Abstract
Background and Aims
Cold stress in rice (Oryza sativa) plants at the reproductive stage prevents normal anther development and causes pollen sterility. Tapetum hypertrophy in anthers has been associated with pollen sterility in response to cold at the booting stage. Here, we re-examined whether the relationships between anther abnormality and pollen sterility caused by cold stress at the booting stage in rice can be explained by a monovalent factor such as tapetum hypertrophy.
Methods
After exposing plants to a 4-d cold treatment at the booting stage, we collected and processed anthers for transverse sectioning immediately and at the flowering stage. We anatomically evaluated the effect of cold treatment on anther internal morphologies, pollen fertilities and pollen numbers in the 13 cultivars with various cold sensitivities.
Key Results
We observed four types of morphological anther abnormalities at each stage. Pollen sterility was positively correlated with the frequency of undeveloped locules, but not with tapetum hypertrophy as commonly believed. In cold-sensitive cultivars grown at low temperatures, pollen sterility was more frequent than anther morphological abnormalities, and some lines showed remarkably high pollen sterility without any anther morphological alterations. Most morphological anomalies occurred only in specific areas within large and small locules. Anther length tended to shorten in response to cold treatment and was positively correlated with pollen number. One cultivar showed a considerably reduced pollen number, but fertile pollen grains under cold stress. We propose three possible relationships to explain anther structure and pollen sterility and reduction due to cold stress.
Conclusions
The pollen sterility caused by cold stress at the booting stage was correlated with the frequency of entire locule-related abnormalities, which might represent a phenotypic consequence, but not a direct cause of pollen abortion. Multivalent factors might underlie the complicated relationships between anther abnormality and pollen sterility in rice.
Keywords: Anther, booting stage, cold stress, flowering stage, morphological abnormality, pollen, rice, sterility, tapetum
INTRODUCTION
Many studies from the 1940s to the 1980s have focused on injury and morphological abnormalities caused by cold stress in rice (Oryza sativa) anthers (Sakai, 1943; Nishiyama, 1970, 1976; Suzuki, 1981; Pacini et al., 1985). Rice plants exposed to low temperatures at the booting stage experience pollen sterility (Hayase et al., 1969; Satake and Hayase, 1970; Satake, 1976). Regions of high latitude where rice is cultivated are characterized by a colder climate, which may lead to a significant reduction of rice grain yield due to pollen sterility (Shimono et al., 2005, 2007; Thakur et al., 2010). Sakai (1943) reported that aborted pollen from rice plants exposed to low temperatures at the booting stage showed a strong association with swelling of the tapetum localized in the innermost layer of the anther wall. The tapetum provides nutrients during the development of pollen microspores, and the thickness of this cell layer gradually diminishes over the course of pollen maturation (Pacini et al., 1985; Li et al., 2006; Niu et al., 2013). Hypertrophy of tapetal tissue, therefore, has long been considered to signal disrupted nutrient supply to pollen microspores. Nishiyama (1970, 1976), who studied morphological changes of the tapetum under chilling conditions, confirmed Sakai’s observations (1943) that cold stress induced tapetum hypertrophy in rice anthers. Indeed, several other reports offered evidence for similar morphological abnormalities in the tapetum during microspore development after exposure to low temperatures (Ito, 1978a, b; Nishiyama, 1978; Oda et al., 2010). However, the causal association between pollen sterility and tapetal swelling was based on observations from typical cold-sensitive rice cultivars, and the frequency of tapetum hypertrophy has not been further explored. Thus, it seemed possible that different cultivars might exhibit varying frequencies and degrees of tapetum hypertrophy (Suzuki, 1978).
To date, clear evidence that tapetal hypertrophy accurately reflects rice pollen sterility caused by cold stress during the booting stage is lacking. The effects of cold stress on rice anthers have been characterized in terms of tapetum ultrastructure, sucrose accumulation, transcriptome profiling and gibberellin metabolism (Nishiyama, 1970; Oliver et al., 2005; Mamun et al., 2006; Oda et al., 2010; Sakata et al., 2014). Each of these studies has investigated rice pollen sterility caused by cold stress, and the underlying mechanisms revealed by each study appeared to be consistent with being causal for pollen sterility. However, these studies only examined one or two rice cultivars with contrasting frequencies of pollen sterility.
Here, we investigated the effects on pollen fertility due to cold stress at the booting stage in 13 rice cultivars. We observed varying degrees of impaired pollen fertility among the cultivars. We also carefully examined anthers from control and cold-stressed plants for all cultivars at the booting and flowering stages and categorized morphological anther abnormalities. The structural changes in anthers were largely divided into eight abnormalities related to anther locule structures at the booting and flowering stages. These locule-related abnormalities (LRAs) occurred at specific locule sites. Although tapetum hypertrophy was detected as one type of LRA, it was not typically correlated with pollen sterility. Rather, the extent of pollen abortion after cold treatment was positively correlated with the frequency of LRAs and with anther length. Our observations demonstrated that tapetum hypertrophy in rice is unlikely to serve as a reliable indicator of pollen sterility in response to cold stress at the booting stage. We also identified cold-sensitive cultivars with pollen sterility but lacking any abnormal anther structures. In contrast to the conventional stereotyped relationship between tapetum hypertrophy and pollen fertility, our comprehensive analyses provide a revised model of multivalent relationships between anther abnormalities and pollen fertility.
MATERIALS AND METHODS
Plant materials
The plant materials used in this study consisted of 13 rice cultivars: 11 japonica cultivars (‘Nipponbare’, ‘Kinmaze’, ‘Taichung 65’ [‘T65’], ‘Nishihomare’, ‘Fukoku’, ‘Sasanishiki’, ‘Hokkai 287’, ‘Shinrei’, ‘Koshihikari’, ‘Kitaake’ and ‘Hoshinoyume’), one tropical japonica cultivar (‘Shilewah’) and one indica cultivar (‘Kasalath’) (Table 1). Twenty rice plants in each plot were grown in Wagner pots (1/5000 100 m2) in the greenhouse at 25/19 °C (day/night cycle); tillers were cut off and only the main culm was retained (Satake et al., 1969; Satake, 1972). When plants entered the booting stage, during which the microspores are between the late tetrad and the uninucleated pollen stage after meiosis, the plants were exposed to the low temperature of 12 °C for 4 d. The timing of the booting stage was estimated from the auricle distance (AD), which is the distance between the auricles from the flag leaf and the penultimate leaf (Matsushima, 1957). In general, the start of the booting stage corresponds to AD = 0 to −2 cm, although in cultivars from Hokkaido, located at one of the highest latitudes for rice production, this corresponds to AD = −2 to −4 cm. At the end of this cold treatment, anthers were collected from a subset of the cold-exposed plants. Anthers from plants maintained under normal growth conditions were collected at the same time as controls. To collect anthers at the flowering stage, the remaining plants in the same pots were maintained under greenhouse conditions after cold treatment. After flowering, anthers were collected from control plants, and plants were exposed to cold treatment. All samples were fixed in FAA (5 % formaldehyde/0.5 % acetic acid/45 % ethanol [v/v/v]) before sectioning and determination of pollen fertility and pollen number.
Table 1.
The materials used in this study
| Cultivar | Ecotype |
|---|---|
| 'Nipponbare' | temperate japonica |
| 'Kinmaze' | temperate japonica |
| 'Taichung 65' ('T65') | temperate japonica |
| 'Nishihomare' | temperate japonica |
| 'Fukoku' | temperate japonica |
| 'Sasanishiki' | temperate japonica |
| 'Hokkai 287' | temperate japonica |
| 'Shinrei' | temperate japonica |
| 'Kitaake' | temperate japonica |
| 'Hoshinoyume' | temperate japonica |
| 'Silewah' | tropical japonica |
| 'Kasalath' | indika |
Pollen fertility
Anthers were collected from at least 15 spikelets at positions 3, 4 and 5 along a primary rachis branch for each cultivar. Pollen grains were stained with Lugol’s iodine (KI-I2) solution, which stains fertile pollen strongly but leaves sterile pollen unstained. Pollen fertility was calculated as the percentage of fertile pollen grains for each cultivar and in each growth condition. In each case, pollen fertility was averaged from at least nine panicles examined.
