed reduction in growth was attributed to apoptosis. Furthermore, hesperetin was shown to dose-dependently increase the amount of Notch1 protein and the downstream markers Hes1 and Hey1 present in ATC cells, indicating a functional increase in Notch signaling. This compound, similar to resveratrol, dose-dependently increased thyrocyte-specific transcription factors, namely TTF1, TTF2, PAX8, TSHR, and NIS (Patel P 2014).
Another compound shown to activate Notch signaling in ATC is chrysin (Yu XM 2013). Chrysin is a natural flavonoid found in honey that inhibited ATC cell growth in a dose- and time-dependent manner. In vitro experiments showed that chrysin activated the Notch1 signaling pathway at micromolar concentrations. In vivo, chrysin reduced ATC xenograft volume. The tumors showed markers of apoptosis, indicating chrysin likely caused cell death through this mechanism (Yu XM 2013).
ATC is particularly difficult treat for many reasons, including the fact these tumors do not concentrate radioiodine due as a result of their undifferentiated features (Patel P 2014). During the process of dedifferentiation, thyrocytes lose the expression of the TSH receptor and thyroglobulin, making the cells unable to absorb radioiodine (Xiao X 2009). The loss of radioiodine is strongly associated with larger tumors and the presence of distant metastases in thyroid cancer (Xiao X 2009, Schlumberger MJ 1998). Therefore, a potential treatment strategy would include inducing re-differentiation in these cells to promote the uptake of iodide. In fact, this strategy has previously been employed by using retinoic acid to induce re-differentiation to promote radioactive iodide uptake in thyroid cancer, as well as breast cancer (Kogai T 2006, Schmutzler C 1997, Grunwald F 1998, Gruning T, 2003).
The final class of compounds shown to modulate Notch signaling in thyroid cancer are histone deacetylase inhibitors (HDAC inhibitors), specifically valproic acid (VPA), suberoyl bis-hydroxamic acid (SBHA), and trichostatin A (TsA) (Damaskos C 2016, Adler JT 2010, Spartalis E 2019, Jang S 2015). In preclinical studies, these HDAC inhibitors decreased thyroid cancer cell growth and induced Notch signaling, primarily shown through an increase in the Notch1 isoform. Due to the abundance of evidence showing the efficacy of HDAC inhibitors to have an anti-cancer effect in thyroid cancer, these compounds have been further tested in several clinical trials. The results of such trials will be discussed in the next section.
CLINICAL IMPACT OF NOTCH SIGNALING IN THYROID CANCER
Most well differentiated thyroid cancers have good prognoses and low mortality rates. These well differentiated and often localized tumors are usually curable by surgery, radioiodine ablation, chemotherapy, or thyroid-stimulating hormone (TSH)-suppressive therapy (Hsu KT 2014). However, these treatments are ineffective for patients with poorly differentiated or metastatic thyroid cancer, making most treatment options palliative (Kim H 2016, Smallridge RC 2010, Hsu KT 2014).
Beyond preclinical studies, targeting and modulating the Notch pathway in thyroid cancer has also been explored in a clinical setting. Notch-targeting mechanisms that have been explored clinically include: anti-Notch receptor antibodies, silencing RNA against Notch genes, and γ-secretase inhibitors (GSIs) to prevent the S3 cleavage of the Notch intracellular domain (Jin S 2016). One study showed that directly inhibiting the Notch1 receptor using a monoclonal antibody against the negative regulatory region inhibited cancer cell growth, but simultaneously inhibiting the Notch1 and Notch2 receptor caused severe gastrointestinal toxicities in vivo (Wu Y 2010). It is important to note that this study was not conducted in any thyroid cancer models. A reversible, non-competitive and selective small molecule designed as a GSI was administered to a patient with advanced PTC. This patient achieved complete remission with decreased levels of HES4 in peripheral blood, indicating a down-regulation of Notch signaling (Messersmith WA 2015).
Several Phase I and Phase II clinical trials have been conducted to explore the efficacy of HDAC inhibitors in patients with advanced thyroid cancer. All of these trials used HDAC inhibitors to reduce tumor burden and induce differentiation in the thyroid cancer cells, in addition to the goal of increasing radioactive iodide uptake.
One Phase II clinical trial investigated the use of the HDAC inhibitor valproic acid (VPA) in patients with advanced stage thyroid cancer of follicular origin that had not responded to conventional treatments (Nilubol N 2010). VPA is currently approved by the Food and Drug Administration (FDA) for the treatment of epilepsy and bipolar disorder. Numerous preclinical studies have shown that VPA is capable of reducing thyroid cancer cell growth and inducing re-differentiation for increased radioiodine uptake, possibly due to the upregulation of Notch signaling (Fortunati N 2004, Catalano MG 2004, Greenblatt DY 2008, Shen WT 2005, Catalano MG 2006). Two objectives cited for this study were to first determine if VPA could reduce tumor growth and cause cancer cell death and secondly determine if VPA increased the uptake of radioiodine by thyroid cancer cells. The results of the trial were disappointing, in that none of the ten patients who completed 10 weeks of treatment had increased radioiodine uptake by their thyroid tumors. No partial or complete responses were observed and six of the ten patients had disease progression. The conclusion of this study was that VPA does not have anti-cancer activity in patients with advanced thyroid cancer originating from follicular thyroid cells. Likewise, VPA does not increase radioiodine uptake in these patients.
Other clinical trials have assessed the effect of HDAC inhibitors on thyroid cancer with variable results. In a Phase I clinical trial using vorinostat (suberoylanilide hydroxamic acid [SAHA]), one patient had a partial response and five patients, including one patient with MTC, had stable disease (Kelly WK 2005). Notably, this study also reported that one patient had higher radioiodine uptake after receiving the drug. A subsequent Phase II clinical trial also investigating the efficacy of vorinostat in patients with advanced thyroid cancer showed that none of the nineteen patients enrolled had neither a partial nor complete response (Woyach JA 2009). This same study examined sixteen patients with differentiated thyroid cancer and three patients with MTC with the overall conclusion that vorinstat is not effective for the treatment of advanced thyroid cancer. Similarly, a Phase II clinical trial that tested another HDAC inhibitior, romidepsin, also ended with disappointing results when none of the sixteen patients with advanced thyroid cancer had neither a partial nor complete response, in addition to only two of the sixteen patients having increased radioiodine uptake (Sherman EJ 2013). Conclusively, HDAC inhibitors have shown promise in preclinical settings, but have not manifested any beneficial clinical effects. Taking these results into consideration, in addition to the strong evidence that HDAC inhibitors activate the Notch pathway, a deeper understanding of Notch signaling in thyroid cancer could lead to improved studies and clinical trials that yield better outcomes for patients.
CONCLUSION
Notch signaling is a multifunctional pathway that canonically plays a critical role in mammalian development. It has also emerged as a key player in many different cancers with various roles ranging from carcinogenesis to cancer cell differentiation. To layer the complexity of Notch signaling, it is widely accepted that the role of this pathway is cell type and context-dependent while also harboring variations in outcomes between the four different receptors and five different ligands. To this extent, Notch signaling has been described to be oncogenic (Reedijk M 2005, Sikandar SS 2010, Ellisen L 1991, Fabbri 2011, Puente 2011, Westoff 2009, Hanlon 2010, Sjölund J 2008, Dantas-Barbosa C 2015) and anti-oncogenic in various types of cancer (Nickoloff BJ 2002, Shou J 2001, Sriuranpong V 2001, Nakakura EK 2005, Kunnimalaiyaan M 2005, Qi R 2003, Viatour 2011, Talora C 2002, Kunni M 2006, Morimura T, Zweidler-McKay 2005, Klinakis A 2011, Stransky N 2011, Zage PE 2012, Rangarajan A 2001). The discrepancy between tumor-promoting and tumor-reducing effects of Notch signaling exists amongst the different subtypes of thyroid cancer.