Transverse sections
At the booting stage, at least 40 anthers were observed in serial transverse sections. At the flowering stage, 13 anthers from cold-treated plants and 15 anthers from control plants were observed per cultivar. Six anthers from three individual plants were used to obtain the result from a single sample. Transverse sections were prepared by paraffin embedding. Anthers were dehydrated in butanol, and then butanol was replaced with paraffin and the anthers were embedded in paraffin. Embedded samples were cut into 7-μm-thick sections with a microtome, and the sections were placed on glass slides using egg white. Samples were stained with 0·05 % toluidine blue after removal of paraffin with xylene. Mounted sections were sealed with Canada balsam.
Determination of anther length and pollen number
For the five cultivars ‘Nipponbare’, ‘Hoshinoyume’, ‘Koshihikari’, ‘T65’ and ‘Kasalath’, anther length was measured and pollen number per anther was determined from at least nine panicles for each cultivar and from each condition (control and cold-treated). Anther length was measured as a three-point distance, the points being both ends and the centre of each anther, on a VHX-1000 digital microscope (Keyence, Osaka, Japan). For pollen number, anthers were crushed and stained in Lugol’s iodine solution. Then, stained pollen grains were counted using a haemocytometer (Erma, Tokyo, Japan).
RESULTS
Varying pollen fertility among 13 cultivars
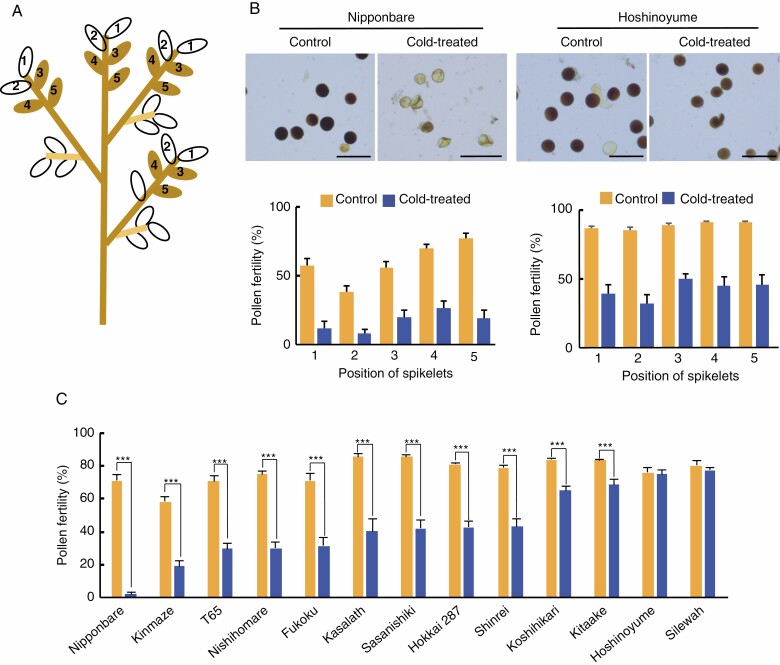
Nishiyama (1982) reported that the seed set rate varied as a function of spikelet position on the panicle. To examine whether pollen fertility might also differ with spikelet position along the panicle, we determined pollen fertility from the top five spikelets in every primary rachis branch from the two rice cultivars ‘Nipponbare’ and ‘Hoshinoyume’ (Fig. 1A). We recorded pollen fertility in control plants maintained in the greenhouse under standard growth conditions and in plants transferred to 12 °C for 4 d at the booting stage (Fig. 1B). Of the five spikelets, the first two from the tip showed relatively lower pollen fertility compared with the lower three spikelets under both normal and cold conditions (Fig. 1B). Therefore, in the remainder of this study we obtained pollen fertility data for the third, fourth and fifth spikelets in four or five primary rachis branches.
Fig. 1.
Evaluation of pollen fertility in 13 rice cultivars. (A) Spikelet positions along panicles used to test pollen fertility. Five spikelets from the top of the primary rachis branch were examined. The first and second spikelets have lower pollen fertility than the other spikelet positions in panicles. (B) Survey of pollen fertility at various spikelet positions in the primary rachis branch. Upper panels show representative images of pollen grains stained with KI-I2 solution from control and cold-treated plants of the cultivars ‘Nipponbare’ and ‘Hoshinoyume’. Lower panels show pollen fertility of spikelets at each of the five positions from control (yellow) and cold-treated (blue) ‘Nipponbare’ and ‘Hoshinoyume’ plants. Error bars represent standard error of the mean. Scale bars = 100 µm. (C) Pollen fertility of control (yellow) and cold-treated (blue) plants from the 13 selected cultivars. More than 15 spikelets were examined for each genotype and treatment. Significant statistical differences according to Student’s t-test are indicated: ***P < 0.001.
Next, we exposed 13 cultivars to the same low temperature of 12 °C for 4 d at the booting stage and compared the pollen fertility of control and cold-treated plants through the analysis of at least 15 spikelets from each primary rachis branch. The cold stress had contrasting effects on pollen fertility across the 13 cultivars tested, with fertility rates ranging from 3·9 to 77·4 % (Fig. 1C). Although cold treatment generally reduced pollen fertility, the cultivars ‘Silewah’, ‘Hoshinoyume’, ‘Kitaake’ and ‘Koshihikari’ appeared more tolerant of cold exposure than the other nine cultivars. The cultivars with varying pollen fertility that responded to the cold treatment were analysed to determine the relationship between anther fertility and pollen structure. There is substantial variability in the relationship between cold stress and pollen fertility between cultivars, confirming our suspicion that elucidating this relationship would require data from multiple cultivars.
Morphological abnormalities of anther locules at the booting stage
We examined and compared the morphology of the internal structure of anthers between control plants and plants exposed to low temperature (12 °C). A typical normal anther morphology at the booting stage is shown in Fig. 2A, B-1. This transverse section represents developing microspores in four anther locules surrounded by tapetum tissues forming a uniform layer. We collected at least 40 anthers from each of the 13 cultivars exposed to the low temperature and compared sections from these anthers (one section per anther) with the normal anther morphology. We observed several types of abnormalities in anther structures in sections from both control and cold-treated plants. Besides tapetum hypertrophy, other abnormalities classified as LRAs were detachment of the tapetum layer, undeveloped locules and empty locules (Fig. 2B). Tapetum detachment was characterized by the partial loss of contact between the tapetum layer and the epidermis layer. An undeveloped locule was defined as one whose development had arrested before the locule reached maturity, while an empty locule was defined as a collapsed locule with degenerated microspores. Comparisons of transverse sections from control and cold-treated plants for the 13 cultivars revealed that the incidence of abnormal anther structures increases immediately after cold treatment, particularly in several cold-sensitive cultivars, but the proportions of LRAs did not correlate precisely with pollen sterility (Fig. 2C, Supplementary Data Tables S1 and S2). Overall, most sections showed normal anther structures. However, samples collected from control plants at the booting stage did show some LRAs in anthers where microspores had been developing in all cultivars. Notably, however, in samples from cold-treated plants, 24·4 % of all sections exhibited some degree of aberrant morphology compared with 17·7 % in sections from control samples (Supplementary Data Table S3). Remarkably, nearly half of the anthers from the cultivars ‘Kinmaze’ and ‘Nishihomare’ had an abnormal structure even under control conditions, although their pollen fertility rates were 58 and 75 %, respectively (Supplementary Data Table S3).
Fig. 2.
Classification of anther morphologies at the booting stage. (A) Schematic representation of anther structure. (Left) Whole anther. (Right) Anther section showing two large locules and two small locules. The tapetum (purple) is located at the innermost layer of the anther. (B) The five internal anther structures at the booting stage defined in this study. (1) Normal structure (orange); (2) detachment of tapetum layer (green); (3) tapetum hypertrophy (red); (4) undeveloped locule (blue); and (5) empty locule (purple). The transverse sections were stained with 0.05 % toluidine blue. Red arrows indicate abnormal locules. Scale bars = 50 µm. Ta, tapetum layer; Mi, microspore. (C) Pie charts showing the proportions of the five anther structures among the 13 cultivars in control (left) and cold-treated (right) plants according to Supplementary Data Tables S1 and S2. The percentage below each pie chart indicates pollen fertility according to Fig. 1C. Anther structures are indicated by the colours defined in (B). For each cultivar, at least 40 samples (40 sections) were observed. Samples that did not belong to any of the five structures above are shown in white (Others).
The relative frequency of each type of abnormal anther structure was specific to each cultivar (Fig. 2C, Supplementary Data Table S2). For example, tapetum detachment was preferentially found in the cultivars ‘T65’ and ‘Nishihomare’, while undeveloped locules were a major structural defect in ‘Kinmaze’, ‘Nishihomare’, ‘Shinrei’, ‘Nipponbare’ and ‘Koshihikari’. Empty locules were frequently observed in cold-treated anthers from ‘Kinmaze’, ‘Fukoku’, ‘Sasanishiki’, ‘Hokkai 287’, ‘Shinrei’ and ‘Hoshinoyume’. Finally, tapetum hypertrophy was preferentially detected in cold-treated anthers from ‘Kinmaze’, ‘Nishihomare’, ‘Shinrei’ and ‘Kitaake’. Although tapetum hypertrophy has generally been proposed to be the major cause of pollen sterility due to cold stress at the booting stage, this type of LRA accounted for only 6 % of all cold-treated samples and for only 18.6 % even in the highly cold-sensitive cultivar ‘Nipponbare’ (Supplementary Data Table S3). These results imply that the relationship between tapetum morphology and fertility under cold stress is more complex than has previously been supposed.
Morphological anomalies of anther locules at the flowering stage
We repeated our microscopy analyses with anthers at the flowering stage prior to anthesis, using 15 anthers collected from control plants and 13 from cold-treated plants. The tapetum layer had disappeared from sections of normal anthers at this nearly mature stage, as expected (Fig. 3A, B-1). As previously described, we classified abnormal anther morphologies into four types: undeveloped locule and empty locule, as well as two new LRA types, designated here as tapetum degeneration retardation (tdr)-like locules and tiny locules (Fig. 3B) (Li et al., 2006). Mutants in the rice TDR gene are male-sterile and show the retardation of the tapetum degeneration that normally has occurred by the flowering stage, which results in a morphology that resembles tapetum hypertrophy at the booting stage (Li et al., 2006). The tdr-like phenotype previously observed is also found in anther locules at the flowering stage (Fig. 3B-2). Tiny locules are characterized by a small inner space in locules, which might result from the prevention of microspore development (Fig. 3B-3). Abnormal anther structures were also more frequent in samples from cold-treated plants than from the respective controls (Fig. 3C, Supplementary Data Tables S4–S6). However, ‘Silewah’, ‘Hoshinoyume’,’ Kitakake’, ‘Kasalath’ and ‘T65’ showed no abnormal structures in either set of samples. Interestingly, this group of cultivars included the two cold-sensitive cultivars ‘Kasalath’ and ‘T65’, along with three others that were among the most cold-tolerant cultivars. In eight cultivars (‘Fukoku’, ‘Nipponbare’, ‘Kinmaze’, Nishihomare, ‘Shinrei’, ‘Hokkai 287’ and ‘Koshihikari’), the LRA-type empty locule constituted the major abnormal anther morphology. We also documented incidences of tdr-like locules appearing in cold-sensitive cultivars, but with no clear influence from the cold treatment, as control anthers from these cultivars also showed the tdr-like phenotype. Overall, abnormal anther morphologies at the flowering stage are likely associated with those at the booting stage.
Fig. 3.
Classification of anther morphologies at the flowering stage. (a) Schematic representation of anther structure. (Left) Whole anther. (Right) Anther section showing two large locules and two small locules. The tapetum has degenerated at the flowering stage and therefore is no longer visible. (B) The five internal anther structures at the flowering stage defined in this study. (1) Normal structure (orange); (2) tiny locule (green); (3) tdr-like locule (red); (4) undeveloped locule (blue); (5) empty locule (purple). The transverse sections were stained with 0.05 % toluidine blue. Red arrows indicate abnormal locules. Scale bars = 50 µm. (C) Pie charts showing the proportions of the five internal anther structures among the 13 cultivars in control (left) and cold-treated (right) plants according to Supplementary Data Tables S4, S5. The percentage below each pie chart indicates pollen fertility according to Fig. 1C. For each cultivar, more than 13 samples (13 sections) and 15 samples (15 sections) were examined for non-treated and cold-treated samples, respectively. Anther structures are indicated by the colours defined in (B). Samples that did not belong to any of the five structures above are shown in white (Others).
Correlation between abnormal locules and cold stress
To investigate the relationship between the observed abnormal anther structures and pollen sterility, we explored the potential for correlation between pollen fertility and the incidence rate of abnormal structures in each cultivar. For both control and cold-treated samples, we obtained negative correlations (with Pearson’s correlation coefficient r around −0·6) between pollen fertility and the total number of abnormal structures in the 13 cultivars at the booting stage (Fig. 4A). However, the degree of correlation between each type of abnormality and pollen fertility varied, with values ranging from −0·64 to 0·03. Of the four abnormal locule structures, undeveloped locules in cold-treated plants were negatively correlated with measured pollen fertility across the 13 cultivars, with r = −0·64. By contrast, tapetum hypertrophy exhibited a low overall Pearson’s correlation coefficient (r = −0·34) with pollen fertility rates from the 13 cultivars under cold conditions. This latter result does not support the notion that tapetum hypertrophy is a major cause of pollen sterility in response to cold stress.
Fig. 4.
Relationships between abnormal anther structures and pollen fertility in control and cold-treated plants. (A, B) Scatter plots of incidence of each abnormal anther structure type as a function of pollen fertility in control and cold-treated samples from the 13 cultivars at the booting stage (A) and the flowering stage (B). Pearson’s correlation coefficient (r) is indicated in each panel. Significant correlations as determined by Student’s t-test are indicated: *P < 0.05; **P < 0.01.
At the flowering stage, the number of abnormal locules across the 13 cultivars was also negatively correlated with pollen fertility, with a Pearson’s correlation coefficient r = −0·59 for anthers collected from control plants and r = −0·66 for those of cold-treated plants (Fig. 4B). Of all Pearson’s correlation coefficients measured between pollen fertility and the four abnormal locules in control plants, the rate of empty locules exhibited the strongest correlation (r = −0·59). For cold-treated cultivars, empty locules and undeveloped locules also had significant Pearson’s correlation coefficients against pollen fertility, with r = −0·62 (significant at P < 0·05) and −0·76 (significant at P < 0·01), respectively. The incidences of the four LRAs at the booting or flowering stage had negative correlations with pollen fertility, but not tapetum hypertrophy.
Relationships of abnormal locule structures between the booting and flowering stages
We further examined the potential for associations of abnormal locule structures between the booting and flowering stages in the 13 cultivars (Fig. 5A). We determined these correlations by plotting the percentage of each abnormal locule structure out of all examined sections along the x-axis for the flowering stage and along the y-axis for the booting stage for each cultivar. Figure 5A summarizes all Pearson’s correlation coefficients from the 16 combinations between abnormal locule structures at the two stages. The strongest association was seen between undeveloped locules at the booting stage and empty locules at the flowering stage (undeveloped locule/empty locule; r = 0·96), followed by tapetum hypertrophy and empty locule (r = 0·80), undeveloped locule and undeveloped locule (r = 0·80), and tapetum hypertrophy and tdr-like locule (r = 0·75) (Fig. 5B). These results suggested that undeveloped locules at the booting stage may remain as empty, undeveloped locules at the flowering stage, while locules showing tapetum hypertrophy may differentiate into tdr-like locules and empty locules at the flowering stage (Fig. 5C). These strong associations also imply that abnormal locule structures at the booting stage form a developmental spectrum in a cultivar-dependent manner and are not corrected at the mature stage. However, the tapetum layer detachment and empty locules at the booting stage had little effect on the development of abnormal structures at the flowering stage. Thus, the two abnormalities detected at the booting stage have detectable effects on morphology at the flowering stage.