The most differentiated thyroid cancer subtype, PTC, is most commonly reported to have a high level of Notch signaling (Yamashita 2013, Gallo 2018, Yuan L 2019, Piana 2019). The elevated activity of Notch in this subtype could be in part due to the well differentiated status of the cells, supported by the widely accepted concept that Notch signaling can directly promote cellular differentiation (Lobry C 2011). Although, a handful of studies conversely report that PTC can be characterized by a low level of Notch signaling (Xiao X 2009, Yu XM 2016). Ultimately, the controversial findings regarding Notch signaling in PTC highlight the complexity of the pathway and the need for continued investigation.
In thyroid cancer subtypes that can be characterized as less differentiated, it appears that Notch signaling is decreased. This observation is supported by evidence that demonstrates lower levels of Notch signaling in FTC, MTC, and ATC in addition to a significant association found between low levels of Notch receptors and more aggressive cases of thyroid cancer (Feretti 2008, Xiao 2009, Yu 2016, Somnay 2017). ATC, the most poorly differentiated subtype, has the lowest expression of the Notch1 receptor when compared to more differentiated thyroid cancers and normal thyroid cells (Feretti 2008, Patel P 2014, Yu XM 2013, Somnay 2017, Piana 2019).
The majority of literature that describes Notch signaling in thyroid cancer focuses on the Notch1 isoform, followed by the Notch3 isoform. There is minimal investigation into the other two known Notch receptors (Notch2 and Notch4). One study on MTC reported that the natural compound resveratrol was able to increase Notch signaling through an induction of Notch2, which is consistent with the study that reported a similar effect on Notch1 (Truong M 2011, Yu XM 2013). The individual and synergistic roles of each Notch receptor (Notch1–4) have yet to be understood in thyroid cancer. A deeper understanding of each receptor and the interplay between them could greatly advance what is known about Notch signaling and potentially lead to new strategies for modulating the pathway.
The paradoxical balance between oncogenic Notch and tumor suppressive Notch impacts the modulation strategy. Various natural compounds have been identified that induce Notch signaling in thyroid cancer, thus exploiting a suspected anti-cancer effect by high levels of Notch (Rashid FA 2017, Tesfazghi S 2013, Yu XM 2013, Patel P 2014, Yu XM 2014, Damaskos C 2016, Adler JT 2010, Spartalis E 2019, Jang S 2015). Such compounds have demonstrated promising preclinical data, but have yet to be explored in a clinical setting. Compounds that have been tested clinically to increase Notch signaling in thyroid cancer, primarily HDAC inhibitors, have resulted in disappointing findings. The use of HDAC inhibitors as a monotherapy for the treatment of thyroid cancer has largely been ineffective. The inconsistency between promising preclinical results and disappointing clinical results could be attributed to additional mechanisms of action by which HDAC inhibitors effect thyroid cancer cells that have yet to be understood. Further understanding how Notch signaling functions in thyroid cancer could lead to advancements in the design of clinical trials that target the pathway. Notch pathway has been cited as a potential prognostic marker in patients with thyroid cancer, although this would also require a more definite role of Notch signaling in these tumors (Jung CW 2017).