Fig. 5.
Correlations between abnormal anther structures at the booting and flowering stages. (A) Pearson’s correlation coefficients (r values) of 16 combinations between abnormal locule structures seen at the booting and flowering stages among 13 cultivars. Significant correlations as determined by Student’s t-test are indicated: **P < 0.01. (B) Scatterplots for the four significant correlations. (1) tapetum hypertrophy versus tdr-like locule; (2) undeveloped locule versus undeveloped locule; (3) tapetum hypertrophy versus empty locule; (4) undeveloped locule versus empty locule. (C) Schematic representation of the developmental links between abnormal locule structures from the booting to the heading stage.
Anther length and pollen fertility are correlated
We noticed that exposure to cold temperature tended to shorten anther length in multiple cultivars relative to control conditions (Fig. 6A). In particular, we noted significant differences for the six cultivars ‘Kitaake’, Koshihikari, ‘Hokkai 287’, ‘Kathalath’, ‘T65’ and ‘Kinmaze’. In addition, we observed a significant correlation between anther length and pollen fertility (r = 0·65) across the 13 cold-treated cultivars (Fig. 6B), although this positive correlation was also present, albeit less pronounced, in samples collected from control plants (r = 0·53) (Fig. 6C). This result suggests that anther length and pollen fertility are correlated regardless of cold exposure. In fact, the correlation between anther length and pollen fertility was r = 0·57 at P < 0.001 when all control and cold-treated samples were combined (Fig. 6D). However, we detected no correlation between the extent of anther shortening and the decrease in pollen fertility due to cold stress (Fig. 6E). Therefore, it was anther length rather than the degree of anther shortening that had a significant effect on pollen fertility. Abnormal anther locule morphologies also affected anther length, as the percentages of abnormal locules at both the booting and the flowering stages were negatively correlated with anther length, with r = −0·78 and r = −0·79, respectively (Fig. 6F, G). In the control samples, similar negative correlations were observed at the booting and flowering stages (Supplementary Data Fig. S1). In particular, undeveloped locules and tapetum hypertrophy at the booting stage showed a significant correlation with anther length (r < −0·65), while undeveloped locules and empty locules were significantly correlated with anther length at the flowering stage (r < −0·75) (Supplementary Data Fig. S2).
Fig. 6.
Association between anther length and pollen fertility in the 13 cultivars tested. (A) Anther lengths of control (yellow) and cold-treated (blue) plants. Error bars represent standard error of the mean. Significant differences according to t-test are indicated: *P < 0.05; ***P < 0.001. (B, C) Correlations between pollen fertility and anther length among cold-treated (B) and control plants (C). (D) Correlation between pollen fertility and anther length among all plants. (E) Correlation between the rate of decrease in pollen fertility and the anther atrophy rate in response to cold treatment. (F) Correlations between incidence of abnormal locules at the booting stage and anther length in cold-treated plants. (G) Correlation between incidence of abnormal locules at the flowering stage and anther length in cold-treated plants. Significant correlations according to Student’s t-test are indicated: *P < 0.05; **P < 0.01.
Morphological changes in whole anthers due to cold treatment
Among the 13 cultivars, we selected five (‘Nipponbare’, ‘Hoshinoyume’, ‘T65’, ‘Kasalath’ and ‘Koshihikari’) in which to perform morphological observations of the entire anther by serial sectioning. Among these cultivars, ‘Nipponbare’ is highly susceptible to cold, while ‘Hoshinoyume’ is highly cold-tolerant, and the remaining three cultivars represent a range of intermediate cold sensitivities. ‘T65’ and ‘Kasalath’ had structurally normal anthers in both control and cold conditions at the booting and flowering stages, but exhibited relatively lower pollen fertility after cold treatment compared with cultivars with similar anther structures. We also included the cultivar ‘Koshihikari’ because, although it is generally considered cold-tolerant, its anthers showed a relatively high proportion of abnormal structures. Since we grew these germplasms independently from the first set of experiments described above, we determined their pollen fertility again (Fig. 7A). The results with these five cultivars were largely congruent with those from the previous experiments, with ‘Nipponbare’ displaying a higher pollen fertility after cold treatment than ‘Kasalath’, whereas ‘Kasalath’ had shown a much lower pollen fertility rate in cold-treated plants earlier.
Fig. 7.
Morphological alterations in whole anthers in response to cold treatment in five cultivars. (A) Pollen fertility of control (yellow) and cold-treated (blue) plants of five cultivars (from at least nine anthers per cultivar and condition). (B) Representative anther shapes: straight (left) and curved (right). The degree of anther curvature was measured as the angle (∠θ) between the three points shown. Scale bars = 250 µm. (C) Effect of cold treatment on anther angles. Control (yellow) and cold-treated (blue) plants from five cultivars. Throughout, error bars represent the standard error of the mean, and significant differences between control and cold-treated plants as determined by Student’s t-test are indicated: *P < 0.05; **P < 0.01; ***P < 0.01.
When examined under a stereoscopic microscope, anthers presented one of two shapes: straight or curved (Fig. 7B). The curved anthers tended to be associated with atrophied small locules. To quantify this effect, we defined an anther angle formed by three points consisting of each end and the centre of the anther. In ‘Hoshinoyume’, a cold-tolerant cultivar, the average anther angle was comparable between control and cold-treated plants and was close to 180°, as expected for straight anthers. By contrast, exposure to cold temperature reduced the anther angle in the cultivars ‘Koshihikari’ and ‘T65’. Interestingly, anthers from control ‘Nipponbare’ plants were the most curved, with an average angle of 143°, while cold exposure increased the angle to an average of 163°, consistent with a straightening of the anther under these conditions (Fig. 7C).
Localization of abnormal structures in anther locules
To examine the structures of entire locules, we generated contiguous serial transverse sections of control and cold-treated anthers from the five selected cultivars. In previous analyses, each anther was represented by a single transverse section. However, one anther is unlikely be formed from uniform sections from end to end, but rather from sections with various shapes. Accordingly, we made contiguous transverse sections every 7 μm from the tip to the base of an anther, resulting in an average of 65 transverse sections. For each cultivar, we excised six anthers from three spikelets (two anthers per spikelet) for each condition (Fig. 8A). These contiguous anther sections presented a mix of structures corresponding to normal and abnormal locules. Locules of normal shape were by far the most common, while the proportion of sections with abnormal structures varied as a function of the cultivar and the growth condition (Fig. 8B, Supplementary Data Table S7). As expected, abnormal anther structures were less frequent in control plants than in plants exposed to cold treatment. At the booting stage, the cold-tolerant cultivar ‘Hoshinoyume’ showed few abnormalities throughout all analysed sections even after cold treatment. By contrast, in the other cold-tolerant cultivar, ‘Koshihikari’, cold treatment greatly increased LRAs, to a rate of ~20 %, compared with 5 % in anthers from control plants. The percentage of abnormal sections seen in ‘Koshihikari’ after cold treatment was comparable to that of ‘Nipponbare’. Notably, the two cultivars most sensitive to cold, ‘T65’ and ‘Kasalath’, almost completely lacked abnormal structures, even after cold treatment. We only observed tapetum hypertrophy in ‘Nipponbare’, but this accounted for only 3 % of abnormalities seen in cold-treated plants. Empty and undeveloped locules were more frequent in cold-treated anthers from ‘Nipponbare’ and ‘Koshihikari’ than tapetum hypertrophy.
Fig. 8.
Characterization of transverse sections of whole anther structures. (A) Representative photographs of continuous transverse sections from four areas of anthers. Anthers from control and cold-treated plants at the booting and flowering stages were collected and processed from the cultivars ‘Nipponbare’ and ‘Kasalath’. The illustration of anther structure on the right indicates the four areas analysed. Red arrows indicate aberrant locules. Scale bars = 50 µm. (B) Average incidence rates of abnormal anther structures for five cultivars. Anthers from control (N) and cold-treated (C) plants at the booting and flowering stages were collected and processed (Supplementary Data Table S7). The different colours in each column refer to aberrant anther structures, as indicated. The pollen fertilities in each cultivar indicated by %.
At the flowering stage, exposure to cold affected locule abnormalities to the same extent as at the booting stage. Anthers from the cultivars ‘Hoshinoyume’, ‘Koshihikari’, ‘T65’ and ‘Kasalath’ largely exhibited normal structures after cold treatment, whereas abnormal locule structures in ‘Nipponbare’ rose from 10 % in control anthers to nearly 20 % after cold treatment. This increase in locule abnormality rate observed in ‘Nipponbare’ was due to empty locules and undeveloped locules.
The contiguous anther sections revealed the preferential position of abnormalities within the vertical axis of the anther (summarized in Fig. 9). We focused on cold-treated anthers from ‘Nipponbare’ and ‘Koshihikari’, in which LRAs were often observed. We defined three regions along each anther: the upper, middle and lower regions. As each anther has four locules, organized as two large and two small locules, we further divided each anther into upper, middle and lower areas, for a total of six regions; we then scored the number of normal and abnormal locules in each of these regions. Abnormal structures in the anthers of ‘Nipponbare’ and ‘Koshihikari’ showed a preference for the middle region of large locules and the upper and bottom areas of small locules, at both the booting and flowering stages (Fig. 9A, B, Supplementary Data Tables S8–S11). By contrast, we identified no obvious abnormalities in the other three areas at either stage (Fig. 9A, B, Supplementary Data Tables S8–S11). Abnormal locule structures in control anthers were mainly detected in the upper and lower areas of small locules (Supplementary Data Fig. S3, Tables S8–S11). These results suggested that the morphological abnormalities in rice anthers likely accumulate in three regions: the middle area of the large locule and the upper and lower areas of the small locule.
Fig. 9.
Frequency of abnormal structures along anther locules from cold-treated plants in ‘Nipponbare’ and ‘Koshihikari’. (A) Abnormal structures of anther locules at the booting stage. Anthers from cold-treated plants were prepared for contiguous transverse sections from tip to base. Anthers consist of two sets of large and small locules and were divided into six areas (1, 2 and 3 for large locules; 4, 5 and 6 for small locules). Pie charts indicate the frequency of each of the five locule structures based on Supplementary Data Table S9. (B) Abnormal structures of anther locules at the flowering stage based on Supplementary Data Table S11.
Temperature effect on pollen number
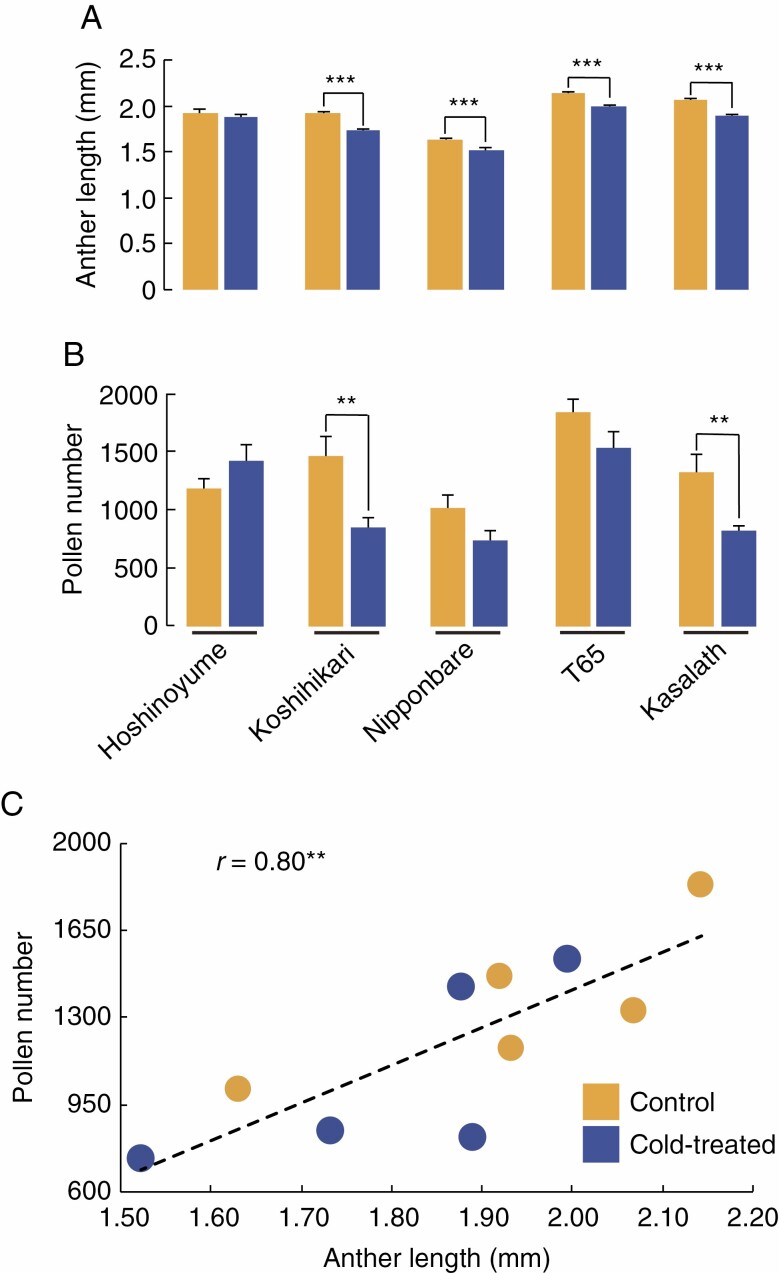
The number of pollen grains (including fertile and sterile pollen) and the anther length decreased across all cultivars in response to cold treatment, with the exception of ‘Hoshinoyume’, which showed a slight increase in pollen number (Fig. 10A, B). Pollen number varied among the five cultivars, although ‘Koshihikari’ and ‘Kasalath’ both showed a significant decrease in pollen number upon cold treatment. Indeed, pollen number in ‘Koshihikari’ plants exposed to cold was only 57·8 % of that measured in control plants (Fig. 10B). However, pollen fertility in ‘Koshihikari’ remained >60 % even after cold treatment, whereas ‘Nipponbare’, ‘T65’ and ‘Kasalath’ had pollen fertility rates of <30 % (Fig. 7A). Therefore, as the total number of pollen grains declined after cold treatment, we hypothesize that fertile pollen in ‘Koshihikari’ survived well, whereas fertile pollen in ‘Nipponbare’, ‘T65’ and ‘Kasalath’ degenerated. As mentioned above, anther lengths were shortened by cold treatment in the four cultivars other than ‘Hoshinoyume’. The number of pollen grains also decreased (Fig. 10A, B). When considering all the anthers from control and cold-treated plants, we obtained a high Pearson’s correlation coefficient (r = 0·80) between anther length and pollen number (Fig. 10C). Thus, it is likely that pollen number in rice is dependent on the anther size.
Fig. 10.
Anther length and pollen number are positively correlated. (A) Anther length from control (yellow) and cold-treated (blue) plants for each of the five cultivars (from at least 46 anthers per genotype and treatment). Error bars represent the standard error of the mean, and significant differences between control and cold-treated plants as determined by Student’s t-test are indicated: **P < 0.01; ***P < 0.001. (B) Pollen number of control (yellow) and cold-treated (blue) plants for each of the five cultivars. (C) Scatter plot between anther length and pollen number. Anther length and pollen number are positively correlated in both control (yellow) and cold-treated (blue) plants.