References
- Adler JT, Hottinger DG, Kunnimalaiyaan M, Chen H. Inhibition of growth in medullary thyroid cancer cells with histone deacetylase inhibitors and lithium chloride. J Surg Res. 2010April;159(2):640–4. doi: 10.1016/j.jss.2008.08.004.Epub 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano MG, Fortunati N, Pugliese M, Costantino L, Poli R, Bosco O, Boccuzzi G. Valproic acid induces apoptosis and cell cycle arrest in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2005March;90(3):1383–9. Epub 2004 Dec 7. [DOI] [PubMed] [Google Scholar]
- Catalano MG, Fortunati N, Pugliese M, Poli R, Bosco O, Mastrocola R, Aragno M, Boccuzzi G. Valproic acid, a histone deacetylase inhibitor, enhances sensitivity to doxorubicin in anaplastic thyroid cancer cells. J Endocrinol. 2006November;191(2):465–72. [DOI] [PubMed] [Google Scholar]
- Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997May13;94(10):5355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983–2002. Am J Clin Oncol 2008October;31(5):460–4. doi: 10.1097/COC.0b013e31816a61f3. [DOI] [PubMed] [Google Scholar]
- Chen W, Cao G, Yuan X, Zhang X, Zhang Q, Zhu Y, Dong Z, Zhang S. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells. J Transl Med. 2015May20;13:167. doi: 10.1186/s12967-015-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Ramu S, Park E, Jung E, Yang S, Jung W, Choi I, Lee S, Kim KE, Seong YJ, Hong M, Daghlian G, Kim D, Shin E, Seo JI, Khatchadourian V, Zou M, Li W, De Filippo R, Kokorowski P, Chang A, Kim S, Bertoni A, Furlanetto TW, Shin S, Li M, Chen Y, Wong A, Koh C, Geliebter J, Hong YK. Aberrant Activation of Notch Signaling Inhibits PROX1 Activity to Enhance the Malignant Behavior of Thyroid Cancer Cells. Cancer Res. 2016February1;76(3):582–93. doi: 10.1158/0008-5472.CAN-15-1199.Epub 2015 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M, Yu XM, Chen H. Notch in the development of thyroid C-cells and the treatment of medullary thyroid cancer. Am J Transl Res. 2010February10;2(1):119–25. [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman S, Tuttle RM; American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006February;16(2):109–42. [DOI] [PubMed] [Google Scholar]
- Damaskos C, Garmpis N, Valsami S, Spartalis E, Antoniou EA, Tomos P, Karamaroudis S, Zoumpou T, Pergialiotis V, Stergios K, Michaelides C, Kontzoglou K, Perrea D, Nikiteas N, Dimitroulis D. Histone Deacetylase Inhibitors: A Novel Therapeutic Weapon Against Medullary Thyroid Cancer? Anticancer Res. 2016October;36(10):5019–5024. [DOI] [PubMed] [Google Scholar]
- Dantas-Barbosa C, Bergthold G, Daudigeos-Dubus E, Blockus H, Boylan JF, Ferreira C, Puget S, Abely M, Vassal G, Grill J, Geoerger B. Inhibition of the NOTCH pathway using γ-secretase inhibitor RO4929097 has limited antitumor activity in established glial tumors. Anticancer Drugs. 2015March;26(3):272–83. doi: 10.1097/CAD.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Dexter JS. The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. Am Nat. 1914;576(48):712–58. [Google Scholar]
- Ellisen L, Bird J, West D, Soreng A, Reynolds T, Smith S, et al. TAN-1, the human homolog of the drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-B. [DOI] [PubMed] [Google Scholar]
- Erba E1 Bergamaschi D, Ronzoni S, Faretta M, Taverna S, Bonfanti M, Catapano CV, Faircloth G, Jimeno J, D’Incalci M. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity. Br J Cancer. 1999June;80(7):971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri G, Rasi S, Rossi D, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208(7):1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti E, Tosi E, Po A, Scipioni A, Morisi R, Espinola MS, Russo D, Durante C, Schlumberger M, Screpanti I, Filetti S, Gulino A. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab. 2008October;93(10):4080–7. doi: 10.1210/jc.2008-0528.Epub 2008 Jul 29. [DOI] [PubMed] [Google Scholar]
- Fortunati N, Catalano MG, Arena K, Brignardello E, Piovesan A, Boccuzzi G. Valproic acid induces the expression of the Na+/I- symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004February;89(2):1006–9. [DOI] [PubMed] [Google Scholar]
- Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, Doi SAR. Prevalence of Differentiated Thyroid Cancer in Autopsy Studies Over Six Decades: A Meta-Analysis. J Clin Oncol. 2016October20;34(30):3672–3679. doi: 10.1200/JCO.2016.67.7419. [DOI] [PubMed] [Google Scholar]
- Gallo C, Fragliasso V, Donati B, Torricelli F, Tameni A, Piana S, Ciarrocchi A. The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis. 2018August29;9(9):871. doi: 10.1038/s41419-018-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers C, Colin IM, Gérard AC. Delta-like 4/Notch pathway is differentially regulated in benign and malignant thyroid tissues. Thyroid. 2011December;21(12):1323–30. doi: 10.1089/thy.2010.0444.Epub 2011 Nov 8. [DOI] [PubMed] [Google Scholar]
- Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008June;247(6):1036–40. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DY, Chen H. Palliation of advanced thyroid malignancies. Surg Oncol. 2007December;16(4):237–47. Epub 2007 Sep 14. [DOI] [PubMed] [Google Scholar]
- Grüning T, Tiepolt C, Zöphel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer--does it hold its promise? Eur J Endocrinol. 2003April;148(4):395–402. [DOI] [PubMed] [Google Scholar]
- Grünwald F, Pakos E, Bender H, Menzel C, Otte R, Palmedo H, Pfeifer U, Biersack HJ. Redifferentiation therapy with retinoic acid in follicular thyroid cancer. J Nucl Med. 1998September;39(9):1555–8. [PubMed] [Google Scholar]
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012September;13(9):654–66. doi: 10.1038/nrg3272.Epub 2012 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon L, Avila JL, Demarest RM, Troutman S, Allen M, Ratti F, Rustgi AK, Stanger BZ, Radtke F, Adsay V, Long F, Capobianco AJ, Kissil JL. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 2010June1;70(11):4280–6. doi: 10.1158/0008-5472.CAN-09-4645.Epub 2010 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KT, Yu XM, Audhya AW, Jaume JC, Lloyd RV, Miyamoto S, Prolla TA, Chen H. Novel approaches in anaplastic thyroid cancer therapy. Oncologist. 2014November;19(11):1148–55. doi: 10.1634/theoncologist.2014-0182.Epub 2014 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Yu XM, Odorico S, Clark M, Jaskula-Sztul R, Schienebeck CM, Kupcho KR, Harrison AD, Winston-McPherson GN, Tang W, Chen H. Novel analogs targeting histone deacetylase suppress aggressive thyroid cancer cell growth and induce re-differentiation. Cancer Gene Ther. 2015August;22(8):410–6. doi: 10.1038/cgt.2015.37.Epub 2015 Aug 7. [DOI] [PubMed] [Google Scholar]
- Jaskula-Sztul R, Pisarnturakit P, Landowski M, Chen H, Kunnimalaiyaan M. Expression of the active Notch1 decreases MTC tumor growth in vivo. J Surg Res. 2011November;171(1):23–7. doi: 10.1016/j.jss.2011.03.035.Epub 2011 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Borkhuu O, Bao W, Yang YT. Signaling Pathways in Thyroid Cancer and Their Therapeutic Implications. J Clin Med Res. 2016April;8(4):284–96. doi: 10.14740/jocmr2480w.Epub 2016 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CW, Kong JS, Seol H, Park S, Koh JS, Lee SS, Kim MJ, Choi IJ, Myung JK. Expression of activated Notch1 and Hey1 in papillary thyroid carcinoma. Histopathology. 2017January;70(2):301–308. doi: 10.1111/his.13065.Epub 2016 Nov 2. [DOI] [PubMed] [Google Scholar]
- Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005June10;23(17):3923–31. Epub 2005 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim MJ, Kim A, Jung CW, Park S, Koh JS, Myung JK. The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma. Cancer Res Treat. 2017April;49(2):509–517. doi: 10.4143/crt.2016.214.Epub 2016 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, van De Walle I, Cathelin S, Trimarchi T, Araldi E, Liu C, Ibrahim S, Beran M, Zavadil J, Efstratiadis A, Taghon T, Michor F, Levine RL, Aifantis I. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011May12;473(7346):230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006September;13(3):797–826. [DOI] [PubMed] [Google Scholar]
- Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005October;289(4):G636–42. [DOI] [PubMed] [Google Scholar]
- Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006December29;281(52):39819–30. Epub 2006 Nov 7. [DOI] [PubMed] [Google Scholar]
- Lin JD, Chao TC, Hsueh C. Follicular thyroid carcinomas with lung metastases: a 23-year retrospective study. Endocr J. 2004April;51(2):219–25. [DOI] [PubMed] [Google Scholar]
- Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011September26;208(10):1931–5. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou I, Odorico S, Yu XM, Harrison A, Jaskula-Sztul R, Chen H. Notch3 as a novel therapeutic target in metastatic medullary thyroid cancer. Surgery. 2018January;163(1):104–111. doi: 10.1016/j.surg.2017.07.039.Epub 2017 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003March;3(3):203–5. [DOI] [PubMed] [Google Scholar]
- Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016April;23(4):313–22. doi: 10.1530/ERC-15-0445.Epub 2016 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, Shaik MN, Cesari R, Zheng X, Reynolds JM, English PA, McLachlan KR, Kern KA, LoRusso PM. A Phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res. 2015January1;21(1):60–7. doi: 10.1158/1078-0432.CCR-14-0607.Epub 2014 Sep 17. [DOI] [PubMed] [Google Scholar]
- Mohr OL. Character changes caused by mutation of an entire region of a chromosome in Drosophila. Genetics. 1919;4:275–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura T, Goitsuka R, Zhang Y, Saito I, Reth M, Kitamura D. Cell cycle arrest and apoptosis induced by Notch1 in B cells. J Biol Chem. 2000November24;275(47):36523–31. [DOI] [PubMed] [Google Scholar]
- Morris LGT, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013July;23(7):885–91. doi: 10.1089/thy.2013.0045.Epub 2013 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, Jin N, Collins BJ, Nelkin BD, Chen H, Ball DW. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005July;90(7):4350–6. Epub 2005 May 3. [DOI] [PubMed] [Google Scholar]
- Negri A, Marco E, García-Hernández V, Domingo A, Llamas-Saiz AL, Porto-Sandá S, et al. Antitumor activity, X-ray crystal structure, and DNA binding properties of thiocoraline A, a natural bisintercalating thiodepsipeptide. J Med Chem. 2007;50:3322–3333. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002August;9(8):842–55. [DOI] [PubMed] [Google Scholar]
- Nilubol N, Merkel R, Yang L, Patel D, Reynolds JC, Sadowski SM, Neychev V, Kebebew E. A phase II trial of valproic acid in patients with advanced, radioiodine-resistant thyroid cancers of follicular cell origin. Clin Endocrinol (Oxf). 2017January;86(1):128–133. doi: 10.1111/cen.13154.Epub 2016 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008February;13(2):98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- Patel PN, Yu XM, Jaskula-Sztul R, Chen H. Hesperetin activates the Notch1 signaling cascade, causes apoptosis, and induces cellular differentiation in anaplastic thyroid cancer. Ann Surg Oncol. 2014;21Suppl 4(0 4):S497–S504. doi: 10.1245/s10434-013-3459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piana S, Zanetti E,Bisagni A, Ciarrocchi A, Giordano D, Torricelli F, Rossi T, Ragazzi M. Expression of NOTCH1 in thyroid cancer is mostly restricted to papillary carcinoma. Endocr Connect. 2019;8(8):1089–1096. doi: 10.1530/EC-19-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AE, Marcadis AR, Lee M, Morris LGT, Marti JL. Changes in Trends in Thyroid Cancer Incidence in the United States, 1992 to 2016. JAMA. 2019December24;322(24):2440–2441. doi: 10.1001/jama.2019.18528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, Escaramis G, Jares P, Beà S, González-Díaz M, Bassaganyas L, Baumann T, Juan M, López-Guerra M, Colomer D, Tubío JM, López C, Navarro A, Tornador C, Aymerich M, Rozman M, Hernández JM, Puente DA, Freije JM, Velasco G, Gutiérrez-Fernández A, Costa D, Carrió A, Guijarro S, Enjuanes A, Hernández L, Yagüe J, Nicolás P, Romeo-Casabona CM, Himmelbauer H, Castillo E, Dohm JC, de Sanjosé S, Piris MA, de Alava E, San Miguel J, Royo R, Gelpí JL, Torrents D, Orozco M, Pisano DG, Valencia A, Guigó R, Bayés M, Heath S, Gut M, Klatt P, Marshall J, Raine K, Stebbings LA, Futreal PA, Stratton MR, Campbell PJ, Gut I, López-Guillermo A, Estivill X, Montserrat E, López-Otín C, Campo E. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011June5;475(7354):101–5. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003December1;63(23):8323–9. [PubMed] [Google Scholar]
- Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834. doi: 10.1155/2014/790834.Epub 2014 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001July2;20(13):3427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid FA, Mansoor Q, Tabassum S, Aziz H, Arfat WO, Naoum GE, Ismail M, Farooqi AA. Signaling cascades in thyroid cancer: Increasing the armory of archers to hit bullseye. J Cell Biochem. 2018May;119(5):3798–3808. doi: 10.1002/jcb.26620.Epub 2018 Jan 22. [DOI] [PubMed] [Google Scholar]
- Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005September15;65(18):8530–7. [DOI] [PubMed] [Google Scholar]
- Romero F, Espliego F, Pérez Baz J, García de Quesada T, Grávalos D, De la Calle F, et al. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot. 1997;50:734–737. [DOI] [PubMed] [Google Scholar]
- Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. Oncologist. 2013;18(10):1093–100. doi: 10.1634/theoncologist.2013-0053.Epub 2013 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998January29;338(5):297–306. [DOI] [PubMed] [Google Scholar]
- Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 1997November26;240(3):832–8. [DOI] [PubMed] [Google Scholar]
- Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013Nov-Dec;63(6):374–94. doi: 10.3322/caac.21195.Epub 2013 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib CD, Sosa JA. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol Metab Clin North Am. 2019March;48(1):23–35. doi: 10.1016/j.ecl.2018.10.002.Epub 2018 Dec 23. [DOI] [PubMed] [Google Scholar]
- Shen WT, Wong TS, Chung WY, Wong MG, Kebebew E, Duh QY, Clark OH. Valproic acid inhibits growth, induces apoptosis, and modulates apoptosis-regulatory and differentiation gene expression in human thyroid cancer cells. Surgery. 2005December;138(6):979–84; discussion 984–5. [DOI] [PubMed] [Google Scholar]
- Sherman EJ, Su YB, Lyall A, Schöder H, Fury MG, Ghossein RA, Haque S, Lisa D, Shaha AR, Tuttle RM, Pfister DG. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013May;23(5):593–9. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 2001October1;61(19):7291–7. [PubMed] [Google Scholar]
- Sikandar SS, Pate KT, Anderson S, Dizon D, Edwards RA, Waterman ML, Lipkin SM. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010February15;70(4):1469–78. doi: 10.1158/0008-5472.CAN-09-2557.Epub 2010 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, Nilsson E, Ljungberg B, Axelson H. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest. 2008January;118(1):217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010August;22(6):486–97. doi: 10.1016/j.clon.2010.03.013.Epub 2010 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somnay YR, Yu XM, Lloyd RV, Leverson G, Aburjania Z, Jang S, Jaskula-Sztul R, Chen H. Notch3 expression correlates with thyroid cancer differentiation, induces apoptosis, and predicts disease prognosis. Cancer. 2017March1;123(5):769–782. doi: 10.1002/cncr.30403.Epub 2016 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartalis E, Athanasiadis DI, Chrysikos D, Spartalis M, Boutzios G, Schizas D, Garmpis N, Damaskos C, Paschou SA, Ioannidis A, Tsourouflis G, Dimitroulis D, Nikiteas NI. Histone Deacetylase Inhibitors and Anaplastic Thyroid Carcinoma. Anticancer Res. 2019March;39(3):1119–1127. doi: 10.21873/anticanres.13220. [DOI] [PubMed] [Google Scholar]
- Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, Ball DW. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001April1;61(7):3200–5. [PubMed] [Google Scholar]
- Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, Nelkin BD, Ball DW. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol. 2002May;22(9):3129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortés ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011August26;333(6046):1157–60. doi: 10.1126/science.1208130.Epub 2011 Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueda R, Imayoshi I, Harima Y, Kageyama R. High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes Dev. 2019May1;33(9–10):511–523. doi: 10.1101/gad.323196.118.Epub 2019 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014February;141(2):140–9. doi: 10.1016/j.pharmthera.2013.09.005.Epub 2013 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talora C, Sgroi DC, Crum CP, Dotto GP. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002September1;16(17):2252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfazghi S, Eide J, Dammalapati A, Korlesky C, Wyche TP, Bugni TS, Chen H, Jaskula-Sztul R. Thiocoraline alters neuroendocrine phenotype and activates the Notch pathway in MTC-TT cell line. Cancer Med. 2013October;2(5):734–43. doi: 10.1002/cam4.118.Epub 2013 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M, Cook MR, Pinchot SN, Kunnimalaiyaan M, Chen H. Resveratrol induces Notch2-mediated apoptosis and suppression of neuroendocrine markers in medullary thyroid cancer. Ann Surg Oncol. 2011May;18(5):1506–11. doi: 10.1245/s10434-010-1488-z.Epub 2010 Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A, Vogel H, Sylvester KG, Thorgeirsson SS, Grompe M, Sage J. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011September26;208(10):1963–76. doi: 10.1084/jem.20110198.Epub 2011 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff B, Colaluca IN, D’Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, Pece S, Di Fiore PP. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A. 2009December29;106(52):22293–8. doi: 10.1073/pnas.0907781106.Epub 2009 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M, Shah MH. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009January;94(1):164–70. doi: 10.1210/jc.2008-1631.Epub 2008 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010April15;464(7291):1052–7. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- Wyche TP, Dammalapati A, Cho H, Harrison AD, Kwon GS, Chen H, Bugni TS, Jaskula-Sztul R. Thiocoraline activates the Notch pathway in carcinoids and reduces tumor progression in vivo. Cancer Gene Ther. 2014December;21(12):518–25. doi: 10.1038/cgt.2014.57.Epub 2014 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009February;8(2):350–6. doi: 10.1158/1535-7163.MCT-08-0585.Epub 2009 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, Baia GS, Kimura ET. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013April;6(2):197–205. doi: 10.1593/tlo.12442.Epub 2013 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Jaskula-Sztul R, Georgen MR, Aburjania Z, Somnay YR, Leverson G, Sippel RS, Lloyd RV, Johnson BP, Chen H. Notch1 Signaling Regulates the Aggressiveness of Differentiated Thyroid Cancer and Inhibits SERPINE1 Expression. Clin Cancer Res. 2016July15;22(14):3582–92. doi: 10.1158/1078-0432.CCR-15-1749.Epub 2016 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol Cancer Ther. 2013;12(7):1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer. 2013February15;119(4):774–81. doi: 10.1002/cncr.27742.Epub 2012 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ma L, Xue H, Song S. Relationship between the upregulation of Notch1 signaling and the clinical characteristics of patients with papillary thyroid carcinoma in East Asia: a systematic review and meta-analysis. Cancer Cell Int. 2019January3;19:5. doi: 10.1186/s12935-018-0723-8.eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zage PE, Nolo R, Fang W, Stewart J, Garcia-Manero G, Zweidler-McKay PA. Notch pathway activation induces neuroblastoma tumor cell growth arrest. Pediatr Blood Cancer. 2012;58:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Qin Y, Zuo B, Gong W, Zhang S, Gong Y, Quan Z, Chu B. Overexpression of NOTCH-regulated Ankyrin Repeat Protein is associated with papillary thyroid carcinoma progression. PLoS One. 2017February16;12(2):e0167782. doi: 10.1371/journal.pone.0167782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, Aster JC, Allman D, Pear WS. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005December1;106(12):3898–906. Epub 2005 Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]