DISCUSSION
Revising the importance of tapetum hypertrophy in pollen sterility at low temperature
Sakai’s reports in 1943 have contributed to the notion that tapetum hypertrophy is a major structural symptom of anthers with sterile pollen after exposure to low temperatures at the booting stage in rice. Zhang et al. (2021) noted that most studies related to tapetum hypertrophy were associated with pollen abortion. Tapetum hypertrophy is thought to result from an accumulation of sucrose, the degradation of which is stalled in the tapetum layer, or from the accumulation of digested sucrose that then cannot be transported to pollen (Mamun et al., 2006). The rice genes involved in the digestion of sucrose (INVERTASE4 [OsINV4]) and transportation of digested sucrose to pollen (MONOSACCHARIDE TRANSPORTER8 [OsMST8]) have been identified (Oliver et al., 2005; Mamun et al., 2006). However, Nishiyama (1970, 1976) pointed out that tapetum hypertrophy is not observed as frequently as would be expected if that were solely responsible for all cases of pollen sterility. Here, we examined 13 cultivars with varying levels of pollen fertility upon exposure to cold. Our results agree with Nishiyama’s statements (Nishiyama, 1970, 1976) and show that tapetum hypertrophy is not a typical characteristic of cold-sensitive cultivars. In fact, we noticed that pollen abortion was often, but not always, associated with various structural changes in anthers. A careful examination of anther sections from 13 cultivars identified eight types of LRAs, including tapetum hypertrophy. The Pearson’s correlation coefficient between tapetum hypertrophy and pollen fertility was r = −0·35, while that between overall LRA and pollen fertility was r = −0·6 (Fig. 4A), indicating that tapetum hypertrophy, though having some effect, is not the sole type of lesion explaining pollen sterility following cold treatment, and that LRA as an overall category contributes more strongly to pollen sterility than tapetum hypertrophy alone.
Relationship between abnormal locule structures and their localizations in anthers
Previous studies compared cold responses for a limited number of cultivars with discrete sensitivities to cold stress (Oliver et al., 2005; Oda et al., 2010). Although such comparative analyses of one tolerant and one susceptible cultivar were useful, these comparisons did not capture the breadth of cold sensitivity among rice cultivars. Here, we showed that there is a broad range of pollen sterility among the 13 rice cultivars that we tested, which may be classified as a mixture of cold-tolerant (four cultivars) and cold-sensitive (nine cultivars). Most of the cold-sensitive cultivars exhibited increased numbers of anthers with abnormal morphology after cold treatment, with the exception of the cultivars ‘T65’ and ‘Kasalath’, which had relatively few malformations affecting anther structure and thus resembled cold-tolerant cultivars in that regard. In the other seven cold-sensitive cultivars, a higher incidence of LRAs after cold treatment at either the booting or the flowering stage was correlated with pollen sterility, although the proportions of abnormal anther morphologies did not clearly predict pollen sterility (Fig. 4). The structural abnormalities in cold-treated plants measured here might be underestimated (Figs 2C and 3C). Indeed, the percentage of pollen sterility in cold-sensitive cultivars appeared to be lower than the proportion of anthers with abnormal locules. Even the most cold-sensitive cultivar, ‘Nipponbare’, displayed 4 % pollen fertility, with abnormal locule structures accounting for no more than 40 % of all sections. One possible reason for this effect is illustrated in Fig. 8, which shows that LRAs are exclusively distributed in three areas of a given anther: the centre region of large locules and the upper and lower areas of small locules. This specific regional accumulation of LRAs might keep the proportion of abnormal sections throughout an anther below 50 %. These three areas may be associated with abnormal pollen development and the appearance of lesions in response to cold stress. The LRAs themselves might be a phenotypic consequence of cold exposure, but not a cause of pollen abortion. How and why anther morphological abnormalities are concentrated in these three areas are questions that remain to be resolved.
Pollen sterility links with LRAs in the booting and flowering stages
We classified locule morphologies observed in anthers into eight types at the booting and flowering stages. Lesions in locules due to cold damage provided quantifiable phenotypes in response to cold sensitivity. As mentioned above, tapetum hypertrophy is the only morphological anther abnormality that has been analysed in relation to pollen sterility. Nishiyama (1976) analysed anther morphological changes caused by cold injury using electron microscopy and classified them into four types, all of which were reported as morphologies derived from tapetum hypertrophy.
Many studies on anther morphological abnormalities have focused on tapetum hypertrophy. However, in this study of 13 cultivars, we observed morphological abnormalities that are clearly distinguishable from tapetum hypertrophy. We divided LRAs into four types each at the booting and flowering stages (Figs 2B and 3B). Among these morphological abnormalities, the type of anther malformation at the booting stage that was most likely to be related to pollen sterility was undeveloped locules (Fig. 4A). Similarly, undeveloped and empty locules contribute to pollen sterility at the flowering stage and may derive directly from undeveloped locules from the earlier stage (Figs 4B and 5B, C). Our results also suggested that tapetum hypertrophy at the booting stage may lead to the formation of tdr-like locules and empty locules at the flowering stage (Fig. 5B, C). Determining the exact relationship between LRAs at the booting and flowering stages based on the measured correlation coefficients requires further investigation.
There have been few reports investigating the changes in anther locules during development. Understanding these changes, which are closely related to pollen development, will be important to clarify the causes of pollen sterility. The genetic control of LRA formation is likely to be complex, as can be inferred from the large collection of genes and quantitative trait loci (QTLs) involved in cold tolerance at the booting stage (Li et al., 1997; Saito et al., 2001, 2004, 2010; Takeuchi et al., 2001; Andaya and Mackill, 2003; Liu et al., 2003; Dai et al., 2004; Oh et al., 2004; Xu et al., 2008; Kuroki et al., 2009; Suh et al., 2010; Ye et al., 2010; Shirasawa et al., 2012; Zhu et al., 2015; Endo et al., 2016; Shimono et al., 2016; Fukushima et al., 2017; Zhang et al., 2017; Xiao et al., 2018).
Distinct locule structures and possible processes leading to pollen sterility and reduction
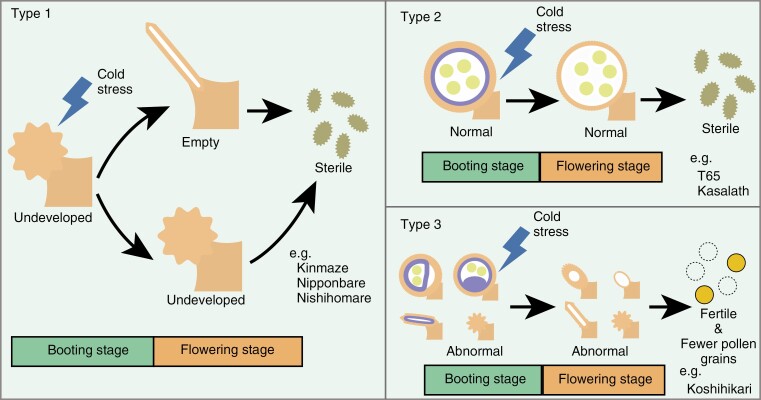
In this study, we found that anthers from 13 rice cultivars showed a variety of shape abnormalities due to cold stress at the booting stage. Overall, we defined three types of abnormalities in relation to pollen sterility (Fig. 11). One type of abnormal anther is characterized by undeveloped locules at the booting stage, leading to undeveloped and empty locules at the flowering stage that were concentrated in the lower part of the anther locule (Fig. 11, Type 1). Anther morphological abnormalities were reported by Satake (1976) to occur frequently in small anther locules. This observation is consistent with the fact that we observed undeveloped locules at both ends of the anther locule, a region that tends to be at a later developmental stage than other regions (Fig. 9). The second type of abnormal anther has a normal appearance at both stages, but produces sterile pollen (Fig. 11, Type 2). Cultivars ‘T65’ and ‘Kasalath’ are examples of this type of abnormality, producing few fertile pollen grains. It seemed clear that other damage due to low temperature, rather than the anther structure as such, causes this decrease in pollen fertility. For example, cold stress might prevent proper synthesis of sucrose, which is normally synthesized outside the anther and is necessary for pollen development (Saini and Westgate, 2000), and low sucrose levels could severely hinder pollen development (Thakur et al., 2010). The third type of abnormal anther is exemplified by cultivar ‘Koshihikari’, which produces ~60 % fertile pollen grains in spite of an overall decrease in total pollen grains due to cold stress, thus ensuring cold tolerance (Fig. 11, Type 3).
Fig. 11.
Three possible scenarios leading to pollen sterility as a function of anther morphology upon cold stress at the booting stage. (Type 1) Cold stress results in undeveloped anther locules at the booting stage, leading to empty or undeveloped locules at the flowering stage and, thus, sterile pollen. Type 1 is found in typical cold-sensitive cultivars such as ‘Kinmaze’, ‘Nipponbare’ and ‘Nishihomare’. (Type 2) After exposure to cold stress at the booting stage, gross anther morphology remains largely intact at both the booting and flowering stages, but the pollen grains are mostly sterile. We identified cultivars ‘T65’ and ‘Kasalath’ as examples of Type 2. (Type 3) Cultivars show frequencies of anther abnormalities that are comparable to those of other cold-sensitive cultivars, and pollen number is reduced, but the remaining pollen is fertile. ‘Koshihikari’ belongs to Type 3.
Validity of multivalent causes of locule anomalies from gene expression analyses
The results obtained in this study suggest that multivalent factors are involved in anther morphological abnormalities linked to pollen sterility caused by cold stress during the booting stage in rice. In fact, several gene expression analyses showed that different networks were associated with pollen sterility due to cold stress. Mamun et al. (2010) described that during anther development in rice, cold stress perturbs the expression of several genes involved in sucrose breakdown/degradation and sucrose transport, leading to excess sucrose accumulation in the endothecium or the tapetum. In anthers, the concentrations of several phytohormones change during pollen development (Hirano et al., 2008). For example, low temperatures lead to increased abscisic acid production and reduced gibberellic acid production in rice anthers (Oliver et al., 2007; Sakata et al., 2014). These two phytohormones act antagonistically during growth and development; hence, a loss of coordination between them due to low temperatures might result in abnormal anther development. Each locule abnormality is considered to be such a signature to disrupt normal developments of the pollen and anther structure. Therefore, our study provided clues to the causes of cold sensitivity in rice pollen and anther development in future.
CONCLUSIONS
Many studies on injury in response to cold exposure at the booting stage have consistently correlated anther morphological abnormalities (particularly tapetum hypertrophy) with pollen sterility in rice. These studies have compared a limited number of genotypes with contrasting cold tolerance and sensitivity. Our study of 13 cultivars exhibiting a spectrum of pollen sterility helped define the different anther structures that can be perturbed in response to cold stress. We identified one type of LRA at the booting stage that is connected to LRA at the flowering stage and may lead to pollen sterility. The frequency of LRAs was globally correlated with pollen sterility, whereas the frequency of tapetum hypertrophy was not. However, LRAs might represent a phenotypic consequence of the cold response, without being a direct cause of pollen abortion, as we found LRA frequencies to be far lower than pollen sterility frequencies. Interestingly, LRAs accumulated in specific regions within anthers, suggesting that the proportion of LRAs may be maintained below 50 %. We propose three possible relationships to explain anther structure and pollen sterility and reduction due to cold stress. Our comprehensive analysis revises the relationships between anther histological structures and pollen sterility, suggesting that different types of cold responses may occur depending on the genetic makeup specific to each cultivar.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: anther length and incidence of abnormal locules are negatively correlated among 13 cultivars. Figure S2: relationship between anther length and each type of abnormal locules observed among 13 cultivars. Figure S3: frequency of abnormal structures along anther locules from control ‘Nipponbare’ and ‘Koshihikari’ plants. Table S1: numbers of each of the six locule structures in the control samples from the 13 cultivars at the booting stage. Table S2: numbers of each of the six locule structures in the cold-treated samples from the 13 cultivars at the booting stage. Table S3: average percentage incidences of the six locule structures in the control and cold-treatment samples from the 13 cultivars at the booting stage. Table S4: numbers of each of the six locule structures in the control samples from the 13 cultivars at the flowering stage. Table S5: numbers of each of the six locule structures in the cold-treated samples from the 13 cultivars at the flowering stage. Table S6: average percentage incidences of the six locule structures in the control and cold-treatment samples from the 13 cultivars at the flowering stage. Table S7: percentage of the five locule structures distributed in a whole anther from the five cultivars. Table S8: numbers of each of the five locule structures distributed in three parts of a whole anther at the booting stage in the five control cultivars. Table S9: numbers of each of the five locule structures distributed in three parts of a whole anther at the booting stage in the five cold-treated cultivars. Table S10: numbers of each of the five locule structures distributed in three parts of a whole anther at the flowering stage in the five control cultivars. Table S11: numbers of each of the five locule structures distributed in three parts of a whole anther at the flowering stage in the five cold-treated cultivars.
ACKNOWLEDGEMENTS
We thank Mr Y. Ota, Mr S. Kikuchi, Mr Y. Kanaoka and Dr D. Kuniyoshi for their assistance and valuable suggestions.
FUNDING
This work was supported by grants from JSPS KAKENHI (no. 19H00937 to Y.Ki.), the Hokkaido University Research and Education Center for Robust Agriculture, Forestry and Fisheries Industry (to Y.Ki.) and the Scientific Technique Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (to Y.Ki.).
LITERATURE CITED
- Andaya VC, Mackill DJ. 2003. QTLs conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica x indica cross. Theoretical and Applied Genetics 106: 1084–1090. [DOI] [PubMed] [Google Scholar]
- Dai LY, Lin XH, Ye CR, et al. 2004. Identification of quantitative trait loci controlling cold tolerance at the reproductive stage in Yunnan landrace of rice, Kunmingxiaobaigu. Breeding Science 54: 253–258. [Google Scholar]
- Endo T, Chiba B, Wagatsuma K, et al. 2016. Detection of QTLs for cold tolerance of rice cultivar ‘Kuchum’ and effect of QTL pyramiding. Theoretical and Applied Genetics 129: 631–640. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Hayashi T, Ohta H, Kaji R, Yokogami N, Tsuda N. 2017. Effects of the number of pollen grains on cold tolerance at the booting stage in rice lines with QTLs for cold tolerance. Plant Production Science 20: 149–155. [Google Scholar]
- Hayase H, Satake T, Nishiyama I, Ito N. 1969. Male sterility caused by cooling treatment at the meiotic stage in rice plants: 2. The most sensitive stage to cooling and the fertilizing ability of pistils. Japanese Journal of Crop Science 38: 706–711. [Google Scholar]
- Hirano K, Aya K, Hobo T, et al. 2008. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant & Cell Physiology 49: 1429–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N. 1978a. Male-sterility caused by cooling treatment at young microspore stage in rice plants. 16. Changes in carbohydrates, nitrogenous and phosphorus-compounds in rice anthers after cooling treatment. Japanese Journal of Crop Science 47: 318–323. [Google Scholar]
- Ito N. 1978b. Male-sterility caused by cooling treatment at young microspore stage in rice plants. 17. Changes in carbohydrates, nitrogenous and phosphorus-compounds in rice anthers during cooling treatment. Japanese Journal of Crop Science 47: 324–329. [Google Scholar]
- Kuroki M, Saito K, Matsuba S, et al.. 2009. Quantitative trait locus analysis for cold tolerance at the booting stage in a rice cultivar, Hatsushizuku. JARQ – Japan Agricultural Research Quarterly 43: 115–121. [Google Scholar]
- Li HB, Wang J, Liu AM, Liu KD, Zhang QF, Zou JS. 1997. Genetic basis of low-temperature-sensitive sterility in indica-japonica hybrids of rice as determined by RFLP analysis. Theoretical and Applied Genetics 95: 1092–1097. [Google Scholar]
- Li N, Zhang DS, Liu HS, et al. 2006. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18: 2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FX, Sun CQ, Tan LB, Fu YC, Li DJ, Wang XK. 2003. Identification and mapping of quantitative trait loci controlling cold-tolerance of Chinese common wild rice (O. rufipogon Griff.) at booting to flowering stages. Chinese Science Bulletin 48: 2068–2071. [Google Scholar]
- Mamun EA, Alfred S, Cantrill LC, Overall RL, Sutton BG. 2006. Effects of chilling on male gametophyte development in rice. Cell Biology International 30: 583–591. [DOI] [PubMed] [Google Scholar]
- Mamun EA, Cantrill LC, Overall RL, Sutton BG. 2010. Mechanism of low-temperature-induced pollen failure in rice. Cell Biology International 34: 469–476. [DOI] [PubMed] [Google Scholar]
- Matsushima S. 1957. Analysis of developmental factors determining yield and yield prediction in lowland rice. Bulletin of the National Institute of Agricultural Sciences in Japan A5: 1–271. [Google Scholar]
- Nishiyama I. 1970. Male sterility caused by cooling treatment at the young microspore stage in rice plants: 7. Electron microscopical observations on tapetal cells dilated by the cooling treatment. Japanese Journal of Crop Science 39: 480–486. [Google Scholar]
- Nishiyama I. 1976. Male sterility caused by cooling treatment at the young microspore stage in rice plants: 12. Classification of tapetal hypertrophy on the basis of ultrastructure. Japanese Journal of Crop Science 45: 254–264. [Google Scholar]
- Nishiyama I. 1978. Male-sterility caused by cooling treatment at young microspore stage in rice plants. 18. Some enzyme-activities in anthers during and after cooling. Japanese Journal of Crop Science 47: 551–556. [Google Scholar]
- Nishiyama I. 1982. Male-sterility caused by cooling treatment at the young microspore stage in rice plants. 22. A method to predict sterility just after the young microspore stage. Japanese Journal of Crop Science 51: 386–392. [Google Scholar]
- Niu NN, Liang WQ, Yang XJ, et al.. 2013. EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nature Communications 4: 1445. [DOI] [PubMed] [Google Scholar]
- Oda S, Kaneko F, Yano K, et al. 2010. Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes & Genetic Systems 85: 107–120. [DOI] [PubMed] [Google Scholar]
- Oh CS, Choi YH, Lee SJ, Yoon DB, Moon HP, Ahn SN. 2004. Mapping of quantitative trait loci for cold tolerance in weedy rice. Breeding Science 54: 373–380. [Google Scholar]
- Oliver SN, Van Dongen JT, Alfred SC, et al. 2005. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell and Environment 28: 1534–1551. [Google Scholar]
- Oliver SN, Dennis ES, Dolferus R. 2007. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant & Cell Physiology 48: 1319–1330. [DOI] [PubMed] [Google Scholar]
- Pacini E, Franchi GG, Hesse M. 1985. The tapetum – its form, function, and possible phylogeny in Embryophyta. Plant Systematics and Evolution 149: 155–185. [Google Scholar]
- Saini HS, Westgate ME. 2000. Reproductive development in grain crops during drought. Advances in Agronomy 68: 59–96. [Google Scholar]
- Saito K, Miura K, Nagano K, Hayano-Saito Y, Araki H, Kato A. 2001. Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theoretical and Applied Genetics 103: 862–868. [Google Scholar]
- Saito K, Hayano-Saito Y, Maruyama-Funatsuki W, Sato Y, Kato A. 2004. Physical mapping and putative candidate gene identification of a quantitative trait locus Ctb1 for cold tolerance at the booting stage of rice. Theoretical and Applied Genetics 109: 515–522. [DOI] [PubMed] [Google Scholar]
- Saito K, Hayano-Saito Y, Kuroki M, Sato Y. 2010. Map-based cloning of the rice cold tolerance gene Ctb1. Plant Science 179: 97–102. [Google Scholar]
- Sakai K. 1943. Zytologisch-histologische Untersuchungen uber die Sterilitatserscheinungen bei Reispflanzen Nordnippons im Jahre 1941. Hokkaido Prefectural Agricultural Experiment Station Report 40: 17. [Google Scholar]
- Sakata T, Oda S, Tsunaga Y, et al. 2014. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiology 164: 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake T. 1972. Circular dense-culture of rice plants in pots, the purpose of obtaining many uniform panicles of main stems. Japanese Journal of Crop Science 41: 2. [Google Scholar]
- Satake T. 1976. Determination of the most sensitive stage to sterile type cool injury in rice plants. Research Bulletin of the Hokkaido National Agricultural Experiment Station 113: 1–44. [Google Scholar]
- Satake T, Hayase H. 1970. Male sterility caused by cooling treatment at the young microspore stage in rice plants: 5. Estimations of pollen developmental stage and the most sensitive stage to coolness. Japanese Journal of Crop Science 39: 468–473. [Google Scholar]
- Satake T, Nishiyama I, Ito N, Hayase H. 1969. male sterility caused by cooling treatment at the meiotic stage in rice plants: 1. Methods of growing rice plants and inducing sterility in the phytotron. Japanese Journal of Crop Science 38: 603–609. [Google Scholar]
- Shimono H, Hasegawa T, Moriyama M, Fujimura S, Nagata T. 2005. Modeling spikelet sterility induced by low temperature in rice. Agronomy Journal 97: 1524–1536. [Google Scholar]
- Shimono H, Hasegawa T, Iwama K. 2007. Modeling the effects of water temperature on rice growth and yield under a cool climate: I. Model development. Agronomy Journal 99: 1327–1337. [Google Scholar]
- Shimono H, Abe A, Aoki N, et al. 2016. Combining mapping of physiological quantitative trait loci and transcriptome for cold tolerance for counteracting male sterility induced by low temperatures during reproductive stage in rice. Physiologia Plantarum 157: 175–192. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Endo T, Nakagomi K, Yamaguchi M, Nishio T. 2012. Delimitation of a QTL region controlling cold tolerance at booting stage of a cultivar, ‘Lijiangxintuanheigu’, in rice, Oryza sativa L. Theoretical and Applied Genetics 124: 937–946. [DOI] [PubMed] [Google Scholar]
- Suh JP, Jeung JU, Lee JI, et al. 2010. Identification and analysis of QTLs controlling cold tolerance at the reproductive stage and validation of effective QTLs in cold-tolerant genotypes of rice (Oryza sativa L.). Theoretical and Applied Genetics 120: 985–995. [DOI] [PubMed] [Google Scholar]
- Suzuki S. 1978. Anther and pollen abnormalities induced by cold treatment and their varietal differences in rice plants. Japanese Journal of Breeding 28: 21–32. [Google Scholar]
- Suzuki S. 1981. Cold tolerance in rice plants with special reference to the floral characters. I. Varietal differences in anther and stigma lengths and the effects of planting densities on these characters. Japanese Journal of Breeding 31: 57–64. [Google Scholar]
- Takeuchi Y, Hayasaka H, Chiba B, et al.. 2001. Mapping quantitative trait loci controlling cool-temperature tolerance at booting stage in temperate japonica rice. Breeding Science 51: 191–197. [Google Scholar]
- Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H. 2010. Cold stress effects on reproductive development in grain crops: an overview. Environmental and Experimental Botany 67: 429–443. [Google Scholar]
- Xiao N, Gao Y, Qian H, et al. 2018. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant Physiology 177: 1108–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LM, Zhou L, Zeng YW, et al.. 2008. Identification and mapping of quantitative trait loci for cold tolerance at the booting stage in a japonica rice near-isogenic line. Plant Science 174: 340–347. [Google Scholar]
- Ye C, Fukai S, Godwin ID, et al.. 2010. A QTL controlling low temperature induced spikelet sterility at booting stage in rice. Euphytica 176: 291–301. [Google Scholar]
- Zhang ZY, Li JJ, Pan YH, et al. 2017. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nature Communications 8: 14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZB, Hu MH, Xu WW, et al.. 2021. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Molecular Biology 105: 1–10. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Chen K, Mi XF, et al.. 2015. Identification and fine mapping of a stably expressed QTL for cold tolerance at the booting stage using an interconnected breeding population in rice. PLoS ONE 10: 0145704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.